Abstract
Aims: Advanced glycation end products (AGEs) were reported to be correlated with the development of diabetes, as well as diabetic vascular complications. Therefore, this study aimed at investigating the association between AGEs and lower-extremity atherosclerotic disease (LEAD).
Methods: A total of 1,013 type 2 diabetes patients were enrolled. LEAD was measured through color Doppler ultrasonography. The non-invasive skin autofluorescence method was performed for AGEs measurement. Considering that age plays an important role in both AGEs and LEAD, age-combined AGEs, i.e., AGEage index (define as AGEs × age/100) was used for related analysis.
Results: The overall prevalence of LEAD was 48.9% (495/1,013). Patients with LEAD showed a significantly higher AGEage (p < 0.001), and the prevalence of LEAD increased with ascending AGEage levels (p for trend < 0.001). Logistic regression analysis revealed that AGEage was significantly positively associated with risk of LEAD, and the odds ratios of presence of LEAD across quartiles of AGEage were 1.00, 1.72 [95% confidence interval (CI) = 1.14–2.61], 2.72 (95% CI = 1.76–4.22), 4.29 (95% CI = 2.69–6.85) for multivariable-adjusted model (both p for trend < 0.001), respectively. The results were similar among patients of different sexes, body mass index, and with or without diabetes family history. Further, AGEage presented a better predictive value for LEAD than glycated hemoglobin A1c (HbA1c), with its sensitivity, specificity, and area under the curve of 75.5% (95% CI = 71.6–79.2%), 59.3% (95% CI = 54.9–63.6%), and 0.731 (0.703–0.758), respectively.
Conclusion: AGEage, the non-invasive measured skin AGEs combined with age, seems to be a more promising approach than HbA1c in identifying patient at high risk of LEAD.
Keywords: advanced glycation end products, lower extremity atherosclerotic disease, non-invasive, type 2 diabetes, biomaker
Introduction
Lower-extremity atherosclerotic disease (LEAD), defined as a buildup of fatty deposits in peripheral vascular (i.e., atherosclerosis) that leads to progressive narrowing of the lower-extremity arteries, is reported to be the primary manifestation of peripheral arterial disease (PAD) (1). Several researches have reported the close relationships between LEAD and cardiac–cerebral vascular events (both non-fatal and fatal) including gangrene, amputation, and death (2, 3). Diabetes is an established important risk factor for LEAD since the prognosis of LEAD is worse in patients with diabetes than those without (4, 5).
LEAD may be silent or present with a variety of symptoms and signs indicative of extremity ischemia, such as claudication and rest pain (6, 7). However, given the common concurrence of neuropathy in patients with diabetes, LEAD often remains clinically imperceptible until the symptoms become aggravated and advance to ulceration or gangrene due to the loss of pain sensation (8). Therefore, early identification and intervention of LEAD should be highlighted to delay its progression and effectively reduce the risk of the related adverse outcomes, thereby improving patients' quality of life.
Doppler ultrasound examination of atherosclerotic stenosis or occlusive lesions of the lower extremities is an importantly auxiliary method for LEAD diagnosis. However, considering the time-consuming and the risk of omissions of plaque due to widely distributed lower-extremity arteries, there is an urgent need for an indicator for early detection of early-stage LEAD, especially in patients with diabetes.
Advanced glycation end products (AGEs) are modifications of proteins or lipids that become non-enzymatically glycated and oxidized (9, 10). AGEs affect nearly every type of cell and molecule in the body and are thought to be one factor in aging, as well as a causative role in diabetic vascular complications (11). Besides, considering the influence of age on LEAD, AGEage index, defined as AGEs × age/100, was constructed to investigate the association of AGEage with LEAD in patients with type 2 diabetes mellitus. In addition, whether AGEage can be used for early screening of patients at high risk of LEAD was also analyzed.
Subjects, Materials, and Methods
Study Population
Individuals diagnosed with type 2 diabetes who were admitted to the Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated to Sixth People's Hospital during May 2017 to November 2019 were recruited. Type 2 diabetes mellitus were diagnosed based on 1999 World Health Organization (WHO) criteria (12). Inclusion criteria include (1) age ≥ 18 years with the presence of type 2 diabetes mellitus; (2) a stable glucose-lowering regimen for the previous 3 months; (3) with valid data on both AGEs assessed by skin autofluorescence and lower limb ultrasound results; and (4) without any megascopic presence of dermopathy. Patients with diabetic ketoacidosis or severe and recurrent hypoglycemic events within the previous 3 months, and prior history of cardiovascular diseases, stroke, malignancy, mental disorders, or severe kidney, or liver dysfunction were excluded. Finally, 1,013 participants were enrolled into the final analysis.
This study was approved by the Shanghai Jiao Tong University Affiliated Sixth People's Hospital Ethics Committees and was in accordance with the Helsinki Declaration principles. Written informed consent was obtained from each participant.
