Abstract
Background and Purpose:
The corticospinal tract (CST) is a crucial brain pathway for distal arm and hand motor control. We aimed to determine whether a diffusion tensor imaging (DTI)-derived CST metric predicts distal upper extremity (UE) motor improvements in chronic stroke survivors.
Methods:
We analyzed clinical and neuroimaging data from a randomized controlled rehabilitation trial. Participants completed clinical assessments and neuroimaging at baseline and clinical assessments four months later, post-intervention. Using univariate linear regression analysis, we determined the linear relationship between the DTI-derived CST fractional anisotropy asymmetry (FAasym) and the percentage of baseline change in log-transformed average Wolf Motor Function Test time for distal items (ΔlnWMFT-distal_%). The Least Absolute Shrinkage and Selection Operator (LASSO) linear regressions with cross-validation and bootstrapping were used to determine the relative weighting of CST FAasym, other brain metrics, clinical outcomes, and demographics on distal motor improvement. Logistic regression analyses were performed to test whether the CST FAasym can predict clinically significant UE motor improvement.
Results:
lnWMFT-distal significantly improved at the group level. Baseline CST FAasym explained 26% of the variance in ΔlnWMFT-distal_%. A multivariate LASSO model including baseline CST FAasym, Age, and UE Fugl-Meyer explained 39% of the variance in ΔlnWMFT-distal_%. Further, CST FAasym explained more variance in ΔlnWMFT-distal_% than the other significant predictors in the LASSO model.
Discussion and Conclusions:
CST microstructure is a significant predictor of improvement in distal UE motor function in the context of an UE rehabilitation trial in chronic stroke survivors with mild-to-moderate motor impairment.
Keywords: stroke rehabilitation, diffusion tensor imaging, corticospinal tract, prediction modeling, chronic stroke, upper extremity motor function
Introduction
Stroke gives rise to long-term disability.1 Distal arm and hand motor function improvement after stroke mainly occurs during the acute and early subacute phases (i.e., within three months after stroke).2 Although several clinical studies have shown distal upper extremity (UE) motor improvement through behavioral and therapeutic interventions during the chronic phase,3–5 results from large-scale clinical trials in these interventions are controversial.6 Experts in stroke rehabilitation research have suggested using biomarkers for selecting and targeting specific patient populations to investigate specific interventions.6,7 Thus, developing an accurate biomarker for chronic stroke is essential to improve the efficacy of rehabilitation clinical trials,7 leading to better interventions for chronic stroke survivors.8
Diffusion tensor imaging (DTI)-derived metrics of sensorimotor pathways are promising biomarkers of stroke recovery.8–11 Recent review articles have suggested that DTI-derived metric of ipsilesional corticospinal tract (CST) is a crucial biomarker of upper extremity motor recovery after stroke,9,12–14 given that the CST is a crucial descending motor pathway for voluntary distal limb motor control.15,16 Specifically, fractional anisotropy (FA) is a reliable DTI metric as a summary measure of white matter microstructural characteristics.17–19 While FA change is not specific to a certain microstructural change,17 decreased DTI-derived FA value of ipsilesional CST may indicate CST microstructural damage.20–24 Previous clinical studies have reported a significant correlation between FA of ipsilesional CST and UE motor impairment in individuals post-stroke.25–28 Longitudinal clinical studies have also shown that the ipsilesional CST FA is a significant predictor for post-stroke UE motor improvement across different recovery phases.13,25,29–36
While evidence supports DTI-derived CST FA as a predictor for UE motor recovery during early phases after stroke, we lack evidence that ipsilesional CST FA is a significant predictor of distal arm and hand motor improvement in chronic stroke survivors, specifically those with mild-to-moderate motor impairment. Previous DTI studies with chronic stroke survivors primarily targeted individuals with severe-to-moderate UE motor impairment10,36,37 and utilized heterogeneous DTI quantification methods.18,38,39
Therefore, our primary aim is to determine whether or not a DTI-derived CST metric can predict improvement in distal arm and hand motor performance after four months of variable dose motor training in chronic stroke survivors who exhibit mild-to-moderate motor impairment. Our secondary aim is to determine which combination of predictors explains the most variance in distal UE motor improvement in this population.
Methods
Participants.
Clinical and neuroimaging data were from a single-site phase IIb randomized rehabilitation trial conducted at the University of Southern California (ClinicalTrials.gov ID: NCT01749358). We utilized clinical and neuroimaging data from 37 of the 42 participants – those who had a full complement of analyzable clinical and neuroimaging data. Details of participant inclusion and exclusion criteria are described in the primary outcome paper.5 Participants were on average three years post-stroke and exhibited mild-to-moderate motor impairment40 (Upper Extremity Fugl-Meyer Score range 25 to 58 with at least some voluntary control of wrist and finger extensors). More details on participant characteristics are in the supplemental document.
The experimental protocol.
Detailed experimental protocol of the parental trial is described in the primary outcome paper.5 With relevance to this project, we utilized clinical and neuroimaging data at baseline (time point 1, T1) and post-therapy (time point 2, T2) time points from the parental trial. There were three 1-week bouts of training with a 3-week wait period between each bout and then a post-test (T2) immediately after the third training bout. The T1 to T2 interval was approximately four months. Participants were randomly assigned to one of four groups that varied in the total number of scheduled motor training hours – 1) active monitoring control group; 2) 15-hour group; 3) 30-hour group; 4) 60-hour group. Details of the UE motor practice program, the Accelerated Skill Acquisition Program (ASAP), are described elsewhere.41,42 In addition to the motor training sessions, all participants engaged in six 2-hour long assessment sessions between T1 and T2: scheduled before and after each of the three 1-week training bouts. Each assessment session included a comprehensive set of upper extremity sensorimotor assessments and biomechanical tests of goal-directed arm reaching movements (~200 arm reaches/assessment session).
Clinical motor outcome measure.
