Abstract
Background:
Time restricted feeding (TRF) involves deliberately restricting the times during which energy is ingested. Preliminary findings suggest that 8–10-h TRF improves sleep. However, the effects of shorter TRF windows (4–6 h) on sleep, remain unknown.
Aims:
This study compared the effects of 4-h versus 6-h TRF on sleep quality, duration, insomnia severity and the risk of obstructive sleep apnea.
Methods:
Adults with obesity (n = 49) were randomized into one of three groups: 4-h TRF (eating only between 3 and 7 p.m.), 6-h TRF (eating only between 1 and 7 p.m.), or a control group (no meal timing restrictions) for 8 weeks.
Results:
After 8 weeks, body weight decreased (p < 0.001) similarly by 4-h TRF (−3.9 ± 0.4 kg) and 6-h TRF (−3.4 ± 0.4 kg), versus controls. Sleep quality, measured by the Pittsburgh Sleep Quality Index (PSQI), did not change by 4-h TRF (baseline: 5.9 ± 0.7; week 8: 4.8 ± 0.6) or 6-h TRF (baseline: 6.4 ± 0.8; week 8: 5.3 ± 0.9), versus controls. Wake time, bedtime, sleep duration and sleep onset latency also remained unchanged. Insomnia severity did not change by 4-h TRF (baseline: 4.4 ± 1.0; week 8: 4.7 ± 0.9) or 6-h TRF (baseline: 8.3 ± 1.2; week 8: 5.5 ± 1.1), versus controls. Percent of participants reporting obstructive sleep apnea symptoms did not change by 4-h TRF (baseline: 44%; week 8: 25%) or 6-h TRF (baseline: 47%; week 8: 20%), versus controls.
Conclusion:
These findings suggest that 4- and 6-h TRF have no effect on sleep quality, duration, insomnia severity, or the risk of obstructive sleep apnea.
Keywords: Intermittent fasting, time restricted feeding, sleep quality, insomnia, obstructive sleep apnea, obesity, weight loss
Introduction
Time restricted feeding (TRF) is a type intermittent fasting that has gained substantial popularity over recent years (Chaix et al., 2019). The diet involves confining the period of eating to 4–10 h and water fasting (with zero calorie beverages permitted) for the rest of the day. Although these diets have shown favorable effects for body weight and metabolic health, only a handful of studies have examined the effect of TRF on sleep (Gabel et al., 2018; Gill and Panda, 2015; Hutchison et al., 2019; Wilkinson et al., 2020). In a recent study by Wilkinson et al. (2020), 10-h TRF improved morning restfulness but had no effect on sleep quality after 12 weeks in participants with metabolic syndrome. In another study of 10-h TRF by Gill and Panda (2015), overweight participants experienced improved sleep quality after 16 weeks of intervention. In contrast, Gabel et al. (2018) demonstrated no change in sleep quality or sleep duration after 12 weeks of 8-h TRF in participants with obesity. Similarly, Hutchison et al. (2019) showed no effect of 9-h TRF on sleep duration in men with obesity. In view of these equivocal findings, the effects of TRF on sleep still remain uncertain.
We recently performed a study to examine the effect of shorter eating windows during TRF (4 h v. 6 h) on energy intake and body weight (Cienfuegos et al., 2020). Results reveal that both TRF interventions reduced energy intake by 550 kcal/day and body weight by 3%, after 8 weeks. Interestingly, these reductions in energy intake occurred without calorie counting. Having to consistently monitor energy intake is a key reason for subject attrition during daily calorie restriction (CR) protocols (Dansinger et al., 2005; Das et al., 2007). TRF diets are able to side-step this requirement by permitting participants to simply ‘watch the clock’ instead of monitoring energy intake. This feature of TRF diets has the potential to greatly increase long-term adherence to this weight loss regimen.
Accumulating evidence suggests that even mild reductions in body weight are related to improved sleep quality and duration (Ashrafian et al., 2015; Koren and Taveras, 2018; Martin et al., 2016). Small decreases in body mass index (BMI) have also been shown to lessen insomnia severity and reduce the risk of obstructive sleep apnea (Ashrafian et al., 2015; Peppard et al., 2000). As such, we were interested in seeing if the mild reductions in body weight by the 4- and 6-h TRF interventions could improve sleep, versus a weight stable control group.