Assessment of Covariates
Family history of diabetes, medical history, smoking status (current smoker or not), and current medication therapy including glucose-lowering drugs, antihypertensive drugs, lipid-lowering drugs, and aspirin were recorded by self-report at baseline interview. Physical examination including height, weight, and blood pressure were performed in each patient. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Fasting venous blood sample was obtained after a 10-h overnight fasting. Biochemical measurements including glycated hemoglobin A1c (HbA1c), glycated albumin (GA), fasting plasma glucose (FPG), fasting C-peptide (FCP), C-reactive protein (CRP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) were assayed as previously reported (13).
Assessment of AGEs
The spectroscopy device (Hefei Institutes of Physical Science, Chinese Academy of Sciences), mainly consisting of an ultraviolet light source, a broadband light source, a trifurcated fiber optic probe, and a compact charge coupled device spectrometer, was used to assess the skin AGEs. This device uses an excitation light with peak wavelength at 370 nm, which excites the AGEs in the skin that have fluorescence properties in a wavelength range of 420–600 nm. Besides, skin diffuse reflectance in a wavelength range of 350–600 nm was also detected to correct tissue absorption and scattering. The measurements were performed by trained nurses (at room temperature in a semidark environment) for three times at a normal skin site of the left volar side of the arm, and the average value was calculated for the analysis. AGEage was defined as AGEs × age/100.
Assessment of LEAD
Color Doppler ultrasonography was used for lower limb artery examination using an Acuson Sequoia 512 scanner (Siemens Medical Solutions, Mountain View, CA, USA) equipped with a linear array transducer with frequencies of 5–13 MHz. Seven arteries including femoral artery, deep femoral artery, superficial femoral artery, popliteal artery, anterior tibial artery, posterior tibial artery, and peroneal artery in each lower limb were measured for atherosclerotic plaque. The definition of atherosclerotic plaque have been described in detail previously, i.e., a focal structure encroaching into the arterial lumen ≥ 0.5 mm, 50% of the surrounding intima-media thickness (IMT) value, or an IMT thickness ≥ 1.5 mm (14–16). The presence of atherosclerotic plaques in any of the lower limb artery segments listed above was defined as LEAD (17).
Statistical Analysis
All statistical analyses were performed using the SPSS Statistics, version 24.0 (SPSS, Inc., Chicago, IL), MedCalc 19.0.4 (MedCalc Software bvba, Ostend, Belgium) and SAS for windows version 9.3 (SAS Institute, Inc., Cary, NC). An unpaired Student's t-test was used for continuous variables with normal distributions for comparisons between groups. For continuous variables with skewed distributions, Wilcoxon rank sum test and Mann–Whitney U-test was conducted. The χ2 test was used for intergroup comparisons of categorical data. The linear regression model was used to examine the independent influencing factors of AGEage. Binary logistic regression analysis was used to assess the association between quartiles of AGEage and LEAD. The receiver operating characteristic curve was used to evaluate the efficacy of AGEage and HbA1c in early detection of LEAD. A restricted cubic spline nested in logistic models was performed to test whether there was a dose–response or non-linear association of AGEage as a continuous variable with the odds of LEAD. A two-tailed p-value of < 0.05 was considered statistically significant.
Results
The clinical characteristics of the 1,013 enrolled type 2 diabetes patients (598 male, 415 female) were listed in Table 1. The mean age was 60 (53–66) years, and the mean diabetes duration was 11 (6-17) years. LEAD was detected in 495 participants, resulting in an overall prevalence of 48.9%. Patients with LEAD were older, more likely to be male, along with longer diabetes duration, higher systolic blood pressure (SBP) and AGEs, and lower diastolic blood pressure (DBP) (all p < 0.05). Moreover, the percentage of hypertension history and current smoker, as well as the percentage of patients receiving antihypertension, lipid-lowering, and anti-coagulant medications increased significantly (all p < 0.05).
Table 1.
Clinical characteristics of study participants by the presence of LEAD.
