Abstract
Nuclear factor-κB (NF-κB) plays a role in the transcriptional regulation of genes involved in inflammation and cell survival. In this report we demonstrate that NF-κB recruits a coactivator complex that has striking similarities to that recruited by nuclear receptors. Inactivation of either cyclic AMP response element binding protein (CREB)-binding protein (CBP), members of the p160 family of coactivators, or the CBP-associated factor (p/CAF) by nuclear antibody microinjection prevents NF-κB-dependent transactivation. Like nuclear receptor-dependent gene expression, NF-κB-dependent gene expression requires specific LXXLL motifs in one of the p160 family members, and enhancement of NF-κB activity requires the histone acetyltransferase (HAT) activity of p/CAF but not that of CBP. This coactivator complex is differentially recruited by members of the Rel family. The p50 homodimer fails to recruit coactivators, although the p50-p65 heterodimeric form of the transcription factor assembles the integrator complex. These findings provide new mechanistic insights into how this family of dimeric transcription factors has a differential effect on gene expression.
Nuclear factor-κB (NF-κB) is a cytokine-inducible transcription factor that plays a key role in the expression of a variety of genes involved in inflammatory responses and cell survival (1, 2, 11). NF-κB is composed of homo- or heterodimeric complexes of members of the Rel family of proteins, consisting of p65 (Rel A), c-Rel, RelB, p50, and p52. These proteins share a 300-amino-acid region, designated the Rel homology domain, which mediates dimerization and DNA binding. The best studied and most abundant of these complexes is the p50-p65 heterodimer. In most cells NF-κB exists in an inactive form in the cytoplasm, bound to an inhibitory protein, such as IκB-α. Phosphorylation of the inhibitor by an IκB kinase complex results in ubiquitination and degradation of the inhibitor and translocation of p50-p65 to the nucleus, followed by a specific up-regulation in gene expression (19).
NF-κB-dependent gene expression involves a growing family of proteins termed transcriptional coactivators that probably function by facilitating or bridging the sequence-specific activators to the basal transcriptional machinery and altering chromatin structure. The p65 component of NF-κB binds to the coactivator CBP (cyclic AMP response element binding protein [CREB]-binding protein) and its structural homolog p300 (10, 27, 43). Phosphorylation of p65 by protein kinase A (PKA) stimulates NF-κB-dependent gene expression by enhancing p65 association with CBP (42, 43). In addition to p65, CBP interacts with a remarkably diverse group of other signal-dependent transcriptional activators. This has led to proposals that the coactivator functions as a signal integrator by coordinating diverse signal transduction events at the transcriptional level (16). This concept is consistent with recent observations that levels of the CBP homolog, p300, are limiting relative to those of p65 and that competition for CBP may regulate p65 transactivation (15, 29).
NF-κB-dependent gene expression involves a second class of transcriptional coactivators. Steroid receptor-coactivator-1 (SRC-1), or nuclear receptor coactivator-1 (NCoA-1), interacts with p50 and potentiates NF-κB-mediated transactivation (22). This coactivator is a member of a group of related coactivators (the p160 family) that includes SRC-1/NCoA-1, NCoA-2 (also known as transcriptional intermediate factor-2 [TIF-2] or glucocorticoid receptor interaction protein [GRIP-1]), and p300/CBP cointegrator-associated protein (p/CIP) (also known as receptor-associated coactivator-3 [RAC-3], amplified in breast carcinoma [AIB-1], activator of the thyroid and retinoic acid receptors [ACTR], and thyroid hormone activator molecule [TRAM-1]). Many of these coactivators were initially identified as ligand-dependent nuclear-receptor-interacting factors (reviewed in references 12 and 35). The p160 coactivators interact with the nuclear receptors through a series of helical domains that contain a core LXXLL consensus sequence, referred to as LXDs. Each of these domains is sufficient for ligand-dependent interaction with the nuclear receptors (14, 20, 36). To date, the p160 family of coactivators is largely restricted to regulating the nuclear receptors, although most of the coactivators will potentiate the transcriptional activity of several types of nuclear hormone receptors.
CBP has also been identified as a crucial component of nuclear receptor transactivation and has been shown to directly interact with numerous members of the nuclear receptor family. CBP can also associate with members of the p160 family of coactivators. SRC-1/NCoA-1 interacts with CBP through two helical domains that contain the core LXXLL consensus sequence (14, 20, 36). CBP can also associate with the CBP-associated factor (p/CAF) (41) and with RNA polymerase II holoenzyme (23). Thus, CBP recruits a series of coactivators and other components of the transcriptional apparatus to form a large complex which appears to function in nuclear hormone receptor-dependent gene expression (6, 32, 36, 41).
Coactivators may also contribute to transcriptional regulation by modifying chromatin structure. CBP (3, 26) and p/CAF (41) contain histone acetyltransferase (HAT) domains and have strong HAT activities, while SRC-1 (33) and ACTR (6) have weak COOH-terminal HAT activity. Hyperacetylated histones have been identified with transcriptionally active chromatin, whereas the opposite is true of hypoacetylated histones (37). CBP can associate with the members of the p160 family of coactivators, as well as with p/CAF, suggesting that complexes with multiple HAT activities can be formed. Several transcription factors which require the HAT activity of one but not the other of these coactivators have been identified. For example, MyoD and some nuclear receptors require the HAT activity of p/CAF but not that of CBP (28), whereas CREB requires the HAT activity of CBP but not that of p/CAF (17). Therefore, not only is there selectivity in the actual components recruited to a coactivator complex, but there is selectivity in the utilization of the specific functions of these proteins.
In this paper we evaluate the in vivo relevance of NF-κB coactivators and demonstrate that SRC-1/NCoA-1, TIF-2/GRIP-1/NCoA-2, and p/CAF, as well as CBP, play a critical role in NF-κB-dependent transcription. Additionally, we examine the role of the coactivator HAT activities in p65-mediated transactivation and show that the HAT activity of p/CAF, but not that of CBP, is required for NF-κB-mediated gene expression. The results demonstrate that NF-κB-dependent gene expression requires multiple coactivators and that the coactivator complex used by p50-p65 closely resembles that used by some of the nuclear receptors.
MATERIALS AND METHODS
Construction of plasmids.
Expression vectors used for transient transfection experiments are as follows: Rous sarcoma virus (RSV)-CBP-hemagglutinin (HA) (provided by Richard Goodman), cytomegalovirus (CMV)-CBPΔ468, -ΔC/H3, and -ΔC1891 and CMV-E1A-H3N (18); CMV-SRC-1NR/CBP (containing amino acids 615 to 1200; constructed from pGEX-SRC-1λ12); and CMV-SRC-1ΔN (constructed by XbaI/XhoI restriction digestion followed by religation into pcDNA3) (Invitrogen, Carlsbad, Calif.). The p/CAF (HAT−) and CBP (HAT−) expression constructs are described in reference 17. PCX–p/CAF was provided by Yoshihro Nakatani. TIF-2 and GRIP-1 expression vectors were provided by Michael Stallcup.
Transient transfections and reporter assays.
COS-7 cells were obtained from the American Type Culture Collection and cultivated in Dulbecco’s modified Eagle medium (GIBCO/BRL, Gaithersburg, Md.) supplemented with 10% fetal calf serum, 2 mM l-glutamine, and antibiotics. Cells were grown on 6-cm dishes and cultured at 37°C in the presence of 5% CO2. The COS-7 cells were transiently transfected with 1 μg of −578 E-selectin promoter-chloramphenicol acetyltransferase (CAT) and 100 ng of CMV-p65 by a modified calcium phosphate method. Varying concentrations of coactivator expression plasmids were transfected, as described in the figure legends. Samples were balanced for total DNA content with the empty expression vector pCR3 (Invitrogen).
Whole-cell extracts were prepared from the transfected cells, and CAT activity was determined, as previously described (24).
DNA affinity purification.
