Abstract
Purpose:
To determine frequency of hypercoagulability testing and hypercoagulable states in CRVO patients under 50 years of age.
Design:
Retrospective cohort study.
Participants:
Deidentified patients under 50 years of age newly diagnosed with CRVO from a national insurance claims database.
Methods:
Optum’s de-identified Clinformatics™ Data Mart Database containing medical claims from a commercial and Medicare Advantage insurance database was used. All outpatient medical claims (office visits, associated diagnoses, and laboratory testing) and demographic data for each beneficiary during their enrollment was accessible.
Main Outcome Measures:
Prevalence of (1) laboratory hypercoagulable work-up within 90 days of CRVO diagnosis; (2) new diagnosis of a hypercoagulable state within 1 year of CRVO diagnosis; (3) diagnosis of hypertension (HTN), diabetes (DM), and hyperlipidemia (HLD).
Results:
1181 patients met inclusion criteria. 671 (56.8%) were men. 450 (38.1%) patients had hypercoagulable testing within 90 days. 136 (11.5%) patients were diagnosed with a hypercoagulable state within 1 year after CRVO. This proportion was similar between those patients with DM, HTN, or HLD (10.5% [65/620]) and those without (12.7% [71/561]) (p=0.28). Of the 136 patients diagnosed with a hypercoagulability state, 68.4% (93/136) had testing done within 90 days of CRVO and 31.6% (43/136) did not. Of those who did not have hypercoagulability testing, 5.9% (43/731) were diagnosed with a hypercoagulable state within 1 year compared to 20.7% (93/450) in those who were tested (p<0.001).
Conclusions:
The prevalence of hypercoagulable state within a year of CRVO diagnosis in patients under 50 was 11.5%, and the prevalence was similar between patients with atherosclerotic risk factors and those without. Rate of testing was only 38.1%. Future research should examine the utility of uniform hypercoagulable testing in young CRVO patients.
Précis:
In a study with 1,181 patients under 50 years of age with new-onset central retinal vein occlusions, a clinically relevant minority of 11.5% were diagnosed with a hypercoagulable disorder within a year of the CRVO.
Central retinal vein occlusions (CRVO) affect approximately 2.5 million people worldwide and can impair vision through macular edema, macular ischemia, retinal hemorrhages, and neovascular glaucoma.1 Despite known systemic risk factor associations, currently no clear guidelines exist to determine how and when ophthalmologists should perform diagnostic work ups on patients with CRVOs.2–4 This is particularly true for younger CRVO patients, those less than 50 years of age, who are frequently approached differently than older adults when they present with a CRVO.
The thrombotic phenomenon underlying CRVO in younger individuals is thought to be more related to hypercoagulable risk factors as opposed to other aspects of Virchow’s triad, namely endothelial cell damage and stasis secondary to so-called “traditional” vasculopathic conditions like hypertension and diabetes which are more prevalent in older patients.5 Younger CRVO patients may receive a hypercoagulability workup, but testing is not uniform in clinical practice, with some experts electing not to endorse routine hypercoagulability screening,4 and some ophthalmologic societies actually discouraging the practice.6 Some studies posit a benefit to screening patients younger than 50 to 60 years,7–9 but one meta-analysis based on a plurality of small observational studies argues otherwise.10 Fundamentally, single studies thus far have been too small to reliably detect the significance of thrombophilic factors in the overall population of young CRVO patients.
Large medical claims databases are rich sources of information on disease prevalence and health care utilization within a population and are well suited to evaluate risk factors in uncommon conditions such as CRVOs in younger patients. The aim of this study was to evaluate the frequency of diagnosed hypercoagulable conditions in patients under the age of 50 who developed new CRVOs, as well as the frequency of hypercoagulability testing in this group. We also studied the incidence of traditional atherosclerotic risk factors among these patients.