We employed the log-transformed average time for the distal control items of the Wolf Motor Function Test (lnWMFT-distal) as the primary outcome measure of distal arm motor performance.43,44 We utilized nine distal control items of WMFT (i.e., the integrative functional tasks) that require both proximal joint control and some level of hand dexterity for object manipulation.45 We chose this outcome measure for three reasons: 1) our sample included those with primarily mild-to-moderate motor impairment whose deficits were primarily in hand dexterity, 2) CST has a crucial role in distal arm and hand function,46,47 and 3) proximal joint control tasks have temporal ceiling effects, especially in our cohort.48 More details on this outcome measure are available in the supplemental document.
We also employed the Upper Extremity Fugl-Meyer Assessment (UEFM) as a well-accepted measure of UE motor impairment.
MRI acquisition.
We described the MRI acquisition procedure previously.38 Briefly, we used a 3 Tesla GE Signa Excite MRI scanner to acquire high-resolution structural MRI and standard research-quality DTI. The total scan time of these MRI sequences was approximately 20 minutes. Supplemental Material includes the detailed MRI acquisition parameters.
MRI data analysis.
Our previous methods paper describes details of the MRI data analysis.38 Briefly, the T1-weighted and diffusion-weighted images were processed using BrainSuite software (http://brainsuite.org/).
We identified the CST from the tractography results for each hemisphere. We defined CST tractography as streamlines passing through the cerebral peduncle (CP), pons on the same side, and originating from M1, primary sensory cortex, or supplementary motor area. We excluded any commissural fibers and fibers projecting to the cerebellum. The first author (BK) visually inspected each CST tractography for accuracy.
DTI-based CST microstructure quantification.
We utilized a 3-dimensional individual CST tractography-based quantification method (Supplemental Figure 2). This method was chosen based on our previous study’s results comparing seven different methods to estimate CST microstructure.38 Using the CST tractography of each participant, 3-D tractography-based CST volume of interest (VOI) was used to calculate the average FA for each side’s entire CST. Then, we computed the FA asymmetry index [(contralesional CST FA – ipsilesional CST FA) / (contralesional CST FA + ipsilesional CST FA)] in order to control for inter-individual variability in FA.37
Other brain structural imaging-derived metrics.
We quantified three other brain structural imaging metrics representing the degree of structural damage to the brain motor system. We included these brain structural metrics in the regression analyses to determine the DTI-derived CST metric’s predictive value compared to these other imaging-derived metrics. These markers include CST-lesion overlap volume,49 Lateral ventricle volume asymmetry (LVA),50 and stroke lesion fractional anisotropy (Lesion FA).51 Supplemental Material includes the details of these metrics and the rationale for choosing them.
Statistical analysis.
Change in motor behavior.
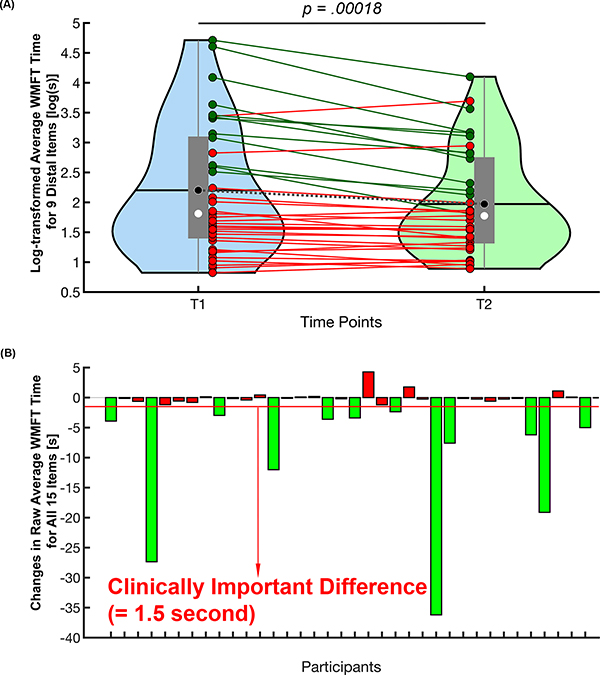
We used a paired T-test to test if there was a statistically significant change in the log-transformed average WMFT-distal time (lnWMFT-distal) between T1 and T2. A one-sample Kolmogorov-Smirnov test 52 confirmed a normal distribution of the motor outcome data at each time point. Further, we examined if each participant had a clinically important difference (CID) in the change in average WMFT time for the entire fifteen-item battery. For our purposes, CID was defined as a decrease in the average raw WMFT time for the fifteen items more than 1.5 seconds, a published minimal clinically important difference (MCID) for the WMFT.53 Given that there is no published MCID for the 9-item distal WMFT, we used that for the 15-item WMFT battery.
Primary Aim. Determine the Relationship between baseline CST metric and change in motor behavior.
We employed simple linear regression analysis to test if baseline CST FA asymmetry can predict motor behavior changes. CST FA asymmetry was the predictor variable of the regression. The dependent variable was the change in log-transformed average WMFT-distal time – a percentage of baseline log-transformed average WMFT-distal time (ΔlnWMFT-distal_%). We checked the linearity assumptions, residual normality, and homoscedasticity by inspecting the case order plot of Cook’s distance and the Q-Q-plot. We identified two influential data points via Cook’s distance value greater than three times the mean Cook’s distance (Supplemental Figure 3).54 So, we refit the model without these data points. We present results from both models with and without influential data. Importantly, we ruled out the effect of training dose on our DTI findings by performing a multiple linear regression with three predictors: CST FA asymmetry, Dose groups, and the interaction between those two variables on ΔlnWMFT-distal_% (See Supplemental Figure 6 for more details).
Secondary Aim: Determine the most significant neuroimaging and clinical predictors of motor improvement using LASSO regression.