Accordingly, the aim of this secondary analysis was to examine the effect of 4- and 6-h TRF on various sleep parameters. We hypothesized that the weight loss induced by 4- and 6-h TRF would increase sleep quality and duration, while decreasing insomnia severity and the risk for obstructive sleep apnea, when compared to weight-stable controls.
Methods
Subject selection
This is a secondary analysis of a 10-week randomized parallel-arm trial comparing the effects of 4- and 6-h TRF versus controls on body weight in adults with obesity (Cienfuegos et al., 2020). Inclusion criteria were as follows: female; male; BMI between 30.0 and 49.9 kg/m2; age between 18 and 65 years; sedentary (light exercise less than 1 h per week) or moderately active (moderate exercise 1–2 h per week); weight stable for > 3 months prior to the beginning of the study (gain or loss < 4 kg); and able to give written informed consent. Participants who were smokers, diabetic, taking weight loss medications, night-shift workers and pregnant were excluded. Perimenopausal women were also excluded since this stage of menopause is associated with altered sleep (Ciano et al., 2017). Menopausal status was determined using the Stages of Reproductive Aging Workshop classification (Harlow et al., 2012). Women were classified in three groups according to self-reported menstruation pattern: premenopausal (regular menses), perimenopausal (irregular menses, with differences on cycle length over seven days or amenorrhea until one year) or postmenopausal (absence of menses for over one year). The University of Illinois Chicago Office for the Protection of Research Participants approved the experimental protocol, and all research participants gave their written informed consent to participate in the trial.
TRF protocol
Participants were randomized by a stratified random sample (based on age, sex and BMI) into one of three groups: 4-h TRF, 6-h TRF, or a no-intervention control group, as previously described (Cienfuegos et al., 2020). Briefly, the trial consisted of a 2-week baseline weight stabilization period followed by an 8-week TRF intervention period. During the 8-week intervention, the 4-h TRF group was instructed to eat ad libitum from 3 to 7 p.m. daily, and fast from 7 to 3 p.m. (20-h fast). The 6-h TRF group was instructed to eat ad libitum from 1 to 7 p.m. daily, and fast from 7 to 1 p.m. (18-h fast). During the feeding windows, TRF participants were not required to monitor caloric intake and there were no restrictions on types or quantities of foods consumed. During the fasting window, TRF participants were encouraged to drink plenty of water and were permitted to consume energy-free beverages, such as black tea, coffee and diet sodas.
Control subject protocol
Controls were instructed to maintain their weight throughout the trial, and not to change their eating, meal timing or physical activity habits. Controls received no diet advice but visited the research center at the same frequency (every week) as the intervention groups to alleviate any investigator-interaction bias.
Body weight, diet compliance, energy intake and physical activity
Body weight was assessed to the nearest 0.25 kg every week without shoes and in light clothing using a digital scale (HealthOMeter, Boca Raton, FL). Body composition (fat mass, lean mass, visceral fat mass) was measured at baseline and at week 8 using dual X-ray absorptiometry (DXA; iDXA, General Electric Inc). Adherence to the 6- and 4-h TRF windows was measured using a daily adherence log, which recorded the times each subject started and stopped eating each day. If the log indicated that the subject ate within the appropriate 6- or 4-h window, that day was labeled ‘adherent’. If the log indicated that the subject consumed food outside of the 6- or 4-h feeding windows, that day was labeled as ‘non-adherent’. Adherence to the TRF diet was assessed as the number of adherent days per week. Energy intake at baseline (pre-intervention) and week 8 was assessed by a 7-day food record. The total daily intake of energy was calculated using the food analysis program, Nutritionist Pro (Axxya Systems, Stafford, TX). All participants were asked to maintain their level of physical activity throughout the entire trial. Activity level (steps/day) was measured over a 7-day period during baseline and at week 8 by Fitbit Alta HR (Fitbit, San Francisco, CA).
Sleep measures
All sleep questionnaires were administered during the baseline period (pre-intervention) and at week 8 (last week of intervention). Sleep quality, timing, duration and sleep onset latency were measured by the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). This 19-item self-report measures total sleep quality in the past month, yielding a total score of 0–21. A PSQI total score greater than 5 indicates poor sleep quality. The PSQI questionnaire was also used to assess usual bedtime, usual wake time, and hours of actual obtained sleep. Sleep onset latency was also measured by the PSQI (component 2 in the questionnaire). The sleep latency score ranges from 0–3, with 0 indicating no problem falling asleep, and 3 indicating a severe problem. The severity of insomnia in the past week was measured by the Insomnia Severity Index (ISI), which is a 7-item questionnaire (Bastien et al., 2001). Each item is rated by a 5-point Likert scale (where 0 indicates no problem, and 4 indicates a very severe problem) yielding a total score of 0–28. The total score for the ISI is interpreted as follows: no clinically significant insomnia (0–7); sub-threshold insomnia (8–14); moderate severity insomnia (15–21); and severe insomnia (22–28). Risk of obstructive sleep apnea (% occurrences) was estimated using the Berlin Questionnaire (Chung et al., 2008).
