Abstract
Using a transgenic mouse model expressing the E2F1 gene under the control of a keratin 5 (K5) promoter, we previously demonstrated that increased E2F1 activity can promote tumorigenesis by cooperating with either a v-Ha-ras transgene to induce benign skin papillomas or p53 deficiency to induce spontaneous skin carcinomas. We now report that as K5 E2F1 transgenic mice age, they are predisposed to develop spontaneous tumors in a variety of K5-expressing tissues, including the skin, vagina, forestomach, and odontogenic epithelium. On the other hand, K5 E2F1 transgenic mice are found to be resistant to skin tumor development following a two-stage carcinogenesis protocol. Additional experiments suggest that this tumor-suppressive effect of E2F1 occurs at the promotion stage and may involve the induction of apoptosis. These findings demonstrate that increased E2F1 activity can either promote or inhibit tumorigenesis, dependent upon the experimental context.
Activation of E2F transcription factors, via perturbation in the p16INK4a-cyclin D-retinoblastoma (Rb) tumor suppressor pathway, may be a key event in the development of most human cancers. This hypothesis is supported by the finding that several members of the E2F gene family, including E2F1, can behave as oncogenes in cell culture-based transformation assays (9, 22, 24). The oncogenic capacity of E2F1 is thought to be related to its ability to regulate the expression of genes critical for cell proliferation (4, 21). There is also accumulating evidence that E2F participates in a protective, apoptotic pathway that functions to eliminate cells that have lost normal cell cycle control. E2F1 has been shown to stimulate the expression of the ARF tumor suppressor, a regulator of p53 protein activity (1, 4, 13). A tumor-suppressive function for E2F1 is demonstrated by the finding that mice lacking E2F1 are predisposed to develop tumors (25).
The mouse skin model of carcinogenesis has been instrumental in developing many concepts currently applied to human neoplasias, including the idea that cancer develops through a multistep process (3). Three mechanistic stages can be defined in this model: initiation, promotion, and progression. Initiation is carried out by administration of a single dose of a carcinogen that results in specific genetic mutations in a subpopulation of cells. In the case of 9,10-dimethyl-1,2-benzanthracene (DMBA), the specific mutation occurs at codon 61 of the c-Ha-ras gene (17). Promotion occurs as a result of exposure of the initiated skin to repetitive treatments with an irritating, nongenotoxic agent such as O-tetradecanoyl-phorbol-13-acetate (TPA). Promotion usually involves hyperplasia and results in the expansion of initiated cells. The endpoint of the promotion stage is the formation of squamous papillomas, which are exophytic, noninvasive lesions. Progression is the conversion of a subset of benign papillomas into malignant carcinomas. Using this model, we and others have recently found that several E2F family members, including E2F1, are overexpressed in late-stage papillomas and squamous cell carcinomas (20, 26).
To study the role of deregulated E2F1 activity in tumor development, we recently developed transgenic mice in which expression of the human E2F1 gene is under the control of a keratin 5 (K5) promoter (15, 16). The K5 promoter is active in several epithelial tissues, including the basal cell layer of the epidermis, hair follicles, oral epithelium, vagina, stomach, esophagus, bladder, and thymus (18). Two transgenic lines were developed, 1.0 and 1.1, that overexpress E2F1 in keratinocytes 80- and 40-fold respectively, as measured by Western blot analysis (15). However, electrophoretic mobility shift assays demonstrate a relatively modest increase in E2F1 DNA-binding activity in transgenic keratinocytes, similar to or below that seen in many tumor cells (16). Deregulated expression of E2F1 was found to induce hyperproliferation, hyperplasia, and p53-dependent apoptosis in the epidermis of transgenic mice (15, 16). Moreover, the K5 E2F1 transgene could promote skin tumor development in combination with other genetic alterations, such as p53 deficiency (16). We now find that older K5 E2F1 transgenic mice develop spontaneous tumors in a variety of K5-expressing tissues, including the skin, vagina, and odontogenic epithelium. To further define the role of E2F1 in cancer development, K5 E2F1 transgenic mice were used in the mouse skin model of multistage carcinogenesis. Surprisingly, we found that E2F1 overexpression inhibits tumor promotion in this model system. This correlates with the induction of apoptosis in the skin of K5 E2F1 transgenic mice following TPA treatment.
MATERIALS AND METHODS
Mice.