|
Total
N = 1,013 |
Without LEAD
N = 518 |
With LEAD
N = 495 |
P | |
|---|---|---|---|---|
| Male/Female | 598/415 | 280/238 | 318/177 | 0.001 |
| Age, years | 60 (53–66) | 56 (46–62) | 63 (58–69) | <0.001 |
| Duration, years | 11 (6–17) | 10 (4–15) | 14 (9–20) | <0.001 |
| BMI, kg/m2 | 24.5 (22.6–27.1) | 24.7 (22.7–27.6) | 24.4 (22.6–26.7) | 0.097 |
| SBP, mmHg | 130 (120–140) | 130 (120–140) | 130 (120–142) | 0.021 |
| DBP, mmHg | 80 (70–85) | 80 (74–88) | 80 (70–85) | 0.003 |
| HbA1c, % | 8.5 (7.2–10.1) | 8.5 (7.1–10.2) | 8.4 (7.3–9.9) | 0.472 |
| GA, % | 21.3 (17.6–26.5) | 21.3 (17.5–26.2) | 21.4 (17.6–26.8) | 0.623 |
| FPG, mmol/L | 7.4 (6.1–9.1) | 7.4 (6.2–9.1) | 7.4 (5.9–9.0) | 0.286 |
| FCP, ng/ml | 1.70 (1.11–2.44) | 1.68 (1.12–2.41) | 1.72 (1.11–2.47) | 0.572 |
| TC, mmol/L | 4.60 (3.87–5.37) | 4.69 (3.96–5.40) | 4.55 (3.79–5.35) | 0.120 |
| TG, mmol/L | 1.44 (1.03–2.16) | 1.50 (1.03–2.29) | 1.38 (1.03–2.03) | 0.061 |
| HDL-c, mmol/L | 1.05 (0.88–1.27) | 1.05 (0.87–1.26) | 1.05 (0.88–1.27) | 0.683 |
| LDL-c, mmol/L | 2.74 (2.13–3.40) | 2.76 (2.15–3.38) | 2.70 (2.12–3.43) | 0.614 |
| CRP, mg/L | 0.83 (0.38–1.75) | 0.84 (0.38–1.87) | 0.83 (0.38–1.66) | 0.640 |
| AGEs | 76.1 (70.2–82.6) | 73.8 (68.9–80.0) | 78.6 (72.4–84.9) | <0.001 |
| AGEage | 45.8 (37.7–53.0) | 41.2 (32.5–48.8) | 49.3 (43.2–56.5) | <0.001 |
| Diabetes family history, n (%) | 606 (59.8) | 307 (59.3) | 299 (60.4) | 0.712 |
| Hypertension history, n (%) | 513 (50.6) | 216 (41.7) | 297 (60.0) | <0.001 |
| Current smoker, n (%) | 245 (24.2) | 111 (21.4) | 134 (27.1) | 0.036 |
| Anti-diabetic agents, n(%) | ||||
| Oral anti-diabetes drugs | 697 (68.8) | 357 (68.9) | 340 (68.7) | 0.936 |
| Metformin | 455 (44.9) | 243 (46.9) | 212 (42.8) | 0.206 |
| Sulfonylureas | 224 (22.1) | 108 (20.8) | 116 (23.4) | 0.326 |
| Thiazolidinediones | 70 (6.9) | 36 (6.9) | 34 (6.9) | 0.993 |
| Glinides | 68 (6.7) | 33 (6.4) | 35 (7.1) | 0.707 |
| DPP-4 inhibitors | 110 (10.9) | 57 (11.0) | 53 (10.7) | 0.920 |
| Glycosidase inhibitors | 375 (37.0) | 175 (33.8) | 196 (39.6) | 0.104 |
| SGLT-2 inhibitors | 5 (0.5) | 2 (0.4) | 3 (0.6) | 0.680 |
| GLP-1 receptor agonists | 15 (1.5) | 9 (1.7) | 6 (1.2) | 0.606 |
| Insulin | 705 (69.6) | 348 (67.2) | 357 (72.1) | 0.088 |
| Antihypertension agents, n(%) | 468 (46.2) | 189 (36.5) | 279 (56.4) | <0.001 |
| ACE inhibitors | 12 (1.2) | 6 (1.2) | 6 (1.2) | 1.000 |
| ARBs | 336 (33.2) | 129 (24.9) | 207 (41.8) | <0.001 |
| CCBs | 235 (23.2) | 104 (20.1) | 131 (26.5) | 0.017 |
| β-Blockers | 106 (10.5) | 46 (8.9) | 60 (12.1) | 0.101 |
| Diuretics | 78 (7.7) | 31 (6.0) | 47 (9.5) | 0.045 |
| Lipid-lowering agents, n(%) | 657 (64.9) | 296 (57.1) | 361 (72.9) | <0.001 |
| Statins | 589 (58.1) | 249 (48.1) | 340 (68.7) | <0.001 |
| Fibrates | 74 (7.3) | 51 (9.8) | 23 (4.6) | 0.002 |
| Ezetimibe | 2 (0.2) | 1 (0.2) | 1 (0.2) | 1.000 |
| Aspirin, n (%) | 305 (30.1) | 107 (20.7) | 198 (40.0) | <0.001 |
Data are expressed as median with interquartile range or n (%).
ACE, angiotension-converting enzyme; ARB, angiotensin receptor blockers; CCB, calcium channel blockers; DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon like peptide-1; SGLT-2, sodium–glucose cotransporter-2.