Methods
Data set
Optum’s de-identified Clinformatics™ Data Mart Database contains the medical claims from a commercial and Medicare Advantage insurance database obtained from a large US insurer and was used for this study. All outpatient medical claims (office visits, associated diagnoses, and laboratory testing) and demographic data for each beneficiary during their enrollment was accessible. The subset of data available for this study included all patients in the database from January 1, 2000 to June 30, 2019. This study was deemed exempt from review by the University of Pennsylvania’s Institutional Review Board due to the deidentified nature of the database.
Study Cohorts
All patients age 50 or younger with a new diagnosis of a CRVO were included for analysis (ICD-9 and ICD-10 codes used for CRVO were 362.35 and H34.81, respectively). Exclusions occurred for those with less than 2 years of continuous data in the insurance plan prior to the date of CRVO diagnosis or less than 1 year of follow-up after the date of CRVO diagnosis. Also excluded were those with a previous history of any retinal vein occlusion (RVO), unspecified RVO or history of a diagnoses thought to possibly be related to a hypercoagulable disorder including primary hypercoagulable state, secondary hypercoagulable state, Factor V Leiden, homocysteinemia, protein C or S deficiency, antiphospholipid antibody syndrome, Lupus anticoagulant, anticardiolipin antibodies, antithrombin III deficiency, venous embolus-thrombosis, pulmonary emboli, polycythemia vera, essential thrombocytosis, paroxysmal nocturnal hemoglobinuria, nephrotic syndrome, HIV/AIDs, pregnancy and cancer. The coding changes to the ICD-10 were an improvement over the ICD-9 in that the diagnosis of “primary hypercoagulable state” was further divided into more specific hypercoagulable disorders as seen in Supplemental Table 1. (See Supplemental Table 1 for all ICD-9/-10 codes used in this study and Figure 1 for flowchart of inclusion and exclusion criteria for the study.)
Figure 1:
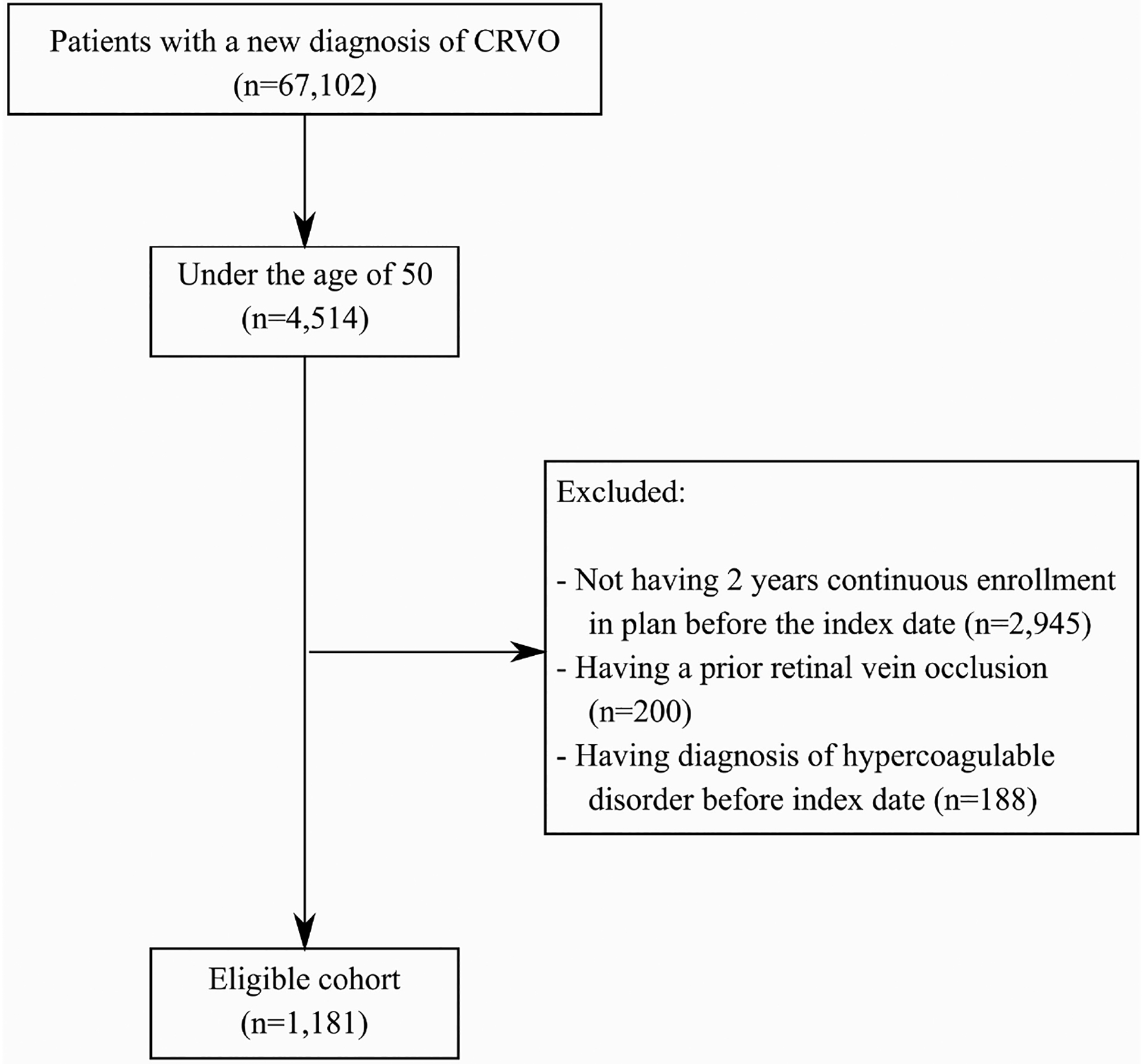
Flowchart of patients who met inclusion and exclusion criteria
Outcome Measures