K5 E2F1 transgenic mice contain the human E2F1 cDNA under the control of the bovine K5 promoter (15). Line 1.1 mice express approximately fourfold lower levels of the transgene than line 1.0 mice. For skin carcinogenesis experiments, transgenic mice were backcrossed to the SENCAR inbred strain SSIn. All of the mice used in this study were at least 90% SSIn. Tg.AC mice carry a v-Ha-ras gene under the control of a ζ-globin promoter, but because of the site of integration, expression can be induced in the skin (11). Tg.AC transgenic mice were also in the SSIn strain background.
Immunohistochemistry assay.
Formalin-fixed sections were deparaffinized and incubated in methanol containing 1% hydrogen peroxide for 20 min. For analysis of odontogenic epithelium, heads were first decalcified in Krajian solution (J. T. Baker) for 2 days with four changes of the solution and washed in water for 6 h. Tissue sections were then rinsed with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) three times. For E2F1 staining, slides were boiled for 5 to 10 min and again rinsed in PBS-BSA. Slides were preincubated with normal goat serum and then incubated with primary rabbit antibody (1:500 dilution) to E2F1 (C-20; Santa Cruz Biotechnology) or K5 (a gift from Dennis Roop) for 30 min at room temperature. After incubation with the primary antibody, slides were rinsed in PBS-BSA, incubated with biotinylated goat anti-rabbit immunoglobulin G for 30 min, and rinsed again. Slides were incubated with streptavidin-horseradish peroxidase conjugate for 30 min, developed with diaminobenzidine tetrahydrochloride solution, rinsed again, and counterstained.
Two-stage mouse skin carcinogenesis model.
The dorsal skin of 6- to 8-week-old line 1.1 K5 E2F1 transgenic mice and wild-type sibling controls was clipped 1 to 2 days before initiation. Mice were initiated by topical application of 10 nM DMBA in 200 μl of acetone to the dorsal skin. Mice were promoted twice weekly with topical TPA in acetone beginning at week 3 after initiation. Mice in experiment 1 initially received 2.5 μg (15 nM) of TPA in 200 μl of acetone per treatment through week 7. At week 8, the TPA dose was reduced to 0.5 μg (3 nM). Mice in experiment 2 received 1.0 μg of TPA in 200 μl of acetone per dose throughout the experiment. Treatment of control mice in each experiment was initiated with DMBA, but the mice were treated twice weekly with the acetone vehicle only. Mice were scored for papillomas weekly. Mice with skin lesions were included in tabulations until their condition mandated their withdrawal from the study.
Tg.AC promotion assay.
Tg.AC mice were crossed with K5 E2F1 transgenic mice (lines 1.0 and 1.1) to obtain single- and double-transgenic mice. Tg.AC mice with and without the K5 E2F1 transgene were treated with 1.0 μg of TPA in 200 μl of acetone applied topically to the dorsal epidermis. Mice were treated twice weekly and observed for papilloma development until an endpoint defined by the tumor load was reached.
TUNEL assay.
K5 E2F1 transgenic mice and wild-type sibling controls were clipped, and the dorsal skin was treated with either a single application of 10 nM DMBA in 200 μl of acetone or four applications of 1.0 μg of TPA in 200 μl of acetone over a 2-week period. Mice were sacrificed 24 h after treatment, and skin sections were fixed in formalin. The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed on skin sections by using the Apoptag kit (Oncor) in accordance with the manufacturer’s protocol.
RESULTS
Tumor development in K5 E2F1 transgenic mice.
Previously, we demonstrated that K5 E2F1 transgenic mice that also contained a v-Ha-ras transgene under the control of a ζ-globin promoter developed benign skin papillomas by 24 weeks of age (15). In addition, K5 E2F1 transgenic mice that were also heterozygous for p53 developed skin carcinomas between 18 and 46 weeks of age (16). A group of K5 E2F1 transgenic mice without additional genetic alterations have been maintained for over 1 year for examination of spontaneous tumor development. Of 34 mice over 1 year of age, 17 (50%) developed spontaneous tumors (Table 1). The majority of these lesions are papillomas, squamous cell carcinomas, or other tumors of the skin. However, tumors have also developed in other K5-expressing tissues, such as the vagina, forestomach, and odontogenic epithelium (Fig. 1).
TABLE 1.
