Abstract
Mutated MCM9 has been associated with primary ovarian insufficiency. Although MCM9 plays a role in genome maintenance and has been reported as a candidate gene in a few patients with inherited colorectal cancer (CRC), it has not been clearly established as a cancer predisposition gene. We re-evaluated family members with MCM9-associated fertility problems. The heterozygote parents had a few colonic polys. Three siblings had early-onset cancer: one had metastatic cervical cancer and two had early-onset CRC. Moreover, a review of the literature on MCM9 carriers revealed that of nine bi-allelic carriers reported, eight had early-onset cancer. We provide clinical evidence for MCM9 as a cancer germline predisposition gene associated with early-onset cancer and polyposis, mainly in a recessive inheritance pattern. These observations, coupled with the phenotype in knockout mice, suggest that diagnostic testing for polyposis, CRC, and infertility should include MCM9 analysis. Early screening protocols may be beneficial for carriers.
Subject terms: Cancer genetics, Molecular medicine
Introduction
Identification of high-risk polyposis and colorectal cancer (CRC) predisposition genes is imperative in order to prevent CRC in carriers and their relatives. The estimated heritability of CRC ranges from 16 to 35%1. Genetic predisposition to CRC has been classically associated with germline mutations or, in nonpolyposis cases, epimutations in the DNA mismatch repair (MMR) genes. Genes associated with dominant polyposis syndromes include APC (FAP MIM #175100), POLE and POLD1 (PPAP MIM #615083 and 612591), GREM1 (HMPS1 MIM #601228), SMAD4 (JPS MIM #175050), BMPR1A (JPS MIM #174900), STK11 (PJS MIM #175200), and PTEN (MIM #158350). Recessive inheritance of polyposis has been associated with mutations in MUTYH (MAP MIM #608456) and NTHL1 (NAP MIM #616415) and the MMR genes (CMMRD MIM #276300). Recently, APC mosaicism was added as a relatively common cause of polyposis in isolated cases2. However, only 4–8% of all patients with CRC are presently found to carry germline pathogenic variants in one of the known high-penetrance genes3, and overall, a considerable proportion of familial aggregation of CRC remains unexplained3. Notably, most cancer predisposition conditions described today are non-syndromic.
The minichromosome maintenance 9 homologous recombination repair factor gene MCM9 (MIM #610098) functions together with MCM8 in a helicase hexameric complex involved in genome maintenance, meiotic recombination, and repair of double-strand breaks via homologous recombination4. The MCM8/9 complex is required for proper localization of the MRN (Mre11-Rad50-Nbs1) complex to DNA double-strand breaks and for DNA resection to enable homologous recombination5,6. Cells lacking MCM8/9 are viable but highly sensitive to inter-strand cross-linking-inducing agents, and they exhibit more chromosome aberrations in the presence of mitomycin C than wild-type cells7. MCM9 is apparently also required for mammalian MMR8 as it co-immunoprecipitates with MCM8, MSH2, and MLH1, and its deficiency has been linked to microsatellite instability (MSI) and MMR alterations8.
MCM9 knockout mice are viable but undergo embryonic germ cell depletion with production of a reduced quantity of spermatozoa due to defective stem-cell renewal. MCM9 impairment induces meiotic recombination defects and oocyte degeneration4. Female mice are sterile, with ovaries that are devoid of oocytes. MCM9 knockout mice were found to be at high risk of hepatocellular carcinomas and ovarian tumors4,9.
We and others have described several families in which bi-allelic mutations in MCM8 or MCM9 were associated with primary ovarian insufficiency (POI)7,10–15. Early-onset polyposis and CRC were reported in only one of them10 (Table 1). Very recently, Golubicki et al.16 reported two patients with CRC who had bi-allelic MCM9 mutations; one also had POI (Table 1). Nevertheless, MCM9 has not been defined as a cancer predisposition gene17,18 and is not tested in targeted panels.
Table 1.