One primary outcome measure for this study was the number of patients who had a new hypercoagulability diagnosis in the year following the CRVO diagnosis. We defined a hypercoagulable disorder as any of the following diagnoses: primary hypercoagulable state, secondary hypercoagulable state, Factor V Leiden, homocysteinemia, protein C or S deficiency, antiphospholipid antibody syndrome, Lupus anticoagulant, anticardiolipin antibodies, antithrombin III deficiency, venous embolus-thrombosis, pulmonary emboli, polycythemia vera, essential thrombocytosis, paroxysmal nocturnal hemoglobinuria, nephrotic syndrome, and cancer. Female hormonal therapy has not been shown to be associated with vein occlusions in the population in this database, and therefore was not considered for its potential association in this study.11 Obesity was similarly not considered because this diagnosis is not uniformly coded, as demonstrated by the fact that only 3% of patients in this database have a diagnosis of obesity while it is well established that 30–40% of the American population is obese.
Another primary outcome measure was the number of patients who had hypercoagulability laboratory work up (defined as having a claim for at least one lab test for a hypercoagulable condition) within 90 days following CRVO diagnosis. To ascertain this, we counted all patients who had a laboratory test done for any of the following conditions: Factor V Leiden mutation, protein C or S, prothrombin (factor II), antithrombin III activity, homocysteine level, anticardiolipin antibody test, ß2-Glycoprotein 1 antibodies, lupus anticoagulant.
Secondary outcome measures included diagnoses of diabetes mellitus (DM), hypertension (HTN), and/or hyperlipidemia (HLD) prior to the date of CRVO diagnosis. The relative prevalence of these diagnoses in those specific patients who had a lab work up as well as in those specific patients that developed a hypercoagulable diagnosis were assessed. All statistical analyses, which consisted of calculating the proportions of patients who fit our predefined outcome measures and the statistical significance of comparisons between subgroups, was performed using SAS (version 9.4; SAS Institute Inc, Cary, NC) and R (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Figure 1 shows the flowchart of inclusion and exclusion criteria for the study. 1181 individuals with a new CRVO at an age younger than 50 met criteria and were included for analysis. Of these, 670 (56.7%) were men. The average age of this group was 40 years old (standard deviation (SD) 8.6). Looking at traditional atherosclerotic risk factors, 620 patients overall (52.5%) had at least one of either DM, HTN, or HLD at the time of diagnosis. Individually, 216 (18.3%) had DM, 439 (37.2%) had HTN, and 462 (39.1%) had HLD at the time of CRVO diagnosis.
