Abstract
Background
Pulses of transcranial magnetic stimulation (TMS) with a predominantly anterior-posterior (AP) or posterior-anterior (PA) current direction over the primary motor cortex appear to activate distinct excitatory inputs to corticospinal neurons. In contrast, very few reports have examined whether the inhibitory neurons responsible for short-interval intracortical inhibition (SICI) are sensitive to TMS current direction.
Objectives
To investigate whether SICI evaluated with AP and PA conditioning stimuli (CSPA and CSAP) activate different inhibitory pathways. SICI was always assessed using a PA-oriented test stimulus (TSPA).
Methods
Using two superimposed TMS coils, CSPA and CSAP were applied at interstimulus intervals (ISI) of 1–5 ms before a TSPA, and at a range of different intensities. Using a triple stimulation design, we then tested whether SICI at ISI of 3 ms using opposite directions of CS (SICICSPA3 and SICICSAP3) interacted differently with three other forms of inhibition, including SICI at ISI of 2 ms (SICICSPA2), cerebellum-motor cortex inhibition (CBI 5 ms) and short-latency afferent inhibition (SAI 22 ms). Finally, we compared the effect of tonic and phasic voluntary contraction on SICICSPA3 and SICICSAP3.
Results
CSAP produced little SICI at ISIs = 1 and 2 ms. However, at ISI = 3 ms, both CSAP and CSPA were equally effective at the same percent of maximum stimulator output. Despite this apparent similarity, combining SICICSPA3 or SICICSAP3 with other forms of inhibition led to quite different results: SICICSPA3 interacted in complex ways with CBI, SAI and SICICSPA2, whereas the effect of SICICSAP3 appeared to be quite independent of them. Although SICICSPA and SICICSAP were both reduced by the same amount during voluntary tonic contraction compared with rest, in a simple reaction time task SICICSAP was disinhibited much earlier following the imperative signal than SICICSPA.
Conclusions
SICICSPA appears to activate a different inhibitory pathway to that activated by SICICSAP. The difference is behaviourally relevant since the pathways are controlled differently during volitional contraction. The results may explain some previous pathological data and open the possibility of testing whether these pathways are differentially recruited in a range of tasks.
Keywords: SICI, TMS, Coil orientation, Triple pulse stimulation, Movement preparation, Inhibitory circuit
Highlights
-
•
Opposite directions of conditioning stimulus (CS) used to suppress MEPs evoked by a conventional test stimulus.
-
•
Different directions of CS have different time courses of short-interval intracortical inhibition (SICI).
-
•
They also interact differently with short-latency afferent inhibition and with cerebellar inhibition.
-
•
They are differently affected in a reaction time task.
-
•
We suggest there are two forms of SICI in motor cortex.
1. Introduction
All movements involve critical interactions between inhibitory and excitatory interneurons within the primary motor cortex (M1). Insights to these interneuronal circuits can be achieved in humans with transcranial magnetic stimulation (TMS) by assessing how different inputs to the cortex influence the excitability of the corticospinal tract via their effects on motor evoked potentials (MEP). Ever since its introduction, it has been known that TMS of the M1 hand area can activate two separate inputs to corticospinal output cells by changing the direction of current from posterior-to-anterior (PA) to anterior-to-posterior (AP). The latter evokes MEPs with longer latency and higher threshold [1,2], and the neural elements that it activates have a different strength-duration time constant than those responding to PA stimulation [3]. The source of these two sets of inputs is unknown, but it has been speculated that AP currents activate inputs from more anterior (premotor) locations than PA currents [[4], [5], [6], [7]].
Double-pulse TMS, in which a subthreshold conditioning stimulus (CS) is followed by a suprathreshold test stimulus (TS) at interstimulus intervals (ISI) of 1–5 ms, can be used to examine a GABAa-ergic cortico-cortical inhibitory process termed short-interval intracortical inhibition (SICI) [8,9]. SICI is implicated, for example, in movement preparation [10,11] and is reduced in a variety of movement disorders, such as dystonia and cortical myoclonus [12,13]. Of note, it is particularly important to use the appropriate CS intensity and ISI, as well as the appropriate intensity of the TS to assess SICI; adjusting these parameters results in different effects that may reflect distinct underlying mechanisms [14,15]. For example, SICI evaluated at an interstimulus interval of 1 ms appears to have quite a different mechanism than at 3 ms [16]; higher intensities of CS can contaminate SICI with a separate phenomenon, short-interval intracortical facilitation [17,18].
Very few papers have investigated whether SICI is sensitive to the orientation of the CS. Conducting these experiments requires either two separate, but overlapping, TMS coils that can be oriented at different angles to the central sulcus, or a special device that allows two stimuli of opposite directions to be delivered through the same coil. Ziemann et al. (1996) [18] used a two-coil approach and found that rotating the CS by 90° to induce latero-medial (CSLM-TSPA) currents in the brain produced the same amount of SICI as conventional CS in PA direction (CSPA-TSPA, SICIPA). The result is compatible with the idea that, unlike the excitatory elements responsible for the MEP, inhibitory elements have no preferred orientation and therefore can be activated by any direction of CS. Hanajima et al. (1998) [14] delivered oppositely directed CS and TS through the same coil and came to a similar conclusion, although they tested participants during slight muscle contraction rather than at rest.
However, one result suggested that opposite CS directions may recruit two separate mechanisms. Hanajima et al. (2008) [12] found that, although SICIPA was reduced or absent in patients with dystonia, it was normal in the same patients if a SICIAP (CSAP-TSAP) was used. Thus, SICIAP seemed to be pathologically different from SICIPA. Unfortunately, Hanajima (2008) [12] did not use a special current reversal device to deliver different directions of CS and TS in this particular set of experiments. Thus, it was never clear whether the dissociation in dystonia was a result of changing the direction of the CS or the TS.
The aim of the present set of experiments was to revisit the orientation sensitivity of SICI using a two-coil method with a constant PA test stimulus (TSPA) and ISIs from 1 to 5 ms. The results showed that the time course of SICI differed for CSAP and CSPA conditioning stimuli, suggesting that there might be two types of SICI (SICICSPA (i.e. CSPA-TSPA) and SICICSAP (i.e. CSAP-TSPA)). To test this further, we made use of the fact that many previous papers have described how SICI interacts with other forms of inhibition such as SICICSPA with different ISI [19], cerebellar-motor cortex inhibition (CBI) [20], and short-latency afferent inhibition (SAI) [[21], [22], [23]]). Our hypothesis was that if SICICSPA and SICICSAP had different mechanisms, then they would interact differently with SICICSPA, CBI and SAI. Finally, to test the functional relevance of these two forms of SICI, behavioural experiments provided further evidence that SICICSPA and SICICSAP have distinct neurophysiological features.
2. Materials and methods
2.1. Participants
A total of 36 healthy participants (18 males and 18 females; mean age: 25.5 ± 4.5 years) without any neurological or other disease were recruited and consented to participate in the study. All experiments were approved by the University College London Ethics Committee, and all experimental designs followed the international safety guidelines for non-invasive brain stimulation [24]. The numbers of participants in each experiment and the overlap between them are tabulated in supplementary material 4 and 5.
2.2. Surface electromyography
Surface electromyography (EMG) signals were recorded using a belly-tendon montage from surface electrodes (WhiteSensor 40713, AmbuR, Denmark) over the right first dorsal interosseous (FDI) muscle. Data were amplified with a gain of 1000, bandpass filtered (5 Hz–3000 Hz) by a Digitimer D360 amplifier (Digitimer Ltd, Welwyn Garden City, Hers, UK), and digitised at 5000 Hz by a Power 1401 data acquisition interface (Cambridge Electronic Design Ltd., Cambridge, UK). All recorded MEPs were stored in the same computer for offline analysis with Signal software version 7.01 (Cambridge Electronic Design Ltd., Cambridge, UK).
2.3. Transcranial magnetic stimulation (TMS)
TMS pulses were delivered by Magstim 200 Monophasic stimulators (Magstim Co., UK). Three different coils were used throughout the experiments: (1) a figure-of-eight coil with an outer diameter of 80 mm (D50, Magstim Co., UK), (2) an oval coil with a long outer diameter of 130 mm and a short outer diameter of 100 mm (Magstim Co., UK) and (3) a 110 mm double cone coil (Magstim Co., UK) used solely for cerebellar stimulation. Note that the oval coil was used in preference to a second figure-of-eight coil because it has a thinner profile, reducing the scalp-coil distance of the overlying figure-of-eight coil.
2.4. Motor cortex stimulation
As previously reported [25], we arranged the coil so that the side of the oval coil resting on the hotspot overlapped with the junction region of the figure-of-eight coil. The coils were then tied securely to each other with the oval coil under the D50 coil. (see Fig. 1A; also see Supplementary material I and Fig. S1 for methodological details). The motor hot spot was defined as the position on the left motor cortex where supra-threshold PA currents in the oval coil produced the largest and most consistent MEPs in the right FDI muscle. AMTs measured in PA direction (AMTPA) were defined separately for both the D50 coil and the oval coil as the minimal intensity to evoke an MEP of more than 200 μV in at least 5 of 10 trials while participants contracted their right FDI muscle by 10% maximum voluntary contraction [26]. AMT for the AP direction (AMTAP) was measured in the D50 coil after connecting a current reversing cable. Whenever the text refers to the intensity of a CS, it is sometimes expressed relative to the AMT (PA or AP as appropriate) of the coil delivering that stimulus and sometimes relative to AMTPA regardless of direction. The text will indicate which was employed.
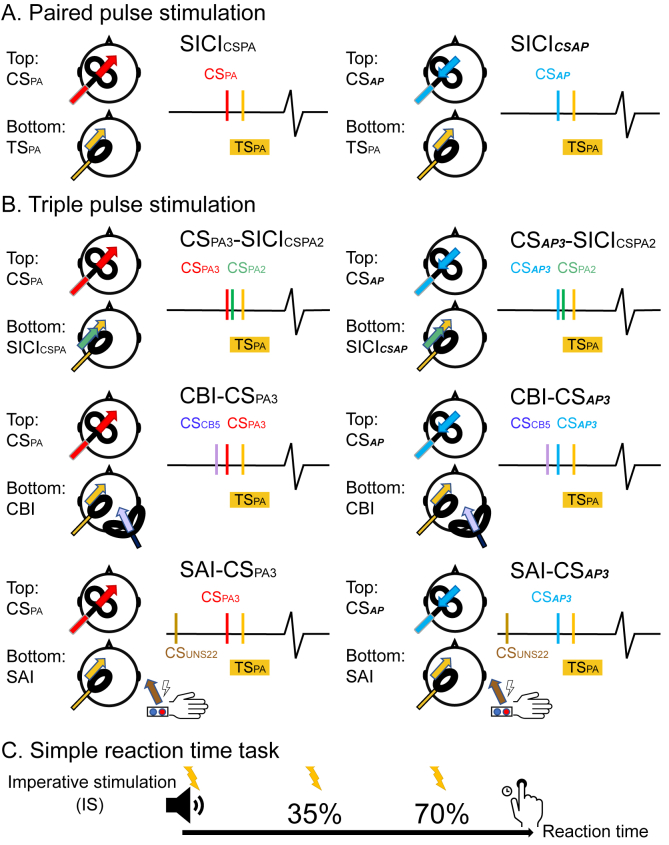
Fig. 1.