We utilized LASSO (least absolute shrinkage and selection operator) linear regression with leave-one-out cross-validation and bootstrapping to regulate and select the best set of variables and enhance prediction accuracy. Predictor variables included age,55 chronicity,10 baseline UEFM,56 CST FA asymmetry,37 lesion FA,51 lateral ventricle asymmetry (LVA),50 CST-lesion overlap volume,49 and Dose group. All variables were analyzed as continuous variables. We chose these variables for two reasons: 1) selected variable had been tested and found to be significant predictors of post-stroke motor recovery, or 2) selected variable is associated with significant stroke brain pathology related to motor impairment. We used z-transformation to standardize all continuous predictor variables. The dependent variable was ΔlnWMFT-distal_%.
Given that the LASSO linear regression does not handle categorical predictors, we performed a separate multiple linear regression to test whether adding a sex variable to the model would improve model accuracy.
We used the beta coefficients of selected variables from the LASSO linear regression to compare the relative impact of neuroimaging and clinical predictors. The beta coefficient indicates how much the dependent variable changes for every one unit of change in the predictor variable.57 Further, we performed simple linear regression analyses for each of the surviving predictor variables to determine how much variance in ΔlnWMFT-distal_% can be explained independently by each of the significant predictor variables.
In addition to linear regressions, we also performed logistic regression analyses for predicting clinically important improvement in average WMFT time for the entire fifteen-item battery (see Supplemental Materials for details of Regression Analyses).
Results
Change in motor behavior.
At the group level, there was a significant decrease in log-transformed average WMFT-distal time at T2 compared to the T1 time point, (t(36)=4.16, p=.00018, Cohen’s d=0.684. Table 1 and Figure 1A). Twelve out of thirty-seven participants (~33%) demonstrated a clinically important difference in average WMFT time for the entire 15-item battery (Figure 1B).
Table 1.
Group Level Demographic and Clinical Characteristics (N=37)
| Characteristics [unit or categories] | Mean (range [min – max]) / Counts |
|---|---|
| Age [years] | 59.43 (30 – 80) |
| Sex [Male/Female] | 27/10 |
| Chronicity [years] | 3.01 (0.5 – 14.4) |
| Hand dominance [Rt/Lt] | 34/3 |
| Affected Hemisphere [Rt/Lt] | 18/19 |
| Lesion Volume [mm3] | 19,841 (40 – 136,180) |
| CST-Lesion Overlap Volume [% of CST Volume] | 4.59 (0 – 25.8) |
| Upper Extremity Fugl-Meyer | 43.49 (25 – 58) |
| Baseline Raw Average Wolf Motor Function Test Time for Nine Distal Items [sec] | 17.37 (2.28 – 111.29) |
| Baseline Log-Transformed Average Wolf Motor Function Test Time for Nine Distal Items [log(sec)] | 2.20 (0.82 – 4.71) |
| Baseline Raw Average Wolf Motor Function Test Time for All Fifteen Items [sec] | 10.74 (1.51 – 87.54) |
| Baseline Log-Transformed Average Wolf Motor Function Test Time for All Fifteen Items [sec] | 1.72 (0.41 – 4.47) |
| 4-Month Post Raw Average Wolf Motor Function Test Time for Nine Distal Items [sec] | 10.99 (2.44 – 60.36) |
| 4-Month Post Log-Transformed Average Wolf Motor Function Test Time for Nine Distal Items [log(sec)] | 1.97 (0.89 – 4.10) |
| 4-Month Post Raw Average Wolf Motor Function Test Time for All Fifteen Items [sec] | 7.27 (1.55 – 51.35) |
| 4-Month Log-Transformed Average Wolf Motor Function Test Time for All Fifteen Items [log(sec)] | 1.51 (0.44 – 3.94) |
Figure 1.
The difference in average WMFT time between baseline (T1) and 4-month post-baseline (T2). (A) Group difference in log-transformed average WMFT time for 9 distal control items between 2 time points. The central gray boxes are the interquartile ranges; black horizontal lines and black circles are the means; white circles are the medians; the gray whiskers above and below the boxes are the locations of the minimum and maximum values; the violin plot indicates the distribution of data for each time point. Each individual solid line indicates each participant’s change in log-transformed average WMFT for distal control items (dark green lines—participants who had clinically important difference (CID) in average WMFT time for all 15 items; red lines—participants who did not show CID). The dotted gray line represents the group mean change in log-transformed average WMFT for distal control items. (B) The CID in average WMFT time for all 15 items. Green bars indicate those participants who demonstrated WMFT CID. Note that we used the CID of raw average WMFT time score from the 15-item battery, as there is no CID defined for log-transformed average WMFT time for the 9 distal control items. WMFT indicates Wolf Motor Function Test. This figure is available in color online (www.jnpt.org).
The linear relationship between baseline CST FA asymmetry and change in motor behavior.
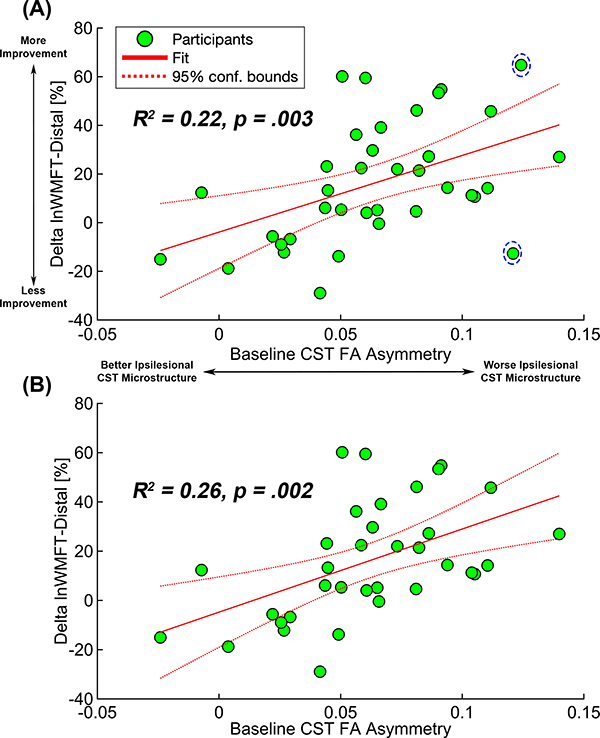
Baseline CST FA asymmetry significantly predicted a change in motor behavior. Baseline CST FA asymmetry alone explained 22% of the variance in ΔlnWMFT-distal_% (F(1,35)=10.085, p=.0031, Figure 2A). This linear regression model’s diagnostic tests showed two influential data points (Supplemental Figure 4). In the linear regression analysis excluding those two data points, baseline CST FA asymmetry alone explained 26% of the variance in ΔlnWMFT-distal_% (F(1,33)=11.4, p=.002, Figure 2B).
