Abstract
It is widely accepted that Hepatitis A virus (HAV) is responsible for liver failure and even death in older people and in people with other serious health issues; so, proposing new compounds with inhibitory activity can help to treated of these disease’s. In current study, a new class of quinolines is proposed with inhibitor activity of the HAV proteinase. So, in the first step, fused quinoline derivatives has been synthesized in short reaction time (12.0 min) and high efficiency yields (94%) in presence of 1-carboxymethyl-2,3-dimethylimidazolium iodide ([cmdmim]I) ionic liquid catalyst using a new method. In the following, chemical reactivity and inhibitory activity of synthesized quinolines were evaluated in density functional theory (DFT) framework and molecular docking methodologies. High global softness (0.67 eV), low HOMOSWBNNT-LUMO4a gap (4.78 eV), and more negative adsorption energy (− 87.9 kJ mol−1) in these quinolines reveal that the 4a and 4b compounds have better delivery than other quinolines using SWBNNT as suitable carrier to target cells. Molecular docking shows that the best cavity of the HAV has − 134.2 kJ mol−1 interaction energy involving bonding and non-bonding interactions. In fact, these interactions are between fused quinolines with especial geometries and sidechain flexibility amino acids residues inside the best binding site of the HAV, as hydrogen bonding, steric, and electrostatic interactions. So, these interactions imply that proposed fused quinolines have good inhibitor activity for the HAV.
Subject terms: Drug discovery, Drug delivery
Introduction
Human Hepatitis A virus (HAV) is regarded as an inflammation of the liver that this infectious or epidemic hepatitis is transmitted by the fecal–oral route1–3. Low time between infection and symptoms, high rate of infection are strong reasons for motivations scientists whose always looking for logical solutions in order to help to liver disease4,5. Albeit, it should not be forgotten that drug design and production are time consuming and complicated. In order to decrease these issues, chemistry research groups partially help with suggestion of design and synthesis new compounds with anti-virus properties. In current study, we propose derivatives of a category of organic compound called fused quinoline.
Quinolines are an important group of heterocyclic compounds that have significant biological properties6–10. In fact, these compounds are used as potential anti-cancer, anti-tumor, and anti-malarials agents11–13. There are several proposed methods to synthesis of these compounds14–37, such as Povarov reaction14, radiation to second type amines15 and so on14–40. For example, for the first time, interaction of quinolines and solid support was carried out using microwaves with solvent free technique6. In another study, compounds based on quinoline were proposed as inhibitor production of the envelope glycoprotein in Dengue Virus Serotype 2 and DNA gyrase through direct binding to the bacterial chromosome40. Also, these compounds were designed and synthesized for DNA-gyrase and topoisomerase-II inhibition41. Another series of quinolines has been prepared as MEK (MAP kinase kinase) inhibitors42. In addition, synthesized quinolines via the Mannich reaction were prepared against Gram-positive (G+), Gram-negative (G−) bacteria39.
Although above mentioned studies have proposed methods with the aim using into pharmaceutical industries, they have low relative yield and high reaction time. So, in current study, we propose a novel method to synthesis of fused quinolines with excellent yield and short reaction time using 1-carboxymethyl-2,3-dimethylimidazolium iodide ([cmdmim]I) ionic liquid catalyst43. In fact, the [cmdmim]I as one of recyclable catalysts mainly results in straightforward work-up procedure, and reusability of the catalyst in about 5 consecutive runs without any appreciable decrease in activity43. In fact, ionic liquids have important role into synthesis of these compounds on pharmaceutical industries. On the other hands, we are going to study quantum mechanics calculations due to introduce suitable quinolines with high chemical reactivity. In addition, their inhibitory activity in interaction with the HAV will study using molecular docking computations.
Experimental section
General
Chemicals were purchased from the Merck chemical companies. Extend of reactions were evaluated by thin layer chromatography (TLC) covered using silica-gel 60, F256 contracted by Merck chemical companies. In the TLC chromatography was used from n-Hexane and Ethyl Acetate solvents. Melting point was recorded using electro thermal devices. FT-IR, 1H-NMR and 13C-NMR spectra were recorded using VERTIX 70 Brucker, a Brucker (400 MHz) Advance DRX in DMSO-d6 solvent. Chemical shift (in ppm) were investigated in related to tetra methyl silane (TMS) as internal standard.
Preparation of the [cmdmim]I ionic liquid
The [cmdmim]I ionic liquid is high impact catalyst with solid state and orange color; whose previously synthesized by our research group43.