TMS Coil Set-up (A, B) and protocol of simple reaction time task (C). The figure-of-eight coil connecting by current reversing cable was applied to induce anterior-posterior direction conditioning current in paired-pulse stimulation (A) and triple-pulse stimulation (B). There were three triple-pulse stimulation protocols here: triple pulse stimulation with two cortical conditioning, with cerebellar conditioning, and with peripheral sensory afferent input. The direction and colour of arrows indicate the orientation and coil selected to induce currents over the cortex. The images on the left side depict scenarios in which both coils (conditioning stimulus: figure-of-eight coil in red; test stimulus: oval-coil in yellow) delivered posterior-anterior (PA) currents. In triple-pulse stimulation, another conditioning stimulus was applied with the oval-coil (green), cerebellar conditioning stimulus (violet), or peripheral sensory afferent input (brown). On the right, the figure-of-eight coil produces an anterior-posterior (AP) current (blue). The bottom figure illustrated the timing giving SICI during a simple reaction time task (C).
2.5. Short-interval intracortical inhibition (SICI)
We used the short-interval intracortical inhibition (SICI) paradigm, where a sub-motor threshold pulse (conditioning stimulus; CS) has the effect of suppressing the MEP generated by a subsequent supra-motor threshold pulse (test stimulus, TS) delivered a few milliseconds later. To simplify interpretation of the data, the TS (from the oval coil) was always delivered in the PA direction (TSPA); the CS stimulus was in either the PA or AP direction (CSPA or CSAP), depending on the direction of current in the figure-of-eight conditioning coil (D50) (Fig. 1A). The current reversal required the insertion of a reversing cable such that it was not possible to randomise the CS direction from trial to trial. Instead, blocks of trials were performed with the same CS direction. The intensity of the TS was adjusted to evoke an MEP of approximately 1 mV peak-to-peak in the relaxed right FDI. SICI was calculated as the ratio between the averaged conditioned responses (CS + TS) and the test (TS) MEP amplitudes. In this study, SICICSPA means SICI consisting of CSPA and TSPA, and SICICSAP indicates SICI was composed of CSAP and TSPA.
2.6. Cerebellar-M1 inhibition (CBI)
To evaluate cerebellar-M1 connectivity (CBI), we used the paired-pulse protocol described by Ugawa et al. [27]. The conditioning pulse was delivered over the right cerebellum, 3 cm lateral to the inion, 5 ms prior to a TSPA over M1 (CSCB5). The conditioning pulse flowed in the superior direction in the cerebellum, and the intensity of the cerebellar CS was set to −5% maximum stimulator output (MSO) below the brainstem motor threshold (AMTBA) [27]. This was measured by stimulating the cerebellum over the inion while subjects contracted their FDI muscle. This threshold was defined as the stimulator output that elicited an MEP of 50 μV in 5 of 10 trials. In 5 participants, there was no response at 80% MSO, which is often perceived as unpleasant. In these cases, the CSCB5 intensity was set to 75% MSO [28]. Stimuli were randomized such that there were 15 trials of conditioned responses for every 15 TS tested. CBI was calculated as the ratio of the average MEP amplitudes conditioned by cerebellar stimulation to the average MEP response elicited by TS alone.
2.7. Short-latency afferent inhibition (SAI)
To investigate how somatosensory input interacts with SICI, we applied a conditioning peripheral nerve stimulation (DS7A; Digitimer Ltd, Welwyn Garden City, Hers, UK) to the ulnar nerve prior to M1 TMS (CSUNS). The inter-stimulus intervals (ISI) between electrical CS and TMS were set according to individual N20 somatosensory-evoked potential latency [29]. We investigated SAI with ISI of ∼22 ms (N20 + 2, CSUNS22) inputs using a square-wave pulse width of 200 μs. The intensity of peripheral CS was adjusted to elicit a 0.2 mV FDI M-wave response (i.e. just above motor threshold [30]). Similar to CBI, SAI was quantified as a ratio of the mean amplitude of 15 CS + TS MEPs to 15 TS MEPs.
2.8. Protocols
2.8.1. Experiment 1. SICI at ISIs = 2 and 3 ms using different intensities and orientations of CS
This experiment investigated how different current directions and conditioning intensities affected SICI. We recruited 11 healthy subjects (28.8 ± 4.2 years old, 4 male and 7 female). We selected ISIs of 2 ms and 3 ms since previous work had found differences in the effect of PA and AP conditioning stimuli at these intervals [12]. First, the AMT was measured in both PA (AMTPA) and AP directions (AMTAP) with the D50 figure-of-eight coil. The intensities used for both CSPA and CSAP were 60, 70, 80, 90, 100 and 110% of AMTPA. These were supplemented for CSAP with the additional intensities of 60, 70, 80, 90, 100 and 110% of AMTAP. This allowed us to assess the entire profile of SICICSAP from low to high intensity since previous studies only used AMTAP when evaluating SICIAP. The ISI was randomized between blocks of trials in which the six different intensities were intermixed. Thus, each block consisted of a total of 105 pulses: 15 pulses per each CS (6 × 15 = 90) and 15 TS pulses. Since each block of trials lasted up to 7–8 min, we had a team member looking at participants during testing and reminding participants to maintain attention.
2.8.2. Experiment 2. SICI at ISIs = 1, 2, 3 and 5 ms using different orientations of CS
In this experiment, we fixed the intensity of the CS to 90% AMTPA (see the result of experiment 1) in order to explore ISIs over a wider range. This experiment included 15 participants (27.3 ± 4.9 years old, 8 male and 7 female). We performed 15 pulses for each ISI state. Within each block of trials, the CS orientation was constant, but the ISIs were randomized.
2.8.3. Experiments 3, 4 and 5
All of these experiments compared the interaction of SICI using opposite orientations of CS (i.e. CSAP and CSPA) with other forms of cortical inhibition. However, we had to first decide the ISI and CS intensity to evoke SICI. The results of experiments 1 and 2 showed that SICI evoked with CSAP and CSPA had different time courses. But at ISI = 3 ms, they both appeared to produce a similar amount of inhibition. Importantly, both SICICSPA3 and SICICSAP3 produced effective inhibition with a CS intensity of 90% AMTPA no matter what the orientation of the CS (see the result of experiments 1 and 2). This raises the question as to whether both current directions activate the same inhibitory system at ISI = 3 ms, even if they do not work at ISI = 2 ms. Thus, in the following experiments, we set the ISI to 3 ms and the CS intensity to 90% AMTPA for both directions of conditioning current (Fig. 1B).
2.8.3.1. Experiment 3. SICI produced by two CS: the interaction of CSPA and CSAP
Previous triple-pulse TMS studies showed that SICICSPA could be enhanced by applying a second CSPA a few ms before the conditioning stimulus and that the amount of inhibition is greater than the expected sum of each stimulus alone (i.e. temporal summation [19]). The aim of experiment 3 was to test whether two conditioning stimuli of opposite directions interacted in the same way as two CSPA.
We refer to the earliest CS as CS1, and the later CS as CS2, with the TS being the last in the series. CS2 was applied through the oval coil in the PA direction using an ISI = 2 ms (SICICSPA2) prior to the TS (also in the PA direction). The intensity of CS2 was expressed relative to the AMTPA in the oval coil (90% AMTPA). CS1 was applied via the D50 coil at an ISI = 3 ms prior to TS. The orientation of current in CS1 was reversed with the reversing cable randomly between blocks (i.e. CSAP3 and CSPA3 at 90%AMTPA). In other words, we measured how SICI evoked by CSPA2 was affected by the presence of either CSPA3 (CSPA3-SICICSPA2) or CSAP3 (CSAP3-SICICSPA2) (Fig. 1B). Thus, we would compare the effect of interacting SICI evoked by either CSPA3 or CSAP3 with a second CS at 2 ms (CSPA2), arguing that if CSPA3 and CSAP3 were both activating the same inhibitory system at ISI = 3 ms, then they would show equal summation with CSPA2. Thirteen subjects (27.5 ± 5.0 years old, 8 male and 5 female) were recruited. For each block, 15 trials of each condition were tested. The conditions in experiments 3 and 4 were intermixed within the same recording session. Details are given in Table 1.
Table 1.
Stimulation conditions in Experiment 3, 4, and 5.
| Experiment | Block | Condition |
|---|---|---|
| Experiment 3, 4 | 1 | Test stimulation, SICICSPA3, CBI-CSPA3 |
| 2 | Test stimulation, SICICSAP3, CBI-CSAP3 | |
| 3 | Test stimulation, CBI | |
| 4 | Test stimulation, SICICSPA2, SICICSPA3, CSPA3-SICICSPA2 | |
| 5 | Test stimulation, CSAP3-SICICSPA2 | |
| Experiment 5 | 6 | Test stimulation, SAI |
| 7 | Test stimulation, SICICSPA3, SAI-CSPA3 | |
| 8 | Test stimulation, SICICSAP3, SAI-CSAP3 |
For experiments 3 and 4, ten participants received blocks 1–5 randomly in a single session. However, this sequence proved time consuming and eleven participants only managed blocks 1, 2, and 3 in a single session. All sixteen individuals contributed data to experiment 4.
Three participants requested to be excused from CBI because of discomfort. They received blocks 2 (but without CBI-CSAP3), 4, and 5 randomly in a single session. These contributed data to experiment 3 together with the ten participants who had received all 5 blocks (i.e. 13 participants in total).
Fourteen individuals performed experiment 5 with blocks 6–8 intermixed randomly in a single session.
For details of participants' distribution in each experiment, please see supplementary material 5.