Figure 2.
Linear regression between baseline CST FA asymmetry and Δ WMFT-distal_%. Green dots indicate individual data points of participants, the solid red line is the fit of the linear regression between baseline CST FA asymmetry and changes in log-transformed average WMFT for distal control items, and the dotted red lines indicate 95% confidence interval of the fit. (A) A regression model with all 37 participants. Two participants in the dashed circle were determined as influential data points. (B) In the regression model with 35 participants, 2 influential data points marked in (A) were excluded. CST FA indicates corticospinal tract fractional anisotropy; WMFT, Wolf Motor Function Test. This figure is available in color online (www.jnpt.org).
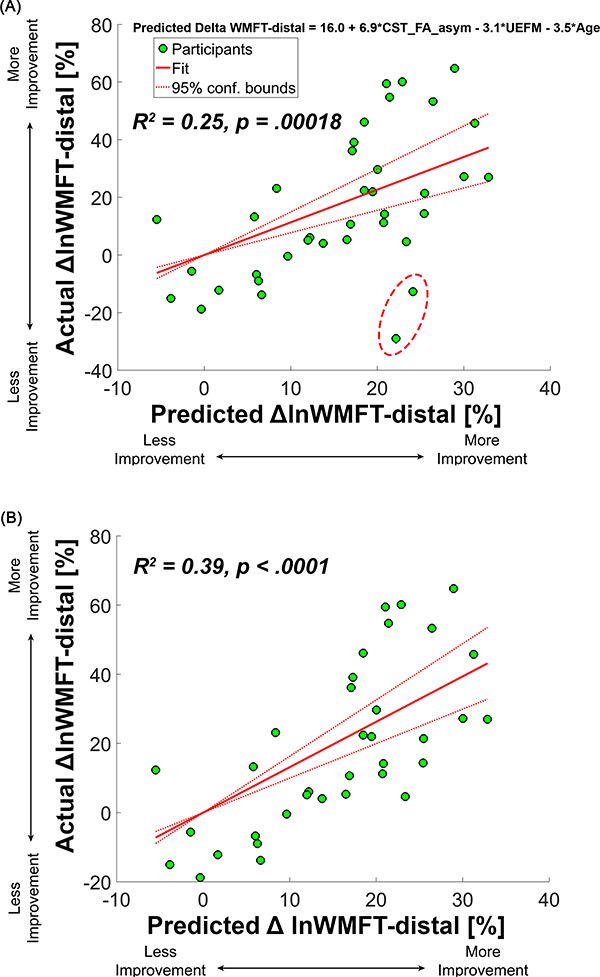
Multimodal prediction model from LASSO regression.
A model, including baseline CST FA asymmetry, UEFM, and Age, is the most accurate for predicting ΔlnWMFT-distal_%. The predicted ΔlnWMFT-distal_% from the LASSO regression model explained 25% of the variance in the actual ΔlnWMFT-distal_% (95% confidence interval of R2=0.23–0.26, p=.00018, Figure 3A). Given that two participants were considered outliers based on statistical rules (See Supplemental Figure 8), we performed the regression between the predicted ΔlnWMFT-distal_% and actual ΔlnWMFT-distal_% without the two outliers. Explained variance increased to 39% (p<.0001, Figure 3B). Predicted ΔlnWMFT-distal_% from LASSO linear regression was calculated as follows:
Figure 3.
Regression between predicted Δ WMFT-distal_% and actual Δ WMFT-distal_% from the Lasso regression model. (A) A regression model with all 37 participants. Two participants in the dashed circle were determined as outliers. (B) In the regression model with 35 participants, 2 influential data points (PID 20 and 34) marked in (A) were excluded. This figure is available in color online (www.jnpt.org).
The beta coefficients of selected predictor variables from the LASSO linear regression were 6.9, −3.1, and −3.5 for CST FA asymmetry, UEFM, and Age, respectively (see above formula). CST FA asymmetry’s beta coefficient absolute value was about two times greater than the other two variables’ beta coefficient absolute value. Baseline UEFM alone explained 15% of the variance in ΔlnWMFT-distal_% (F(1,35)=6.19, p=.018). Baseline Age alone explained 12% of the variance in ΔlnWMFT-distal_% (F(1,35)=4.83, p=.035).
A separate multiple linear regression model including CST FA asymmetry, UEFM, Age, and Sex explained 53.6% of the variance in the actual ΔlnWMFT-distal_% (F(4,30)=8.66, p<.001, Supplemental Figure 9). However, the coefficient of the Sex variable in this multiple regression model was not significant (t=0.697, p=.49). Sex variable alone explained 17% of the variance in ΔlnWMFT-distal_% (F(1,35)=7.24, p=.01) in a simple linear regression between the sex and ΔlnWMFT-distal_%.
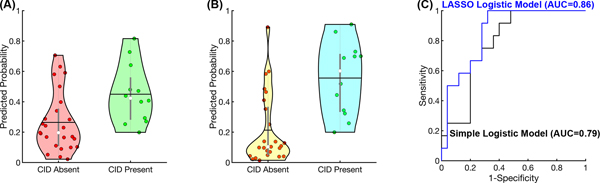
The logistic regressions also showed that baseline CST FA asymmetry, baseline UEFM, and Age were significant predictors for the presence of a CID in average WMFT time for the entire fifteen-item battery (Figure 4).
Figure 4.