Synthesis of the 7-(3-Nitro-Phenyle)-H8-Benzo [h] Indo [1.2-b] quinoline-8-on (4a)
In balloon flask (100 mL) equipped with an electrical condenser, a mixture consisting of 1, 3-indandeion (0.146 g, 1 mmol), 3-Nitro Benzaldehide (0.1510 g, 1 mmol), and 1-Naphtyle Amine (0.143 g, 1 mmol), in presence of the [cmdmim]I ionic liquid catalyst (10% molar), water solvent (10.0 mL) was heated for 15 min at reflux conditions. The progress of the reaction was followed by the TLC (eluting with a mixture of Petroleum Ether, Ethyl Acetate (3:10), accompanied one drop of Methanol). After cooling and solvent evaporation, the mixture of reaction was dissolved in Ethanol, Ethyl Acetate, and Chloroform (30:30:10 mL) and then, it was purified and dried. The yellow powder of the synthesized compound (4a; 0.361 g) was produced in 90.0% yield and 326–328 °C. Other quinoline derivatives are produced using proposed method, see Table S1 and S2 (S means supplementary file).
The 4a synthesis has been carried out in various conditions such as six different solvents, 10% molar of catalyst, and reflux condition, see Table S3. Influence of different catalyst and their value have been evaluated after investigation of reliable solvent, see Tables S2 to S5. According to Tables S4 and S5, the most favorable condition for the 4a synthesis was using 10% molar catalyst in water solvent and reflux condition.
To confirmation of produced structures, 4a, the IR, 1H-NMR and 13C-NMR spectroscopies were used. IR (KBr): 3062 (C–H stretch, aromatic), 1710 (C=O stretch), 1639 (C=N stretch), 1608, 1571, 1469 (C–C stretch, aromatic), 1519, 1346 (NO2 stretch), 844, 815, 763, 730 (C–H out of plane bending, aromatic) cm−1. 1H-NMR (400 MHz, DMSO) δ (ppm): He or Hh 9.46 (dd, J = 2 and 8.2 Hz, 1H), He or Hh and Hk 8.47–8.50 (m, 2H), Hl 8.27 (d, J = 7.2 Hz, 1H), Ha or Hd 8.04 (d, J = 7.6 Hz, 1H), Hj 7.98 (d, J = 8.8 Hz, 1H), Hc 7.93 (t, J = 6.2 Hz, 1H), Hm, Hb and Hf or Hg 7.86–7.93 (m, 3H), Hn 7.73 (d, J = 7.2 Hz, 1H), Hg or Hf 7.66 (t, J = 7.2 Hz, 1H), Hi 7.52 (d, J = 8.8 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): C=O ketone 192, 24 aromatic Carbone: 162.5, 160.5, 154.2, 150.7, 148.4, 146.5, 144.0, 141.9, 139.9, 139.7, 137.6, 135.9, 135.4, 133.1, 131.3, 129.1, 128.7, 128.3, 125.6, 125.0, 124.6, 124.5, 123.6, 122.9, and 121.9.
Theoretical section
Low lying structure have been investigated in the framework of hybrid-DFT (H-DFT) by using Gaussian09 program package44. The ωB97-XD/6-31g(d) level of theory was selected using DFT calibration method45,46; which combines long range correlation functionals, with all electron basis set, 6-31g(d)47. Geometry optimization of structures carried out without any symmetry constrains (C1 symmetry). Vibrational frequency test guarantees that the optimized geometries are in real minima.
Tendency and role of the SWBNNT, the 4a, and derivatives in their interactions have been investigated using global chemical reactivity descriptors such as chemical potential (μ = − (IE + EA)/2), hardness (η = (IE − EA)/2), softness (S = 1/η), and electrophilicity (ω = μ2/2η)48. In fact, the μ is the escaping tendency of electrons, the η is a resistance to charge transfer, the ω is floating of electron between nucleophile and electrophile. The IE and EA are adiabatic ionization energy and electron affinity, respectively; they calculated using tree approach point methods:
| 1 |
| 2 |
where , , and are total energy of neutral, cationic, and anionic compounds (SWBNNT and fused quinoline derivatives), respectively. Thermodynamic stability of complexes was calculated using adsorption energy equation:
| 3 |
where , , and are total energy of complexes, SWBNNT, and molecule, respectively. In order to achievement to realistic value of the Eads, zero-point vibrational energy (ZPVE) and basis set supper position error (BSSE) was considered. Therefore, the counterpoise correlation was used to correct BSSE error. Geometric information is completed by results of natural bond orbital (NBO) analysis. Type of bonds, depletion of occupancies, percent of Lewis and non-Lewis and stabilization energy () are obtained by NBO analysis:
| 4 |
where is interaction Hamiltonian, and are orbital energies, and is matrix element.