2.8.3.2. Experiment 4. Interaction of SICI using either CSPA or CSAP with CBI
The interaction between CBI and SICI evoked by CSPA has been reported in a previous publication by Daskalakis and colleagues [20]. In experiment 4, we used the CBI protocol to activate cerebello-thalamo-cortical inputs to M1 and measured the effects on SICI with different conditioning stimulus directions (CSPA3 and CSAP3). The question addressed here is whether CBI interacts differently with SICI evoked by CSPA3 or CSAP3. Sixteen healthy volunteers were recruited (26.5 ± 4.6 years old, 9 male and 7 female). The paradigm was similar to the protocol described by Daskalakis et al. [20] and involved three stimuli. The first stimulus was applied to the cerebellum (CSCB5) 5 ms prior to the TS over M1 (CBI). SICI was produced by either CSPA3 and CSAP3. In other words, the triple pulse combinations were CBI-CSPA3 and CBI-CSAP3 (Fig. 1B), and paired pulse combinations were SICICSPA3, SICICSAP3 and CBI. Fifteen responses of each condition were averaged and compared. As mentioned in experiment 3, the conditions in experiments 3 and 4 were intermixed within the same recording session. Details are given in Table 1.
2.8.3.3. Experiment 5. Interaction of SICI using either CSPA or CSAP with SAI
Since previous work has reported the interaction of SAI with SICICSPA [[21], [22], [23]], the question here was whether SAI interacts differently with SICI evoked with CSPA3 and CSAP3. Fourteen subjects (27.1 ± 4.9 years old, 7 male and 7 female) participated in this session with a triple-pulse stimulation protocol similar to experiment 4. Here, a peripheral nerve CS was applied 22 ms (CSUNS22) prior to the TS over M1. SICI again was either produced by CSPA3 or CSAP3. Thus, conditions in this session included two different combinations of triple pulse stimulation (SAI-CSPA3 and SAI-CSAP3) (Fig. 1B) and paired stimulation (SAI, SICICSPA3 and SICICSAP3). In addition, the set of CSPA3 and the set of CSAP3 were tested separately with randomized order of conditions. Each block included 15 trials of each condition. Conditions tested in this experiment are listed in Table 1.
2.8.4. Experiment 6. Changes of SICI in a simple reaction time task
Thirteen healthy participants (25.2 ± 5.5 years old, 7 male and 6 female) were enrolled in this experiment. We probed SICICSPA3 and SICICSAP3 in 3 different brain states: at rest, during tonic muscle activation, and while subjects performed a simple reaction time task (SRTT). During tonic activation of muscle, participants were asked to make an isometric (approximately 5% MVC) contraction of FDI (in one block) or abductor digiti minimi muscle (ADM, in another block) muscle while SICI was measured. In SRTT, there was no warning cue; the imperative (“go”) stimulus (IS) was an auditory tone burst (100 ms) of 500 Hz given randomly every 5 ± 0.5 s. Individuals had to tap their index finger as quickly as possible on hearing the tone. SICI was tested at 3 time points in the reaction time period: at the cue, the early stage of reaction time (RT35%), the late stage of reaction time (RT70%) (Fig. 1C). RT timings were adjusted to each individual's mean reaction time, taken from a practice session consisting of 20 trials.
The test pulse intensity was set to elicit a ∼1 mV peak-to-peak amplitude for all conditions (rest, tonic and SRTT). Both conditioning pulse directions (CSPA and CSAP) were set to 90% AMTPA and the ISI = 3 ms. Throughout all experimental conditions, we recorded 20 test pulses and 20 CS trials. For the SRTT, participants performed the task twice for each muscle, and a total of 140 trials were performed (120 trials with TMS; 20 trials with just the auditory cue).
2.9. Data analysis and statistics
EMG recordings were analyzed using Signal software version 7.01 (Cambridge Electronic Design Ltd., Cambridge, UK). The MEPs amplitudes from both unconditioned and conditioned MEPs were considered for analysis. As in previous studies [[31], [32], [33], [34]], we excluded extreme MEP amplitudes in which responses were more than 1.5 times above the third quartile or below the first quartile of the interquartile range. Trials were also excluded if ongoing muscle activity was detected (EMG signal >50 μV within 100 ms before TMS pulse) [34]. The total number of discarded MEPs amounted to 4.9% of all recordings in this study. In experiment 1, the SICI data is plotted against both relative (% of AMT) and absolute intensities (% of Maximum Stimulator Output, MSO) of the CS. Absolute intensities of 70% AMTAP (48 ± 7.6%) and 100% AMTPA (48 ± 9.1%) did not significantly differ (48% MSO) (t = 0.052, df = 10, p = 0.959). SPSS version 22 (IBM Co., US) was used for all statistical analyses.
All measures of inhibition in this paper used the conditioned/test MEP amplitude ratio as the variable of interest, where values lower than 1 indicate inhibition. In experiment 1, we compared the effect of CS intensity on SICICSPA and SICICSAP separately at each ISI (i.e. 2 or 3 ms) with a two-way repeated-measures ANOVA (RM-ANOVA) with factors “Intensity” (60%, 70%, 80%, 90%,100%, and 110% AMTPA) and “Orientation” (CSPA and CSAP). The data from this experiment were also used to select the optimal CS intensity to use at ISI = 3 ms in all subsequent experiments. To do this, additional one-tailed t-tests (to avoid type II error) were used to compare the amount of inhibition (SICI) at each CS intensity with baseline (ratio = 1) (see results Experiment 2). In four cases (SICICSAP3 with 100% and 110% AMTAP, SICICSAP3 with 60% AMTPA, SICICSAP3 with 110%AMTPA), a Shapiro-Wilk test showed a violation of normality. In these instances, we used a one-tailed Wilcoxon signed-rank test in experiment 1.
In experiment 2, a two-way RM-ANOVA with factors “Orientation” (CSPA and CSAP) and “ISI” (1, 2, 3, and 5 ms) was used to compare SICICSPA and SICICSAP. Here one-tailed t-tests were done again to examine the effectivity of inhibition, and a Shapiro-Wilk test was done for checking normality.
In experiments 3, 4 and 5, we compared the amount of inhibition produced by SICICSAP3 and SICICSPA3 alone with that seen during the presence of other forms of inhibition (SICICSPA2, CBI, SAI). To do so, we used two separate one-way RM-ANOVAs and a two-way RM-ANOVA in each experiment. For example, in experiment 4, where CBI interacted with SICI, we first performed 2 separate one-way RM-ANOVAs: one compared CBI, SICICSAP3 and CBI-CSAP3, and the other compared CBI, SICICSPA3, and CBI-CSPA3. The question here was whether the inhibition produced by CBI combined with SICI differed from that produced by SICI alone. A two-way ANOVA determined whether CBI interacted with SICICSPA3 and SICICSAP3 in different ways. It had “Orientation” (CSAP and CSPA) and “Condition” (SICI alone (SICICSPA3 and SICICSAP3) and SICI in the presence of CBI (CBI-CSPA3 and CBI-CSAP3) as main factors. Therefore, the structure to access interaction in the two-way RM-ANOVA was (SICICSPA3, CBI-CSPA3) x (SICICSAP3, CBI-CSAP3). These correspond to the 4 right-hand columns of Fig. 5. Additionally, in order to check the consistency of our data with those of previous triple pulse studies [20,21,23], changes in SICI during CBI or SAI were calculated using the method proposed by Daskalakis and co-workers [20] (See Supplementary material 6 for details). Daskalakis et al. calculated the change in SICICSPA2 in the presence of CBI as by [amplitude of MEP conditioned by CBI-CSPA2]/[amplitude of MEP by CBI only]. We used the same method to compute the change in SICICSPA3 (when CBI or SAI was presented). Normality was tested by the Shapiro-Wilk test. Paired t-tests were applied to compare SICICSPA3 with the change in SICICSPA3 when CBI or SAI was also performed.
Fig. 5.
Interaction between SICI and CBI. The left panel depicts the effect of cerebellar stimulation alone (CBI: ISI = 5 ms) compared to the responses elicited when combined with CSAP3 (middle-panel) or CSPA3 (right-panel). The combination of CBI and CSAP3 produced more inhibition than either conditioning stimulus alone, whereas the combination of CBI and CSPA3 produced approximately the same amount of inhibition as either conditioning stimulus alone. Double asterisks indicate p < 0.05 in post hoc pairwise comparison. CS1 indicates the earlier CS, and CS2 means the later CS.
In experiment 6, two separate two-way RM-ANOVAs with “Brain state” (Rest, Tonic muscle contraction) and “Orientation” (SICICSPA3 and SICICSAP3) as main factors were used to investigate changes in SICI in different brain states in each muscle (FDI and ADM). Then, we used a further two-way RM-ANOVA with factors “Orientation” (SICICSPA3 and SICICSAP3) and “Time” (on the cue, RT35% and RT70%) to assess changes in SICI during the preparation of movement, separately in each muscle (FDI and ADM).
Mauchly's test was used to check sphericity in all RM-ANOVAs, and Greenhouse-Geisser correction was used for non-sphericity conditions. Although RM-ANOVA is quite robust to violations of normality, we nevertheless checked the normality of residuals with Quantile-Quantile Plots (Q-Q plots) in each experiment. There were no obvious violations of normality in these datasets. The critical value of significance was 0.05 in all statistics. Post hoc pairwise comparisons employed a Bonferroni correction.
3. Results
3.1. Experiment 1
This experiment investigated how SICI at ISIs = 2 and 3 ms varied with the intensity and orientation of the CS. Fig. 2A and Fig. 2B gives an overall picture of the results. CS intensities are given in MSO in order to display all data using the same x-axis; the two curves on each graph display effects of the two orientations of CS. Note that x-error bars (±SEM) are necessary since in the actual experimental sessions, individual CS intensities were expressed relative to AMT, which differed in terms of MSO for each person. In addition, since there was no obvious SICI at an ISI = 2 ms with CSAP, we extended the range of intensities for this orientation to 60–110% AMTAP. These two ranges are indicated in the graphs by dark and light symbols, respectively. The absolute intensities of the conditioning stimuli may appear to be quite high; this is because the conditioning coil was placed over the test coil which increased the distance between the scalp surface and the conditioning (D50) coil.