Spontaneous tumor development in K5 E2F1 transgenic mice
| Mouse no. | Line | Sexa | Ageb | Tumor location | Histological appearance | E2F1c |
|---|---|---|---|---|---|---|
| 1 | 1.1 | F | 53 | Vagina | Carcinoma | + |
| 2 | 1.1 | F | 54 | Vagina | SCCd | + |
| 3 | 1.1 | F | 47 | Skin | SCC | + |
| 4 | 1.1 | F | 63 | Skin | SCC | + |
| 4 | 1.1 | F | 63 | Skin | Adenocarcinoma | + |
| 5 | 1.1 | F | 51 | Skin | Papilloma | + |
| 6 | 1.1 | F | 48 | Skin | Papilloma | + |
| 6 | 1.1 | F | 48 | Mammary gland | Carcinoma | − |
| 7 | 1.1 | M | 88 | Skin | SCC | + |
| 8 | 1.1 | M | 55 | Forestomach | SCC | + |
| 9 | 1.0 | M | 94 | Penis | SCC | + |
| 9 | 1.0 | M | 94 | Mouth | Ameleoblastoma | NDe |
| 10 | 1.0 | M | 95 | Mouth | Complex composite odontoma | ND |
| 11 | 1.0 | M | 62 | Forestomach | Papilloma | + |
| 12 | 1.0 | F | 68 | Mouth | Ameleoblastoma | + |
| 13 | 1.0 | M | 86 | Skin | Adenosquamous carcinoma | + |
| 14 | 1.0 | M | 74 | Skin | SCC | + |
| 14 | 1.0 | M | 79 | Skin | SCC | + |
| 15 | 1.0 | F | 52 | Skin | Carcinoma | + |
| 16 | 1.0 | M | 86 | Head | Carcinoma | + |
| 17 | 1.0 | M | 91 | Mouth | Ameleoblastoma | ND |
F, female; M, male.
Age in weeks at time tumor was detected.
Immunohistological staining for E2F1 overexpression.
SCC, squamous cell carcinoma.
ND, not determined.
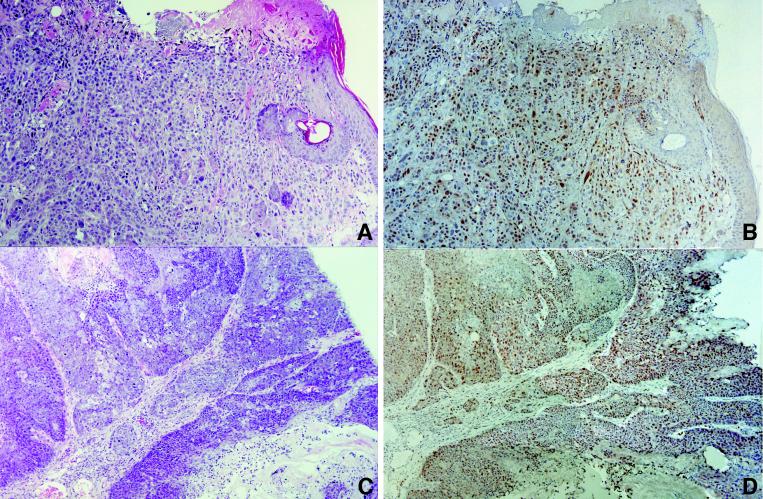
FIG. 1.
Histopathology of tumors from K5 E2F1 transgenic mice. Shown are squamous cell carcinoma of the skin of a 47-week-old line 1.1 mouse (A and B) and vaginal carcinoma from a 54-week-old line 1.1 mouse (C and D) either stained with hematoxylin and eosin (A and C) or immunostained with E2F1 antibody (B and D).