Thirty picomoles of a biotinylated oligonucleotide (Integrated DNA Technologies, Inc., Coralville, Iowa) containing the two NF-κB binding sites from the beta interferon gene promoter were bound to 30 pM streptavidin-paramagnetic beads (Promega, Madison, Wis.) for 15 min at room temperature in a buffer containing 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 1 M sodium chloride. The beads were washed three times in this buffer and twice in 20 mM HEPES (pH 7.9)–0.5 mM EDTA–100 mM KCl–10% glycerol and were incubated for 30 min at room temperature with either 1 or 3 μg of either His-p50 or His-p50–His-p65. The beads were washed an additional three times and then incubated with 2 mg of K562 nuclear extract (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) for 2 h at 4°C in 20 mM HEPES (pH 7.9)–0.5 mM EDTA–150 mM KCl–10% glycerol. The paramagnetic beads were washed an additional three times with 20 mM HEPES (pH 7.9)–0.5 mM EDTA–150 mM KCl–10% glycerol–0.1% Triton X-100 and then boiled in sodium dodecyl sulfate (SDS) sample buffer for 5 min. The samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide) followed by Western blotting with the following antibodies: rabbit anti-CBP (Santa Cruz Biotechnology Inc.), rabbit anti-p/CAF (Yoshihiro Nakatani), mouse anti-SRC-1/NCoA-1 (Myles Brown), and rabbit anti-c-JUN (Santa Cruz Biotechnology Inc.). Detection was carried out by enhanced chemiluminescence (Amersham Life Science Inc., Arlington Heights, Ill.).
Single-cell microinjection assay.
Rat-1 fibroblasts were seeded on acid-washed glass coverslips at a subconfluent density and grown in MNE–F-12 medium supplemented with 10% fetal bovine serum, gentacin, and methotrexate. Before injection, cells were rendered quiescent by incubation in serum-free medium for 24 to 26 h. Plasmids were injected into the nuclei of cells at 100 μg ml−1. Either preimmune immunoglobulin G (IgG) of the appropriate species or antibodies directed against CBP, SRC-1/NCoA-1, or p/CAF were coinjected, and the injected cells were unambiguously identified. Microinjections were performed with an Eppendorf semiautomated microinjection system mounted on an inverted Zeiss microscope. After overnight incubation, cells were fixed and stained to detect injected IgG and β-galactosidase expression. Injected cells were identified by staining with tetramethylrhodamine-conjugated donkey anti-rabbit IgG.
RESULTS
Multiple functional domains of CBP are required for p65-dependent transactivation.
The p65 subunit of the NF-κB transcription factor was shown to interact with the N terminus of CBP (10, 43). Several functional approaches were taken to establish the relevance of the p65-CBP interaction in vivo and to determine if additional domains of CBP are required for NF-κB-dependent gene expression. These studies included overexpression experiments with intact and mutant forms of CBP, inhibition analysis with the CBP binding protein E1A, and antibody microinjection studies.
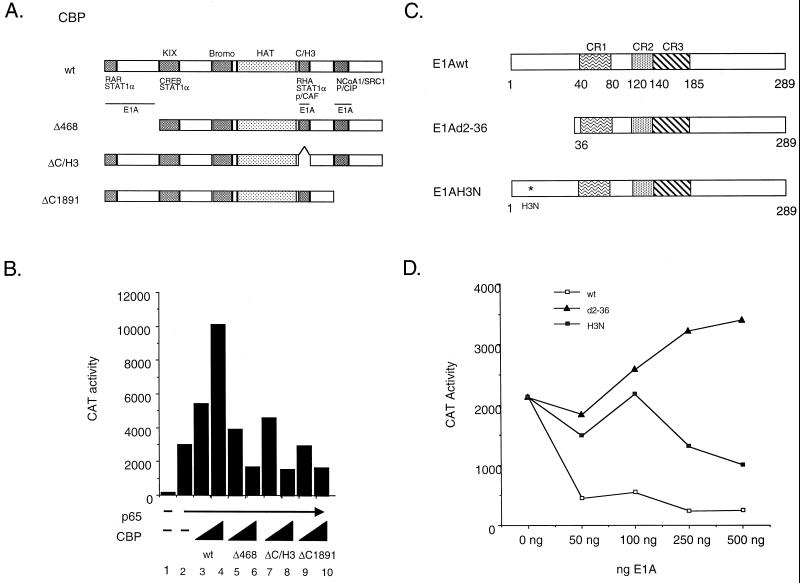
In the first approach, CBP overexpression experiments were performed with intact and specifically altered forms of the coactivator (Fig. 1A). Overexpression of p65 activated transcription from an E-selectin promoter-CAT reporter construct approximately 10-fold (Fig. 1B). As expected, cotransfection of increasing concentrations of intact CBP further stimulated transcription from this reporter construct about fourfold (Fig. 1B). Interestingly, each of the CBP deletion mutants (Δ468, ΔC/H3, and ΔC1891) was incapable of stimulating transcription above that with p65 alone (Fig. 1B; compare lanes 5, 7, and 9 with lane 2). Moreover, at higher concentrations, each of the mutants appeared to have a dominant-negative effect on p65-stimulated transcription (Fig. 1B, lanes 6, 8, and 10). These findings suggest that multiple domains in CBP are required to potentiate p65-dependent transcription.
FIG. 1.
Stimulation of p65-dependent transactivation requires multiple functional domains of CBP. (A) Schematic of CBP wild-type (wt) and mutant expression constructs used in the transient transfection assays. (B) Expression of CBP deletion mutants block p65 transactivation. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT, 250 ng of pcDNA-p65 (lanes 2 to 10), and either 1 or 10 μg of the indicated CBP expression construct. Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The data are representative of three independent experiments performed in duplicate. (C) Schematic of E1A wild-type and mutant expression constructs used in the transient transfection assays. (D) Differential effects of mutated forms of E1A on p65-stimulated reporter gene expression. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT, 250 ng of pcDNA-p65, and the indicated concentrations of the wild-type E1A, E1Ad2-36, or E1AH3N expression construct. Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The data are representative of three independent experiments performed in duplicate.
A second approach to determine the relevance and complexity of the CBP-p65 interaction involves the viral protein E1A. Transcriptional activators whose activity is enhanced by CBP are repressed by the 12S E1A oncoprotein. This inhibitory effect is mediated through direct binding of CBP by E1A. We have shown previously that the activity of the NF-κB transcription factor is inhibited by intact E1A and that this inhibition could be rescued upon overexpression of CBP (10). The E1A-CBP interaction utilizes the highly conserved C/H3 region of CBP (Fig. 1A). In addition, Kurokawa et al. (18) recently identified two additional E1A interaction domains on the CBP molecule, an N-terminal domain located in the first 450 amino acids of the protein, and a C-terminal region (amino acids 2058 to 2163) that corresponds to the SRC-1/NCoA-1 and p/CIP binding sites of CBP (Fig. 1A). We used a previously characterized E1A point mutant (E1A-H3N) which eliminated interaction with the C/H3 region of CBP but not with the N- or C-terminal domain (18) to assess the functional importance of specific CBP domains in stimulating p65-dependent gene expression (Fig. 1C). As expected, the wild-type E1A protein completely inhibited p65 activation of an E-selectin CAT-reporter construct (Fig. 1D). A deletion mutant of E1A (E1Ad2-36), which completely destroys the CBP interaction domain, no longer inhibited p65-mediated transcriptional activity (Fig. 1D). However, the E1A-H3N point mutant was capable of only partially inhibiting p65 activation of the E-selectin CAT-reporter construct. This suggests that E1A inhibits p65-dependent transcription by binding to the C/H3 region of CBP, as well as to either of the two additional E1A interaction domains on the CBP molecule, and inhibiting coactivator function. The findings are consistent with the results of the overexpression studies (Fig. 1B) demonstrating the functional significance of the N-terminal, C/H3, and C-terminal regions of CBP in p65-dependent gene expression. Collectively, these results suggest that multiple domains in CBP are required to potentiate p65-dependent transcription.