Statistical analysis
All data are presented as means ± SEM. Statistical analyses were performed using SPSS v.25.0 for Mac (SPSS Inc.). A two-tailed p value of less than 0.05 was considered statistically significant. Data were analyzed for completers only. Tests for normality were included in the model, and all data were found to be normally distributed. At baseline, differences between treatment arms (4-h TRF, 6-h TRF and control) were tested by a one-way ANOVA with a Tukey post-hoc test (continuous variables) or McNemar test (categorical variables). Repeated measures ANOVA with groups (4-h TRF, 6-h TRF and control) as the between-subject factor and time (baseline and 8) as the within-subject factor was used to compare changes in dependent variables between the groups over time.
Results
Subject baseline characteristics and dropouts
As previously reported (Cienfuegos et al., 2020), 82 participants were assessed for eligibility and 24 were excluded because they did not meet one or more inclusion criteria. A total of 58 participants were randomized into the 4-h TRF group (n = 19), 6-h TRF group (n = 20), or the control group (n = 19). At the end of the trial, the number of completers was as follows: 4-h TRF group, 16; 6-h TRF group, 19; the control group, 14. At baseline, there were no significant differences between groups for any parameter (i.e. body weight, body composition, compliance, physical activity, or sleep (Table 1)). Participants who completed the study were primarily middle-age, women with obesity who were normotensive and normocholesterolemic but insulin resistant (Cienfuegos et al., 2020).
Table 1.
Body weight, body composition and sleep variables after 8 weeks of time restricted feeding (TRF).
| 4-h TRF (n = 16) | 6-h TRF (n = 19) | Controls (n = 14) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 8 | Changea | Baseline | Week 8 | Changea | Baseline | Week 8 | Changea | |
| Demographics (n) | |||||||||
| Age | 49 ± 2 | 46 ± 3 | 45 ± 2 | ||||||
| Sex (female/male) | 14/2 | 18/1 | 12/2 | ||||||
| Anthropometries | |||||||||
| Body weight (kg) | 101.0 ± 4.8 | 97.1 ± 3.6* | −3.9 ± 0.4 | 99.3 ± 4.6 | 95.9 ± 4.4* | −3.4 ± 0.4 | 92.7 ± 4.5 | 92.9 ± 4.4 | 0.2 ± 0.5 |
| Fat mass (kg) | 48.4 ± 2.8 | 45.6 ± 3.4* | −2.8 ± 0.4 | 47.5 ± 3.4 | 46.1 ± 3.4* | −1.4 ± 0.3 | 42.5 ± 3.3 | 41.9 ± 3.2 | −0.6 ± 0.4 |
| Lean mass (kg) | 52.4 ± 2.3 | 51.6 ± 2.2 | −0.8 ± 0.4 | 50.2 ± 2.6 | 48.7 ± 2.6 | −1.5 ± 0.2 | 47.6 ± 2.8 | 47.3 ± 2.8 | −0.3 ± 0.2 |
| Visceral fat mass (kg) | 1.4 ± 0.2 | 1.2 ± 0.1 | −0.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | −0.1 ± 0.1 | l.l ± 0.2 | l.l ± 0.2 | 0 ± 0.1 |
| Compliance with TRF (day/week) | 6.2 ± 0.2 | 6.2 ± 0.1 | |||||||
| Energy intake (kcal/day) | 1752 ± 196 | 1224 ± 185* | −528 ± 102 | 1931 ± 222 | 1365 ± 126* | −566 ± 142 | 1638 ± 121 | 1533 ± 125 | −105 ± 52 |