Of all those newly diagnosed with a CRVO, only 38.1% (450) patients had any form of hypercoagulable testing within 90 days of diagnosis. The percentage of patients without DM, HTN, or HLD who were tested for hypercoagulable risks factors was 41.0% (230/561). The proportion of testing was significantly lower for the CRVO patients who did have a history of DM, HTN, or HLD (35.4% (220/620); (p=0.049)). In the full cohort of young CRVO patients, 11.5% (136/1181) were diagnosed with a hypercoagulable condition within 1 year after diagnosis. Supplemental Table 2 shows the baseline characteristics of those who went on to have testing or progressed to a hypercoagulable state. No difference was seen in the proportion of patients who progressed to a hypercoagulable state within 1 year in the cohort with a history of DM, HTN and HLD individually or collectively compared to the cohort without these risk factors (p>0.166 for all comparisons). Of the patients with CRVO who were found to have a hypercoagulable state within a year, 68.4% (93/136) had hypercoagulability lab testing done, whereas 31.6% (43/136) of patients diagnosed with a hypercoagulable state did not have testing done. Of those out of the total cohort who did not have testing, 5.9% (43/731) went on to be diagnosed with a hypercoagulable state within 1 year of CRVO diagnosis compared to 20.7% (93/450) diagnosed among those who had testing (p<0.001).
The frequency of various hypercoagulability diagnoses in the entire cohort of patients, with stratification for the presence or absence of pre-existing DM, HTN, or HLD, is listed in Supplemental Table 2. Primary hypercoagulable state (45.1%) and venous embolus-thrombosis (32.4%) were the two most common diagnoses. Supplemental Table 3 shows the rates of the different hypercoagulable disorders that were diagnosed.
Discussion
In this study, 11.5% of patients younger than 50 with a new onset CRVO were found to be newly diagnosed with a hypercoagulable state within 1 year, whether through testing or a clinical event. While not perfectly synonymous with hypercoagulable states, the percentage of thromboembolic disorders reported in the general population is roughly 0.1%,12 suggesting the percentage found in our study vastly exceeds would be expected to be found in the general population. Despite this, surprisingly, only 38.1% of all young CRVO patients without a prior pro-thrombotic diagnosis had a hypercoagulable laboratory workup done. While less testing occurred in patients with atherosclerotic risk factors, our results show that the percentage of patients diagnosed with a hypercoagulable condition was similar between the cohort who had systemic vascular risk factors, such as HTN, HLD, and DM, and the cohort that did not at the time of diagnosis. Our findings lead us to conclude that a decision to test a young CRVO patient for hypercoagulable states should not be based on whether a patient has a history of “classic” atherosclerotic risk factors.
Unfortunately, the decision to test for hypercoagulable states in young patients with central retinal vein occlusions is not straightforward. There is presently no consensus on the role of hypercoagulability testing in the internal medicine and primary care literature for patients presenting with retinal vein occlusions, which is likely a reflection of our own specialty’s lack of clarity on the subject. Currently, conflicting studies exist demonstrating data for and against the impact of hypercoagulable disorders on young CRVOs patients and, by proxy, the role for diagnostic testing, especially in those with known cardiovascular risk factors.5,8,9,18,19 Interestingly, it seems to be the smaller studies (50 or fewer cases of CRVO) that argue against testing, with larger ones arguing for it.8,9,18,19 It is unclear if the smaller size of some studies limited their ability to assess the prevalence of hypercoagulable disorders due to the randomness that is sometimes found in small numbers and the lack of power to detect this infrequent pathology. One strength of this study is the large number of patients that were evaluated, which assessed 1181 young CRVO patients.