MCM9 carriers with reported polyposis and/or cancer.
| Study | Subject ID in publication | Variant | Zygosity | Sex | Polyps, no, (age at diagnosis) | Cancer (age at diagnosis) | MMR status | Fertility (age at diagnosis) |
|---|---|---|---|---|---|---|---|---|
| Current | III-3 | c.1483G > T, (p.E495*) | Mono-allelic | F | Yes, 3 (68) | No | Normal | |
| III-2 | c.1483G > T, (p.E495*) | Mono-allelic | M | Yes, 3 (66–68) | No | Normal | ||
| IV-1 | c.1483G > T, (p.E495*) | Bi-allelic | F | Yes (NA) | CRC (31) | NT | POI (15) | |
| IV-2 | c.1483G > T, (p.E495*) | Bi-allelic | F | NT | Clear cell carcinoma of cervix (37) | NT | POI (15) | |
| IV-3 | c.1483G > T, (p.E495*) | Mono-allelic | M | Yes (NA) | CRC (35) | MSS | OTA | |
| Goldberg et al.10 | II-1 | c.672_673delGGinsC, (p.Glu225Lysfs*4) | Mono-allelic | F | Yes, >10 (53–65) | No | Normal | |
| II-2 | c.672_673delGGinsC, (p.Glu225Lysfs*4) | Mono-allelic | M | Yes, 1 (83) | CRC (83) | MSS | Normal | |
| III-3 | c.672_673delGGinsC, (p.Glu225Lysfs*4) | Bi-allelic | F | Yes, >40 (34) | CRC (34) | MSS | POI (<20) | |
| III-4 | c.672_673delGGinsC, (p.Glu225Lysfs*4) | Bi-allelic | F | Yes >20 (37) | CRC X 2 (37) | NT | POI (<20) | |
| III-1 | c.672_673delGGinsC, (p.Glu225Lysfs*4) | Mono-allelic | F | No | No | Normal | ||
| III-2 | c.672_673delGGinsC, (p.Glu225Lysfs*4) | Mono-allelic | F | Yes, 2 (39) | No | Normal | ||
| Golubicki et al.16 | 011-69294-1 | c.1642C > T, p.(Arg548Trp) c.152A > T, p.(Asn51Ile) | Bi-allelic | F | Yes (>40) | CRC (45) | MSI | POI (<40) |
| 011-69294-2 | c.1642C > T, p.(Arg548Trp) c.152A>T, p.(Asn51Ile) | Bi-allelic | M | Yes (>40) | CRC (55) | MSS | NA | |
| MSS13-1961 | c.3425A > G, p.(Lys1142Arg); c.1640T > C, p.(Leu547Pro) | Bi-allelic | F | No | CRC (42) | MSS | ||
| Alvarez-Mora et al.13 | POI_02 | c.1473dupT (p. T492Yfs*4) | Bi-allelic | F | NT | germ cell tumor (14) | NT | POI (20) |
| POI_03 | c.1473dupT (p. T492Yfs*4) | Bi-allelic | F | NT | POI (14) |
All ages reported in years.
CRC colorectal cancer, MSI microsatellite instability, MSS microsatellite stability, NA not available, NT not tested, OTA oligoteratoasthenozoospermia, POI primary ovarian insufficiency.
Prompted by these findings, we re-evaluated a family with an MCM9 pathogenic variant, previously evaluated at our medical center for POI, and revealed that three siblings had early-onset cancer.
Results and discussion
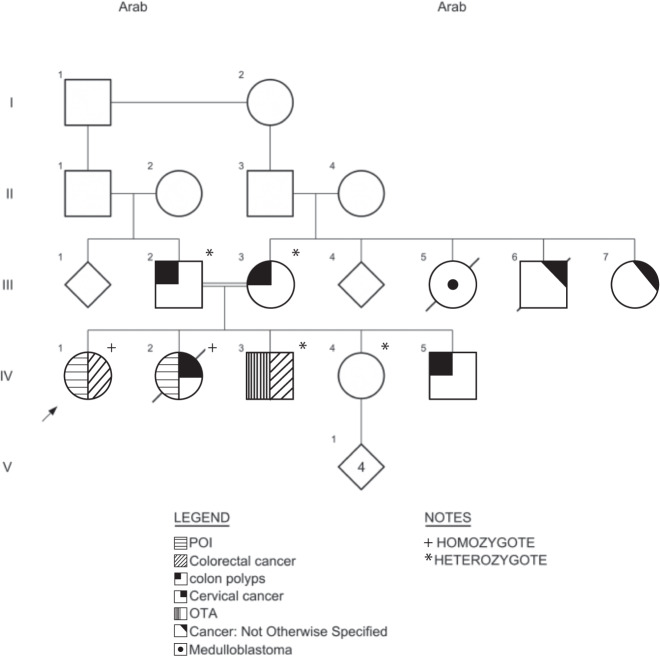
The clinical and pathological data of the family described in the present study are shown in Table 1 and Fig. 1.