CBP, SRC-1/NCoA-1, and p/CAF are required for p65 transcriptional activation.
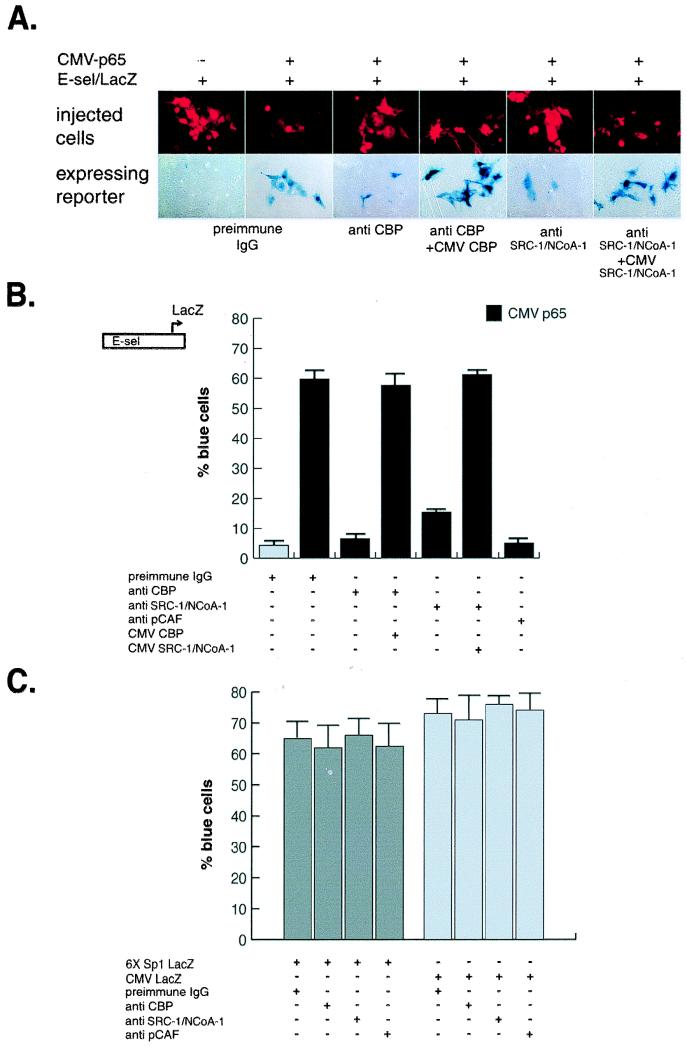
The third approach to demonstrate the in vivo relevance of coactivators to NF-κB-dependent gene expression involved single-cell microinjection studies. Microinjection of a highly specific antibody against CBP completely blocked p65-stimulated expression of an E-selectin promoter-reporter gene (Fig. 2A and B). Coinjection of a CBP expression plasmid reversed the blocking effect of the anti-CBP IgG (Fig. 2A and B). Similar approaches were used to investigate the role of the p160 family of coactivators and p/CAF in p65-dependent gene expression. Microinjection of an anti-SRC-1/NCoA-1 (Fig. 2A and B) antibody inhibited transcriptional activation of the κB-dependent reporter construct. Coinjection of an SRC-1/NCoA-1 expression vector reversed the blocking effect of the anti-SRC-1/NCoA-1 IgG (Fig. 2A and B). These findings suggest that p65-dependent gene expression requires the p160 family member, SRC-1/NCoA-1, as well as CBP. The results are consistent with the observation that overexpression of a CBP mutant lacking the SRC-1/NCoA-1-interacting domain inhibited p65-dependent gene expression (Fig. 1B). Likewise, in the E1A blocking studies (Fig. 1D), binding to the carboxy-terminal p160 interaction domain in CBP (18) partially inhibited p65-dependent gene expression.
FIG. 2.
Coactivator requirements for NF-κB-dependent gene expression. Plasmids consisting of a LacZ reporter under the transcriptional control of the E-selectin promoter were injected into the nuclei of Rat-1 cells in the presence of either preimmune IgG or affinity-purified antibodies to the indicated coactivators. The expression of the reporter plasmid was monitored by staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and quantitated based on the percentage of injected cells that stained blue. (A) Effects of nuclear microinjection of anti-CBP, SRC-1/NCoA-1, and pCAF antibodies on p65-induced E-selectin-lacZ reporter gene expression. Photomicrographs of rhodamine-stained injected cells (top panel) and the corresponding phase-contrast pictures (lower panel) display typical results. (B) Coactivator requirements of NF-κB-dependent gene expression. Results were repeated in three separate experiments with more than 200 cells injected for each data point; data are expressed as means, and error bars represent standard errors of the means. (C) Effect of nuclear microinjection of anti-CBP, SRC-1/NCoA-1, and p/CAF antibodies on Sp1-LacZ and CMV-LacZ reporter constructs.
Microinjection of a specific blocking antibody against p/CAF (amino acids 465 through 832) revealed that this coactivator is also required for p65-stimulated gene expression (Fig. 2B). In control studies, a promoter that was under the control of multiple SP1 sites was unaffected by anti-CBP, anti-SRC-1/NCoA-1 IgG, or anti-p/CAF IgG (Fig. 2C), suggesting that these coactivators are not required for transcription of all promoters. When no specific antibodies were used, preimmune rabbit IgG was used to identify the injected cells and served as a preimmune control (Fig. 2A and B).
Simultaneous overexpression of CBP or some of the members of the p160 family of coactivators enhances p65 transcriptional activity.
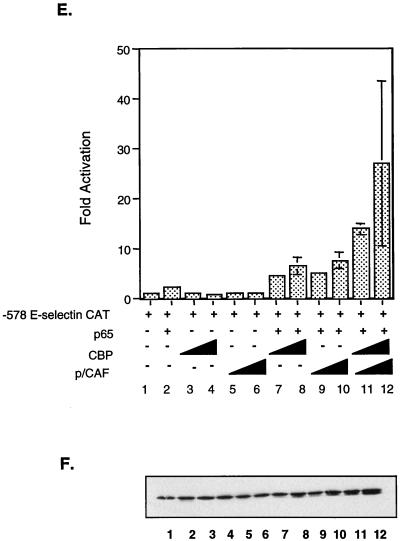
The results from the microinjection experiments described above demonstrate that SRC-1/NCoA-1 and p/CAF, in addition to CBP, are required for p65-dependent transcriptional activity. Exogenous expression of CBP has been shown to augment NF-κB-dependent transcriptional activity through direct binding of the p65 subunit. In an effort to further elucidate the role of these coactivators in p65-mediated transcriptional regulation of the E-selectin gene, we performed transient transfection studies and monitored reporter activity from an E-selectin-promoter-CAT construct in the presence of p65 and the indicated coactivators. Overexpression of SRC-1/NCoA-1 augmented p65-dependent transcriptional activation approximately threefold (Fig. 3A, bar 10), consistent with recent results that demonstrated a role for SRC-1/NCoA-1 in the activation of a reporter construct containing isolated κB sites (22). As with CBP, enhancement of transcription by SRC-1/NCoA-1 was observed only upon coexpression of p65. Overexpression of both CBP and SRC-1/NCoA-1 enhanced transcription approximately 20-fold (Fig. 3A, bar 12), a more-than-additive effect over the activation observed upon transfection of either coactivator alone. Control studies demonstrate that overexpressed coactivators did not increase production of p65 from the corresponding expression construct (Fig. 3B).
FIG. 3.