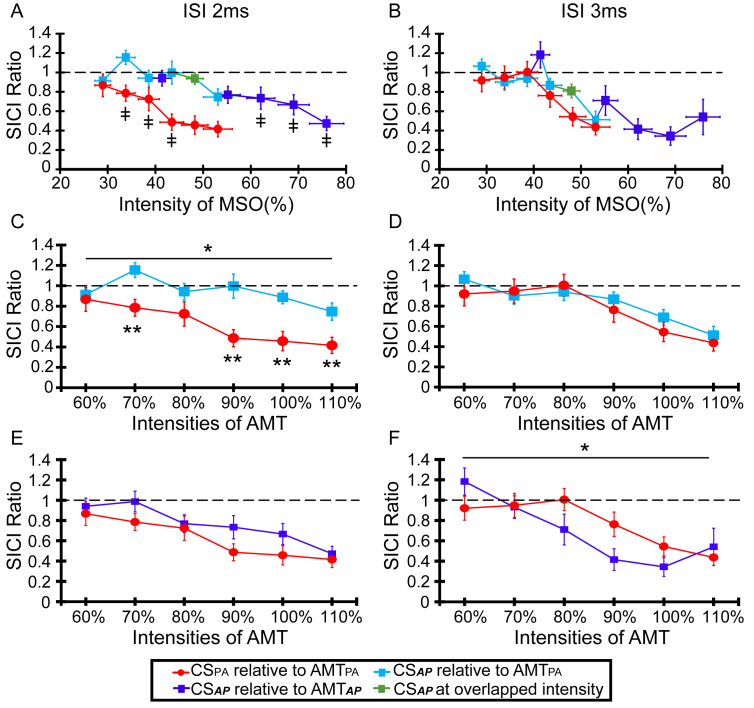
Fig. 2.
SICI at 2 and 3 ms ISI: effect of different conditioning stimulus intensities for CSAP and CSPA. The left column (A, C, E) depicts SICI at ISI = 2 ms and in the right column (B, D, F) SICI at ISI = 3 ms. Panels in the top row (A, B) plot the CS intensity in percent MSO; panels in the middle row (C, D) plot CS intensity in percent of AMTPA; panels (E, F) in the bottom raw plot CS intensity in percent of direction-appropriate AMT (i.e. AMTAP for CSAP, AMTPA for CAPA). When intensities of conditioning stimulation were given based on AMTPA, panel D shows that at ISI = 3 ms, both CSAP (light blue) and CSPA (red) have approximately the same threshold (i.e. 90% AMTPA). In contrast, at ISI = 2 ms (panel C), inhibition is evident only with CSPA but not with CSAP. Because of the lack of SICI over these intensities with CSAP, an additional run was performed in which the intensity of CSAP was expressed in percent AMTAP. When intensities of conditioning stimulation were giving based on the AMT of the direction of conditioning stimulation (i.e. CSAP by AMTAP), panel F reveals that SICICSAP3 by AMTAP (deep blue) is significantly different from SICICSPA3 by AMTPA (red). In contrast, panel E shows that SICICSAP2 by AMTAP (deep blue) is not different from SICICSPA2 by AMTPA (red). The data from both these runs are combined in panels A and B, in which the intensity of the conditioning stimuli is expressed in %MSO in order to plot all data on the same x-axis. Because AMT varies (in terms of %MSO) across individuals, the data are plotted with x-error bars. In each graph, the data points in red and light blue replot the data in panels C and D; the dark blue points and line plot data from the additional runs at higher intensities of CSAP. Green points are data from points in the two runs of CSAP in which the absolute intensities of CS were the same, and thus the data was combined. The graphs show that at ISI = 3 ms, the threshold for producing SICI is approximately the same for both directions of CS. However, at ISI = 2 ms, SICI was evident only with high intensities of CSAP. The single asterisk indicates the significant (p < 0.05) “Orientation” X “Intensity” interaction in a two-way RM-ANOVA; double asterisks indicate intensities with a significant difference (post hoc pairwise comparison) in the amount of inhibition produced by CSPA and CSAP. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
This is illustrated more clearly in Fig. 2C and D, which directly compare CS intensities in those blocks where intensity was expressed relative to AMTPA (for both current directions). The x-axis plots CS intensity as a percent of AMTPA. At ISI = 3 ms (Fig. 2D), the recruitment of SICI is similar for both directions of CS. In contrast, at ISI = 2 ms (Fig. 2C), CSAP evokes very little SICI at any intensity whilst it is clear for CSPA. For completeness, Fig. 2E and F plot the comparison between CSPA and CSAP in terms of the AMT of each direction of CS. Fig. 2E (ISI = 2 ms) shows that the threshold for producing SICI (relative to AMT of the CS) is the same for each CS direction. However, the situation at ISI = 3 ms is quite different. When expressed in terms of the direction-appropriate AMT, the threshold for SICI with CSAP was lower than that for CSPA.
The impression given by the graphs was borne out in the statistical analysis. In Fig. 2C, a two-way RM-ANOVA between CSPA and CSAP at ISI = 2 ms and CS intensities expressed for both orientations in terms of AMTPA, revealed a main effect of “Orientation” (F1,10 = 12.119, p = 0.006), and a significant “Orientation x Intensity” interaction (F5,50 = 3.113, p = 0.016). Post hoc pairwise comparison showed that this was due to the fact that SICICSPA2 was stronger than SICICSAP2 (p = 0.02 at 70%, p = 0.006 at 90%, p = 0.002 at 100%, and p = 0.013 at 110%AMT) (Fig. 2C). In contrast, no differences were found between CS directions at ISI = 3 ms (Orientation: F1,10 = 1.000, p = 0.341; Orientation x Intensity: F5,50 = 0.509, p = 0.768) (Fig. 2D). In Fig. 2E (ISI = 2 ms), there were no main or interaction effects. In contrast, in Fig. 2F (ISI = 3 ms), there was a significant “Orientation x Intensity” interaction (F5,50 = 3.690, p = 0.006), which was due to the fact that CSAP evoked inhibition at a lower threshold relative to the AMT of the CS than CSPA.
To summarise, at ISI = 3 ms, the threshold for recruiting SICI was the same with both CS orientations, whereas at ISI = 2 ms, the absolute threshold was much higher for CSAP than CSPA, although it was approximately the same in terms of the relative AMT for each direction.
3.2. Experiment 2
In this experiment, the intensity of the CS was the same for both directions (90% AMTPA), while we tested ISIs shorter and longer than in experiment 1. The data in Fig. 2D show that 90% AMTPA was the lowest intensity that produced significant inhibition (one-tailed t-test compared to baseline) with both orientations of CS (t = −1.84, df = 10, p = 0.048 for SICICSAP3 and t = −1.97, df = 10, p = 0.039 in SICICSPA3). At ISIs = 2, 3 ms, the results are very similar to those in experiment 1: both directions of CS produced the same amount of SICI at ISI = 3 ms, whereas SICI was absent at ISI = 2 ms for CSAP, whilst it was clear for CSPA. An even greater difference was seen at ISI = 1 ms, where there was strong inhibition for CSPA but much less for CSAP. Neither direction of CS produced inhibition at ISI = 5 ms.
The two-way RM-ANOVA revealed a significant “Orientation x ISI” interaction (F3,42 = 13.902, p < 0.001); post hoc pairwise comparison showed a stronger SICI with CSPA than CSAP at 1 ms (p < 0.001) and 2 ms (p = 0.020). Compared with baseline, there was a small amount of inhibition with CSAP1 (one-sample t-test, df = 14, t = −2.623, p = 0.01) but no detectable inhibition with CSAP2. In summary, the results confirm those of experiment 1 (also see supplementary material II and Fig. S2) and extend the differences in CS direction to ISI = 1 ms.
3.3. Experiment 3: Interaction between SICICSPA and SICICSAP
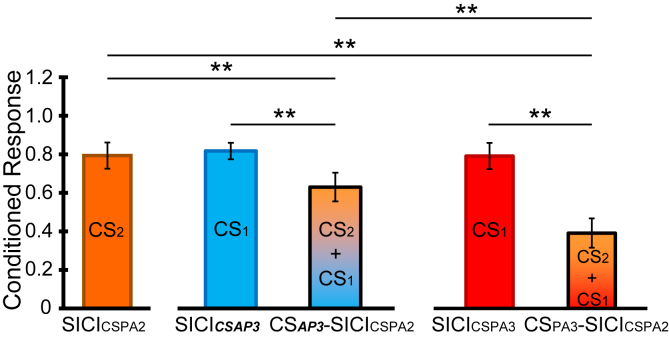
Fig. 4 shows that when given alone, all three CS (CSPA3, CSAP3, and CSPA2) evoked approximately the same amount of SICI. However, CSPA3-SICICSPA2 evoked much greater inhibition than CSAP3-SICICSPA2. One way of comparing these values is to ask how much SICI we might expect to observe from combining the effects of two CS. The level of SICI evoked by CSAP3 and CSPA2 alone was 0.82 and 0.79, respectively. Thus if the effects were independent, we should expect that the result of combining them should be 0.82 ∗ 0.79 = 0.65. This prediction is similar to the observed value of 0.63 when CSAP3 and CSPA2 were combined experimentally (i.e. CSAP3-SICICSPA2). This contrasts with the combination of CSPA2 and CSPA3 (i.e. CSPA3-SICICSPA2). This combination produced SICI = 0.39 which was much more powerful than expected from independently combining SICICSPA3 (0.79) and SICICSPA2 (0.79 ∗ 0.79 = 0.62). Thus, rather than acting independently, CSPA2 and CSPA3 show temporal facilitation and produce more SICI than expected.
Fig. 4.
Interaction between CSPA and CSAP. This experiment was designed to compare the interaction of CSPA3 and CSAP3 with CSPA2. The left panel shows the amount of SICI produced by CSPA2 alone. The middle panel shows the amount of SICI produced by CSAP3 alone and the amount produced by the combination of CSAP3 and SICICSPA2. The right panel shows the amount of SICI produced by CSPA3 alone and the amount produced by the combination of CSAP3 and SICICSPA2. Both combinations produce more inhibition than either conditioning stimulus alone, but the effect is much larger with CSPA3-SICICSPA2 than with CSAP3-SICICSPA2. Double asterisks indicate p < 0.05 in post hoc pairwise comparison. CS1 indicates the earlier CS, and CS2 means the later CS.
In the statistical analysis, we compared the level of inhibition produced by CSPA3-SICICSPA2, SICICSPA3, and SICICSPA2. A one-way RM-ANOVA revealed a significant, global difference among them (F2,24 = 21.387, p < 0.001). Post hoc pairwise comparison showed that CSPA3-SICICSPA2 was significantly different from both SICICSPA3 (p < 0.001) and SICICSPA2 (p = 0.001). Similarly, another one-way RM-ANOVA on the level of SICI produced by CSAP3-SICIPA2, SICICSAP3, and SICICSPA2 revealed a significant difference among them (F2,24 = 4.668, p = 0.019). Post hoc pairwise comparison showed that CSAP3-SICIPA2 was different from both SICICSAP3 (p = 0.039) and SICICSPA2 (p = 0.035). Crucially, the two-way RM-ANOVA with “Orientation” (CSPA and CSAP) and “Condition” (SICI at ISI of 3 ms alone (SICICSPA3 and SICICSAP3) and SICI at ISI of 3 ms in the presence of SICICSPA2 (CSPA3-SICICSPA2 and CSAP3-SICICSPA2)) as main factors revealed an “Orientation x Condition” interaction (F1,12 = 4.943, p = 0.046), which post hoc pairwise comparison showed was due to the fact that CSPA3-SICICSPA2 induced stronger cortical inhibition than CSAP3-SICIPA2 (p = 0.004).