Simple and LASSO logistic regression models for prediction of the presence of WMFT CID. (A) Simple logistic regression results. Predicted probability was calculated from the baseline CST FA asymmetry. The central gray boxes are the interquartile ranges; black horizontal lines are the means; white circles are the medians; the gray whiskers above and below the boxes are the locations of the minimum and maximum values, except outliers; the violin plot indicates the distribution of data for each time point. (B) LASSO logistic regression results. Predicted probability was calculated from 3 variables selected by LASSO logistic regression: baseline CST FA asymmetry, UEFM, and age. (C) Receiver operating characteristic (ROC) curves of simple and LASSO logistic regression models. Black line indicates the ROC curve of the simple logistic regression model; blue line indicates the ROC curve of the LASSO logistic regression model. CID indicates clinically important difference; CST FA, corticospinal tract fractional anisotropy; LASSO, least absolute shrinkage and selection operator; UEFM, Upper Extremity Fugl-Meyer Assessment; WMFT, Wolf Motor Function Test. This figure is available in color online (www.jnpt.org).
Discussion
This study provides evidence that DTI-derived CST FA asymmetry predicts distal UE motor improvement at the chronic stage in response to an UE behavioral intervention and/or six two-hour assessments in individuals with post-stroke mild-to-moderate motor impairment. Thus, we believe our findings support the significance of CST microstructure in predicting distal UE motor improvement in chronic stroke survivors.
Relationship between CST microstructure and motor improvement.
Our findings indicate that chronic stroke survivors with worse ipsilesional CST microstructure at baseline (i.e., greater CST FA asymmetry) had greater motor improvement, with at least a minimum amount of practice than individuals with better CST microstructure.
Stroke damage to the CST leads to cell death, axonal damage, and demyelination of corticospinal neurons (i.e., pyramidal cells from ipsilesional M1) during the acute and early subacute phases.58,59 Insufficient ipsilesional CST microstructure will portend more limited restitution of distal arm and hand motor control. Defective CST microstructure at the chronic stage is indicative of fewer cortico-spinal projections, the poor transmission of the efferent drive from M1,60 and fewer residual motor neurons in ipsilesional M1 that can undergo experience-dependent functional and structural plasticity (i.e., reorganization of the hand representation area of the ipsilesional primary motor cortex and axonal sprouting at the spinal cord). Thus, we expected individuals with milder CST microstructural damage at baseline would show more distal UE motor improvement after four months, a finding reported by previous studies.26,36 However, the pattern of our results was opposite to our expectation and inconsistent with previous studies.
Lindenberg and colleagues36 reported that chronic stroke survivors with greater fractional anisotropy of the ipsilesional pyramidal tract (i.e., better ipsilesional pyramidal tract microstructure) at baseline showed a greater change in the WMFT after a five-day non-invasive brain stimulation and rehabilitation therapy program. Similarly, Borich and colleagues26 found that chronic stroke individuals with less FA asymmetry (i.e., better ipsilesional CST microstructure) of the posterior limb of the internal capsule demonstrated greater motor learning capability following UE training.
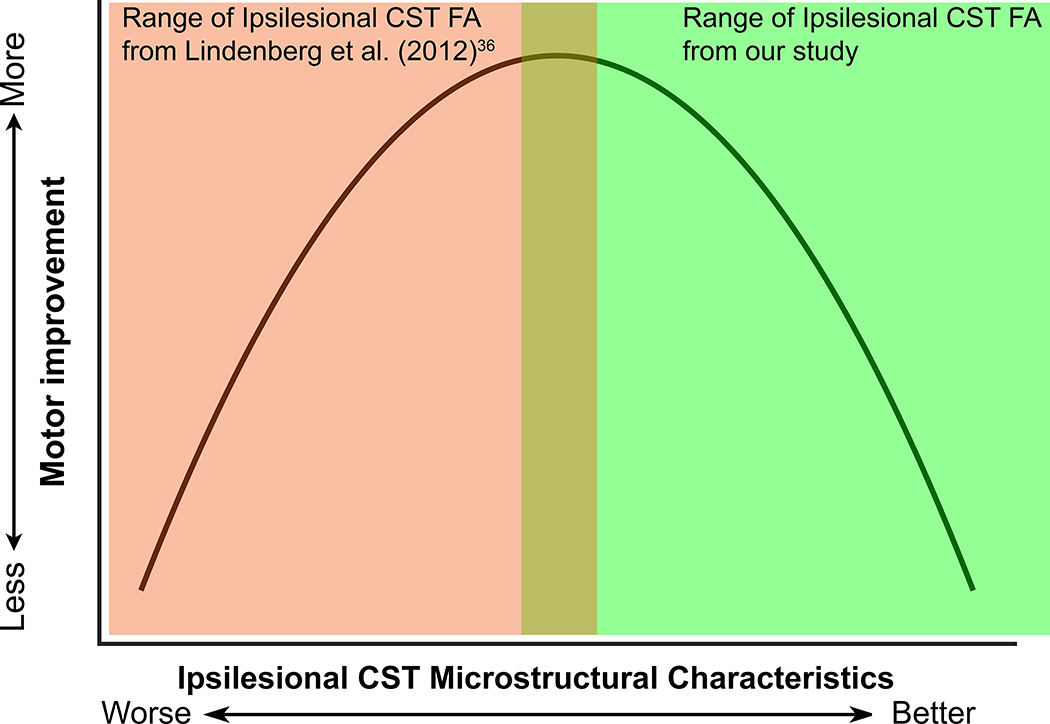
We believe that this inconsistency is, first and foremost, a function of the different range of motor severity in each cohort. Specifically, Lindenberg and colleagues36 recruited fifteen chronic stroke survivors with moderate to severe motor impairment (Medical Research Council [MRC] strength grade of ≤ 3/5 in the hemiparetic arm extensor muscles), whereas our sample included individuals with predominantly mild-to-moderate motor impairment (UEFM score range 25 to 58; mean 43.5).