Fig. 1. MCM9 mutated pedigree.
Clinical manifestations, polyps, and cancers are indicated. Genotypes are indicated as: + for homozygotes; * for heterozygotes. Tumor spectrum included cancer—not otherwise specified, cervical cancer, colon polyps, colorectal cancer, medulloblastoma, oligo-terato-asthenozoospermia (OTA), and primary ovarian insufficiency (POI).
The parents (III-2, III-3) are first paternal cousins of Middle Eastern Arabic origin. They have five children, three daughters (IV-1, IV-2, IV-4) and two sons (IV-3, IV-5). Two daughters were diagnosed with POI around age 15 years. Karyotype was normal (46, XX), and there was no premutation in the FMR1 gene. Both were infertile. One of the two sons was diagnosed with severe oligoteratoasthenozoospermia (OTA) at age 35 years. Whole-exome sequencing detected the c.1483G > T [p.E495*] variant in the MCM9 gene14.
The c.1483G > T [p.E495*] variant introduces a premature stop codon in coding exon 8 and is expected to lead to the loss of a functional protein. The variant is not found in gnomAD exomes and genomes. The pathogenic computational verdict is based on five pathogenic predictions. Segregation analysis indicated autosomal recessive inheritance in the family.
Re-evaluation of the family for the present study revealed that three of the siblings had cancer and the parents had a few colonic polyps. Current clinical and pathological data are shown in Fig. 1 and Table 1.
Patient IV-1, homozygous for the MCM9 variant, is currently 40 years old. She was diagnosed at age 15 years with primary amenorrhea secondary to hypogonadism, and was treated with hormonal replacement. At age 31 years, colonoscopy performed to evaluate rectal bleeding revealed a villous adenoma with high-grade dysplasia and foci of well-differentiated adenocarcinoma.
Patient IV-2, homozygous for the MCM9 variant, was, like her sister, diagnosed with primary amenorrhea around age 15 years. At age 37 years, she was found to have metastatic clear cell carcinoma of the cervix. PET-CT scan showed massive involvement. She died 2 months after diagnosis.
Patient IV-3, heterozygous brother, was diagnosed with OTA. Karyotype, Y micro-deletions, and CFTR gene testing were normal. At age 35 years, he presented with rectal bleeding and weight loss and was ultimately diagnosed with moderately differentiated colon adenocarcinoma with liver involvement. Findings on immunohistochemistry for the four MMR proteins were normal. Surveillance revealed recurrent polyps.
Patient IV-4, heterozygous for the MCM9 variant, is healthy at age 42 years. She has four healthy children and did not report any fertility problems. She has not undergone colonoscopy.
Patient IV-5 has never gone for genetic counseling but has recurrent polyps.
Both parents (III-2 and III-3) are cancer free at age 69 and 74 years. They did not report fertility problems. Colonoscopy performed in the mother at age 68 years for evaluation of rectal bleeding revealed three polyps. The father had one tubular adenoma at age 66 years and one tubulo-villous adenoma with low-grade dysplasia and one hyperplastic polyp at age 68 years. The family history was remarkable for early cancer in three family members, one with suspected medulloblastoma and one reported to have died of an “abdominal tumor” (Fig. 1).
Updated review of published cases
Our review of previous publications on MCM9 carriers from families with cancer (Table 1) yielded 11 additional patients. Of the nine bi-allelic carriers, eight reported fertility problems and eight had early-onset cancer, including six with CRC (ages 31–55 years; mean 41). Three of the four tumors analyzed for MSI were stable. Multiple polyps were documented in four bi-allelic carriers, and gynecologic cancer was reported in two bi-allelic women (ages 14 and 37 years). Among the seven mono-allelic carriers, two (ages 35 and 83 years) had CRC and six (ages 39–83 years) had a few colonic polyps.