| Steps/day | 7787 ± 859 | 7190 ± 669 | −597 ± 702 | 7312 ± 659 | 7365 ± 778 | 53 ± 457 | 9477 ± 736 | 9836 ± 883 | 359 ± 533 |
| Pittsburgh Sleep Quality Index (PSQI) | |||||||||
| Total PSQI sleep quality score | 5.9 ± 0.7 | 4.8 ± 0.6 | −l.l ± 0.8 | 6.4 ± 0.8 | 5.3 ± 0.9 | −l.l ± 0.5 | 6.7 ± 0.7 | 6.5 ± 0.7 | −0.2 ± 1.1 |
| Wake time (h:min) | 5:40 ± 0:20 | 5:40 ± 0:20 | 0:00 ± 0:20 | 6:30 ± 0:25 | 6:25 ± 0:20 | −0:05 ± 0:25 | 5:35 ± 0:30 | 5:45 ± 0:25 | 0:10 ± 0:25 |
| Bedtime (h:min) | 22:40 ± 0:20 | 22:30 ± 0:20 | −0:10 ± 0:20 | 22:40 ± 0:25 | 22:35 ± 0:30 | −0:05 ± 0:30 | 22:50 ± 0:30 | 22:45 ± 0:30 | −0:05 ± 0:30 |
| Sleep duration (h) | 7.0 ± 0.3 | 7.2 ± 0.2 | 0.2 ± 0.2 | 7.8 ± 0.3 | 7.8 ± 0.3 | 0 ± 0.2 | 6.8 ± 0.4 | 7.0 ± 0.3 | 0.2 ± 0.3 |
| Sleep latency score | 0.67 ± 0.16 | 0.79 ± 0.15 | 0.12 ± 0.18 | 1.00 ± 0.23 | 1.00 ± 0.24 | 0 ± 0.13 | 0.85 ± 0.15 | 0.62 ± 0.21 | −0.23 ±0.17 |
| Insomnia severity index (ISI) | |||||||||
| Total score | 4.4 ± 1.0 | 4.7 ± 0.9 | 0.3 ± 0.9 | 8.3 ± 1.2 | 5.5 ± l.l | −2.8 ± 1.0 | 6.5 ± 1.2 | 6.9 ± 1.5 | 0.4 ± 1.2 |
| Berlin questionnaire | |||||||||
| High risk of obstructive sleep apnea (%)b | 44 | 25 | −19 | 47 | 20 | −27 | 46 | 54 | 8 |
Continuous variables reported as mean ± SEM.
Absolute change score from baseline to week 8.
Risk of obstructive sleep apnea reported as % occurrences.
No significant differences between groups for any parameter at baseline (ANOVA for continuous variables; McNemar test for categorical variables).
Significantly different from control group using repeated measures ANOVA (p < 0.05, group × time interaction).
Body weight, diet compliance, energy intake, and physical activity
Body weight decreased (p < 0.001, group × time interaction) in the 4- and 6-h TRF groups, relative to controls, from baseline to week 8 (Figure 1(a)). Fat mass decreased (p < 0.05, group × time interaction) in the 4- and 6-h TRF groups, relative to controls, from baseline to week 8. Lean mass and visceral fat mass remained unchanged (no group × time interaction) in the TRF groups, versus controls. Compliance with the TRF interventions was excellent. On average, participants in the 4- and 6-h TRF groups reported being compliant with their feeding windows on 6.2 ± 0.2 day/week and 6.2 ± 0.1 day/week, respectively, during the 8-week trial (Table 1). Energy intake decreased (p < 0.01, group × time interaction) in the TRF groups, versus controls, from baseline to week 8 (Table 1). Physical activity, measured as steps/day, did not change (no group × time interaction) over the course of the trial in any group (Table 1). Physical activity level was ‘low to somewhat active’ (5000–9999 steps/day) (Sisson et al., 2012) at baseline and week 8, in each group.
Figure 1.