One of the larger studies to argue for testing found that of those with a hypercoagulable disorder and CRVO, 64% developed the CRVO prior to age 60.8 A German study found that the presence of prothrombotic factors was an independent risk factor for development of retinal vein occlusions among patients under 45 years.7 Contrary to our findings, however, this study also argued that the absence of cardiovascular risk factors was a strong predictor for the presence of coagulation disorders in both young and old CRVO patients, and suggested targeted testing would be more beneficial. Supporting a similar position, a combined case-series and meta-analysis found increased prevalence of Factor V Leiden in CRVO patients, but only moderately increased compared to controls, thereby concluding that the association was not large enough to warrant broad testing, especially in those with known systemic vascular disease.19 However, in their meta-analysis, the authors did not stratify for age nor for the presence or absence of vascular risk factors. By contrast, our study was designed to look specifically at younger patients and stratified for the presence of HTN, HLD, or DM. This allowed us to conclude that the presence of a systemic vascular risk factor does not obviate the risk of having a hypercoagulable state.
These results and their implication for targeted versus broad testing are open to interpretation. The higher percentage of newly diagnosed hypercoagulable states in those tested (20.7%) compared to those not tested (5.9%) could suggest that physicians are being appropriately “selective” in who should be tested. However, one argument in favor of testing more broadly is that it is impossible to know the true prevalence of hypercoagulable disorders in the untested group. In our study, 5.9% of patients were diagnosed without testing within the first year, which suggests that there may be many other patients who may have remained undetected due to the lack of testing and/or not having a second thromboembolic event until after the first year post-CRVO. The 5.9% of patients with a hypercoagulable state diagnosed within a year is likely an underestimate of the true prevalence. Additional evidence for this point was offered by Liu et al. who found that 28% of patients in a small cohort of CRVO patients under 56 years of age who were tested for hypercoagulable states at the outset of the study had at least one positive test result, a number comparable to the 20.7% of tested patients in our study who progressed to a hypercoagulability diagnosis within a year.5 A further argument for broader testing can be made in light of the similarity in incidence of hypercoaguability states between those with atherosclerotic comorbidities and those without appears to show that the presence of classic risk factors. Unfortunately, this issue may not be settled until a large enough database with both ocular and systemic health findings can be assessed, not just for whether a hypercoagulable test was performed, but also the results.
Another interesting result we found was that of the patients who went on to have an underlying hypercoagulable state, 10.3% of them developed a new diagnosis of cancer (see Supplemental table 2). Numerous case reports have reported an association between solid tumors and CRVO.13,14 Hematologic malignancies are also a well-established cause of retinal vein occlusions.15,16 A recent nation-wide cohort study from South Korea supports the association between malignancy and retinal vein occlusions, particularly hematologic malignancy.17 The association between cancer and CRVO warrants consideration by providers when seeing a young CRVO patient. It also warrants further research to identify specific cancers that may be associated with retinal vein occlusions in young patients.
Unfortunately, no studies exist that demonstrate the most efficient testing for hypercoagulable disorders specific to young CRVO patients. As such, for those inclined to perform hypercoagulable testing, we recommend an adapted combination of tests to cover both acquired and inherited causes of thrombophilia. Included in this work up should be a complete blood count (CBC) with smear, an activated partial thromboplastin time (aPTT), an international normalized ratio (INR), an erythrocyte sedimentation rate (ESR), a renal function test, a urinalysis, Protein C and S, Factor V Leiden, prothrombin gene mutation (G20210A), antithrombin, antiphospholipid antibodies (anticardiolipin and beta2-glycoprotein I antibodies) and the lupus anticoagulant (LA).20–22
Limitations
Limitations of our study include our inability to know the overall prevalence of positive hypercoagulability states in the entire cohort of young CRVO patients, as opposed to the incidence in the specific cohort that underwent testing plus the remainder of untested patients that were subsequently found to have a hypercoagulable state within a year. Moreover, it is impossible to know whether there were any inherent differences on presentation between the patients who did ultimately undergo a workup per their providers, as compared to those that did not. Nevertheless, our results still support our assertion that hypercoagulability plays a role in the pathogenesis of young CRVO. Next, due to the nature of medical claims data, we are unable to verify with chart level data the diagnoses used within this study. Similarly, while we are able to know a hypercoagulable test was done, we do not necessarily know which specific test or panel of tests was done due to the use of a single billing code that could be used for multiple tests. Nor do we know the results of that test and therefore are unable to determine who exactly was diagnosed based on testing or based on other clinical presentation that arose within the year after CRVO diagnosis.