3.4. Experiment 4. Interaction of SICI using either CSPA or CSAP with CBI
Since the results in experiment 3 suggested that there are differences in the inhibition produced by AP and PA conditioning stimuli, we next tested whether these would interact in different ways with another input to M1.
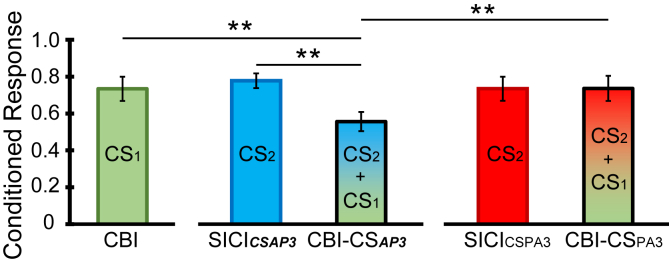
Fig. 5 shows that CBI produced approximately the same amount of inhibition as SICI with CSAP3 or CSPA3. CBI-CSPA3 yielded no greater inhibition than either alone, whereas CBI-CSAP3 produced more inhibition than either stimulus alone.
In the statistical analysis, we compared the level of inhibition evoked by CBI, SICICSAP3 and CBI-CSAP3. A one-way RM-ANOVA with Greenhouse-Geisser correction revealed a significant difference between them (F1.480,22.199 = 5.902, p = 0.014). Post hoc pairwise comparison showed that CBI-CSAP3 was significantly different from both SICICSAP3 (p = 0.003) and CBI (p = 0.027). However, there were no significant differences in another one-way ANOVA including CBI, SICICSPA3 and CBI-CSPA3 (F1.459,21.883 = 0.001, p = 0.996). Crucially, the two-way RM-ANOVA with “Orientation” (CSPA and CSAP) and “Condition” (SICI alone and SICI in the presence of CBI) as main factors revealed an “Orientation x Condition” interaction (F1,15 = 14.756, p = 0.002), which post hoc pairwise comparison showed was due to the fact that CBI-CSAP3 produced more inhibition than CBI-CSPA3 (p = 0.001). Interestingly, we also found that the calculated expected sum of CBI-CSAP3 (i.e. [CBI (0.73)] ∗ [SICICSAP3 (0.78)] = 0.58) was similar to the observed value (0.56).
3.5. Experiment 5. Interaction of SICI using either CSPA or CSAP with SAI
As in experiment 4, we next examined how sensory afferent inputs to the motor cortex interact with SICI produced by different directions of CS.
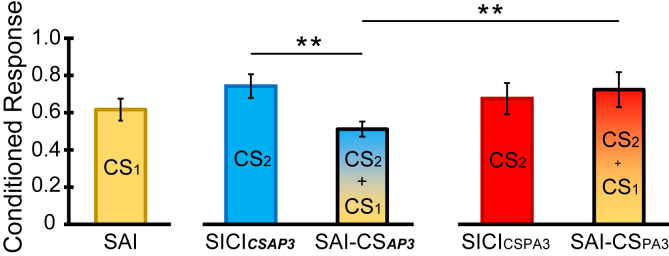
Fig. 6 shows that SAI produced approximately the same amount of inhibition as SICI with CSAP3 or CSPA3. SAI-CSPA3 yielded no greater inhibition than either alone, whereas SAI-CSAP3 appears to produce more inhibition than SICICSAP3.
Fig. 6.
Interaction of SICI with SAI. The left panel shows the MEP conditioned by ulnar nerve stimulation input (SAI; ISI = 22 ms). The middle panel represents SICI obtained with CSAP3, either alone or combined with ulnar nerve stimulation (SAI-CSAP3). The right panel plots SICI induced by CSPA3 alone and combined with ulnar nerve stimulation (SAI-CSPA3). The combination SAI-CSAP3 produced more inhibition than either conditioning stimulus alone, whereas SAI-CSPA3 produced approximately the same amount of inhibition as each conditioning stimulus alone. Double asterisks indicate p < 0.05 in post hoc pairwise comparison. CS1 indicates the earlier CS, and CS2 means the later CS.
In the statistical analysis, we compared the level of inhibition evoked by SAI, SICICSAP3 and SAI-CSAP3. The one-way RM-ANOVA revealed a significant difference among them (F2,26 = 6.920, p = 0.004). Post hoc pairwise comparison showed that SAI-CSAP3 was significantly different from SICICSAP3 (p = 0.01) but not different to SAI alone (p = 0.264). However, there were no significant differences in the one-way RM-ANOVA comparing SAI, SICICSPA3 and SAI-CSPA3 (F2,26 = 1.251, p = 0.303). Crucially, the two-way RM-ANOVA with “Orientation” (CSPA and CSAP) and “Condition” (SICI alone and SICI in the presence of SAI) as main factors revealed an “Orientation x Condition” interaction (F1,13 = 15.561, p = 0.002), which post hoc pairwise comparison showed was due to the fact that SAI-CSAP3 produced more inhibition than SAI-CSPA3 (p = 0.001). Again, the expected combination of SAI-CSAP3 (i.e. [SAI (0.62)] x [SICICSAP3 (0.74)] = 0.46) was not far from the observed value of 0.51.
Lastly, the change in SICICSPA3 when CBI was performed was calculated (1.36 ± 0.59). A paired t-test revealed that CBI suppressed SICICSPA3 significantly (t = −4.128, df = 15, p = 0.001, Fig. 7A). This result is consistent with Daskalakis et al. [20]. Suppression of SICI also occurred when this was tested together with SAI (2.13 ± 0.29): A paired t-test revealed SICICSPA3 was significantly facilitated in the presence of SAI (t = −4.441, df = 13, p = 0.001, Fig. 7B). These results are in agreement with those of Udupa and co-workers [23].
Fig. 7.
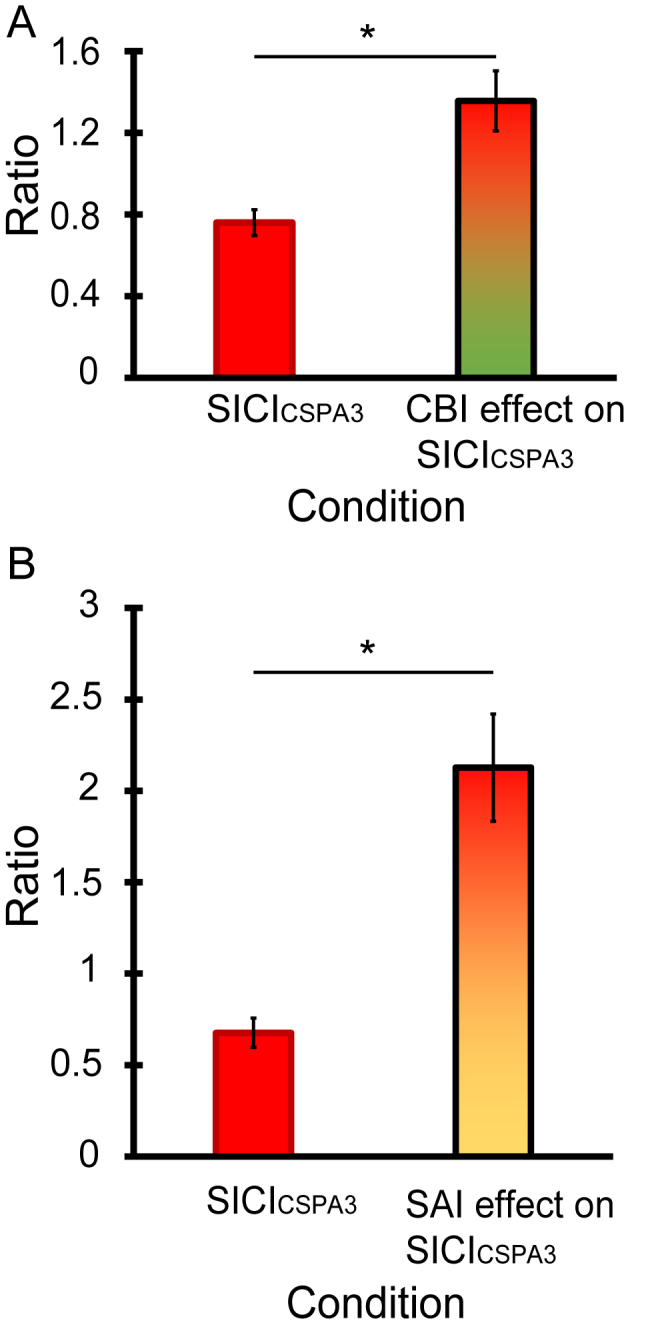
Change of SICI in triple pulses stimulation. Upper panel shows SICICSPA3 and the change of SICICSPA3 in CBI-CSPA3 (A). SICICSPA3 was suppressed significantly when CBI was presented in the same trial. Lower panel plots SICICSPA3 and the change of SICICSPA3 when presented with SAI-CSPA3 (B). Again, SICICSPA3 was suppressed significantly when SAI was presented at the same trial. Asterisks indicate p < 0.05 in paired t-test.
3.6. Experiment 6. Changes in SICI in a simple reaction time task
For each muscle, we first compared SICI across rest and tonic activation states when probed with different conditioning currents. In the FDI muscle, a two-way RM-ANOVA with “Brain state” and “Orientation” as main factors revealed that there was no effect of “Orientation” (F1,12 = 0.155, p = 0.701) or “Orientation x Brain state” interaction (F1,12 = 0.412, p = 0.201). However, there was a significant main effect of “Brain state” (F1,12 = 9.492, p = 0.010), indicating that contraction reduced SICI. Similar results were seen in ADM. A two-way RM-ANOVA with “Brain state” and “Orientation” as main factors revealed there was no effect of “Orientation” (F1,12 = 3.336, p = 0.093) or “Orientation x Brain state” interaction (F1,12 = 1.929, p = 0.190), but there was a significant effect of “Brain state” (F1,12 = 5.528, p = 0.037) (Fig. 8A and B). In other words, we found no evidence that SICICSPA3 and SICICSAP3 respond differently during rest or tonic activation.
Fig. 8.