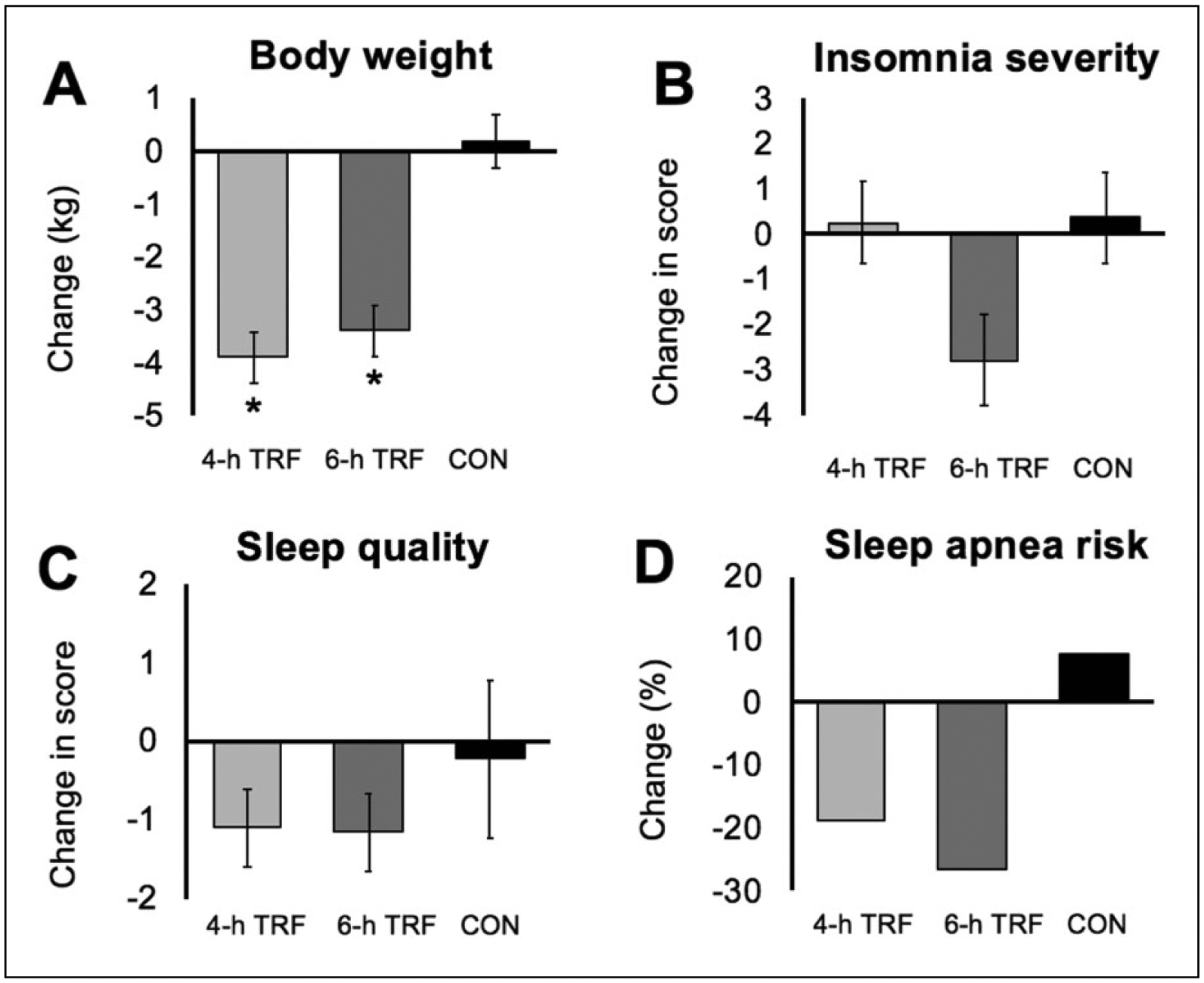
Change in (a) body weight, (b) insomnia severity, (c) sleep quality and (d) risk of obstructive sleep apnea after 8 weeks of 4-h versus 6-h time restricted feeding (TRF). Continuous variables reported as mean ± SEM. Risk of obstructive sleep apnea reported as % occurrences. (a) Body weight decreased similarly (p < 0.001, group × time interaction) in the 4- and 6-h TRF, versus controls. (b) Insomnia severity did not change in the 4- and 6-h TRF groups, versus controls (no group × time interaction). (c) Sleep quality (Pittsburgh Sleep Quality Index, PSQI) score did not change in the 4- and 6-h TRF groups, versus controls (no group × time interaction). (d) Risk of obstructive sleep apnea did not change in the 4- and 6-h TRF groups versus controls (no group × time interaction).