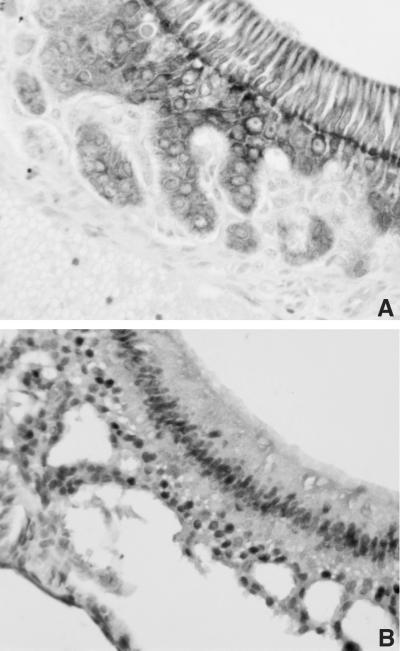
The vast majority of the tumors arising in K5 E2F1 transgenic mice stain positive for E2F1 protein expression (Fig. 1). Our immunohistochemistry assay detects E2F1 protein only in tissues expressing the transgene and does not detect endogenous E2F1. The one tumor that did not stain positive for E2F1 is a mammary carcinoma. K5 is not expressed in the luminal epithelium, the tissue that gives rise to most mammary tumors, but it is expressed in the myoepithelium. It is possible that this mammary tumor arose independently of the E2F1 transgene or that a paracrine interaction with the myoepithelium was involved in tumor development. It was not possible to examine E2F1 staining in some of the odontogenic tumors because the original decalcification method used for processing of these samples interfered with the E2F1 immunostaining assay. However, as shown in Fig. 2, K5 is expressed in the odontogenic epithelium and, in the K5 E2F1 transgenic mice, ectopic E2F1 expression can be detected in this tissue after use of an improved decalcification method.
FIG. 2.
Expression of K5 and the K5 E2F1 transgene in odontogenic epithelium. (A) Immunohistochemistry assay of a head section from a K5 E2F1 transgenic mouse using an antibody specific for K5. (B) Immunohistochemistry assay of a section similar to that in panel A using an antibody specific for E2F1.
Inhibition of tumor promotion by E2F1.
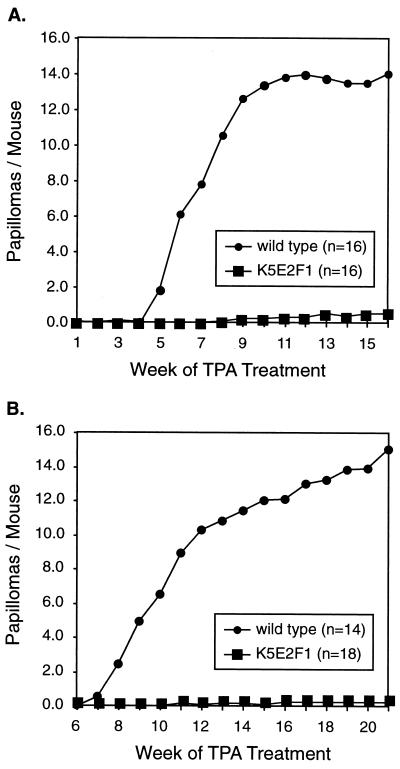
K5 E2F1 (line 1.1) transgenic mice and wild-type sibling controls were used in the mouse skin carcinogenesis two-stage protocol (Fig. 3). In experiment 1, 16 transgenic and 16 nontransgenic mice were initiated with DMBA and promoted with 2.5 μg of TPA twice weekly. At week 8, the development of skin lesions in some mice necessitated a reduction in the TPA dose to 0.5 μg for the remainder of the experiment. Papillomas were first observed in the wild-type controls at week 5 of TPA treatment and rapidly increased in number and size until the termination of the experiment (Fig. 3A). Wild-type mice developed an average of 14 papillomas per mouse by 16 weeks of TPA treatment. In sharp contrast, K5 E2F1 transgenic mice averaged less than one papilloma per mouse. In experiment 2, 18 K5 E2F1 transgenic mice (lane 1.1) and 14 wild-type sibling controls were initiated with DMBA and followed by promotion with 1.0 μg of TPA twice weekly for 21 weeks. Again, wild-type mice developed numerous papillomas (an average of 15 per mouse) while K5 E2F1 transgenic mice were almost completely resistant to tumor development (Fig. 3B). Control mice for both groups, which were initiated with DMBA but treated with the acetone vehicle only, did not develop papillomas.
FIG. 3.
Resistance of K5 E2F1 transgenic mice to two-stage carcinogenesis. (A) Initiation of carcinogenesis in line 1.1 mice and wild-type sibling controls with DMBA and promotion with 2.5 μg of TPA until week 7, followed by promotion with 0.5 μg of TPA until week 16. (B) Two-stage carcinogenesis induction as described above, except that promotion was performed by using a dose of 1.0 μg of TPA throughout the experiment.