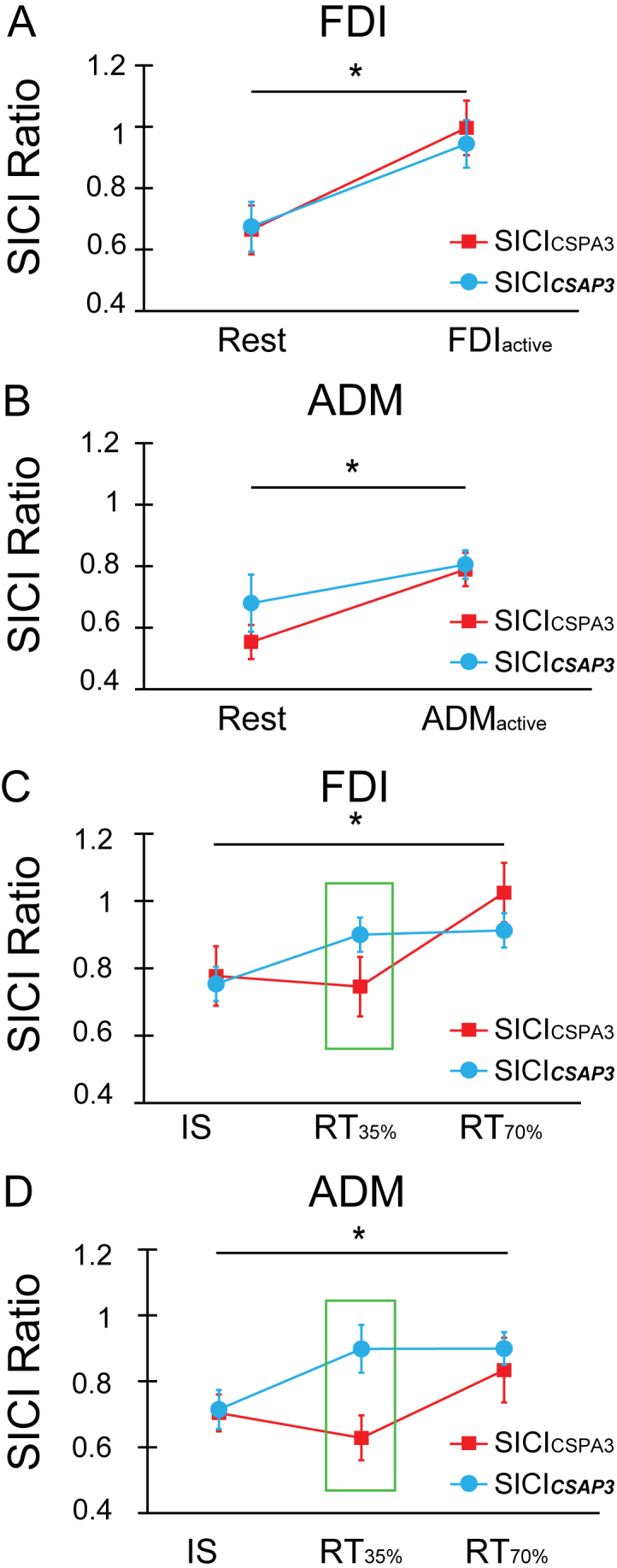
Results from experiment 6. SICICSPA3 and SICICSAP3 in both FDI (A) or ADM (B) were equally effective when measured at rest or during tonic contraction. However, there was significantly less SICI during tonic movement than at rest in both muscles. Panels C and D show how SICI changed during the SRTT (C, D). Compared to MEPs evoked at the time of the imperative stimulus (auditory tone), SICI became less effective when probed during the reaction period, although the time course differed between CSPA and CSAP. SICI obtained with CSAP decreased earlier (RT35%) than SICI tested with CSPA, which only declined at RT70% regardless of whether FDI (C) or ADM (D) were involved in the task. This result suggests that at least two inhibitory networks are involved in controlling action responses, each showing specific temporal dynamics. Asterisks indicate significant main effects of “Brain state” (panels A and B) and significant “Orientation x time” interactions (panels C and D) (p < 0.05). The green box in panels C and D indicate significant differences in post hoc pairwise comparisons (p < 0.05).
The results were different when we investigated how SICI changed during the reaction period of an SRTT. Two-way RM-ANOVAs with “Orientation” and “Time” as main factors revealed a significant “Orientation x Time” interaction in both muscles (FDI: F2,24 = 3.675, p = 0.041; ADM: F2,24 = 5.670, p = 0.001). Post hoc pairwise comparison revealed that SICICSAP3 decreased much earlier during the reaction time (RT35%) than SICICSPA3 (FDI: p = 0.035; ADM: p = 0.005) (Fig. 8C and D). The amplitude of the test MEP within blocks of SICICSPA3 did not differ from SICICSAP3 in equivalent blocks (see supplementary material III and Fig. S3).
4. Discussion
The present experiments explored the effect of reversing the direction of the conditioning stimulus (CS) on short-interval intracortical inhibition (SICI) at interstimulus intervals (ISIs) of 1–5 ms using a range of CS intensities. Conventionally, SICI is produced using a sub-motor threshold posterior-anterior (PA) conditioning stimulus (CSPA). The present results suggest that an anterior-posterior conditioning stimulus (CSAP) of the same intensity can activate a different, and perhaps even independent, inhibitory circuit. We discuss the evidence for this conclusion in the following paragraphs.
4.1. SICI with different orientations of CS
Using different coils for the conditioning and test stimuli allows independent adjustment of the direction of CS and TS. This allowed us to maintain a conventional PA test stimulus while reversing the direction of the conditioning stimulus. Most previous investigations [12,14,34,35] have been limited to a single coil, which means SICI can only be assessed with conditioning and test currents applied in the same direction (i.e. AP to AP; PA to PA). The problem with this is that AP and PA test stimuli evoke different combinations of I-waves which are differentially sensitive to SICI [14], which confounds interpretation of any differences in the effect of the CS. In addition, some authors (e.g. Hanajima et al. (1998) [14]) have also conducted experiments during slight muscle contraction rather than at rest, which also affects the recruitment of I-waves. In the present experiments, we ensured stability of I-wave recruitment by always using a PA test stimulus in participants at rest; we only changed the orientation of the CS pulse.
A potential disadvantage of this method is that one coil (conditioning coil in the present experiments) rests on top of the other coil, which means that there is an additional distance from the scalp surface. This will reduce its focality and result in activation of a larger cortical area than if the coil were placed in contact with the scalp. However, this is unlikely to have had an important influence on the results since in the past SICI has been tested with a large number of different coil types of varying focality with little noticeable difference in outcome [18,25].
4.2. Inhibition at interstimulus intervals of 2 ms and 3 ms
Experiment 1 showed that at 3 ms, the absolute threshold for evoking SICI was approximately the same for both directions of CS (CSPA and CSAP). This is perhaps unexpected since we usually express the intensity of a CS relative to its own AMT. Nevertheless, it is similar to the conclusion of Ziemann et al. [18], who found that a CS with latero-medial orientation produced the same amount of SICI as CSPA at ISI = 3 ms even though the CS intensity was the same in each direction. In contrast, at 2 ms, CSAP was much less effective than (conventional) CSPA. Hanajima [12] had also found that CSAP was less effective in evoking inhibition at 2 ms than 3 ms, although a direct comparison with the present data is difficult because both CS and TS were in the AP direction.
The difference in the time course of SICI produced by CSPA and CSAP could be because CSAP has a delayed onset. The onset of MEPs evoked with a single AP pulse (AP-MEPs) is usually 2–3 ms later than those evoked with a PA pulse (PA-MEPs), so maybe the onset of SICI evoked with CSAP is also delayed and begins at 3 ms. However, while this accounts for the difference in latency, it does not explain why the thresholds for CSAP and CSPA are approximately equal whereas the threshold for AP-MEPs is higher than for PA-MEPs. The answers may depend on precisely which population of neurons is activated by AP and PA pulses and their relative locations.
Finally, it is interesting to note that SICI could be evoked at 2 ms with high-intensity CSAP that was approximately equal to 90% of the direction-appropriate AMT. This is the same relative intensity required when using a (conventional) CSPA. However, it is quite different to the behaviour at ISI = 3 ms, where the intensity of CSAP can be much lower than 90%AMTAP (also see Supplementary material 7 and Fig. S4). This implies that the circuits involved in SICI at these two intervals are different, at least when using CSAP.
4.3. Inhibition at 1 ms
Fig. 3 not only replicates the observation that SICICSPA is more effective than SICICSAP at 2 ms but also shows that this is true at 1 ms, even though both directions are equally effective at 3 ms. The origin of SICI at 1 ms is debated. Some authors have suggested that it is due to a synaptic mechanism [16,36,37], whereas others attribute it to axonal refractoriness since CS recruits some axons normally recruited by the TS [25]. We do not know which axons these are, but the assumption is that they provide excitatory input that drives corticospinal discharge and the MEP. In the present result, both SICICSPA1 and SICICSAP1 produced effective inhibition, but SICICSPA1 was more effective than SICICSAP1. Since MEPs were evoked with a PA test stimulus, CSPA might activate some excitatory inputs that then become refractory to the TSPA, resulting in a smaller conditioned response. In contrast, CSAP would be unable to activate them since their threshold is much higher for the AP current. Therefore, it is possible that both theories of SICI at 1 ms contribute to SICICSPA1 but that axonal refractoriness is the predominant mechanism. At first sight, our data seem to differ from those of Hanajima et al. [12] since they reported good inhibition at 1 ms. However, in those experiments, both the CS and TS were in the AP direction and would therefore activate the same set of axons involved in producing the test MEP, again consistent with the idea that inhibition at 1 ms is due, at least partially, to axonal refractoriness.
Fig. 3.