Coexpression of CBP and SRC-1/NCoA-1, GRIP-1, TIF-2, or p/CAF enhances p65-mediated transcriptional activity. (A) SRC-1/NCoA-1 potentiates NF-κB-dependent gene expression. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT and 100 ng of pcDNA-p65 (lanes 2, 7, and 8) and either 3.25 μg (lanes 5, 9, and 11) or 6.5 μg (lanes 6, 10, and 12) of CMV-SRC-1/NCoA-1 and/or 3.25 μg (lanes 3, 7, and 11) or 6.5 μg (lanes 4, 8, and 12) of RSV-CBP. Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The level of activity observed upon transfection of E-selectin CAT alone was set at one. The data are presented as means; error bars, standard deviations. (B) Western blot analysis of p65 levels in transfected cell extracts. A portion of each whole-cell extract was separated by SDS–10% PAGE, transferred to nitrocellulose, and probed with a rabbit anti-p65 antibody (Rockland) as described in Materials and Methods. Following incubation with a horseradish peroxidase-conjugated donkey anti-rabbit secondary antibody, the bands were visualized by enhanced chemiluminescence (Amersham Life Science). (C) GRIP-1 and TIF-2 increase p65-dependent gene expression. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT and 100 ng of pcDNA-p65 (lanes 4 to 16) and 1, 2.5, 4, or 6.6 μg of simian virus 40 (SV40)-GRIP-1, SV40-TIF-2, or RSV-CBP. Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The level of activity observed upon cotransfection of E-selectin CAT and p65 was set at one. Data are presented as means; error bars, standard deviations. (D) Western blot analysis of p65 levels in transfected cell extracts performed as described above and in Materials and Methods. (E) p/CAF potentiates p65-dependent transactivation and synergizes with CBP. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin–CAT and 100 ng of pcDNA-p65 (lanes 2 and 7 to 12) and either 3.25 μg (lanes 5, 9, and 11) or 6.5 μg (lanes 6, 10, and 12) of CMV-p/CAF and/or 3.25 μg (lanes 3, 7, and 11) or 6.5 μg (lanes 4, 8, and 12) of RSV-CBP. Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The level of activity observed upon transfection of E-selectin CAT alone was set at one. Data are presented as means; error bars, standard deviations. (F) Western blot analysis of p65 levels in cell extracts performed as described above and in Materials and Methods.
TIF-2 and GRIP-1 (or NCoA-2) are functional homologues of SRC-1/NCoA-1 which have been shown to enhance the ligand-dependent transcriptional activity of some members of the nuclear receptor family (7). We used overexpression studies in an attempt to understand the role of these p160 family members in p65-mediated transcriptional activation. As demonstrated in Fig. 3C, overexpression of either GRIP-1 or TIF-2 augmented p65 activation of an E-selectin CAT reporter. These results indicate that TIF-2 (GRIP-1) and SRC-1/NCoA-1 may be functionally redundant with respect to NF-κB activity. This redundancy has been observed in SRC-1/NCoA-1 knockout mice, where elevated TIF-2 levels may compensate for the loss of SRC-1/NCoA-1, thereby resulting in only a partial resistance to hormone (40).
Since the microinjection experiments demonstrated that p/CAF had a role in p65-dependent activation of an E-selectin reporter construct, we tested the possibility that this protein might also potentiate p65-dependent transcription. We performed transient transfection studies and monitored reporter activity from an E-selectin-promoter-CAT construct in the presence of p65 and increasing amounts of p/CAF. As shown in Fig. 3E (bars 9 and 10), cotransfection of p/CAF potentiated p65-dependent transcription fivefold. The stimulation by the coactivators was observed only upon coexpression of p65 and was similar to the level of stimulation seen with either SRC-1/NCoA-1 or CBP. Cotransfection of both p/CAF and CBP resulted in stimulation of transcription 20- to 30-fold over that observed upon transfection of p65 alone (Fig. 3E, bars 11 and 12). The synergistic response was similar to that seen with SRC-1/NCoA-1 and CBP (Fig. 3A, bar 12). These findings demonstrate that overexpression of either SRC-1/NCoA-1 or p/CAF increases p65-dependent gene expression, and they suggest that both of these coactivators are present in limiting amounts.
Multiple functional domains of SRC-1/NCoA-1 are required for p65 activation of an E-selectin CAT reporter.
The transcriptional coactivator SRC-1/NCoA-1 was originally identified as a nuclear receptor-specific coactivator. However, the data described above demonstrate that SRC-1/NCoA-1 is required for p65-dependent transactivation. Recently this coactivator was shown to interact directly with the p50 subunit of the NF-κB transcription factor (22). The p50-interacting domain was mapped to amino acids 759 to 1141 of SRC-1/NCoA-1, a region overlapping with the CBP-interacting domain (Fig. 4A). In addition to the CBP binding site, SRC-1/NCoA-1 has other functional domains which may contribute to NF-κB-dependent gene expression. The coactivator may stimulate NF-κB-dependent gene expression by providing a transcriptional activating domain or performing an enzymatic function.
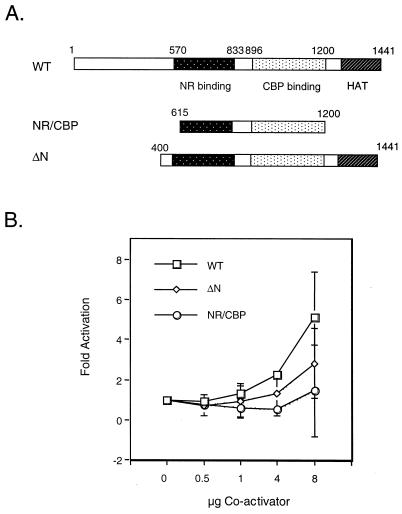
FIG. 4.
NF-κB-dependent transactivation in vitro requires specific functional domains of SRC-1/NCoA-1. (A) Schematic representation of SRC-1/NCoA-1 expression plasmids used in the overexpression experiments. (B) The N terminus of SRC-1/NCoA-1 is required for potentiation of p65-dependent transactivation. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT, 100 ng of pcDNA-p65, and 0.5, 1, 4, or 8 μg of either wild-type CMV-SRC-1/NCoA-1 (WT), CMV-SRC-1-NR/CBP, or CMV-SRC-1 ΔN. Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The level of activity observed upon cotransfection of E-selectin CAT and p65 was set at one. Data are presented as means; error bars, standard deviations.
To test whether additional regions of SRC-1/NCoA-1 are required for activation of NF-κB-mediated transcription, we performed transient transfection studies with a series of SRC-1/NCoA-1 deletion mutants (Fig. 4A). Each mutant was tested for its ability to stimulate transcription of an E-selectin promoter-reporter construct in the presence of overexpressed p65. As shown in Fig. 4B, expression of wild-type SRC-1/NCoA-1 stimulated transcription approximately fivefold over that with p65 alone. Analysis of the SRC-1/NCoA-1 mutants indicated that deletion of both the N and C termini of SRC-1/NCoA-1 resulted in a loss of the coactivator’s ability to stimulate p65-dependent transcription. In addition, deletion of only the N terminus of SRC-1/NCoA-1 destroyed the stimulatory effect seen with the full-length protein. This suggests that either the C-terminal HAT domain is not required for p65-dependent expression of this reporter construct or both the N-terminal activation domain and the HAT region are required.
LXD2 and LXD4 in SRC-1/NCoA-1 are required for NF-κB-mediated transcription.
SRC-1/NCoA-1 interacts with the nuclear receptors and CBP through a series of helical domains that contain a core LXXLL consensus sequence, referred to as LXDs (36). There are three such LXXLL motifs in the nuclear receptor interaction domain (LXD1 to LXD3) and two in the CBP interaction domain (LXD4 and LXD5). The region encompassing LXD1, LXD2, and LXD3 does not interact with CBP, and conversely, the region containing LXD4 and LXD5 does not interact with liganded nuclear receptors (36). Recently it has been reported that the LXDs are differentially utilized by the nuclear receptors (20). For example, the estrogen receptor utilizes a single LXD, LXD2, whereas the progesterone receptor and peroxisome proliferator-activated receptor γ (PPARγ) utilize LXD1 and LXD2. The amino acid sequence carboxy-terminal to the LXXLL motif, as well as proper spacing between the LXDs, is critical in mediating the interaction with the nuclear receptor AF2 domains (20).