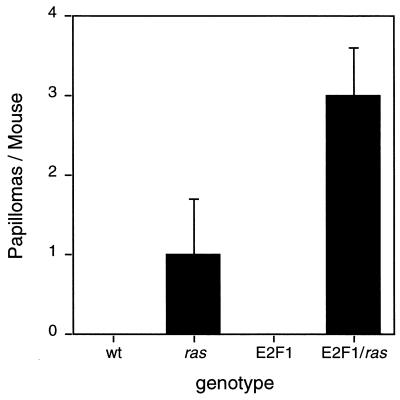
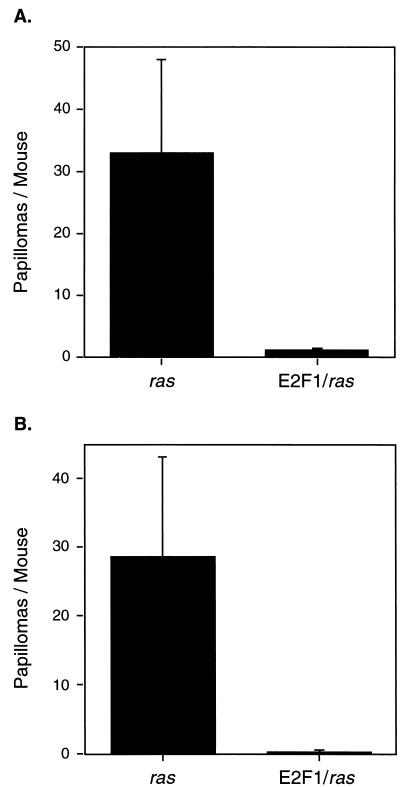
A transgenic mouse line, termed Tg.AC, containing a v-Ha-ras gene under the control of a ζ-globin promoter has been described (11). When treated repeatedly with TPA, these mice rapidly develop multiple skin papillomas. Thus, these transgenic mice serve as a “preinitiated” model for mouse skin carcinogenesis. We previously demonstrated that double-transgenic Tg.AC/K5 E2F1 line 1.0 mice developed spontaneous papillomas. K5 E2F1 line 1.1 transgenic mice, which express lower levels of E2F1, were also crossed with Tg.AC mice. As before, the K5 E2F1 transgene cooperated with the v-Ha-ras gene to induce tumors (Fig. 4). Double-transgenic mice developed an average of three spontaneous papillomas per mouse by 32 weeks of age. Tg.AC single-transgenic mice developed an average of one papilloma per mouse, while wild-type and K5 E2F1 transgenic mice did not develop spontaneous papillomas.
FIG. 4.
Cooperation of the K5 E2F1 transgene with a v-Ha-ras transgene to induce spontaneous papillomas. A female Tg.AC transgenic mouse was crossed with a male K5 E2F1 (line 1.1) transgenic mouse to generate wild-type (wt) mice (n = 2), Tg.AC ras (n = 4), K5 E2F1 (n = 4), and double K5 E2F1/Tg.AC ras (n = 3) transgenic mice. The average numbers of spontaneous papillomas these mice developed at 32 weeks of age are presented.
The finding that the K5 E2F1 transgene cooperates with a v-Ha-ras transgene to induce spontaneous papillomas but inhibits papilloma development in the two-stage carcinogenesis model is puzzling given that initiation with DMBA results in a point mutation in codon 61 of the mouse Ha-ras gene (17). Thus, both models involve an activated ras oncogene as an initiating event in tumor development. One difference in the experiments, however, is that papillomas were allowed to develop spontaneously in the K5 E2F1/Tg.AC double-transgenic mice while TPA was used to promote papillomas in the two-stage carcinogenesis model. To determine if overexpression of E2F1 could also inhibit TPA-promoted tumorigenesis in Tg.AC mice, double K5 E2F1/Tg.AC transgenic mice and single-transgenic Tg.AC controls were treated twice weekly with TPA. As expected, Tg.AC single-transgenic mice developed numerous papillomas in two separate experiments (Fig. 5). In sharp contrast, double-transgenic mice generated with either K5 E2F1 line were resistant to tumor promotion by TPA. Consistent with previous findings, some K5 E2F1/Tg.AC double-transgenic mice in these experiments did develop spontaneous papillomas outside the area of TPA treatment.
FIG. 5.