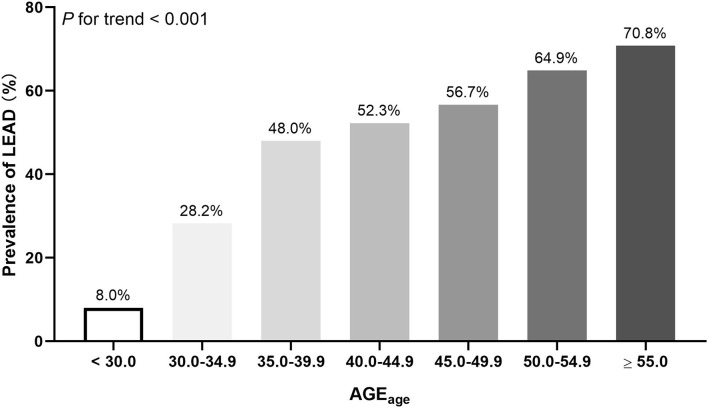
Besides, we also found an increase in AGEage in patients with LEAD compared with those without LEAD [49.3 (43.2–56.5) vs. 41.2 (32.5–48.8), p < 0.001]. Similarly, as shown in Figure 1, the prevalence of LEAD increased progressively across the categories of increasing AGEage (p for trend < 0.001). Then, the participants were stratified according to AGEage levels (AGEage < 43.2 and AGEage ≥ 43.2). Supplementary Table 1 depicts the characteristics of subjects by AGEage levels.
Figure 1.
The prevalence of LEAD stratified by AGEage levels.
Multivariate linear regression analysis defined the AGEage levels as the dependent variable, and sex, diabetes duration, BMI, SBP, family history of diabetes, HbA1c, FPG, lipid profile, CRP, smoking status, antidiabetic therapy, antihypertensive medication, lipid-lowering medication, and aspirin use were designated as the independent variables. The results showed an independently positive association between diabetes duration, SBP, HDL-c, antihypertensive medication, lipid-lowering medication, and AGEage (all p < 0.01). Besides, BMI and TC were negatively associated with the AGEage levels (all p < 0.001).
Logistic regression analysis revealed that AGEage was significantly positively associated with risk of LEAD, that is, the odds ratios of presence of LEAD across quartiles of AGEage were 1.00, 2.43 [95% confidence interval (CI) = 1.66–3.57], 4.71 (95% CI = 3.21–6.92), and 7.95 (95% CI = 5.34–11.84) for crude model, and 1.00, 1.72 (95% CI = 1.14–2.61), 2.72 (95% CI = 1.76–4.22), and 4.29 (95% CI = 2.69–6.85) for multivariable-adjusted duration, BMI, SBP, DBP, hypertension history, current smoker, use of angiotensin receptor blockers, calcium channel blockers, diuretics, statins, fibrates, and aspirin model (both p for trend <0.001), respectively. The results were similar among patients of different sexes, BMI, and with or without diabetes family history (Table 2).
Table 2.
Association of AGEage with LEAD among patients with diabetes.
| AGE age | P for trend | Per SD increase | ||||
|---|---|---|---|---|---|---|
| <37.7 | 37.7–45.8 | 45.9–52.8 | ≥52.9 | |||
| Total | ||||||
| No. of patients | 253 | 254 | 253 | 253 | ||
| No. of cases | 59 | 108 | 149 | 179 | ||
| Odds ratios | 1.00 | 2.43 (1.66–3.57) | 4.71 (3.21–6.92) | 7.95 (5.34–11.84) | <0.001 | 2.50 (2.13–2.92) |
| Multivariable-adjusted odds ratios | 1.00 | 1.72 (1.14–2.61) | 2.72 (1.76–4.22) | 4.29 (2.69–6.85) | <0.001 | 2.00 (1.66–2.42) |
| Male | ||||||
| No. of patients | 184 | 128 | 138 | 148 | ||
| No. of cases | 48 | 62 | 93 | 115 | ||
| Odds ratios | 1.00 | 2.66 (1.65–4.29) | 5.86 (3.61–9.51) | 9.87 (5.94–16.41) | <0.001 | 2.80 (2.28–3.43) |
| Multivariable-adjusted odds ratios | 1.00 | 1.63 (0.97–2.76) | 2.69 (1.53–4.71) | 4.64 (2.54–8.48) | <0.001 | 2.10 (1.64–2.69) |
| Female | ||||||
| No. of patients | 69 | 126 | 115 | 105 | ||
| No. of cases | 11 | 46 | 56 | 64 | ||
| Odds ratios | 1.00 | 3.03 (1.45–6.35) | 5.01 (2.39–10.50) | 8.23 (3.87–17.50) | <0.001 | 2.35 (1.79–3.07) |