This large national study reveals that the rate of “classic” atherosclerotic risk factors was similar between young CRVO patients that did and did not progress to be diagnosed with a hypercoagulable state the year after diagnosis. We found that the incidence of newly diagnosed hypercoagulable states was 11.5% in the overall cohort of young CRVO patients. Our results support the argument that CRVOs in young people are associated with hypercoagulable conditions in a considerable proportion of patients. Future research should continue to examine the utility of uniform hypercoagulable testing in young CRVO patients.
Supplementary Material
Supplemental Table 2: Baseline characteristics of young CRVO patients who had hypercoagulable testing or progressed to being diagnosed with a hypercoagulable state at 1 year
Supplemental Table 1: International Classifications of Diseases (ICD), Ninth and Tenth Revisions (ICD-9 and ICD-10) codes used within this study
Supplemental Table 3: Frequency and percentage of hypercoagulability diagnoses among young CRVO patients
Financial Support:
National Institutes of Health K23 Award (1K23EY025729 - 01) and University of Pennsylvania Core Grant for Vision Research (2P30EY001583). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness, Karen & Herbert Lotman Fund for Macular Vision Research Foundation, and the Paul and Evanina Mackall Foundation. None of the funding organizations had any role in the design or conduction of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–9.e1. doi: 10.1016/j.ophtha.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAllister IL. Central retinal vein occlusion: a review. Clin Experiment Ophthalmol. 2012;40(1):48–58. doi: 10.1111/j.1442-9071.2011.02713.x [DOI] [PubMed] [Google Scholar]
- 3.Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost JTH. 2010;8(9):1886–1894. doi: 10.1111/j.1538-7836.2010.03909.x [DOI] [PubMed] [Google Scholar]
- 4.Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal Vein Occlusions Preferred Practice Pattern®. Ophthalmology. 2020;127(2):P288–P320. doi: 10.1016/j.ophtha.2019.09.029 [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Lahey JM, Karlen R, Stewart JM. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Retina Phila Pa. 2018;38(6):1175–1179. doi: 10.1097/IAE.0000000000001661 [DOI] [PubMed] [Google Scholar]
- 6.Sivaprasad S, Amoaku WM, Hykin P, Group RVOG. The Royal College of Ophthalmologists Guidelines on retinal vein occlusions: executive summary. Eye Lond Engl. 2015;29(12):1633–1638. doi: 10.1038/eye.2015.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhli-Hattenbach C, Scharrer I, Luchtenberg M, Hattenbach L-O. Coagulation disorders and the risk of retinal vein occlusion. Thromb Haemost. 2010;103(2):299–305. doi: 10.1160/TH09-05-0331 [DOI] [PubMed] [Google Scholar]
- 8.Arsene S, Delahousse B, Regina S, Le Lez M-L, Pisella P-J, Gruel Y. Increased prevalence of factor V Leiden in patients with retinal vein occlusion and under 60 years of age. Thromb Haemost. 2005;94(1):101–106. doi: 10.1160/TH04-10-0659 [DOI] [PubMed] [Google Scholar]
- 9.Kuhli C, Hattenbach L-O, Scharrer I, Koch F, Ohrloff C. High prevalence of resistance to APC in young patients with retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240(3):163–168. doi: 10.1007/s00417-001-0415-1 [DOI] [PubMed] [Google Scholar]
- 10.Kirkegaard K, Heegaard S, Hvas A-M. No evidence for thrombophilia in patients with retinal venous occlusion: a systematic GRADE-based review. Acta Ophthalmol (Copenh). 2017;95(1):12–19. doi: 10.1111/aos.13214 [DOI] [PubMed] [Google Scholar]