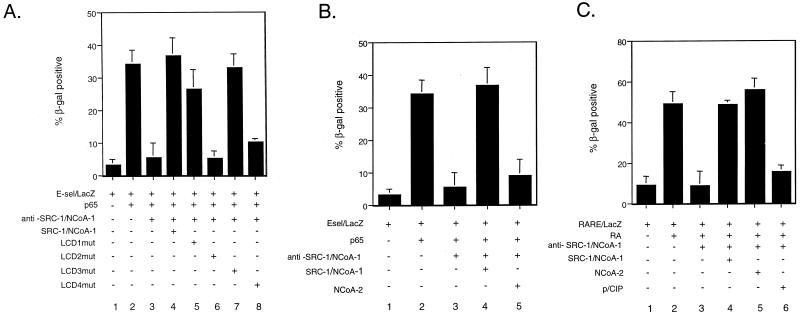
In an effort to understand the role of the SRC-1/NCoA-1 LXDs in NF-κB-dependent transcription, we tested a series of SRC-1/NCoA-1 LXD mutants (LXXLL to LAAAA) for their ability to rescue the inhibitory effect of the anti-SRC-1/NCoA-1 antibody (20). Coinjection of an anti-SRC-1/NCoA-1 antibody blocked the transcription of the E-selectin–LacZ reporter in the presence of p65 (Fig. 5A, bar 3). As expected, the inhibition was completely rescued by coexpression of wild-type SRC-1/NCoA-1 (Fig. 5A, bar 4). Mutation of either LXD1 or LXD3 did not effect the ability of SRC-1/NCoA-1 to rescue the block (Fig. 5A, bars 5 and 7). However, mutation of LXD2 in the nuclear receptor-interacting domain abolished SRC-1/NCoA-1’s ability to rescue the inhibition (Fig. 5A, bar 6). The absolute requirement of LXD2 in NF-κB-mediated transcription is similar to that observed with the estrogen receptor, which also requires LXD2 but not LXD1 or LXD3.
FIG. 5.
Coactivator LXXLL motif specificity in NF-κB-dependent gene expression. (A) The LXD2 and LXD4 domains of SRC-1/NCoA-1 are required for NF-κB-mediated transcription. Plasmids consisting of a LacZ reporter under the transcriptional control of the E-selectin promoter were injected into the nuclei of Rat-1 cells in the presence of either preimmune IgG or an affinity-purified antibody to SRC-1/NCoA-1. The expression of the reporter plasmid was monitored by X-Gal staining and quantitated based on the percentage of injected cells that stained blue. Rescue experiments were performed by coinjecting the indicated expression plasmids. (B) Coinjection of NCoA-2 expression plasmid does not rescue the inhibitory effect of the anti-SRC-1/NCoA-1 antibody on p65-dependent transcription. (C) Coinjection of NCoA-2 expression plasmid rescues the inhibitory effect of the anti-SRC-1/NCoA-1 antibody on RAR-dependent transcription. In all panels, data are means from three separate experiments; error bars represent standard errors of the means.
LXXLL motifs are also required for CBP recruitment to the coactivator complex. As shown in Fig. 5A, mutation of LXD4 in the CBP-interacting domain of SRC-1/NCoA-1 abolished the coactivator’s ability to rescue the inhibition (Fig. 5A, bar 8). The functional requirement of LXD4 in NF-κB-mediated transcription is similar to that observed for thyroid hormone (TR), retinoic acid receptor (RAR), and PPARγ function (20). Taken together, these findings suggest that LXD4 of SRC-1/NCoA-1 is required for both nuclear receptor- and NF-κB-dependent gene expression and that the functional importance of this domain is likely to be linked to the requirement for interaction with CBP.
SRC-1/NCoA-1 and TIF-2/GRIP-1/NCoA-2 have separate and distinct roles in NF-κB-mediated transcription.
The p160 family of coactivators includes SRC-1/NCoA-1, TIF-2/GRIP-1/NCoA-2, and p/CIP/RAC-3/AIB-1/ACTR. The family members are highly homologous, most noticeably in the central LXXLL motifs (LXD1, LXD2, and LXD3). Here, we have demonstrated that both TIF-2 and GRIP-1 (or NCoA-2) enhance p65-dependent transcription in a manner similar to that of SRC-1/NCoA-1. These family members are also able to augment nuclear receptor function. Given the similarity between the family members, the question of whether the proteins were performing redundant functions was examined by antibody microinjection experiments. In Fig. 5B (and Fig. 2), microinjection of anti-SRC-1/NCoA-1 inhibited p65-dependent activation of an E-selectin–LacZ reporter construct. As predicted, the inhibition was rescued upon coinjection of an SRC-1/NCoA-1 expression plasmid (Fig. 5B, bar 4, and Fig. 2). Surprisingly, coinjection of a NCoA-2 expression plasmid was unable to rescue the inhibition (Fig. 5B, bar 5). This is in direct contrast to the ligand-dependent activation of a RAR–LacZ reporter construct (Fig. 5C), where coinjection of either an SRC-1/NCoA-1 or a NCoA-2 expression plasmid rescued the inhibition (Fig. 5C, bars 4 and 5). The third member of the p160 family, p/CIP, was unable to rescue the function (Fig. 5C, bar 6). These data indicate that SRC-1/NCoA-1 and NCoA-2 are functionally distinct with respect to NF-κB transcriptional activation, in contrast to the similar function these p160 family members have in nuclear receptor function.
The HAT activity of p/CAF, but not that of CBP, is required to coactivate p65-dependent transcription.
We have demonstrated that both CBP and p/CAF are required for p65-mediated activation of an E-selectin reporter construct (Fig. 2). Additionally, we have shown that overexpression of these coactivators in the presence of p65 will further stimulate transcription (Fig. 3E). Each of these transcriptional coactivators possesses intrinsic HAT activity (26, 33, 41). Histone acetylation is believed to play a role in transcriptional activation by altering chromatin structure and thereby providing transcription factors with access to the DNA template (34).
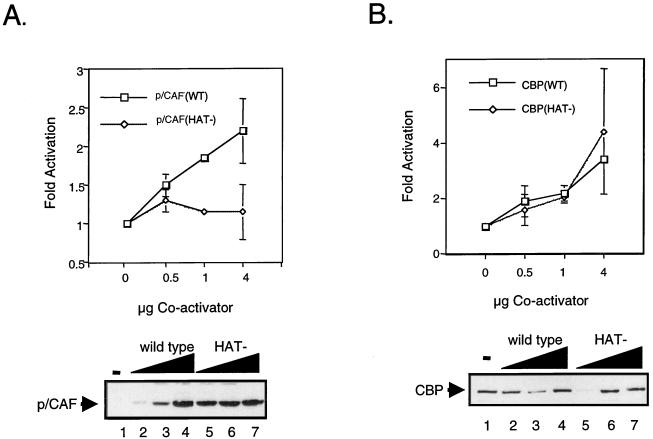
In an effort to understand the role of histone acetylation in p65-dependent transcription, we used mutants of CBP and p/CAF that lacked HAT activity. A substitution of two conserved residues in the acetyl coenzyme A binding site of each protein resulted in complete loss of HAT activity (17). p/CAFHAT− and CBPHAT− mutants were transfected into COS-7 cells in the presence of p65, and transcriptional activity was determined by monitoring CAT activity driven from an E-selectin promoter-reporter construct. Cotransfection of wild-type p/CAF stimulated transcription in a dose-dependent manner (Fig. 6A). The stimulation was more than twofold (with 4 μg of p/CAF) that observed with p65 alone. Cotransfection of p/CAFHAT− failed to augment p65-dependent transactivation of the E-selectin reporter construct (Fig. 6A). Conversely, both wild-type CBP and CBPHAT− (Fig. 6B) were capable of stimulating transcription of the E-selectin reporter construct upon cotransfection of p65 in a dose-dependent manner. In addition, unlike intact p/CAF, the CBP HAT mutant potentiated p65-dependent transcription to approximately the same level as wild-type CBP (more than threefold that of p65 alone at a concentration of 4 μg). Western blot analysis revealed that the mutant proteins were expressed to levels comparable to those of their wild-type counterparts (Fig. 6A and B, lower panels). These data indicate a role for p/CAF-mediated histone acetylation in NF-κB-dependent transactivation.