| Multivariable-adjusted odds ratios | 1.00 | 2.39 (1.08–5.29) | 3.28 (1.45–7.43) | 4.82 (2.05–11.34) | 0.001 | 1.92 (1.40–2.61) |
| BMI < 25.0 | ||||||
| No. of patients | 121 | 145 | 145 | 154 | ||
| No. of cases | 25 | 57 | 80 | 114 | ||
| Odds ratios | 1.00 | 2.49 (1.43–4.32) | 4.73 (2.73–8.18) | 10.94 (6.20–19.33) | <0.001 | 2.84 (2.25–3.58) |
| Multivariable-adjusted odds ratios | 1.00 | 1.89 (1.05–3.41) | 3.00 (1.63–5.51) | 6.64 (3.43–12.84) | <0.001 | 2.41 (1.84–3.16) |
| BMI ≥ 25.0 | ||||||
| No. of patients | 132 | 109 | 108 | 99 | ||
| No. of cases | 34 | 51 | 69 | 65 | ||
| Odds ratios | 1.00 | 2.53 (1.47–4.36) | 5.10 (2.93–8.87) | 5.51 (3.12–9.74) | <0.001 | 2.25 (1.81–2.80) |
| Multivariable-adjusted odds ratios | 1.00 | 1.90 (1.02–3.52) | 3.08 (1.59–5.98) | 3.05 (1.53–6.07) | <0.001 | 1.78 (1.36–2.32) |
| Without diabetes family history | ||||||
| No. of patients | 117 | 100 | 87 | 103 | ||
| No. of cases | 26 | 44 | 54 | 72 | ||
| Odds ratios | 1.00 | 2.75 (1.53–4.95) | 5.73 (3.10–10.59) | 8.13 (4.44–14.90) | <0.001 | 2.42 (1.91–3.06) |
| Multivariable-adjusted odds ratios | 1.00 | 2.61 (1.37–4.98) | 4.64 (2.26–9.52) | 5.73 (2.76–11.90) | <0.001 | 2.12 (1.59–2.82) |
| With diabetes family history | ||||||
| No. of patients | 136 | 154 | 166 | 150 | ||
| No. of cases | 33 | 64 | 95 | 107 | ||
| Odds ratios | 1.00 | 2.22 (1.34–3.68) | 4.18 (2.54–6.87) | 7.77 (4.58–13.17) | <0.001 | 2.56 (2.06–3.18) |
| Multivariable-adjusted odds ratios | 1.00 | 1.25 (0.71–2.19) | 1.91 (1.08–3.39) | 3.26 (1.75–6.09) | <0.001 | 1.89 (1.46–2.44) |
| Age < 65.0 | ||||||
| No. of patients | 253 | 245 | 187 | 58 | ||
| No. of cases | 59 | 100 | 102 | 32 | ||
| Odds ratios | 1.00 | 2.27 (1.54–3.34) | 3.95 (2.62–5.94) | 4.05 (2.24–7.33) | <0.001 | 1.07 (1.05–1.09) |
| Multivariable-adjusted odds ratios | 1.00 | 1.66 (1.08–2.54) | 2.24 (1.40–3.61) | 2.28 (1.17–4.44) | <0.001 | 1.05 (1.03–1.07) |
| Age ≥ 65.0 | ||||||
| No. of patients | - | 8 | 67 | 195 | ||
| No. of cases | - | 7 | 48 | 147 | ||
| Odds ratios | - | 1.00 | 0.36 (0.04–3.13) | 0.44 (0.05–3.65) | 0.718 | 1.02 (0.99–1.06) |
| Multivariable-adjusted odds ratios | - | 1.00 | 0.30 (0.03–2.86) | 0.32 (0.03–2.95) | 0.577 | 1.02 (0.98–1.06) |
SD, standard deviation.
Adjusted for duration, BMI, SBP, DBP, hypertension history, current smoker, use of ARB, CCB, diuretics, statins, fibrates, and aspirin other than the variable for stratification.
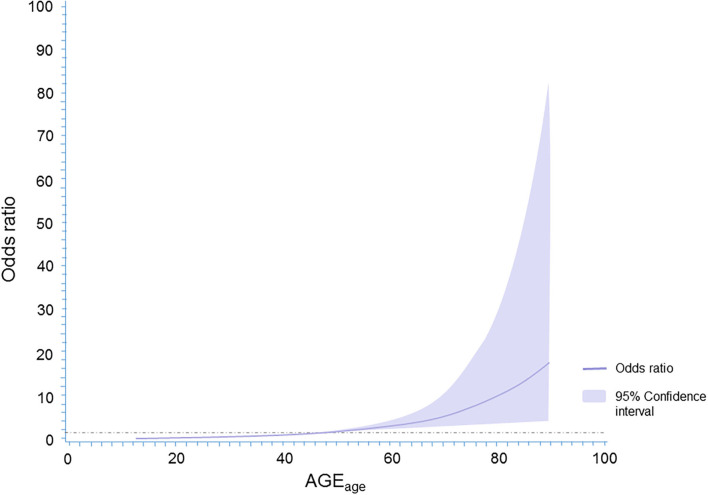
When AGEage was considered as a continuous variable by using restricted cubic splines, a graded positive association of AGEage with the odds of presence of LEAD was observed (p for trend < 0.001; Figure 2). This curve trend was consistent with the findings in Table 2 when AGEage was considered as a categorical variable.
Figure 2.
The spline association between AGEage and LEAD in patients with type 2 diabetes mellitus. Adjustments were made for sex, diabetes duration, BMI, blood pressure, lipid panels, HbA1c, CRP, family history of diabetes, smoking status, antidiabetic therapy, antihypertensive medication, lipid-lowering medication, and aspirin use.