Molecular docking
Secondary structures of Hepatitis A virus (PDB ID: 1HAV) were retrieved from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (http://www.rcsb.org/), see Figure S4. In geometry minimization processing, the best of their potential ligand binding site (cavity) has X: 11.96, Y: 5.87, Z: 10.08 Cartesian coordinate, radius 15 Å, volume 1438.2 Å3, and surface 2420.5 Å2, respectively. Maximum global minimization step for molecular docking was set at 1500 steps. The side chain flexibility of the amino acid residues of these proteins inside the cavity with tolerance of 1.00 and strength of 0.90 were considered, see Fig. 1.
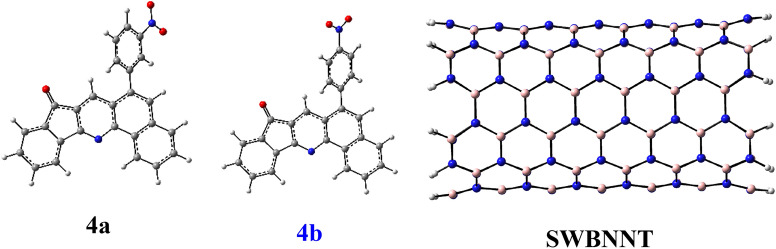
Figure 1.
Ground state structure of the 4a, 4b, and single walled boron nitride nanotube (SWBNNT) in ωB97-XD/6-31g* level of theory.
Then, molecular docking was simulated using Molegro Virtual Docker (MVD) 6.0149. For this purpose, MolDock algorithm as docking method based on guided differential evolution (combination differential evolution optimization technique with cavity prediction algorithm) and force field based scoring function [extension of Piecewise Lenear Potential (PLP)]50 identify the potential binding site of proteins and binding orientation of ligands50. Re-ranking of top ranked conformations mainly results in improvement of docking accuracy51. The MolDock scoring function iteration was set 1500 with a simplex evolution size of 50 and a minimum of 10 runs. In addition, the simplex evolution was set for 300 steps with a neighbor distance factor of 1.00. The MolDock grid score was set with a grid resolution of 0.30 Å.
Results and discussion
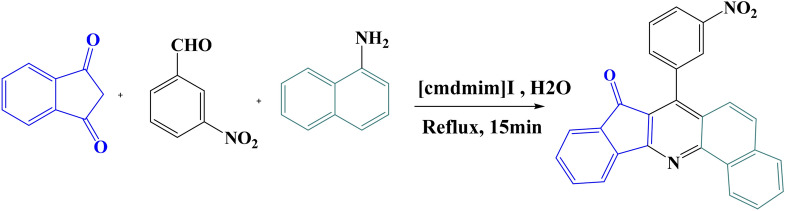
In starting point, optimization processing of reaction condition for synthesis of Indo-quinoline derivatives was carried out in different solvent and catalysts; see Table S1. The most efficiency yields (90.0%) was obtained in 10% molar the [cmdmim]I ionic liquid catalyst and reflux condition in the water as green solvent. This efficiency mainly results from selection of the 1,3-indandione, aryl aldehydes and 1-naphthylamine with the same ratio in one pot reaction, see Scheme 1.
Scheme 1.
The synthetic pathway toward synthesis of 4a compound in reflux condition.
In the proposed reaction mechanism, Scheme S1, aryl aldehydes are the key component in this reaction due to especial geometry. More importantly, role of ionic liquid in proposed mechanism (Scheme S1) is activation of aldehyde and ketone carbonyl and facilitating of reaction cyclo addition step, respectively, see Scheme S2.
The main aim of current study is to provide a conceptual theoretical framework based on ωB97-XD/6-31g* method. Geometry of quinoline derivatives in the real minima are given in Fig. 1 and Figure S1. In fact, synthesized fused quinolines are susceptible non-covalent interactions.