FIG. 6.
NF-κB-dependent transactivation in vitro requires p/CAF HAT activity. (A) The HAT activity of p/CAF is required for potentiation of p65-dependent transactivation (upper panel). COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT and 100 ng of pcDNA-p65 and 0.5, 1, or 4 μg of either wild-type (WT) CMV-p/CAF or CMV-p/CAF (HAT−). Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The level of activity observed upon cotransfection of E-selectin CAT and p65 was set at one. Data are presented as means; error bars, standard deviations. Representative Western blot analyses of wild-type p/CAF (lower panel, lanes 2 to 4) and HAT− p/CAF (lower panel, lanes 5 to 7) are shown. (B) The HAT activity of CBP/p300 is not required for potentiation of p65-dependent transactivation. COS-7 cells were transiently transfected with 1 μg of −578 E-selectin-CAT, 100 ng of pcDNA-p65, and 0.5, 1, or 4 μg of either wild-type (WT) RSV-CBP or CMV-CBP (HAT−). Forty-eight hours posttransfection, the cells were harvested and CAT activity was assayed as described in Materials and Methods. The level of activity observed upon cotransfection of E-selectin CAT and p65 was set at one. Data are presented as the means; error bars, standard deviations. Representative Western blots of wild-type CBP (lower panel, lanes 2 to 6) and HAT− CBP (lower panel, lanes 7 to 11) are shown. (C) HAT requirements for NF-κB-dependent gene expression. Plasmids consisting of a LacZ reporter under the transcriptional control of the E-selectin promoter were injected in the nuclei of Rat-1 cells in the presence of either an anti-CBP or an anti-p/CAF antibody. The expression of the reporter plasmid was monitored by X-Gal staining and quantitated based on the percentage of injected cells that stained blue. Rescue experiments were performed by coinjecting the indicated p/CAF or CBP expression plasmid.
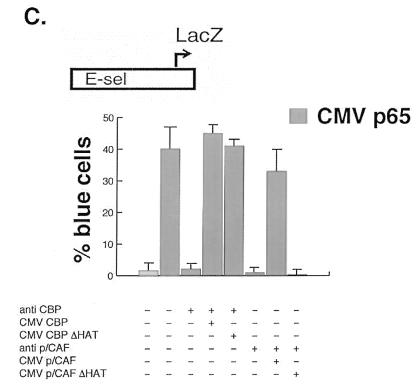
To demonstrate the in vivo relevance of the selective requirement for coactivator HAT activity in NF-κB-dependent gene expression, single-cell nuclear microinjection studies were used. Microinjection of a highly specific antibody against CBP completely blocked p65-stimulated expression of an E-selectin promoter-reporter gene (Fig. 6C; also see Fig. 2). Coinjection of a CBP expression plasmid reversed the blocking effect of the anti-CBP IgG (Fig. 6C). Coinjection of a vector directing expression of the CBP HAT mutant also reversed the blocking effect of the CBP antibody and fully rescued p65-stimulated reporter gene expression (Fig. 6C). Similar approaches were used to investigate the role of p/CAF HAT activity in p65-dependent gene expression. Microinjection of a specific blocking antibody against p/CAF revealed that this coactivator is also required for p65-stimulated gene expression (Fig. 6C; also see Fig. 2). Coinjection of a p/CAF expression plasmid reversed the blocking effect of the anti-p/CAF IgG (Fig. 6C). In contrast to the findings with CBP, coinjection of a vector directing expression of the p/CAF HAT mutant did not overcome the effects of the antibody and did not rescue p65-stimulated gene expression (Fig. 6C). The results of the microinjection experiments are consistent with those of the overexpression studies and demonstrate a role for p/CAF-mediated histone acetylation in NF-κB-dependent transactivation. This selective requirement for p/CAF HAT activity is similar to that found for nuclear receptor-dependent gene expression (17).
CBP, SRC-1, and p/CAF are recruited to the DNA-bound p50-p65 heterodimer.
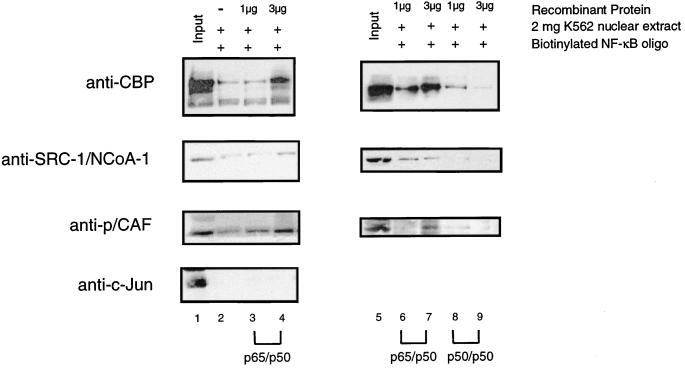
The Rel family members form a series of dimers with different abilities to activate transcription. For example, the p50-p65 heterodimer activates transcription, while the p50 homodimer has been associated with transcriptional repression (11). To determine whether members of the Rel family could differentially recruit the coactivator complex, we performed DNA affinity purification experiments with an oligonucleotide containing two NF-κB binding sites. The biotinylated oligonucleotide was bound to streptavidin-conjugated paramagnetic beads and incubated with recombinant histidine-tagged p50 and p65 at varying concentrations. Following removal of any unbound p50-p65, the magnetic beads were incubated with K562 nuclear extract and the bound proteins were identified by Western blotting. As shown in Fig. 7, lane 1, each of the coactivators was detected by Western blotting in the K562 nuclear extract. Upon addition of increasing concentrations of p50-p65 heterodimer, we observed an increase in the amount of bound CBP, SRC-1/NCoA-1, and p/CAF (Fig. 7, lanes 2 to 4). As a control for nonspecific binding, we blotted for c-Jun, and although there was a significant amount of this protein present in the input, we observed very little binding to the DNA-bound p50-p65. In order to rule out nonspecific binding to the streptavidin-paramagnetic beads, we incubated the K562 nuclear extract with the paramagnetic beads in the absence of any p50-p65 protein. Very little binding of CBP, SRC-1/NCoA-1, or p/CAF to the paramagnetic beads was observed (data not shown). These observations indicate that CBP, SRC-1/NCoA-1, and p/CAF are recruited to DNA through NF-κB sites, thereby forming a complex containing multiple coactivator proteins.
FIG. 7.
CBP/p300, SRC-1/NCoA-1, and p/CAF are recruited to a DNA-bound p65-p50 heterodimer. (Left panel) Coactivators are recruited to a DNA-bound p65-p50 heterodimer. A biotinylated oligonucleotide containing two NF-κB binding sites was bound to streptavidin-paramagnetic beads and loaded with 0 μg (lane 2), 1 μg (lane 3), or 3 μg of His-p50–His-p65 (lane 4). After a wash, the DNA-bound heterodimers were incubated with 2 mg of K562 nuclear extracts and then washed, and bound proteins were identified by Western blotting as described in Materials and Methods. Lane 1 represents 200 μg, or 1/10 of the total K562 input. The antibodies used for Western blotting are indicated on the left. (Right panel) The p50 homodimer inhibits recruitment of CBP/p300, SRC-1/NCoA-1, and p/CAF. A biotinylated oligonucleotide containing two NF-κB binding sites was bound to streptavidin-paramagnetic beads and loaded with 1 or 3 μg of either p50-p65 (lanes 6 and 7) or the p50–p50 homodimer (lanes 8 and 9). After a wash, the DNA bound dimers were incubated with 2 mg of K562 nuclear extracts and then washed, and bound proteins were identified by Western blot analysis, as described in Materials and Methods. Lane 5 (Input) represents 200 μg, or 1/10, of the total K562 input.