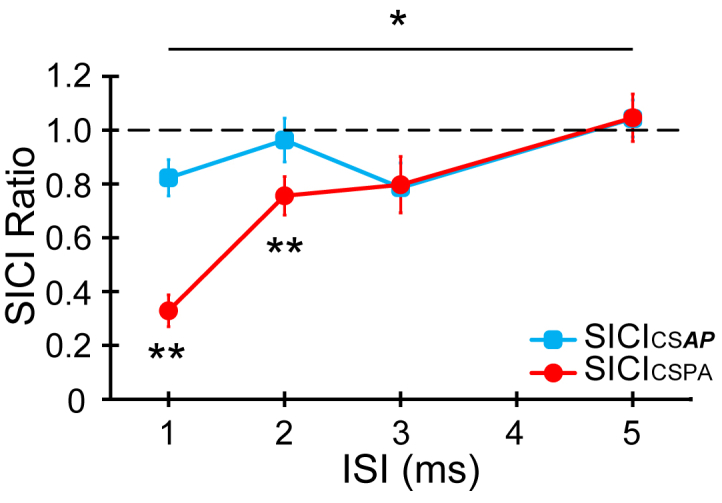
Comparison of SICI produced by the same intensity of CSPA and CSAP over a range of ISIs. The x-axis depicts the ISI selected between the CS and TS pulses. Both CSPA (red) and CSAP (blue) intensities were set to 90% AMTPA. We found significant differences between the two different CS at ISIs = 1 ms and 2 ms but not at other intervals. Asterisks indicate a significant orientation × ISI interaction in a two-way ANOVA, p < 0.05. Double asterisks indicate p < 0.05 in post hoc pairwise comparison between the different orientations at each ISI. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4.4. Interaction of separate cortical inhibitory networks
At 3 ms, both directions of CS evoke a similar amount of SICI and have the same (absolute) threshold. The simplest explanation is that they both activate the same set of inhibitory interneurons, which are insensitive to the direction of the TMS pulse. Experiments 3–5 were conducted to test this. The rationale was that if CSAP and CSPA activated the same set of inhibitory neurons, then they would interact with other forms of inhibition in the same way. The design of these experiments was less complex than the classic studies of interactions between SICI and SAI or CBI [20,21,23], which explored a range of conditioning and test intensities, as well as interstimulus intervals. The question here was simply whether SICI evoked with CS of different directions, but otherwise of the same intensity, interstimulus interval, and depth of inhibition interacts in the same way with SICI itself, CBI and SAI.
Previous work showed that combining two (conventional) subthreshold CSPA could produce a SICI-like effect if the interval between them was 1–5 ms [19]. The authors suggested that the conditioning stimuli activate excitatory synaptic input to inhibitory neurons [21]. Each CS on its own might fail to generate an excitatory postsynaptic potential (EPSP) large enough to reach the firing threshold in the inhibitory interneurons, and thus no SICI would occur. In contrast, if two CS are applied with short interstimulus intervals, the EPSP evoked by the second CS could summate with the EPSP evoked by the first CS so that the threshold for SICI was reached.
The results of experiment 3 were consistent with this idea of temporal facilitation since they showed that two CSPA stimuli (at 2 and 3 ms) produce an effect larger than the predicted sum of each stimulus alone. However, temporal facilitation was not evident when CSAP3 was paired with CSPA2. Importantly, the amount of SICI produced by CSAP3 alone was the same as when CSPA3 was used, and thus we might have expected to see the same amount of temporal summation with CSPA2. In fact, inhibition was only equal to the expected sum of the effect of each CS alone. This is consistent with the idea that CSPA and CSAP have an additive effect such as would be expected from activating two separate inhibitory circuits. This does not necessarily mean that they activate two different sets of inhibitory interneurons. It could be, for example, that excitatory inputs activated by CSPA and CSAP activate different fractions of the same inhibitory population, or that their inputs target different, non-interacting parts of the dendritic tree of a single neuron. Whatever the mechanistic details, the result suggests that inhibition by CSAP at 3 ms is not a time-delayed version of CSPA2.
Note that our CS intensities were above the threshold for producing some SICI and therefore slightly higher than in the original experiments of Bestmann et al. [19]. However, any discharge of inhibitory neurones by the first stimulus would have made them refractory to the second stimulus, and if anything, this would have underestimated any temporal facilitation occurring within the subliminal fringe of non-activated neurones.
4.5. Interaction of CBI and SICI
The interaction of CBI and SICICSPA2 was first examined by Daskalakis et al. [20]. As in the present study, they found that combined stimulation did not produce more inhibition than either CS alone. However, in their experiments, they described less inhibition, or even facilitation, when both CS were applied. One possible reason that we failed to observe a significant reduction in inhibition is that we used SICI at 3 ms, whereas they used 2 ms. In addition, we express the amount of inhibition as a percent of the response to the TS alone. However, Daskalakis used a test amplitude of 0.5 mV to assess SICI alone and CBI alone, but in order to assess the combination of CBI + SICI, they increased the intensity of the test stimulus such that it produced a 0.5 mV MEP in the presence of CBI.
Although we did not perform a condition with this change in test intensity, we can still analyse the data similarly to Daskalakis et al. by expressing the amplitude of the CBI-SICI conditioned response as a percent of the amplitude of the conditioned response to CBI alone. Fig. 7A shows that this type of analysis appears to reduce the amount of SICIPA when measured in the presence of CBI (see supplementary material 6).
The crucial finding, however, was that the effect of combining CBI with CSAP3 was completely different: there was more inhibition from combined stimulation than to each stimulus alone. In fact, the expected sum of CBI and CSAP3 was equal to the combined effect of each stimulus alone. As above, this is consistent with the idea that the two independent inhibitory pathways converge on corticospinal output. In contrast, the pathways responsible for CBI and SICICSPA3 interact with each other, as suggested by Daskalakis et al. [20]. This reinforces the conclusion that CSAP3 and CSPA3 produce SICI via different pathways.
4.6. Interaction of SAI and SICI
These results and their interpretation are very similar to those for CBI. The combination of SAI and CSPA3 produced a similar amount of inhibition to each stimulus given alone, whereas SAI-CSAP3 produced more inhibition than SICICSAP3. As above, this suggests that inhibition produced by SAI and SICICSAP3 co-exist as two separate effects on corticospinal excitability. Indeed, inhibition produced by combined stimulation was approximately the same as the expected sum of each conditioning stimulus alone. Interaction of SAI and conventional (CSPA) SICI has been investigated previously by Alle et al. and Udupa et al. [21,23]. They found that the SICI became facilitation when SAI presented but the effect of combined stimulation varied with the intensity of the CS and the interstimulus interval used for SICI. Stimuli like those used here produced similar results (Fig. 7B). However, no combination of stimulus parameters produced more inhibition than either stimulus alone, as we observed with SAI-CSAP3. We conclude that the pathways responsible for SAI and SICICSPA3 interact with each other, whereas those responsible for SAI and SICICSAP3 are likely to be independent.
4.7. The role of different inhibitory circuits in behaviour
The results of experiment 6 show for the first time that distinct SICI circuits are differentially modulated throughout the time course of movement preparation. The implication is, again, compatible with the hypothesis that CSPA and CSAP probe different inhibitory circuits and that these circuits are modulated at specific times during the course of movement. The effects do not differ across the effector probed and are state-dependent, as no differences were found across rest or tonic activation. While speculative, changes in SICI probed with CSAP may reflect the influence of premotor area [7,38] which are modulated earlier in the reaction period than the circuits probed by CSPA, which may reflect direct involvement of M1 [4,6].
What is activated by different directions of CS?
The results are consistent with the notion that opposite directions of CS activate two different populations of inhibitory neurons, but they give no information about where and what these neurons are. Nevertheless, they do give some clues.
-
1)
The threshold for producing inhibition is the same in each direction when ISI is 3 ms or longer.
-
2)
Inhibition starts at shorter ISIs with CSPA compared with CSAP.
In addition, since there are no long-range inhibitory connections in the cortex, the neurons that inhibit the MEP are likely to be near to the corticospinal neurones that conduct the final motor output to the cord. Possibilities include neurons that monosynaptically inhibit corticospinal neurons (i.e. direct inhibition) or neurons that inhibit excitatory inputs to corticospinal neurons (i.e. dis-facilitation) (Fig. 9). Realistic neuronal modelling [6] favours the former, suggesting that inhibition produced by CSPA is caused by stimulation of synaptic terminals of basket cells that monosynaptically inhibit corticospinal neurons and/or activation of excitatory synapses onto the basket neurons (as in Fig. 9).
Fig. 9.
Possible neural circuit to account for the interactions between CSAP and CSPA and other forms of cortical inhibition. Neuron P is a large pyramidal cell in the motor cortex with an axon projecting to spinal cord. It receives excitatory (open circles) and inhibitory (filled circles) synaptic inputs, representing I-wave inputs and SICI, respectively. Following recent modelling (Aberra et al. [6]), we assume the lowest threshold sites of activation are presynaptic terminals. The lightning bolts represent the sites activated by subthreshold CSPA and CSAP. The former activates excitatory inputs to a GABAa-ergic inhibitory interneuron (B) that causes SICICSPA. The latter activates synapses in a more rostral location (e.g., premotor cortex) that activate a different set of GABAa-ergic inhibitory interneurons (A) that produce SICICSAP. Neuron B also receives excitatory and inhibitory inputs from SAI and CBI that are responsible for the interaction of these forms of inhibition with SICICSPA. However, they have no direct effect on neuron A which mediates SICICSAP. Higher intensities of TMS activate excitatory inputs: PA-TMS activates I1-wave input to the proximal portion of neuron P at a site where it is relatively unaffected by dendritic SICI. Later I-wave inputs, activated by PA-TMS or AP-TMS, are shown as targeting dendritic sites near to the SICI inputs.
The same models suggest that AP-TMS (single-pulse TMS with suprathreshold intensity in AP direction) shifts the site of neural activation anteriorly compared with PA-TMS (single-pulse TMS with suprathreshold intensity in PA direction). If CSAP activates at a more anterior site, it may discharge a cortico-cortical neuron that excites inhibitory neurons in the posterior location near to the corticospinal neurones. Because these inhibitory neurones interact with CBI and SAI in a different way to those activated by CSPA, they may represent a different population (signified in Fig. 9 as neurones A and B). Conduction delay and an extra synapse might account for the delay (3 ms) in the onset of inhibition. But why would the threshold for evoking SICI from this anterior site be the same as for CSPA, particularly since the threshold for evoking AP-MEPs is higher than for PA-MEPs? One possibility relates to the fact that AP-TMS recruits late I-waves, which could have a high threshold and require a greater amount of excitatory input to discharge than small, inhibitory interneurons responsible for SICI. Thus the AP stimulus intensity required to produce sufficient excitatory input to generate an MEP would be greater than that required to produce inhibition. Another factor that might raise the threshold for AP-MEPs is that AP stimulation does not recruit I1 waves [38]; MEPs only occur when the stimulus intensity is sufficient to evoke late I-waves. In contrast, PA stimulation recruits I1 waves at a lower intensity than I3 waves. These I1 waves can evoke an MEP during a background voluntary contraction. The result is that AMTPA is lower than AMTAP, whereas the thresholds may be similar for SICI.
However, there are alternative explanations. It could be that the anterior site targeted by CSAP provides background excitatory input to the posterior site (e.g. AP-TMS might target premotor cortex while PA-TMS might target M1 [6,7,38]). In this case, SICICSAP could evoke local inhibition in the premotor cortex and remove ongoing facilitation from M1.