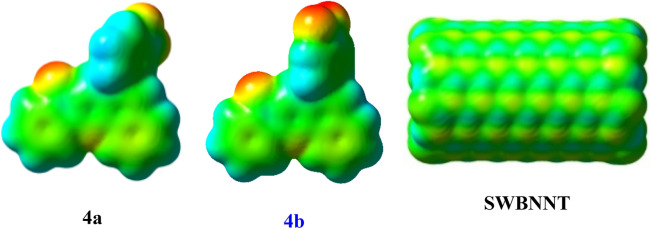
High electron density in the molecular electrostatic potential (MEP) maps52, confirms non bond interaction, as shown in Fig. 2 and Figure S1. This property can give into inhibitory activity on Hepatitis A virus. The MEP maps52, investigate and predict active sites and strength of interactions in the 4a, derivatives and SWBNNT. According to this figure, despite areas without electron density (green color) in the 4a and SWBNNT, Oxygen and Nitrogen centers in the 4a molecule show negative electrostatic potential. In fact, these parts with electron delocalization can introduce as involve sites in their interactions.
Figure 2.
Molecular electrostatic potential (MEP) maps of the 4a, 4b, and single walled boron nitride nanotube (SWBNNT) in ωB97-XD/6-31g* level of theory.
The more charge transfer is carried out from SWBNNT to 4a molecule due to lower the HOMOSWBNNT-LUMO4a gap than others, see Figure S2. This issue confirms by 125 kcal mol−1 in total second order stabilization energy.
With investigating of their role, they were interacted in suitable orientations, see Figure S3. Energy of stable configurations was considered to calculate of adsorption energy (Eads). Based on the more negative value of the Eads, 4a and 4b complex has adsorption strength and stable configuration than others, as shown in Table S8.
Density of state (DOS) analysis has been carried out due to better understanding of these interactions. Total DOS of the 4a molecule, SWBNNT, and outside complexes are given in Figure S1. The reduction of energy gaps in complexes can be attributed to better interactions. These interactions may influence on electron excitation and excited states spectrum.
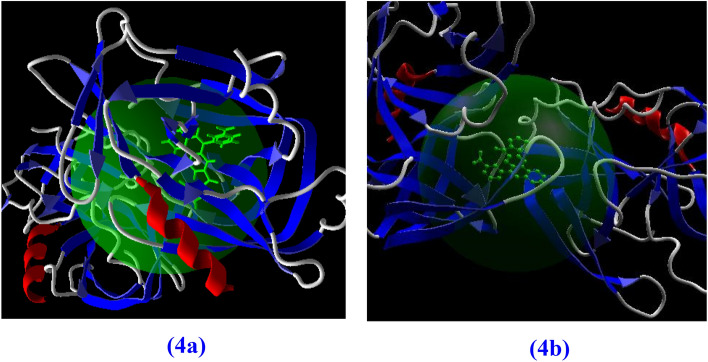
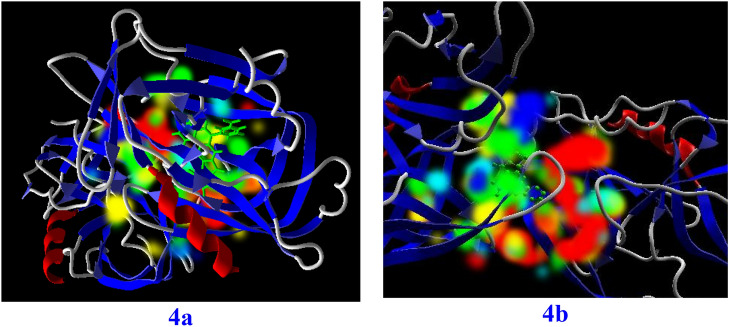
After delivery of drug molecule to target cells involving Hepatitis A virus, their interactions are important. The best geometry of docking has more negative value in Gibbs free energy at 25 ns, see Fig. 3. The best favorable sites of fused quinoline rings as drug molecule interact with Hepatitis A virus from the hydrogen bonding, electrostatic, and steric interactions.
Figure 3.
Molecular simulation of the 4a, 4b, and Hepatitis virus.