A possible explanation of our finding would be that people with moderate CST impairment (i.e., worse CST microstructure) may not have reached their maximal recovery potential, a potential that could be tapped by either the ASAP intervention or the multiple assessment sessions. Alternatively, it is also possible that individuals with moderate CST impairment had reached their maximum recovery potential well before study participation and subsequently declined in upper extremity performance at the time of the baseline assessment, possibly due to non-use. We cannot rule this possibility out, as we have no motor performance assessments made at the sub-acute stage. In contrast, those with milder impairment are closer to their full capacity and, therefore, might express little to no improvement even with multiple assessments and motor practice. Combining results from Lindenberg’s study with ours along a hypothetical motor severity continuum, we envision a non-linear relationship (inverted “U” shape) between CST microstructure status and motor improvement in chronic stroke survivors (Figure 5). This non-linear relationship suggests a specific range of impairment in chronic stroke survivors that is more responsive to interventions that promote deliberate practice than others. A recent study by Senesh and Reinkensmeyer46 reported such an inverted “U” shape relationship between baseline Fugl-Meyer score (i.e., impairment) and changes in Fugl-Meyer score after a robotic movement training in people with chronic stroke. They found that people with moderate motor impairment (UEFM motor score between 22 and 40) are more responsive to the intensive UE movement training than those with mild or severe motor impairment (i.e., those on the ends of this inverted “U” shape). This UEFM motor score range matches our participants who had greater CST FA asymmetry and a greater improvement in distal UE motor performance.
Figure 5.
A hypothesized inverted “U” shape relationship between CST structural damage and motor improvement in chronic stroke survivors. Green shaded area indicates the relationship between DTI-derived ipsilesional CST metric and motor improvement from this study; orange shaded area indicates the relationship between DTI-derived ipsilesional CST metric and motor improvement from Lindenberg et al.36 CST indicates corticospinal tract; DTI, diffusion tensor imaging. This figure is available in color online (www.jnpt.org).
A better understanding of this relationship may inform more precise screening procedures that use diffusion imaging to target the people who have the most potential for improvement at the chronic stage.
LASSO regression with cross-validation and bootstrapping informed a multimodal prediction model.
The LASSO regression with cross-validation and bootstrapping is advantageous in that it avoids overfitting and multicollinearity of the model,61 and as such, this approach aligns well with recent recommendations.13 We used LASSO regression to directly compare a DTI-derived CST metric’s relative impact to other relevant brain structural imaging metrics and clinical predictors.
Using these state-of-the-art statistical analysis methods, we demonstrate that baseline CST FA asymmetry was the most important variable among several clinical and neuroimaging variables in predicting UE motor improvement in response to ASAP practice and/or intensive UE motor assessments in chronic stroke survivors. This finding is consistent with previous findings both in acute and chronic stroke that indicate CST structural damage is one of the most critical factors associated with motor impairment after stroke.13,62 In addition to the DTI-derived CST metric, age, and UEFM comprised the other significant predictors of motor improvement in this population. This result is also consistent with previous findings that age and UE motor impairment impact UE motor improvement in chronic stroke survivors.63,64
A separate multiple regression model, including four predictors – CST FA asymmetry, age, UEFM, and sex, explained more variance in the change in distal motor function than the LASSO linear regression model. However, the coefficient of the sex variable in the model was not significant. Further, as the multiple linear regression does not regulate multicollinearity, there would likely be an inflation of the R-squared value in this model.57
Study limitations.
This study has several limitations. First, we did not account for the effects of repeated intensive UE motor assessments. Participants in the 0-hour dose group (active monitoring control group) showed significant paretic UE motor function improvement after four months. We speculate that this results from repeated UE motor assessments, each consisting of approximately 200 goal-directed paretic arm reaching movements and several other sensorimotor assessments. All these motor outcome assessments require participants to use their paretic arm and hand. Thus, we believe this high volume of UE motor assessments may explain the UE motor improvement in the 0-hour dose group. The high volume of UE motor assessments may enable participants to use and focus attention on their paretic arm and hand. This fact confounds the effects of ASAP training on UE motor improvement in our participants. Alternatively, we cannot rule out the possibility that the zero-dose group’s improvement may represent a placebo effect.65
Second, we employed a subset of a time-based clinical motor outcome measure that lacks information about how participants achieved motor improvement—the quality of movement. Distal upper extremity motor performance should be assessed in various domains, including clinical outcome measures and kinematic measures, given that post-stroke individuals can achieve motor improvement by learning different movement strategies.66
Third, there are several other potential predictors of motor improvement in chronic stroke survivors that we did not consider here. Given that our final prediction model only explains 38% of the variance in distal UE motor function, other potential predictors should be included to improve the model’s accuracy. These variables would include, but are not limited to, CST functional integrity measured by transcranial magnetic stimulation (TMS),8 DTI-derived metrics of other white matter fibers, such as corpus callosum,11 brain functional imaging markers,62 advanced diffusion imaging markers,33,67 brain-derived neurotrophic factor (BDNF) polymorphism,68 and social-cognitive psychological factors. Given that this is a secondary data analysis of an existing rehabilitation trial dataset not intended to develop a predictive model, these promising predictive variables were not available. Thus, future prospectively designed studies with a larger and more varied sample and including these additional predictor variables may improve the predictive model in chronic stroke survivors.
Conclusions
Using robust brain imaging and statistical methods, we provide evidence that DTI-derived CST FA asymmetry is a significant predictor of distal UE motor improvement for chronic stroke survivors with mild-to-moderate motor impairment. We would caution against generalizing these findings to post-stroke individuals in the acute/subacute stage or chronic stroke survivors with severe motor impairment, given that our participants were limited to individuals in the chronic stage post-stroke and with predominantly mild-to-moderate motor impairment. Further, we would caution against using the CST metric to make predictions for individual chronic stroke survivors. Future studies with a larger sample size and more extensive inclusion of potential predictors are needed across a broader range of impairment levels to directly test the relationship between CST microstructure and upper extremity motor improvement in chronic stroke survivors. Understanding this relationship would inform us about who will more likely have distal UE motor improvement after motor practice among chronic stroke survivors.