When stratified by sexes, BMI, HbA1c, never and past or current smokers, as well as taking antidiabetic, lipid-lowering, antihypertensive, and antiplatelet medication or not, the graded positive association between AGEage and the odds of presence of LEAD were consistent across all subgroups. Subgroup analyses were then performed to examine potential effect modifiers, and we observed an interaction of HbA1c and using antiplatelet medication or not with a p for interaction <0.01 (Supplementary Table 2). Supplementary Table 3 described the confounding factors associated with LEAD.
Areas under the curve (AUCs) of AGEage and HbA1c for early detection of LEAD were measured. The results showed that AGEage had a better predictive value for LEAD than HbA1c, concretely, 0.731 (0.703–0.758) for AGEage and 0.513 (0.482–0.544) for HbA1c, respectively (p < 0.01). The optimal cutoff point for AGEage in early detecting LEAD was 43.2, with a sensitivity of 75.5% (95% CI = 71.6–79.2%) and a specificity of 59.3% (95% CI = 54.9–63.6%). The study participants were stratified based on sex, BMI, diabetes family history, and current smoker or not, and the results showed an acceptable efficacy of AGEage < 43.2 in early identifying LEAD in all related subgroups. In addition, the efficacy of AGEage in early detection of LEAD seems more pronounced in male and BMI < 25 kg/m2 subgroup type 2 diabetic patients (Table 3).
Table 3.
The efficacy of AGEage ≥ 43.2 in early identifying LEAD in type 2 diabetic patients.
| AUC | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| Total | 0.731 (0.703–0.758) | 75.5 (71.6–79.2) | 59.3 (54.9–63.6) | 65.0 (62.3–67.6) | 70.8 (67.1–74.1) |
| Sex | |||||
| Male | 0.768 (0.732–0.801) | 73.8 (68.7–78.5) | 68.5 (62.6–74.0) | 73.6 (69.8–77.1) | 68.8 (64.3–72.9) |
| Female | 0.696 (0.650–0.740) | 78.6 (71.9–84.3) | 48.5 (41.9–55.1) | 54.4 (50.7–58.0) | 74.3 (68.0–79.8) |
| BMI | |||||
| <25 | 0.750 (0.712–0.785) | 82.1 (77.1–86.4) | 56.8 (50.9–62.7) | 65.2 (61.8–68.4) | 76.4 (71.2–80.9) |
| ≥25 | 0.715 (0.670–0.756) | 67.4 (60.9–73.5) | 62.4 (55.7–68.8) | 64.8 (60.3–69.1) | 65.1 (60.1–69.8) |
| Diabetes family history | |||||
| Yes | 0.721 (0.683–0.756) | 77.0 (71.9–81.6) | 55.2 (49.3–60.9) | 63.5 (60.2–66.7) | 70.3 (65.3–74.9) |
| No | 0.748 (0.702–0.789) | 73.3 (66.6–79.2) | 65.4 (58.4–71.9) | 67.6 (62.9–71.9) | 71.3 (65.9–76.1) |
| Current smoker | |||||
| Yes | 0.711 (0.650–0.767) | 70.1 (61.7–77.6) | 66.7 (56.9–75.4) | 72.7 (66.7–78.1) | 63.7 (56.8–70.1) |
| No | 0.744 (0.711–0.774) | 77.6 (73.0–81.7) | 57.3 (52.3–62.2) | 62.8 (59.8–65.7) | 73.3 (69.1–77.2) |
NPV, negative predictive value; PPV, positive predictive value.
Discussion
In this observational study, the AGEage index was proposed for the first time. We observed a positive association of AGEage with the odds of presence of LEAD among patients with type 2 diabetes mellitus, independent of traditional risk factors for LEAD that include HbA1c. Besides, AGEage may be a suitable indicator for early identification of patients at high risk of LEAD, with its optimal cutoff point of 43.2.
LEAD is an emerging public health burden with an endemic progression worldwide that affects over 200 million people worldwide (18). Related studies reported that LEAD was two to four times more frequent in people with type 2 diabetes than in the general population (19, 20), and the prevalence of LEAD also increased along with the rising diabetes duration as shown in the United Kingdom Prospective Diabetes Study (UKPDS), concretely, 1.2% when first diagnosed with diabetes, and increased up to 12.5% after 18 years of evolution (21). Besides, LEAD is particularly frequent in diabetic patients with worse outcomes, especially the risk of lower limb amputation, four to five times higher, compared with non-diabetic subjects (22, 23), suggesting that poor glycemic control may play an important role in LEAD progression.
HbA1c is a well-established marker for assessment of glycemic control. In the UKPDS trial, each 1% reduction in HbA1c was associated with a 43% reduction in the risk of major LEAD (amputation or death induced by peripheral vascular event) (24). However, HbA1c is insufficient in terms of the overall evaluation of glycemic control, i.e., patients with similar HbA1c could have totally distinct glucose profiles. The results of the ADVANCE trial demonstrated that the incidence of major LEAD (lower-limb ulceration, amputation, revascularization requirement, or death following a PAD) was comparable between intensive and standard glucose control groups (25, 26). Moreover, type 2 diabetic patients with or without LEAD in the current study share similar HbA1c levels. Therefore, other factors related to glycemic control beyond HbA1c may be related to LEAD.