Molecular docking
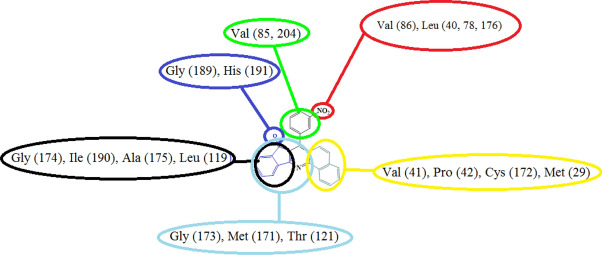
The best binding cavity for each molecular docking simulation using the MVD program package are shown in Fig. 4. Docking scoring results in best selected cavity are presented in Table 1. More negative re-rank score showing better docking of ligand into active site of Hepatitis A virus. According to this table, re-rank score in Hepatitis A virus is negative. Molecular interaction energy between ligand and Hepatitis A virus is − 134.6 kJ mol−1. Besides, deep bonded and non-bonded interaction ligand inside the binding pocket of Hepatitis A virus indicating strong molecular interaction involving both interactions. Although these interactions are with Val (41, 85, 86, 204), Leu (40, 78, 119, 176), Gly (173, 174, 189), Met (29, 30), His (191), Ile (190), Ala (175), Thr(121), Cys (172), Pro (42) residues of Hepatitis A virus. These interactions at the active site of ligand are presented in Fig. 5. Indeed, introduced active sites of proteins have high-affinity for binding into ligand molecule at binding region. This result is in good agreement with chemical reactivity descriptors. Green, turquoise, yellow, red and blue colors in the energy map of Hepatitis virus might contribute in favorable steric interaction, hydrogen acceptor, hydrogen donor, electrostatic potential with the ligand51. While steric, hydrogen donor and electrostatic potential interaction are the most probably interaction in Hepatitis A virus, steric interaction is the most probably interaction, see Fig. 6. So, these results imply favorable ligand–protein interaction energy at the binding cavity of Hepatitis virus. In addition, it can be proposed this ligand with relatively good inhibitor.
Figure 4.
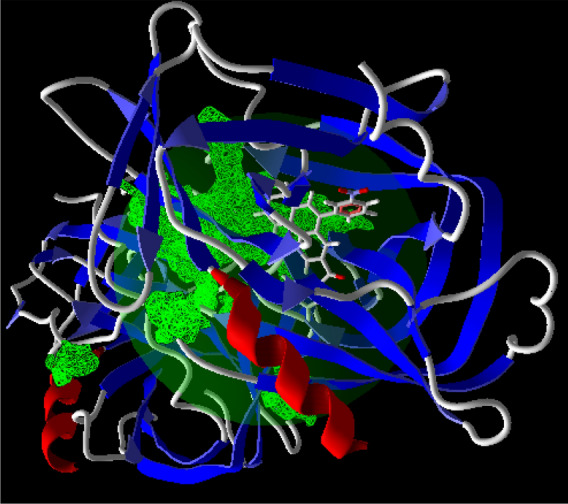
The potential ligand binding cavities of Hepatitis virus.
Table 1.
Molecular docking score of Hepatitis A virus with ligand (4a and 4b) in the favorable cavity.
| Protein–ligand | RS | Etot | IE | EHB |
|---|---|---|---|---|
| HAV-4a | − 43.31 | − 134.60 | − 16.76 | − 5.23 |
| HAV-4b | − 39.02 | − 127.04 | − 13.43 | − 3.23 |
RS Re-rank score is linear combination of internal (Steric, Van der Waals, Hydrogen bonding, and Electrostatic) and external (Torsion strain, Torsion strain sp2-sp2, Hydrogen bonding, Van der Waals, and Electrostatic) energies. Etot total interaction energy between protein and pose, IE internal energy of pose, EHB hydrogen bonding energy.
Figure 5.
Molecular interaction between fused quinoline (4a, 4b) and active site of Hepatitis virus.
Figure 6.
Energy map of fused quinoline (4a, 4b) at the binding cavity of Hepatitis virus.
Conclusion
In summary, one pot three component reaction in presence mentioned ionic liquid catalyst and condition is good strategies to synthesis of fused quinolines with excellent efficiency. As well as that, using of this catalyst satisfies green chemistry aims. SWBNNT is introduced as a good carrier to transform synthesized compounds without side effects. With entrance of drug molecule into target cell involving Hepatitis virus, fused quinolones interact from oxygen and nitrogen sites. So, introduced fused quinolines are susceptible these interactions due to existence five aromatic rings.
Supplementary Information
Acknowledgements
The authors are grateful to Dr. Fazlolah Eshghi due to his high suggestion, doing theoretical and molecular docking studies in this work.
Author contributions
M.M. conceived of the presented idea. M.R.J. carried out computational jobs and experiments. M.R.J. and P.J. wrote the manuscript and discussed the results and contributed to the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98529-0.
References
- 1.Baron S. Medical Microbiology. 4. University of Texas Medical Branch; 1996. [PubMed] [Google Scholar]
- 2.Louten, J. Virus Structure and Classification. in Essential Human Virology 19–29 (Elsevier, 2016). 10.1016/B978-0-12-800947-5.00002-8.