Sleep measures
Sleep quality, timing, duration and sleep latency were measured by the PSQI: a total score greater than 5 indicates poor sleep quality (Buysse et al., 1989). The average scores for PSQI were 5.9 ± 0.7 for 4-h TRF, 6.4 ± 0.8 for 6-h TRF and 6.7 ± 0.7 for controls, indicating poor sleep quality in all groups at baseline (Table 1). After 8 weeks of intervention, sleep quality scores did not change significantly (no group × time interaction) in either TRF group relative to controls (Figure 1(c)). Wake time, bedtime and sleep duration did not change (no group × time interaction) over the course of the study in any group (Table 1). The PSQI sleep latency score indicated little trouble falling asleep in all groups at baseline (Table 1). There was no significant change in sleep latency (no group × time interaction) by week 8 in the 4- and 6-h TRF groups, versus controls. After 8 weeks of intervention, insomnia severity scores did not change significantly (no group × time interaction) in the 4- or 6-h TRF group, relative to controls (Figure 1(b)). Risk for obstructive sleep apnea was present in 44% of 4-h TRF participants, 47% of 6-h TRF participants and 46% of controls, at baseline (Table 1). By week 8, the risk of obstructive sleep apnea did not change (no group × time interaction) in the 4- or 6-h TRF groups, versus controls (Figure 1(d)).
Discussion
This study is the first to compare the effects of 4-h versus 6-h TRF on sleep in adults with obesity. Contrary to our hypothesis, the mild weight loss (3%) induced by 4- and 6- h TRF did not increase sleep quality and duration, or decrease insomnia severity and the risk for obstructive sleep apnea, versus weight-stable controls.
The goal of this exploratory analysis was to compare the effects of two popular forms of TRF (4-h versus 6-h TRF) on sleep. Weight loss has been shown to improve sleep in adults with obesity (Ashrafian et al., 2015; Koren and Taveras, 2018; Martin et al., 2016). Since our original study (Cienfuegos et al., 2020) observed body weight reductions of 3% with 4- and 6-h TRF, we were interested in seeing if this degree of weight loss would improve sleep. After 8 weeks of intervention, sleep quality remained unchanged in both the 4- and 6-h TRF groups. This finding is similar to what has been reported in other TRF studies. For instance, Gabel et al. (2018) observed no effect on sleep quality after 12 weeks of 8-h TRF, despite 3% weight loss. Wilkinson et al. (2020) also reported no change in sleep quality during 10-weeks of 10-h TRF, with 3% weight loss. There are several reasons that may explain why sleep quality was not affected by these fasting interventions. First, the weight loss observed by TRF is quite minimal (3–4% weight loss over 8–12 weeks (Cienfuegos et al., 2020; Gabel et al., 2018; Wilkinson et al., 2020)). It is possible that at least 5% weight loss may be necessary to see changes in sleep quality (Alfaris et al., 2015; Martin et al., 2016). Second, the participants in previous TRF studies were, for the most part, ‘good sleepers’ at baseline (PSQI score < 5 (Buysse et al., 1989)). As such, it is not surprising that their sleep habits did not further improve by the end of the study. Sleep duration also did not change during either the 4- or 6-h TRF intervention. However, our participants had a mean sleep duration of 7 h per night, which is in line with the 7 h minimum recommended by the National Sleep Foundation (Hirshkowitz et al., 2015). Sleep duration also remained unaltered in the 8-h TRF study by Gabel et al. (2018) and the 9-h TRF study by Hutchison et al. (2019).
The impact of these TRF regimens on insomnia severity and sleep onset latency was also assessed. After 8 weeks of intervention, we observed no change in insomnia severity or sleep onset latency in either the 4- or 6-h TRF groups, versus controls. We postulated that TRF may improve insomnia, as fasting for 2–3 hours before bedtime can have beneficial effects on one’s ability to fall asleep (Chaix et al., 2019). More specifically, a short fast before sleep can help an individual abstain from eating fatty or acidic foods, which could help to reduce acid reflux and nighttime heartburn. This, in turn, could lower rates of insomnia (Lim et al., 2018; Surdea-Blaga et al., 2019). On the other hand, it is possible that TRF may have some negative effects on sleep. Not eating for several hours before bed could lead a person to become very hungry. This increase in hunger could hinder their ability to fall asleep, as they may be preoccupied with thoughts of wanting to eat. Whether TRF has positive or negative effects on insomnia severity remains unclear due to the paucity of data in this area. It will be important for future studies of TRF to evaluate how various durations of fasting before sleep impact insomnia severity and sleep onset latency.
The risk of obstructive sleep apnea also did not change in the present study, relative to controls. The lack of a significant effect for this parameter was somewhat surprising as the percent of participants reporting sleep apnea decreased from 40% to 20% in both TRF groups by week 8. It is possible, however, that our study was underpowered to detect significant differences versus controls.