AGEs are considered as one factor in aging and some age-related chronic diseases including Alzheimer's disease, cardiovascular disease, stroke, and diabetes. Studies have reported that hyperglycemic status may promote the accumulation of AGEs, while AGEs can cause vascular stiffening and entrapment of LDL particles in the artery walls by inducing crosslinking of collagen in the context of cardiovascular disease (27, 28). AGEs can also cause LDL glycation, thereby further promoting its oxidation, while oxidized LDL is one of the major factors in the development of atherosclerosis. In addition, the interaction between AGEs and RAGE (receptor for AGEs) induced oxidative stress, and activation of inflammatory pathways in vascular endothelial cells also plays an important role in the development of systemic atherosclerosis including LEAD (28). These findings raised the possibility that AGEs and AGE-generated indicators may be an alternative indicator reflecting LEAD.
Considering the fact that AGEs are closely correlated with age, while LEAD is reported to be discovered during the fifth decade of life and the prevalence of LEAD increased exponentially after 65 years (29), we proposed the AGEage index for the first time, which combines AGEs and age organically. Consistent with our hypothesis, in the current study, the level of AGEage was significantly increased in LEAD patients with type 2 diabetes mellitus. Accordingly, we also found a graded positive association of AGEage with the odds of presence of LEAD, even after adjusting for clinical risk factors, including HbA1c, which imply the value of AGEage in assessing the risk of diabetic complications independent of HbA1c. Particularly, skin autofluorescence is a non-invasive method for AGEs detection. All the above-mentioned suggest that AGEage may be a simple and effective indicator for predicting LEAD.
Meanwhile, we proposed that in type 2 diabetes patients, AGEage might be a more suitable indicator than HbA1c for mimicking the poor prognosis of diabetes, i.e., LEAD, regardless of gender, BMI, and family history of diabetes. Our results showed that AGEage had a significantly higher predictive value for LEAD than HbA1c. With the cutoff point of 43.2, around 3/4 patients with LEAD (384/591) were successfully identified. Based on the current study, we recommend type 2 diabetic patients whose AGEage ≥ 43.2 are considered at high risk for LEAD that needs ultrasound for further confirmation.
The relatively large sample size and well-documented clinical information of the current study makes our findings more reliable. Nevertheless, there are some limitations that should be pointed out. First, since the current study was a cross-sectional study, the cause-and-effect relationship between AGEs and LEAD could not be clarified. Second, the participants enrolled in the current study were Chinese type 2 diabetic hospitalized patients. Considering the racial difference in circulating AGEs, as well as the promotion of hyperglycemia on AGE accumulation, whether our results can be generalized to all diabetic patients and even patients with cardiovascular diseases needs further study.
Conclusion
In conclusion, we provide evidence that AGEage is associated with the prevalence of LEAD in patients with type 2 diabetes mellitus independent of HbA1c. AGEage, the non-invasive measurement of accumulated AGEs combined with age, seems a promising approach than HbA1c to mimic the poor prognosis of hyperglycemia, i.e., triage for patient at high risk of LEAD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Shanghai Jiao Tong University Affiliated Sixth People's Hospital Ethics Committees. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JZ and YiW designed the study. LY and YS collected the data. LY, YS, and YZ performed statistical analysis and wrote the paper. YuW, JiY, and WZ help with the AGEs measurement. JZ, YB, JuY, YL, and YiW revised the paper and contributed to discussion. LY, YS, and YZ had equal contribution to this paper and were the guarantors. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Key R&D Program of China (2018YFC2000802), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161430), the Bureau of International Cooperation, Chinese Academy of Sciences (Grant no. 116134KYSB20170018), and the Natural Science Foundation of Anhui Province of China (1908085QH365).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer NW has declared a shared affiliation, with no collaboration, with several of the authors LY, YS, JuY, YuW, JiY, WZ, YB, and JZ, to the handling editor at the time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all of the involved clinicians, nurses, and technicians for dedicating their time and skill to the completion of this study. We would also like to thank all participants for their dedication in data collection and laboratory measurements.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.696156/full#supplementary-material
References
- 1.Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease. Creager MA, Belkin M, Bluth EI, Casey DE, Jr., Chaturvedi S, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease). Circulation. (2012) 125:395–467. 10.1161/CIR.0b013e31823299a1 [Erratum in: Circulation. (2012) 125:e387–8. Erratum in: Circulation. (2013) 128:e179.] [DOI] [PubMed] [Google Scholar]