- 3.Ryu, W.-S. New emerging viruses. in Molecular Virology of Human Pathogenic Viruses 289–302 (Elsevier, 2017). 10.1016/B978-0-12-800838-6.00021-7.
- 4.Vos T, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global hepatitis report, 2017 (2017).
- 6.Kidwai M, Bhushan KR, Sapra P, Saxena RK, Gupta R. Alumina-supported synthesis of antibacterial quinolines using microwaves. Bioorg. Med. Chem. 2000;8:69–72. doi: 10.1016/S0968-0896(99)00256-4. [DOI] [PubMed] [Google Scholar]
- 7.Madrid PB, et al. Synthesis of ring-substituted 4-aminoquinolines and evaluation of their antimalarial activities. Bioorg. Med. Chem. Lett. 2005;15:1015–1018. doi: 10.1016/j.bmcl.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Yamato M, Takeuchi Y, Hashigaki K, Ikeda Y, Ming-rong M, Takeuch K, Matsushima M, Tsuruo T, Tashiro T, Tsukagoshi S, Yamashita Y. Synthesis and antitumor activity of fused tetracyclic quinoline derivatives. 1. J. Med. Chem. 1989;32:1295–1300. doi: 10.1021/jm00126a025. [DOI] [PubMed] [Google Scholar]
- 9.Loddo R, Briguglio I, Corona P, Piras S, Loriga M, Paglietti G, Carta A, Sanna G, Giliberti G, Ibba C, Farci P. Synthesis and antiviral activity of new phenylimidazopyridines and N-benzylidenequinolinamines derived by molecular simplification of phenylimidazo[4,5-g]quinolines. J. Med. Chem. 2014;84:8–16. doi: 10.1016/j.ejmech.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Matada BS, Pattanashettar R, Yernale NG. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021;32:115973. doi: 10.1016/j.bmc.2020.115973. [DOI] [PubMed] [Google Scholar]
- 11.Abbas SH, Abd El-Hafeez AA, Shoman ME, Montano MM, Hassan HA. New quinoline/chalcone hybrids as anti-cancer agents: Design, synthesis, and evaluations of cytotoxicity and PI3K inhibitory activity. Bioorg. Chem. 2019;82:360–377. doi: 10.1016/j.bioorg.2018.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, et al. Discovery of novel quinoline-chalcone derivatives as potent antitumor agents with microtubule polymerization inhibitory activity. J. Med. Chem. 2019;62:993–1013. doi: 10.1021/acs.jmedchem.8b01755. [DOI] [PubMed] [Google Scholar]
- 13.Kaur K, Jain M, Reddy RP, Jain R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010;45:3245–3264. doi: 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Sridhaan V, Avendano C, Convenient JCM. two-step synthesis of 2-styrylquinolines: An application of the CAN-catalyzed vinylogous type-II Povarov reaction. Tetrahedron. 2009;65:2087–2096. doi: 10.1016/j.tet.2008.12.077. [DOI] [Google Scholar]
- 15.Wilson NS, Sarko CR, Roth GP. Microwave-assisted synthesis of 2-aminoquinolines. Tetrahedron Lett. 2002;43:581–583. doi: 10.1016/S0040-4039(01)02242-0. [DOI] [Google Scholar]
- 16.Vasamsetty S, Medidi S, Ampolu S, Majji RK, Kotupalli MR, Satyanarayana CC, Nowduri A, Sanasi P. Free one-pot synthesis of chromeno quinolines and their antibacterial activity. Chem. Green Sustain. 2017;7:141–152. doi: 10.4236/gsc.2017.72011. [DOI] [Google Scholar]
- 17.Kouznetsoy VV, Bohorquez ARR, Saavedra LA. Convenient procedure for the synthesis of new α-pyridinyl-substituted 7H-indeno [2, 1-c] quinoline derivatives based on a three-component imino Diels–Alder reaction. Synthesis (Stuttg) 2009;24:4219–4225. [Google Scholar]
- 18.Khaligh NG, Mihankhah T. Poly(4-vinylpyridinium) hydrogen sulfate catalyzed synthesis of 12-aryl-12-hydro-5H-benzo[g]indeno[2,1-b]quinoline-6,11,13-trione derivatives. Res. Chem. Intermed. 2015;41:4569–4579. doi: 10.1007/s11164-014-1552-6. [DOI] [Google Scholar]