Future studies are also needed to explore the possibility that CST microstructural changes represent a potential neural mechanism underlying distal UE motor improvement in chronic stroke survivors. Determining the role of microstructural plasticity of the CST in distal UE motor improvement after motor practice may guide the development of novel rehabilitation interventions to improve UE motor function.
Supplementary Material
Acknowledgments
We thank Drs. James Gordon and Beth Fisher for their invaluable comments and suggestions on an earlier version of this manuscript, and Dr. Nerses Sanoosian for confirming lesion location on the MRI images.
Source of Funding:
National Institute of Neurological Disorders and Stroke of the National Institutes of Health under R01 HD065438 and R56 NS100528.
Footnotes
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil Neural Repair. 2017;31(9):793–799. [DOI] [PubMed] [Google Scholar]
- 3.Kelly K, Brander F, Strawson A, Ward N, Hayward K. Pushing the limits of recovery in chronic stroke survivors: a descriptive qualitative study of users perceptions of the Queen Square Upper Limb Neurorehabilitation Programme. BMJ Open. 2020;10(10):e036481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer SC, Le V, Saver JL, et al. Intense Arm Rehabilitation Therapy Improves the Modified Rankin Scale Score: Association Between Gains in Impairment and Function. Neurology. Published online February 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winstein C, Kim B, Kim S, Martinez C, Schweighofer N. Dosage Matters. Stroke. 2019;50(7):1831–1837. [DOI] [PubMed] [Google Scholar]
- 6.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348–360. [DOI] [PubMed] [Google Scholar]
- 7.Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12(5):480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stinear CM, Byblow WD, Ackerley SJ, Barber PA, Smith M-C. Predicting Recovery Potential for Individual Stroke Patients Increases Rehabilitation Efficiency. Stroke. 2017;48(4):1011–1019. [DOI] [PubMed] [Google Scholar]
- 9.Puig J, Blasco G, Schlaug G, et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology. 2017;59(4):343–351. [DOI] [PubMed] [Google Scholar]
- 10.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. [DOI] [PubMed] [Google Scholar]
- 11.Mang CS, Borich MR, Brodie SM, et al. Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clin Neurophysiol. 2015;126(10):1959–1971. [DOI] [PubMed] [Google Scholar]
- 12.Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol. 2017;16(10):826–836. [DOI] [PubMed] [Google Scholar]
- 13.Kim B, Winstein C. Can Neurological Biomarkers of Brain Impairment Be Used to Predict Poststroke Motor Recovery? A Systematic Review. Neurorehabil Neural Repair. 2017;31(1):3–24. [DOI] [PubMed] [Google Scholar]
- 14.Moura LM, Luccas R, Paiva JPQ de, et al. Diffusion Tensor Imaging Biomarkers to Predict Motor Outcomes in Stroke: A Narrative Review. Front Neurol. 2019;10(May):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemon RN, Johansson RS, Westling G. Modulation of corticospinal influence over hand muscles during gripping tasks in man and monkey. Can J Physiol Pharmacol. 1996;74(4):547–558. [PubMed] [Google Scholar]
- 16.Lemon RN, Baker SN, Davis JA, Kirkwood PA, Maier MA, Yang HS. The importance of the cortico-motoneuronal system for control of grasp. Sens Guid Mov. 1998;218:202–218. [DOI] [PubMed] [Google Scholar]
- 17.Alexander AL, Hurley SA, Samsonov AA, et al. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connect. 2011;1(6):423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis AF, Myers M, Heiser J, Kolar M, Baird JF, Stewart JC. Test–retest reliability and minimal detectable change of corticospinal tract integrity in chronic stroke. Hum Brain Mapp. 2020;41(9):2514–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zolkefley MKI, Firwana YMS, Hatta HZM, et al. An overview of fractional anisotropy as a reliable quantitative measurement for the corticospinal tract (CST) integrity in correlation with a Fugl-Meyer assessment in stroke rehabilitation. J Phys Ther Sci. 2021;33(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15(4):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. [DOI] [PubMed] [Google Scholar]
- 22.Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. [DOI] [PubMed] [Google Scholar]
- 23.Shereen A, Nemkul N, Yang D, et al. Ex Vivo Diffusion Tensor Imaging and Neuropathological Correlation in a Murine Model of Hypoxia–Ischemia-Induced Thrombotic Stroke. J Cereb Blood Flow Metab. 2011;31(4):1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L, et al. Brain-Derived Neurotrophic Factor Administration Mediated Oligodendrocyte Differentiation and Myelin Formation in Subcortical Ischemic Stroke. Stroke. 2015;46(1):221–228. [DOI] [PubMed] [Google Scholar]
- 25.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30(11):3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borich MR, Brown KE, Boyd L a. Motor skill learning is associated with diffusion characteristics of white matter in individuals with chronic stroke. J Neurol Phys Ther. 2014;38(3):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74(4):280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo YJ, Kim JW, Kim JS, Hong BY, Lee KB, Lim SH. Corticospinal Tract Integrity and Long-Term Hand Function Prognosis in Patients With Stroke. Front Neurol. 2019;10:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front Hum Neurosci. 2015;9(April). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boggio PS, Castro LO, Savagim EA, et al. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404(1–2):232–236. [DOI] [PubMed] [Google Scholar]
- 31.Kumar P, Kathuria P, Nair P, Prasad K. Prediction of Upper Limb Motor Recovery after Subacute Ischemic Stroke Using Diffusion Tensor Imaging: A Systematic Review and Meta-Analysis. J stroke. 2016;18(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groisser BN, Copen W a, Singhal AB, Hirai KK, Schaechter JD. Corticospinal Tract Diffusion Abnormalities Early After Stroke Predict Motor Outcome. Neurorehabil Neural Repair. 2014;28(8):1545968314521896–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Jiaerken Y, Wang S, et al. Changes in the Corticospinal Tract Beyond the Ischemic Lesion Following Acute Hemispheric Stroke: A Diffusion Kurtosis Imaging Study. J Magn Reson Imaging. 2020;52(2):512–519. [DOI] [PubMed] [Google Scholar]