This report complements our previous description14 and, together with the work of Golubicki et al.16, supports the recognition of MCM9 as a cancer predisposition gene associated with early-onset cancer, mainly CRC and gynecologic tumors, in addition to infertility. The mode of inheritance is autosomal recessive, with some evidence of a milder mono-allelic, phenotype.
The co-occurrence of infertility and early-onset cancer, both conditions associated with mutated MCM9, in the heterozygote brother (Patient IV-3) is intriguing because it may indicate substantial malfunction of MCM9 also in the mono-allelic state. A heterozygous variant in MCM9 leading to Y511* was reported in ClinVar in a patient with POI (ClinVar 156586), and in three MCM heterozygotes with POI15. Moreover, MCM8-9 heterozygous variants were identified in 16/131 Dutch cases of polyposis and familial cancer16, and CRC and a few polyps at a later age were reported in heterozygote parents10. Thus, mono-allelic carriers may be at increased risk, with low penetrance or late onset. The effect of MCM9 mutations might be dosage-dependent whereas bi-allelic variations lead to more severe defects.
However, given the relative high frequency of both infertility and cancer in the general population, incidental irrelevant MCM9 heterozygous findings could be argued.
Of the four tumors analyzed for MSI, three were stable. MCM9 is required for mammalian MMR18. MCM8/9 deficiency impairs HR-mediated DNA repair, and deficient cells are particularly sensitive to DNA cross-linking agents and inhibition of poly-ADP ribose polymerase19,20. This might vindicate both MSI signatures.
To conclude, we provide clinical supporting evidence for MCM9 as a cancer germline predisposition gene associated with early-onset CRC and polyposis, mainly in a recessive inheritance pattern. The new data presented here, by itself not sufficient to conclude that MCM9 is causal for either polyposis or CRC, however, these observations, coupled with the phenotype in knockout mice10,16, suggest that early screening protocols may be beneficial for carriers. Diagnostic testing for polyposis, CRC, and infertility should include analysis of MCM9. Additional studies in other cohorts including patients affected with familial cancer, colonic polyposis, and/or fertility problems could help to further define the role of MCM9 in predisposing to these pathologies.
Methods
Background
In 2016, a report from the HaEmek Medical Center in Israel, by Fauchereau et al.14 described the genetic findings in a consanguineous family with POI. Whole-exome sequencing of two patients and a non-affected sister yielded a homozygous causal variant in the MCM9 gene, c.1483G > T [p.E495*], apparently leading to loss of a functional protein.
Patient re-evaluation
For the present study, to investigate the possible association among mutant MCM9, infertility, and a predisposition to cancer, we retrieved the clinical and pathological data from the files of the patients evaluated by Fauchereau et al.14 at our medical center 5 years previously. We searched for additional morbidities and assessed the clinical, oncologic, and pathologic findings. All patients described in this manuscript provided written informed consent. The study was approved by the HaEmek Medical Center Research Ethics Committee.
Literature review
Public resources such as Pubmed and ClinVar were searched for publications on MCM9 carriers using the keyword “MCM9”. Data on cancer and colonic polyps among MCM9 carriers and/or family members were retrieved and analyzed.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This article is based upon work from COST Action CA17118, supported by COST (European Cooperation in Science and Technology) www.cost.eu.
Author contributions
All authors made substantial contributions to the work, as follows: O.A., L.P.-P. and S.A.S. contributed to acquisition of data. S.A.S., M.N. and Y.G. substantially contributed to study concept and design; analysis and interpretation of data as well as drafting of the manuscript. All authors contributed in revising the work critically approved the final version and account for all aspects of the work.
Data availability
The authors declare that data supporting the findings of this study are available within the paper. Further information may be found in the online version of the original article describing the genetic analysis of the patients (family MO4)14 https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1111%2Fcge.12736&file=cge12736-sup-0003-AppendixS1.pdf. All other data are available on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the local institutional review board, Helsinki approval #: EMC0094-16.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41525-021-00242-4.