- 19.Sandaroos R, Vadi M, Damavandi S. Efficient example of cross-linked polymeric catalysed synthesis of 7H-benzo[h]indeno[1,2-b]quinolin-8-one and 8H-naphtho[2,3-h]indeno[1,2-b]quinolin-9-one. J. Chem. Sci. 2013;125:1497–1501. doi: 10.1007/s12039-013-0507-6. [DOI] [Google Scholar]
- 20.Azizian J, Delbari AS, Yadollahzadeh K. One-pot, three-component synthesis of pyrimido[4,5-b]quinoline-tetraone derivatives in water. Synth. Commun. 2014;44:3277–3286. doi: 10.1080/00397911.2011.626139. [DOI] [Google Scholar]
- 21.Lanza F, et al. Development of a semiautomated procedure for the synthesis and evaluation of molecularly imprinted polymers applied to the search for functional monomers for phenytoin and nifedipine. Anal. Chim. Acta. 2001;435:91–106. doi: 10.1016/S0003-2670(01)00905-9. [DOI] [Google Scholar]
- 22.Sunkel CE, Fau de Casa-Juana M, Cillero FJ, Priego JG, Ortega MP. Synthesis, platelet aggregation inhibitory activity, and in vivo antithrombotic activity of new 1,4-dihydropyridines. J. Med. Chem. 1988;31:1886–1890. doi: 10.1021/jm00118a004. [DOI] [PubMed] [Google Scholar]
- 23.Budriesi R, et al. 1,4-Dihydropyridine derivatives as calcium channel modulators: The role of 3-methoxy-flavone moiety. Bioorg. Med. Chem. 2005;13:3423–3430. doi: 10.1016/j.bmc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, et al. New 4-aryl-1,4-dihydropyridines and 4-arylpyridines as p-glycoprotein inhibitors. Drug Metab. Dispos. 2005;33:321–328. doi: 10.1124/dmd.104.002089. [DOI] [PubMed] [Google Scholar]
- 25.Ozer EK, et al. Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers. Turk. J. Biochem. 2018;43:578–586. doi: 10.1515/tjb-2016-0247. [DOI] [Google Scholar]
- 26.Yadav JS, Reddy BVS, Rao RS, Naveenkumar V, Nagaiah K. Microwave-assisted one-pot synthesis of 2,4-disubstituted quinolines under solvent-free conditions. Synthesis (Stuttg.) 2003;10:1610–1614. doi: 10.1055/s-2003-40531. [DOI] [Google Scholar]
- 27.Kundu A, Pramanik A. Novel synthesis of a series of spiro 1,3-indanedione-fused dihydropyridines through the condensation of a tetrone with N-aryl/alkylenamines in presence of solid support silica sulfuric acid. Mol. Divers. 2015;19:459–471. doi: 10.1007/s11030-015-9582-6. [DOI] [PubMed] [Google Scholar]
- 28.Nikpassand M, Mamaghani M, Tabatabaeian K. An efficient one-pot three-component synthesis of fused 1,4-dihydropyridines using HY-zeolite. Molecules. 2009;14:1468–1474. doi: 10.3390/molecules14041468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko S, Sastry MNV, Lin C, Yao CF. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005;46:5771–5774. doi: 10.1016/j.tetlet.2005.05.148. [DOI] [Google Scholar]
- 30.Moghaddam FM, Saeidian H, Mirjafary Z, Sadeghi A. Rapid and efficient one-pot synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives through the Hantzsch four component condensation by zinc oxide. J. Iran. Chem. Soc. 2009;6:317–324. doi: 10.1007/BF03245840. [DOI] [Google Scholar]
- 31.Shestopalov AM, et al. Synthesis of 3-cyano-2-fluoropyridines. J. Fluor. Chem. 2009;130:236–240. doi: 10.1016/j.jfluchem.2008.10.005. [DOI] [Google Scholar]
- 32.Wang XM, Ye HL, Qaun ZJ. One-pot synthesis of benzoquinoline and coumarin derivatives using Meldrum’s acid in three-component reactions. Res. Chem. Intermed. 2012;39:2357–2367. doi: 10.1007/s11164-012-0762-z. [DOI] [Google Scholar]
- 33.Albagli D, et al. Measurement and analysis of neutron and gamma-ray emission rates, other fusion products, and power in electrochemical cells having Pd cathodes. J. Fusion Energy. 1990;9:133–148. doi: 10.1007/BF02627578. [DOI] [Google Scholar]
- 34.Chen D-S, Li Y-L, Liu Y, Wang X-S. Synthesis of bis-benzoquinoline derivatives catalyzed by iodine via ring-opening of furan. Tetrahedron. 2013;69:7045–7050. doi: 10.1016/j.tet.2013.06.042. [DOI] [Google Scholar]