- 2.Olivier CB, Mulder H, Hiatt WR, Jones WS, Fowkes FGR, Rockhold FW, et al. Incidence, characteristics, and outcomes of myocardial infarction in patients with peripheral artery disease: insights from the EUCLID trial. JAMA Cardiol. (2019) 4:7–15. 10.1001/jamacardio.2018.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pourghaderi P, Yuquimpo KM, Roginski Guetter C, Mansfield L, Park HS. Outcomes following lower extremity amputation in patients with diabetes mellitus and peripheral arterial disease. Ann Vasc Surg. (2020) 63:259–68. 10.1016/j.avsg.2019.08.084 [DOI] [PubMed] [Google Scholar]
- 4.Kamil S, Sehested TSG, Carlson N, Houlind K, Lassen JF, Bang CN, et al. Diabetes and risk of peripheral artery disease in patients undergoing first-time coronary angiography between 2000 and 2012 - a nationwide study. BMC Cardiovasc Disord. (2019) 19:234. 10.1186/s12872-019-1213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang SL, Zhu LY, Han R, Sun LL, Li JX, Dou JT. Pathophysiology of peripheral arterial disease in diabetes mellitus. J Diabetes. (2017) 9:133–40. 10.1111/1753-0407.12474 [DOI] [PubMed] [Google Scholar]
- 6.Layden J, Michaels J, Bermingham S, Higgins B, Guideline Development Group . Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ. (2012) 345:e4947. 10.1136/bmj.e4947 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. (2001) 286:1317–24. 10.1001/jama.286.11.1317 [DOI] [PubMed] [Google Scholar]
- 8.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes–a review. Diabet Med. (2010) 27:4–14. 10.1111/j.1464-5491.2009.02866.x [DOI] [PubMed] [Google Scholar]
- 9.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. (2006) 114:597–605. 10.1161/CIRCULATIONAHA.106.621854 [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. (2002) 44:129–46. 10.1007/s001250051591 [Erratum in: Diabetologia. (2002) 45:293.] [DOI] [PubMed] [Google Scholar]
- 11.Yan SF, D'Agati V, Schmidt AM, Ramasamy R. Receptor for advanced glycation endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes and aging. Curr Mol Med. (2007) 7:699–710. 10.2174/156652407783220732 [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. (2018) 41:2370–6. 10.2337/dc18-1131 [DOI] [PubMed] [Google Scholar]
- 14.He X, Hu X, Ma X, Su H, Ying L, Peng J, et al. Elevated serum fibroblast growth factor 23 levels as an indicator of lower extremity atherosclerotic disease in Chinese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. (2017) 16:77. 10.1186/s12933-017-0559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X, Su J, Ma X, Lu W, Zhu W, Wang Y, et al. The association between serum growth differentiation factor 15 levels and lower extremity atherosclerotic disease is independent of body mass index in type 2 diabetes. Cardiovasc Diabetol. (2020) 19:40. 10.1186/s12933-020-01020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Y, Dai D, Lu J, Wang Y, Zhu W, Bao Y, et al. Visit-to-visit variability of glycated albumin was associated with incidence or progression of lower extremity atherosclerotic disease. Cardiovasc Diabetol. (2020) 19:211. 10.1186/s12933-020-01187-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. (2018) 39:763–816. 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 18.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. (2013) 382:1329–40. 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. (2015) 116:1509–26. 10.1161/CIRCRESAHA.116.303849 [Erratum in: Circ Res. (2015) 117:e12.] [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. (2004) 110:738–43. 10.1161/01.CIR.0000137913.26087.F0 [DOI] [PubMed] [Google Scholar]
- 21.Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. (2002) 25:894–9. 10.2337/diacare.25.5.894 [DOI] [PubMed] [Google Scholar]
- 22.Li MF, Zhao CC, Li TT, Tu YF, Lu JX, Zhang R, et al. The coexistence of carotid and lower extremity atherosclerosis further increases cardio-cerebrovascular risk in type 2 diabetes. Cardiovasc Diabetol. (2016) 15:43. 10.1186/s12933-016-0360-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyko EJ, Seelig AD, Ahroni JH. Limb- and person-level risk factors for lower-limb amputation in the prospective seattle diabetic foot study. Diabetes Care. (2018) 41:891–8. 10.2337/dc17-2210 [DOI] [PubMed] [Google Scholar]
- 24.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. (2000) 321:405–12. 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, ADVANCE Collaborative Group et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. (2008) 358:2560–72. 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 26.Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Williams B, Lisheng L, et al. Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care. (2016) 39:1796–803. 10.2337/dc16-0588 [DOI] [PubMed] [Google Scholar]
- 27.Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. (2012) 20:177–83. 10.1097/CRD.0b013e318244e57c [DOI] [PubMed] [Google Scholar]
- 28.Di Marco E, Gray SP, Jandeleit-Dahm K. Diabetes alters activation and repression of pro- and anti-inflammatory signaling pathways in the vasculature. Front Endocrinol. (2013) 4:68. 10.3389/fendo.2013.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nativel M, Potier L, Alexandre L, Baillet-Blanco L, Ducasse E, Velho G, et al. Lower extremity arterial disease in patients with diabetes: a contemporary narrative review. Cardiovasc Diabetol. (2018) 17:138. 10.1186/s12933-018-0781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.