References
- 1.Jiao S, et al. Estimating the heritability of colorectal cancer. Hum. Mol. Genet. 2014;23:3898–38905. doi: 10.1093/hmg/ddu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen AM, et al. Distinct patterns of somatic mosaicism in the APC gene in neoplasms from patients with unexplained adenomatous polyposis. Gastroenterology. 2017;152:546–549. doi: 10.1053/j.gastro.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 3.You YN, et al. Detection of pathogenic germline variants among patients with advanced colorectal cancer undergoing tumor genomic profiling for precision medicine. Dis. Colon Rectum. 2019;62:429–437. doi: 10.1097/DCR.0000000000001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutzmann M, et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol. Cell. 2012;47:523–534. doi: 10.1016/j.molcel.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 5.Park J, et al. The MCM8-MCM9 complex promotes RAD51 recruitment at DNA damage sites to facilitate homologous recombination. Mol. Cell Biol. 2013;33:1632–1644. doi: 10.1128/MCB.01503-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KY, et al. MCM8-9 complex promotes resection of double-strand break ends by MRE11-RAD50-NBS1 complex. Nat. Commun. 2015;6:7744. doi: 10.1038/ncomms8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouali N, et al. New MCM8 mutation associated with premature ovarian insufficiency and chromosomal instability in a highly consanguineous Tunisian family. Fertil. Steril. 2017;108:694–702. doi: 10.1016/j.fertnstert.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Traver S, et al. MCM9 is required for mammalian DNA mismatch repair. Mol. Cell. 2015;59:831–839. doi: 10.1016/j.molcel.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Hartford SA, et al. Minichromosome maintenance helicase paralog MCM9 is dispensible for DNA replication but functions in germ-line stem cells and tumor suppression. Proc. Natl Acad. Sci. USA. 2011;108:17702–17707. doi: 10.1073/pnas.1113524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg Y. Mutated MCM9 is associated with predisposition to hereditary mixed polyposis and colorectal cancer in addition to primary ovarian failure. Cancer Genet. 2015;208:621–624. doi: 10.1016/j.cancergen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YX, et al. Novel loss-of-function mutation in MCM8 causes premature ovarian insufficiency. Mol. Genet. Genom. Med. 2020;8:e1165. doi: 10.1002/mgg3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood-Trageser MA, et al. MCM9 mutations are associated with ovarian failure, short stature, and chromosomal instability. Am. J. Hum. Genet. 2014;95:754–762. doi: 10.1016/j.ajhg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez-Mora MI, et al. An exome-wide exploration of cases of primary ovarian insufficiency uncovers novel sequence variants and candidate genes. Clin. Genet. 2020;98:293–298. doi: 10.1111/cge.13803. [DOI] [PubMed] [Google Scholar]
- 14.Fauchereau F, et al. A non-sense MCM9 mutation in a familial case of primary ovarian insufficiency. Clin. Genet. 2016;89:603–607. doi: 10.1111/cge.12736. [DOI] [PubMed] [Google Scholar]
- 15.Guo T, et al. Novel pathogenic mutations in minichromosome maintenance complex component 9 (MCM9) responsible for premature ovarian insufficiency. Fertil. Steril. 2020;113:845–852. doi: 10.1016/j.fertnstert.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Golubicki M, et al. Germline biallelic Mcm8 variants are associated with early-onset Lynch-like syndrome. JCI Insight. 2020;5:e140698. doi: 10.1172/jci.insight.140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belhadj S, et al. Candidate genes for hereditary colorectal cancer: mutational screening and systematic review. Hum. Mutat. 2020;41:1563–1576. doi: 10.1002/humu.24057. [DOI] [PubMed] [Google Scholar]
- 18.Terradas M, et al. Contribution to colonic polyposis of recently proposed predisposing genes and assessment of the prevalence of NTHL1- and MSH3-associated polyposes. Hum. Mutat. 2019;40:1910–1923. doi: 10.1002/humu.23853. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura K, et al. Mcm8 and Mcm9 form a complex that functions in homologous recombination repair induced by DNA interstrand crosslinks. Mol. Cell. 2012;47:511–522. doi: 10.1016/j.molcel.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Morii I, et al. Inhibiting the MCM8-9 complex selectively sensitizes cancer cells to cisplatin and olaparib. Cancer Sci. 2019;110:1044–1053. doi: 10.1111/cas.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the paper. Further information may be found in the online version of the original article describing the genetic analysis of the patients (family MO4)14 https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1111%2Fcge.12736&file=cge12736-sup-0003-AppendixS1.pdf. All other data are available on reasonable request.