- 35.Maiti G, Karmakar R, Kayal U. One pot imino Diels–Alder reaction for the synthesis of 3-aryl-3,4-dihydrobenzo[f]quinoline derivatives catalyzed by antimony trichloride. Tetrahedron Lett. 2013;54:2920–2923. doi: 10.1016/j.tetlet.2013.03.093. [DOI] [Google Scholar]
- 36.Wang XS, Zhou J, Yang K, Yao CS. Yb(OTf)3: An efficient catalyst for the synthesis of 3-arylbenzo [f]quinoline-1,2-dicarboxylate derivatives via imino-Diels–Alder reaction. Tetrahedron Lett. 2010;51:5721–5723. doi: 10.1016/j.tetlet.2010.08.074. [DOI] [Google Scholar]
- 37.Khaligh NG. Three-component, one-pot synthesis of benzo[f]indenoquinoline derivatives catalyzed by poly(4-vinylpyridinium) hydrogen sulfate. Chin. J. Catal. 2014;35:474–480. doi: 10.1016/S1872-2067(12)60752-9. [DOI] [Google Scholar]
- 38.Fu HG, et al. Synthesis and biological evaluation of quinoline derivatives as a novel class of broad-spectrum antibacterial agents. Molecules. 2019;24:1–11. doi: 10.3390/molecules24030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter S, Parolin C, Palumbo M, Palù G. Antiviral properties of quinolone-based drugs. Curr. Drug Targets Infect. Disord. 2004;4:111–116. doi: 10.2174/1568005043340920. [DOI] [PubMed] [Google Scholar]
- 40.de la Guardia C, et al. Antiviral activity of novel quinoline derivatives against dengue virus serotype 2. Molecules. 2018;23:1–11. doi: 10.3390/molecules23030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui Z, et al. Synthesis and inhibitory activity of novel tri- and tetracyclic quinolines against topoisomerases. Bioorg. Med. Chem. 1998;6:735–742. doi: 10.1016/S0968-0896(98)00030-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang N, et al. Synthesis and structure-activity relationships of 3-cyano-4-(phenoxyanilino)quinolines as MEK (MAPKK) inhibitors. Bioorg. Med. Chem. Lett. 2000;10:2825–2828. doi: 10.1016/S0960-894X(00)00580-1. [DOI] [PubMed] [Google Scholar]
- 43.Jahanshahi P, Mamaghani M. Chemodivergent, multicomponent-tandem facile synthesis of novel 1 H-pyrazolo[1,2-b]phthalazine-5,10-dione using acetic acid functionalized imidazolium salt [cmdmim]I as a recyclable catalyst. New J. Chem. 2019;43:8266–8278. doi: 10.1039/C9NJ00993K. [DOI] [Google Scholar]
- 44.Frisch, M. et al. Gaussian 09, Revision A.1 (2009).
- 45.Pakiari AH, Eshghi F. Geometric and electronic structures of vanadium sub-nano clusters, Vn (n = 2–5), and their adsorption complexes with CO and O2 ligands: A DFT-NBO study. Phys. Chem. Res. 2017;5:601–615. [Google Scholar]
- 46.Chai J-D, Head-Gordon M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008;10:6615–6620. doi: 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- 47.McLean AD, Chandler GS. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980;72:5639–5648. doi: 10.1063/1.438980. [DOI] [Google Scholar]
- 48.Parr RG, Wang Y. Density-Functional Theory of Atoms and Molecules. Oxford University Press; 1989. [Google Scholar]
- 49.Molegro, A. P. S. MVD 5.0 Molegro virtual docker. DK-8000 Aarhus C, Denmark (2011).
- 50.Thomsen R, Christensen MH. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 51.De Azevedo J, Walter F. MolDock applied to structure-based virtual screening. Curr. Drug Targets. 2010;11:327–334. doi: 10.2174/138945010790711941. [DOI] [PubMed] [Google Scholar]
- 52.Tasi G, Palinko I, Nyerges L, Fejes P, Foerster H. Calculation of electrostatic potential maps and atomic charges for large molecules. J. Chem. Inf. Comput. Sci. 1993;33:296–299. doi: 10.1021/ci00013a003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.