The ability of the p50 homodimer to recruit CBP, SRC-1/NCoA-1, and p/CAF was directly compared with that of the p50–p65 heterodimer by using the DNA affinity purification approach described above. The biotinylated oligonucleotide containing two NF-κB binding sites was bound to streptavidin-paramagnetic beads and preloaded with recombinant dimers of either p50-p65 or p50-p50. In the presence of increasing amounts of the heterodimer, increasing concentrations of CBP, SRC-1/NCoA-1, and p/CAF were recruited to the DNA (Fig. 7, lanes 6 and 7). In contrast, the presence of increasing concentrations of the p50 homodimer was not associated with binding of either CBP, SRC-1/NCoA-1, or p/CAF (Fig. 7, lanes 8 and 9), suggesting that a similar coactivator complex was not recruited. Therefore, the ability of the Rel family members to recruit a coactivator complex is quite different.
DISCUSSION
In this study we have taken several approaches to demonstrate that multiple coactivators are required for NF-κB-dependent gene expression. Notably, microinjection of antibodies against SRC-1/NCoA-1 demonstrated that this member of the p160 family is required for NF-κB-dependent gene expression. Similar in vivo approaches were used to establish that both CBP and p/CAF are essential coactivators. Additionally, we showed that the HAT activity of p/CAF, but not that of CBP, is required for NF-κB-mediated gene expression.
Multiple interactions are involved in the assembly of the NF-κB transcription complex. Transcriptional activation by p65 requires CBP, or its homolog p300, which also exerts an essential coactivator role for many other classes of regulated transcription factors (reviewed in references 5 and 31). Three distinct regions of CBP are required for NF-κB-dependent gene expression. The N terminus of CBP interacts directly with the p65 component of the transcription factor (10, 27, 42). The phosphorylation of p65 by PKA stimulates p65 transcriptional activity by promoting an interaction with two regions of the N terminus of CBP (43). The second critical region of CBP is the C/H3 segment, which interacts with the adenoviral E1A oncoprotein (9); RNA helicase A, which may function in the recruitment of RNA polymerase II holoenzyme complex (23); and p/CAF, which is described below. The third important region of CBP is the C-terminal section of the protein, which interacts with the p160 family of coactivators. Thus, CBP provides a platform for a variety of proteins that are important in NF-κB-dependent gene expression. CBP also has a HAT function that is required for the function of some transcription factors (3, 26), although NF-κB, like some of the nuclear receptors (17), does not require CBP’s HAT activity.
Members of the p160 family of coactivators are also essential for NF-κB-dependent gene expression. To date several distinct but related p160 family members have been characterized, including SRC-1/NCoA-1, TIF-2/GRIP-1/NCoA-2, and p/CIP (also called RAC-3, AIB-1, ACTR, and TRAM-1). SRC-1/NCoA-1 potentiates the transcriptional activity of NF-κB, consistent with recent findings (22). Additionally, SRC-1/NCoA-1 was reported to interact with the p50 component of NF-κB (22). Microinjection of anti-SRC-1/NCoA-1 demonstrates that this coactivator is essential for p65-dependent transactivation in vivo. SRC-1/NCoA-1, like the other members of the p160 family, has a strong transactivation domain located in the amino terminus and a CBP interaction domain. It is possible that binding of the coactivator to the p50 component of NF-κB provides an activation function which facilitates recruitment of CBP. Additionally, interaction of CBP with the p65 subunit of NF-κB may facilitate recruitment of SRC-1/NCoA-1. The second member of the p160 family also stimulates NF-κB-dependent gene expression. Both GRIP-1 and TIF-2 potentiate the transcriptional activity of NF-κB (Fig. 3C). The level of coactivation observed in the presence of either GRIP-1 or TIF-2 alone (Fig. 3C) is higher than that observed for SRC-1/NCoA-1 alone (Fig. 3A). Although, within each experiment, GRIP-1, TIF-2, and SRC-1/NCoA-1 coactivate to the same level as CBP, and Western blot analysis demonstrates that all the coactivators are expressed to similar levels (data not shown), we cannot exclude the possibility that the increased activity is an intrinsic quality of GRIP-1/TIF-2, a result of increased expression or experimental variability. Clearly, however, the function of NCoA-2 (GRIP-1/TIF-2) in NF-κB-dependent gene expression is not redundant with that of SRC-1/NCoA-1 (Fig. 5B). Thus, members of the p160 family of coactivators can increase p65 transactivation as they do transcription by multiple members of the nuclear receptor family.
The CBP-associated protein p/CAF is another important component of the NF-κB coactivator complex. p/CAF is found in a complex with more than 20 associated proteins (38). This protein has a unique amino-terminal domain that is capable of interacting with a variety of proteins, including CBP, SRC-1/NCoA-1, and the nuclear receptors; p/CAF also contains a p/CIP interaction region and a carboxy-terminal HAT domain (18). p/CAF potentiates NF-κB-dependent transactivation, and this effect requires the HAT domain. Microinjection of anti-p/CAF antibodies into living cells blocked p65 transactivation. Collectively, these findings suggest that the p/CAF complex is essential in NF-κB-dependent gene expression both in vitro and in vivo.
Different classes of signal-activated transcription factors require distinct coactivator components, including CBP, the p160 family members, and p/CAF. For example, the steroid-activated nuclear receptors require CBP, SRC-1/NCoA-1, p/CIP, and p/CAF (reviewed in references 12, 30, and 35), whereas cyclic AMP-activated CREB requires CBP, p/CIP, and p/CAF but does not require SRC-1/NCoA-1 (17). Gamma interferon-activated STAT-1 requires the action of CBP and pCIP but does not require either p/CAF or SRC-1/NCoA-1 (36). The NF-κB-dependent coactivator complex requires CBP, SRC-1/NCoA-1, and p/CAF. Interestingly, none of these coactivators can function alone, which suggests that these proteins may be forming functional complexes in vivo. Additionally, overexpression of any of the coactivators leads to activation of transcription, suggesting that these coactivators are limiting in vivo. It is possible that there are inactive, partial coactivator complexes lacking one of the coactivators in vivo and that overexpression of any of these components drives the equilibrium toward formation of the fully competent coactivator complex(es).
NF-κB and nuclear receptors show similarities in their specific requirements for coactivators and their acetyltransferase functions. First, these studies demonstrate that NF-κB activity, like nuclear receptor-dependent gene expression, requires a coactivator complex with the p160 family members. These coactivators have LXDs containing consensus LXXLL motifs that are important for the interaction of the coactivators with both nuclear receptors and CBP (8, 20, 25, 39). For example, a single LXD in SRC-1/NCoA-1 is sufficient for activation of the estrogen receptor, while different combinations of two appropriately spaced LXDs are required for the actions of several of the other nuclear receptors (20). NF-κB-dependent gene expression, like estrogen-dependent gene expression, requires only LXD2 in the nuclear receptor interaction region of SRC-1/NCoA-1. It is possible that the specificity of LXD usage in these two instances may be dictated by a similar mechanism of interaction (8, 20, 25). Additionally, both nuclear receptor- and NF-κB-dependent gene expression involve the LXD4 motif in the CBP recognition domain of SRC-1/NCoA-1. These findings suggest that LXXLL-mediated interactions between the activators and SRC-1/NCoA-1, as well as between the p160 family members and CBP, underlie the assembly of both the NF-κB and the nuclear receptor coactivator complexes. Second, NF-κB and nuclear receptor-dependent gene expression show the same selectivity in the type of HAT activity required for function. The HAT activity of p/CAF, but not that of CBP, appears to be important for activation of NF-κB-dependent gene expression, for nuclear receptor activation (17), and for the muscle-specific transcription factor, MyoD (28). In contrast, the HAT activity of CBP is required for the transcriptional functions of CREB and STAT-1 (17). This suggests that there are common themes in the content of coactivators and the requirements for specific HAT activities among groups of transcription factors. The presence of multiple HAT activities in the coactivator complexes suggests that these HAT activities are restricted to specific substrates, which may include nonhistone proteins. The HAT activity of p300 can acetylate p53, increasing its sequence-specific DNA binding activity (13). Similarly, GATA-1 is acetylated in vitro by the HAT activity of p300 (4). The HAT activities of CBP and p/CAF do not acetylate either the p50 or the p65 component of NF-κB (21) (data not shown). Interestingly, acetylation of the architectural protein high mobility group I(Y) by CBP may decrease its DNA binding activity and destabilize an NF-κB-dependent enhancer complex (21). This may provide an important negative regulatory signal decreasing the expression of a variety of signal-dependent genes.