4.8. Limitations
The experiments used a limited range of stimulus intensities and interstimulus intervals. Thus, our conclusions are also limited to the parameters we have investigated. It is possible that, with other parameters, the effects of CSAP3 and CSPA3 may be more similar, and it will be important to perform more studies in future in order to know if it is possible to generalize the conclusions. For example, by analogy with the latency difference between MEPs evoked by AP and PA test pulses, it is possible that inhibition using CSAP is a delayed version of CSPA. Thus, it could be argued that we should have compared CSAP3 with earlier timings of CSPA rather than CSPA3. Udupa et al. [23] found that there were subtle differences in the way CSPA2 and CSPA3 interacted with SAI, but such effects would not be sufficient to explain the very different interactions we saw here.
A second limitation of the present study was the potential contamination of SICI at ISI = 3 ms by short-interval intracortical facilitation (SICF). Using two pulses of the same direction, the second peak of SICF occurs at around 2.8 ms [39], and a similar timing was noted for pulses of opposite direction [40] (although Delvendahl and coworkers [40] used biphasic pulses for this part of their experiment). Nevertheless, we think any interaction would have been minimal since, when SICF is present, it usually shows up as a reduction in inhibition at higher intensities of CS [39]. When we used an AP conditioning stimulus to suppress a PA test response (Fig. 2B), reduced inhibition only occurs at around 80% MSO (in the AP direction), which again is much higher than the range of CS intensities we used in the experiments.
The experiments were also limited technically by the fact that we could not randomize the direction of the CS from trial to trial and had to use two separate, overlapping coils, rather than a single one. Achieving these would probably have reduced the variability of the results, but we think it unlikely to change the main conclusions. Finally, we note that the differences between PA and AP conditioning stimuli differ between individuals, which probably indicates that it is not possible with a TMS pulse to isolate completely one set of inhibitory neurones from another.
5. Conclusion
We show that different directions of TMS-induced currents in the brain are capable of recruiting two independent sets of inhibitory inputs over a range of ISIs used to probe SICI. Fig. 9 illustrates a possible mechanism consistent with the results of this and other studies [6,[14], [15], [16],20,21,23,38]. It will be interesting in future studies to test whether these two inhibitory pathways are preferentially active in different types of movement and are affected differently in neurological disorders.
CRediT authorship contribution statement
Po-Yu Fong: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Danny Spampinato: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Lorenzo Rocchi: Conceptualization, Methodology, Writing – review & editing. Ricci Hannah: Conceptualization, Methodology, Writing – review & editing. Yinghui Teng: Writing – original draft, Investigation. Alessandro Di Santo: Investigation. Mohamed Shoura: Investigation. Kailash Bhatia: Conceptualization, Resources, Supervision. John C. Rothwell: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Thanks to Mr. Paul Hammond for helping us to measure the induced electric fields produced by the coils used in this study. PYF was supported by the Taiwan Government Scholarship to Study Abroad (GSSA). The work was supported by MRC UK grant MR/P006671/1 to JCR.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2021.08.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Day B.L., Dressler D., Maertens de Noordhout A., Marsden C.D., Nakashima K., Rothwell J.C. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannah R. Transcranial magnetic stimulation: a non-invasive window into the excitatory circuits involved in human motor behavior. Exp Brain Res. 2020;238:1637–1644. doi: 10.1007/s00221-020-05803-0. [DOI] [PubMed] [Google Scholar]
- 3.D'Ostilio K., Goetz S.M., Hannah R., Ciocca M., Chieffo R., Chen J.A. Effect of coil orientation on strength-duration time constant and I-wave activation with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2016;127(1):675–683. doi: 10.1016/j.clinph.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volz L.J., Hamada M., Rothwell J.C., Grefkes C. What makes the muscle twitch: motor system connectivity and TMS-induced activity. Cereb Cortex. 2015;25(9):2346–2353. doi: 10.1093/cercor/bhu032. [DOI] [PubMed] [Google Scholar]
- 5.Siebner H.R. Does TMS of the precentral motor hand knob primarily stimulate the dorsal premotor cortex or the primary motor hand area? Brain Stimul. 2020;13(2):517–518. doi: 10.1016/j.brs.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Aberra A.S., Wang B., Grill W.M., Peterchev A.V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020;13(1):175–189. doi: 10.1016/j.brs.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimazu H., Maier M.A., Cerri G., Kirkwood P.A., Lemon R.N. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24(5):1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lazzaro V., Pilato F., Dileone M., Ranieri F., Ricci V., Profice P. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575(Pt 3):721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opie G.M., Ridding M.C., Semmler J.G. Task-related changes in intracortical inhibition assessed with paired- and triple-pulse transcranial magnetic stimulation. J Neurophysiol. 2015;113(5):1470–1479. doi: 10.1152/jn.00651.2014. [DOI] [PubMed] [Google Scholar]
- 11.Hendy A.M., Ekblom M.M., Latella C., Teo W.P. Investigating the effects of muscle contraction and conditioning stimulus intensity on short-interval intracortical inhibition. Eur J Neurosci. 2019;50(7):3133–3140. doi: 10.1111/ejn.14488. [DOI] [PubMed] [Google Scholar]
- 12.Hanajima R., Okabe S., Terao Y., Furubayashi T., Arai N., Inomata-Terada S. Difference in intracortical inhibition of the motor cortex between cortical myoclonus and focal hand dystonia. Clin Neurophysiol. 2008;119(6):1400–1407. doi: 10.1016/j.clinph.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Latorre A., Rocchi L., Berardelli A., Bhatia K.P., Rothwell J.C. The interindividual variability of transcranial magnetic stimulation effects: implications for diagnostic use in movement disorders. Mov Disord. 2019;34(7):936–949. doi: 10.1002/mds.27736. [DOI] [PubMed] [Google Scholar]
- 14.Hanajima R., Ugawa Y., Terao Y., Sakai K., Furubayashi T., Machii K. Paired-pulse magnetic stimulation of the human motor cortex : differences among I waves. J Physiol. 1998;509(2):607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanajima R., Furubayashi T., Iwata N.K., Shiio Y., Okabe S., Kanazawa I. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151(4):427–434. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- 16.Fisher R.J., Nakamura Y., Bestmann S., Rothwell J.C., Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143(2):240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- 17.Tokimura H., Ridding M.C., Tokimura Y., Amassian V.E., Rothwell J.C. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control. 1996;101(4):263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- 18.Ziemann U., Rothwell J., Ridding M.C. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestmann S., Siebner H.R., Modugno N., Amassian V.E., Rothwell J.C. Inhibitory interactions between pairs of subthreshold conditioning stimuli in the human motor cortex. Clin Neurophysiol. 2004;115(4):755–764. doi: 10.1016/j.clinph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Daskalakis Z.J., Paradiso G.O., Christensen B.K., Fitzgerald P.B., Gunraj C., Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557(Pt 2):689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alle H., Heidegger T., Krivanekova L., Ziemann U. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol. 2009;587(Pt 21):5163–5176. doi: 10.1113/jphysiol.2009.179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown M.J.N., Weissbach A., Pauly M.G., Vesia M., Gunraj C., Baarbe J. Somatosensory-motor cortex interactions measured using dual-site transcranial magnetic stimulation. Brain Stimul. 2019;12(5):1229–1243. doi: 10.1016/j.brs.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Udupa K., Ni Z., Gunraj C., Chen R. Effects of short-latency afferent inhibition on short-interval intracortical inhibition. J Neurophysiol. 2014;111(6):1350–1361. doi: 10.1152/jn.00613.2013. [DOI] [PubMed] [Google Scholar]
- 24.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Safety of T.M.S.C.G. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannah R., Rocchi L., Tremblay S., Wilson E., Rothwell J.C. Pulse width biases the balance of excitation and inhibition recruited by transcranial magnetic stimulation. Brain Stimulation. 2020;13(3):536–538. doi: 10.1016/j.brs.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell J.C., Hallett M., Berardelli A., Eisen A., Rossini P., Paulus W. Magnetic stimulation: motor evoked potentials. The international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- 27.Ugawa Y., Uesaka Y., Terao Y., Hanajima R., Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37(6):703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 28.Schlerf J.E., Galea J.M., Spampinato D., Celnik P.A. Laterality differences in cerebellar-motor cortex connectivity. Cereb Cortex. 2015;25(7):1827–1834. doi: 10.1093/cercor/bht422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokimura H., Di Lazzaro V., Tokimura Y., Oliviero A., Profice P., Insola A. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523 Pt 2:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannah R., Rothwell J.C. Pulse duration as well as current direction determines the specificity of transcranial magnetic stimulation of motor cortex during contraction. Brain Stimul. 2017;10(1):106–115. doi: 10.1016/j.brs.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak S.K., Kim J.H. Statistical data preparation: management of missing values and outliers. Korean J Anesthesiol. 2017;70(4):407–411. doi: 10.4097/kjae.2017.70.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spampinato D., Ibanez J., Spanoudakis M., Hammond P., Rothwell J.C. Cerebellar transcranial magnetic stimulation: the role of coil type from distinct manufacturers. Brain Stimul. 2020;13:153–156. doi: 10.1016/j.brs.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123(5):858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wessel M.J., Draaisma L.R., Morishita T., Hummel F.C. The effects of stimulator, waveform, and current direction on intracortical inhibition and facilitation: a TMS comparison study. Front Neurosci. 2019;13:703. doi: 10.3389/fnins.2019.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sale M.V., Lavender A.P., Opie G.M., Nordstrom M.A., Semmler J.G. Increased intracortical inhibition in elderly adults with anterior-posterior current flow: a TMS study. Clin Neurophysiol. 2016;127(1):635–640. doi: 10.1016/j.clinph.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 36.Vucic S., Cheah B.C., Kiernan M.C. Dissecting the mechanisms underlying short-interval intracortical inhibition using exercise. Cereb Cortex. 2011;21(7):1639–1644. doi: 10.1093/cercor/bhq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roshan L., Paradiso G.O., Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151(3):330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- 38.Di Lazzaro V., Profice P., Ranieri F., Capone F., Dileone M., Oliviero A. I-wave origin and modulation. Brain Stimul. 2012;5(4):512–525. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Peurala S.H., Muller-Dahlhaus J.F., Arai N., Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119(10):2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Delvendahl I., Lindemann H., Jung N.H., Pechmann A., Siebner H.R., Mall V. Influence of waveform and current direction on short-interval intracortical facilitation: a paired-pulse TMS study. Brain Stimul. 2014;7(1):49–58. doi: 10.1016/j.brs.2013.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.