- 34.Nazarova M, Kulikova S, Piradov MA, et al. Multimodal Assessment of the Motor System in Patients with Chronic Ischemic Stroke. Stroke. Published online 2021:241–249. [DOI] [PubMed] [Google Scholar]
- 35.Lindenberg R, Zhu LL, Schlaug G. Corticospinal Tract Integrity Predicts Recovery Potential in Chronic Stroke Patients. Ann Neurol. 2009;66:S30–S30. [Google Scholar]
- 36.Lindenberg R, Zhu LL, Rüber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012;33(5):1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135(8):2527–2535. [DOI] [PubMed] [Google Scholar]
- 38.Kim B, Fisher BE, Schweighofer N, et al. A comparison of seven different DTI-derived estimates of corticospinal tract structural characteristics in chronic stroke survivors. J Neurosci Methods. 2018;304:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park C-H, Kou N, Boudrias M-H, Playford ED, Ward NS. Assessing a standardised approach to measuring corticospinal integrity after stroke with DTI. NeuroImage Clin. 2013;2:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woytowicz EJ, Rietschel JC, Goodman RN, et al. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Arch Phys Med Rehabil. 2017;98(3):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winstein CJ, Wolf SL, Dromerick AW, et al. Effect of a Task-Oriented Rehabilitation Program on Upper Extremity Recovery Following Motor Stroke. JAMA. 2016;315(6):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart JC, Gordon J, Winstein CJ. Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke II: planning and adjustments to control movement distance. Exp Brain Res. Published online 2014:3431–3443. [DOI] [PubMed] [Google Scholar]
- 43.Tan C, Tretriluxana J, Pitsch E, Runnarong N, Winstein CJ. Anticipatory Planning of Functional Reach-to-Grasp. Neurorehabil Neural Repair. 2012;26(8):957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varghese R, Winstein CJ. Relationship Between Motor Capacity of the Contralesional and Ipsilesional Hand Depends on the Side of Stroke in Chronic Stroke Survivors With Mild-to-Moderate Impairment. Front Neurol. 2020;10(January):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf SL, Catlin P a., Ellis M, Archer a. L, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as Outcome Measure for Research in Patients After Stroke. Stroke. 2001;32(7):1635–1639. [DOI] [PubMed] [Google Scholar]
- 46.Senesh MR, Reinkensmeyer DJ. Breaking Proportional Recovery After Stroke. Neurorehabil Neural Repair. 2019;33(11):888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Neurosci. 2009;10(861–72):861–872. [DOI] [PubMed] [Google Scholar]
- 48.Woodbury M, Velozo CA, Thompson P a, et al. Measurement Structure of the Wolf Motor Function Test: Implications for Motor Control Theory. Neurorehabil Neural Repair. 2010;24(9):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41(5):910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mok VCT, Liu T, Lam WWM, et al. Neuroimaging predictors of cognitive impairment in confluent white matter lesion: volumetric analyses of 99 brain regions. Dement Geriatr Cogn Disord. 2008;25(1):67–73. [DOI] [PubMed] [Google Scholar]
- 51.Lo R, Gitelman D, Levy R, Hulvershorn J, Parrish T. Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage. 2010;49(1):9–18. [DOI] [PubMed] [Google Scholar]
- 52.Massey FJ. The Kolmogorov-Smirnov Test for Goodness of Fit. J Am Stat Assoc. 1951;46(253):68–78. [Google Scholar]
- 53.Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Liu JS. Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabil Neural Repair. 2009;23(5):429–434. [DOI] [PubMed] [Google Scholar]
- 54.Neter J, Kutner M, Wasserman W, Nachtsheim C. Applied Linear Statistical Models. 4th Editio. Irwin; 1996. [Google Scholar]
- 55.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272–287. [DOI] [PubMed] [Google Scholar]
- 56.Kwakkel G, Kollen B. Predicting improvement in the upper paretic limb after stroke: a longitudinal prospective study. Restor Neurol Neurosci. 2007;25(5–6):453–460. [PubMed] [Google Scholar]
- 57.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Routledge; 2013. [Google Scholar]
- 58.Nudo. Neuroscientific basis of therapy. Published online 2007.
- 59.Sato Y, Chin Y, Kato T, et al. White matter activated glial cells produce BDNF in a stroke model of monkeys. Neurosci Res. 2009;65(1):71–78. [DOI] [PubMed] [Google Scholar]
- 60.Warbrick T, Rosenberg J, Shah NJ. The relationship between BOLD fMRI response and the underlying white matter as measured by fractional anisotropy (FA): A systematic review. Neuroimage. 2017;153(2017):369–381. [DOI] [PubMed] [Google Scholar]
- 61.Majeed Id YA, Awadalla SS, Patton Id JL. Regression techniques employing feature selection to predict clinical outcomes in stroke. Published online 2018. [DOI] [PMC free article] [PubMed]
- 62.Burke Quinlan E, Dodakian L, See J, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77(1):132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thakkar HK, Liao WW, Wu CY, Hsieh YW, Lee TH. Predicting clinically significant motor function improvement after contemporary task-oriented interventions using machine learning approaches. J Neuroeng Rehabil. 2020;17(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stinear CM, Byblow WD, Ackerley SJ, Smith M-C, Borges VM, Barber PA. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol. 2017;4(11):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16(7):403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwakkel G, Van Wegen EEH, Burridge JH, et al. Standardized measurement of quality of upper limb movement after stroke: Consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2019;14(8):783–791. [DOI] [PubMed] [Google Scholar]
- 67.Hodgson K, Adluru G, Richards LG, et al. Predicting Motor Outcomes in Stroke Patients Using Diffusion Spectrum MRI Microstructural Measures. Front Neurol. 2019;10(FEB):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang WH, Park E, Lee J, Lee A, Kim Y-H. Association Between Brain-Derived Neurotrophic Factor Genotype and Upper Extremity Motor Outcome After Stroke. Stroke. 2017;48(6):1457–1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.