Although it is possible that the nuclear receptors and NF-κB utilize the same coactivator complex, there could be subtle qualitative differences in the complex or distinct configurations of the specific components of the coactivator complex recruited by the two classes of activators. Several observations support this proposal. First, the p160 family members do not directly interact with p65, and the affinity of the direct interaction between these family members and the p50 component of NF-κB may be less than that seen with the nuclear receptors (data not shown). This suggests that the affinity of the interaction between the individual components is low. It is possible that a complex of factors is formed which is more stable or that additional components are required to stabilize the association. Second, two of the members of the p160 family, SRC-1/NCoA-1 and NCoA-2/TIF-2/GRIP-1, are functionally distinct with respect to NF-κB transcriptional activation (Fig. 5B). This is in striking contrast to the nuclear receptors, where the functions of these two family members overlap (Fig. 5C). Collectively, these findings suggest that the structure and specificity of NF-κB-coactivator interactions may have some unique features not shared with the nuclear receptors.
The members of the Rel family of transcription factor dimers have distinct abilities to recruit this coactivator complex. Although many dimeric transcription factors have similar abilities to activate gene expression, the Rel family members can either activate or repress gene expression depending upon the composition of the dimer. For example, in some promoter contexts the p50 homodimer can repress gene expression. In our studies we determined whether this functional effect on gene expression correlated with the ability to recruit the coactivator complex. Although the p50 component of NF-κB was previously reported to interact directly with the SRC-1/NCoA-1 member of the p160 family in vitro (22), the DNA-bound homodimer was unable to recruit either CBP, the p160 family member, or p/CAF. This is in striking contrast to the p50-p65 heterodimeric form of the transcription factor, which could recruit the integrator complex (Fig. 7). These findings provide new mechanistic insights into how this family of transcription factors has a differential effect on gene expression.
ACKNOWLEDGMENTS
We thank Yoshihiro Nakatani, Michael Stallcup, William Chin, and Myles Brown for providing the indicated reagents. We acknowledge the insightful comments and advice provided by Lou Schiltz and Akira Takeshita. Amy Williams assembled constructs used in some of these studies, and her efforts are appreciated. Kay Case provided excellent assistance with some of the cell culture.
This work was supported by research grants from the National Institutes of Health to D.T., C.K.G., M.G.R., and T.C. D.T. is a member of the Pew Scholars Program, C.K.G., is an Established Investigator of the American Heart Association, and M.G.R. is an Investigator in the Howard Hughes Medical Institute.
REFERENCES
- 1.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 4.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 5.Brownell J E, Allis C D. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184. doi: 10.1016/s0959-437x(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor co-activator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen J D, Li H. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit Rev Eukaryot Gene Expr. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 8.Darimont B D, Wagner R L, Apriletti J W, Stallcup M R, Dushner P J, Baxter J D, Fletterick R J, Yamamoto K R. Structure and specificity of nuclear receptor-co-activator interactions. Genes Dev. 1998;12:3369–3382. doi: 10.1101/gad.12.21.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 10.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional co-activators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh S, May M J, Kopp E B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 12.Glass C K, Rose D W, Rosenfeld M G. Nuclear receptor co-activators. Curr Opin Cell Biol. 1997;9:222–232. doi: 10.1016/s0955-0674(97)80066-x. [DOI] [PubMed] [Google Scholar]
- 13.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 14.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to the nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 15.Hottigen M O, Felzien L K, Nabel G J. Modulation of cytokine-induced HIV gene expression by competitive binding of transcription factors to the co-activator p300. EMBO J. 1998;17:3124–3134. doi: 10.1093/emboj/17.11.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 17.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T M, Glass C K, Rosenfeld M G. Transcription factor-specific recruitment of co-activators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 18.Kurokawa R, Kalafus D, Ogliastro M H, Kioussi C, Xu L, Torchia J, Rosenfeld M G, Glass C K. Differential use of CREB binding protein-coactivator complexes. Science. 1998;279:700–703. doi: 10.1126/science.279.5351.700. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis T. Catalysis by a multiprotein IκB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- 20.McInerney E M, Rose D W, Flynn S E, Westin S, Mullen T M, Krones A, Inostroza J, Torchia J, Nolte R T, Assa-Munt N, Milburn M V, Glass C K, Rosenfeld M G. Determinants of co-activator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998;12:3357–3368. doi: 10.1101/gad.12.21.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN-β expression by disrupting the enhanceosome. Mol Cell. 1998;4:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 22.Na S-Y, Lee S-K, Han S-J, Choi H-S, Im S-Y, Lee J W. Steroid receptor co-activator-1 interacts with the p50 subunit and coactivates nuclear factor κB-mediated transactivation. J Biol Chem. 1998;273:10831–10834. doi: 10.1074/jbc.273.18.10831. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima T, Uchida C, Anderson S F, Le C G, Hurwitz J, Parvin J D, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 24.Neish A S, Read M A, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-κB as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995;15:2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolte R T, Wisely G B, Westin S, Cobb J E, Lambert M H, Kurokawa R, Rosenfeld M G, Wilson T M, Glass C K, Milburn M V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 26.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional co-activators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 27.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 co-activator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 28.Puri P L, Sartorelli V, Yang X-J, Hamaori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y J, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and pCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard K A, Phelps K M, Williams A J, Thanos D, Glass C K, Rosenfeld M G, Gerritsen M E, Collins T. Nuclear integration of glucocorticoid receptor and nuclear factor-κB signaling by CREB-binding protein and steroid receptor co-activator-1. J Biol Chem. 1998;273:29291–29294. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- 30.Shibata H, Spencer T E, Onate S A, Jenster G, Tsai S Y, Tsai M J, O’Malley B W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- 31.Shikama N, Lyon J, La Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 32.Smith C L, Onate S A, Tsai M J, O’Malley B W. CREB binding protein acts synergistically with steroid receptor co-activator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer T E, Jenster G, Burcin M M, Assis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Steroid receptor co-activator-1 is a histone acetyltransferase. Nature. 1997;289:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 34.Struhl K. Chromatin structure and RNA polymerase II connection: implications for transcription. Cell. 1996;84:179–182. doi: 10.1016/s0092-8674(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 35.Torchia J, Glass C K, Rosenfeld M G. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 36.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 37.Turner B M. Histone acetylation as an epigenetic determinant of long-term transcriptional competence. Cell Mol Life Sci. 1998;54:21–31. doi: 10.1007/s000180050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vassilev A, Yamauchi J, Kotani T, Prives C, Avantaggiati M L, Qin J, Nakatani Y. The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol Cell. 1998;2:869–875. doi: 10.1016/s1097-2765(00)80301-9. [DOI] [PubMed] [Google Scholar]
- 39.Westin S, Kurokowa R, Nolte R T, Wisely G B, McInerney E M, Rose D W, Milburn M V, Rosenfeld M G, Glass C K. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature. 1998;305:199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Qiu Y, DeMayo F J, Tsai S Y, Tsai M-J, O’Malley B W. Partial hormone resistance in mice with disruption of the steroid receptor co-activator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 41.Yang X J, Ogryzkao V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 42.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Gosh S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Voll R E, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the co-activator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]