- 11.Song D, Nadelmann J, Yu Y, VanderBeek BL. Association of Retinal Vascular Occlusion With Women Filling a Prescription for Female Hormone Therapy. JAMA Ophthalmol. Published online November 12, 2020. doi: 10.1001/jamaophthalmol.2020.4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan GS, Rodgers GM, Ware Branch D. The inherited thrombophilias: genetics, epidemiology, and laboratory evaluation. Best Pract Res Clin Obstet Gynaecol. 2003;17(3):397–411. doi: 10.1016/s1521-6934(03)00010-5 [DOI] [PubMed] [Google Scholar]
- 13.Segev F, Segev A, Livne A, Assia EI, Mekori YA. Bilateral central retinal vein occlusion in a patient with occult colon cancer. Arch Ophthalmol Chic Ill 1960. 2001;119(10):1552–1553. [PubMed] [Google Scholar]
- 14.Madanagopalan VG, Paneer Selvam V, Sarath Sivan NV, Govindaraju NV. Central retinal vein occlusion in a patient with breast carcinoma. GMS Ophthalmol Cases. 2019;9:Doc04. doi: 10.3205/oc000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bawankar P, Bhattacharjee H, Paulbuddhe V. Simultaneous Bilateral Central Retinal Vein Occlusion as the First Manifestation of Chronic Myeloid Leukemia. Ophthalmol Retina. 2018;2(4):378. doi: 10.1016/j.oret.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 16.Narang S, Gupta P, Sharma A, Sood S, Palta A, Goyal S. Bilateral Central Retinal Vein Occlusion as Presenting Feature of Chronic Myeloid Leukemia. Middle East Afr J Ophthalmol. 2016;23(3):253–255. doi: 10.4103/0974-9233.186113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MS, Cho JH, Byun SJ, Oh C-M, Park KH, Park SJ. Increased risk of cancer in patients with retinal vein occlusion: a 12-year nationwide cohort study. Br J Ophthalmol. Published online September 26, 2020. doi: 10.1136/bjophthalmol-2020-316947 [DOI] [PubMed] [Google Scholar]
- 18.Ahluwalia J, Rao S, Varma S, et al. Thrombophilic risk factors are uncommon in young patients with retinal vein occlusion. Retina Phila Pa. 2015;35(4):715–719. doi: 10.1097/IAE.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 19.Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Thromb Haemost. 2008;99(11):925–929. doi: 10.1160/TH07-11-0658 [DOI] [PubMed] [Google Scholar]
- 20.Caprini JA, Glase CJ, Anderson CB, Hathaway K. Laboratory markers in the diagnosis of venous thromboembolism. Circulation. 2004. March 30;109(12 Suppl 1):I4–8. doi: 10.1161/01.CIR.0000122869.59485.36. [DOI] [PubMed] [Google Scholar]
- 21.Weingarz L, Schwonberg J, Schindewolf M, et al. Laboratory markers in the diagnosis of venous thromboembolism. Br J Haematol. 2013. December;163(5):655–65. doi: 10.1111/bjh.12575. [DOI] [PubMed] [Google Scholar]
- 22.Baur KA, Lip GYH. Evaluating adult patients with established venous thromboembolism for acquired and inherited risk factors. https://www.uptodate.com/contents/evaluating-adult-patients-with-established-venous-thromboembolism-for-acquired-and-inherited-risk-factors. Accessed March 17, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 2: Baseline characteristics of young CRVO patients who had hypercoagulable testing or progressed to being diagnosed with a hypercoagulable state at 1 year
Supplemental Table 1: International Classifications of Diseases (ICD), Ninth and Tenth Revisions (ICD-9 and ICD-10) codes used within this study
Supplemental Table 3: Frequency and percentage of hypercoagulability diagnoses among young CRVO patients