Our study has several limitations. First, our sample size was small (n = 49). Since our power calculation was based exclusively on body weight, it is likely that this study was not powered adequately to identify significant differences between groups at baseline or at week 8 for these secondary outcome measures of sleep. Second, all measures of sleep were assessed via self-report. This study would have benefitted from the use of wrist actigraphy to provide more objective assessments of rest and activity patterns. Third, although we attempted to exclude perimenopausal women, there is the possibility that some of these women were accidentally included. Since perimenopause is associated with increased insomnia (Ciano et al., 2017), the accidental inclusion of these women could have had a confounding effect on our insomnia severity data. Fourth, we did not control the time-of-day physical activity was performed. Exercising earlier in the day may improve the quality of nocturnal sleep (Dolezal et al., 2017). These points should be taken into consideration when interpreting these data.
Conclusion
In summary, these preliminary findings suggest that 4- and 6-h TRF have no effect on sleep quality, duration, insomnia severity or the risk for obstructive sleep apnea. Although this study showed no positive effects on sleep, it is important to note that these fasting interventions did not negatively impact sleep by worsening sleep quality or increasing insomnia severity. Thus, TRF can be viewed as an effective weight loss strategy that has no adverse impact on sleep in adults with obesity. Nevertheless, a well-powered clinical trial that specifically aims to assess the effect of TRF on sleep will be needed before solid conclusions can be reached.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health, NIDDK, R01DK119783.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical statement
The University of Illinois Chicago Office for the Protection of Research Subjects approved the experimental protocol (IRB #2018-1525), and all research participants gave their written informed consent to participate in the trial.
Trial registration: Clinicaltrials.gov NCT03867773
References
- Alfaris N, Wadden TA, Sarwer DB, et al. (2015) Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity (Silver Spring) 23: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, Toma T, Rowland SP, et al. (2015) Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses. Obesity Surgery 25: 1239–1250. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A and Morin CM (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2: 297–307. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, et al. (1989) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Chaix A, Manoogian ENC, Melkani GC, et al. (2019) Time-restricted eating to prevent and manage chronic metabolic diseases. Annual Review of Nutrition 39: 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Yegneswaran B, Liao P, et al. (2008) Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology 108: 822–830. [DOI] [PubMed] [Google Scholar]
- Ciano C, King TS, Wright RR, et al. (2017) Longitudinal study of insomnia symptoms among women during perimenopause. Journal of Obstetric, Gynecologic and Neonatal Nursing 46: 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos S, Gabel K, Kalam F, et al. (2020) Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metabolism 32: 366–378 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansinger ML, Gleason JA, Griffith JL, et al. (2005) Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 293: 43–53. [DOI] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, et al. (2007) Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. American Journal of Clinical Nutrition 85: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Dolezal BA, Neufeld EV, Boland DM, et al. (2017) Interrelationship between sleep and exercise: A systematic review. Advances in Preventive Medicine 2017: 1364387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, Haggerty N, et al. (2018) Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutrition and Healthy Aging 4: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S and Panda S (2015) A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metabolism 22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, et al. (2012) Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 19: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, et al. (2015) National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 1: 40–43. [DOI] [PubMed] [Google Scholar]
- Hutchison AT, Liu B, Wood RE, et al. (2019) Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity (Silver Spring) 27: 50–58. [DOI] [PubMed] [Google Scholar]
- Koren D and Taveras EM (2018) Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism 84: 67–75. [DOI] [PubMed] [Google Scholar]
- Lim KG, Morgenthaler TI and Katzka DA (2018) Sleep and nocturnal gastroesophageal reflux: An update. Chest 154: 963–971. [DOI] [PubMed] [Google Scholar]
- Martin CK, Bhapkar M, Pittas AG, et al. (2016) Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: The CALERIE 2 randomized clinical trial. JAMA: Internal Medicine 176: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, et al. (2000) Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 284: 3015–3021. [DOI] [PubMed] [Google Scholar]
- Sisson SB, Camhi SM, Tudor-Locke C, et al. (2012) Characteristics of step-defined physical activity categories in US adults. American Journal of Health Promotion 26: 152–159. [DOI] [PubMed] [Google Scholar]
- Surdea-Blaga T, Negrutiu DE, Palage M, et al. (2019) Food and gastroesophageal reflux disease. Current Medical Chemistry 26: 3497–3511. [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Manoogian ENC, Zadourian A, et al. (2020) Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metabolism 31: 92–104.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]