Inhibition of TPA-induced papilloma development in Tg.AC mice by overexpression of E2F1. Two Tg.AC (ras) and two line 1.1 (E2F1/Tg.AC ras) transgenic mice were treated twice weekly with 1.0 μg of TPA. The number of papillomas per mouse after 12 weeks of treatment is presented (A). Three Tg.AC (ras) and three line 1.0 (E2F1/Tg.AC ras) transgenic mice were treated twice weekly with 1.0 μg of TPA. The number of papillomas per mouse after 20 weeks of treatment is presented (B).
Induction of apoptosis in K5 E2F1 epidermis by TPA treatment.
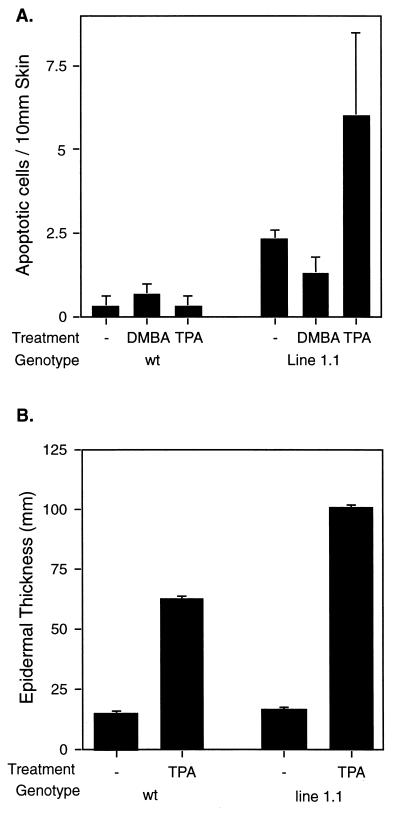
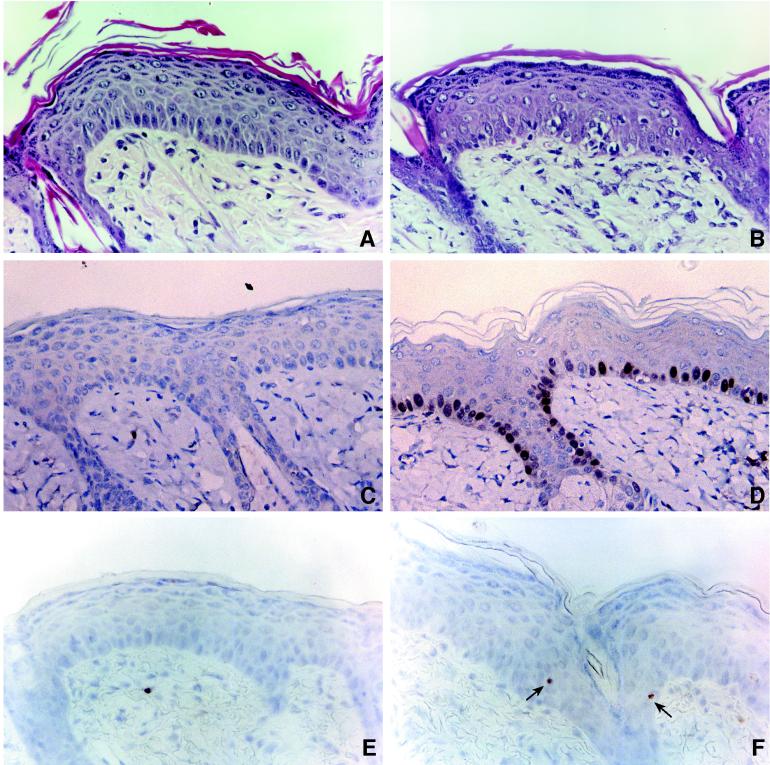
It has been suggested that the ability of E2F1 to function as a tumor suppressor is related to its ability to induce apoptosis. To determine if E2F1-mediated apoptosis could account for the ability of E2F1 to inhibit tumor development in the two-stage carcinogenesis model, skin sections were examined for apoptotic cells by the TUNEL assay following either treatment initiation or tumor promotion. Transgenic (line 1.1) and nontransgenic mice were treated with 10 nM DMBA, and skin sections were taken 24 h later. DMBA has been shown to cause DNA damage and to induce apoptosis in the mouse epidermis (14). However, at the concentration of DMBA used for treatment initiation in our experiments, we found no evidence for significant levels of apoptosis occurring in treated skin (Fig. 6A). This was true for both wild-type and K5 E2F1 transgenic mice. K5 E2F1 and wild-type controls were also treated with TPA twice weekly for 2 weeks and sacrificed 24 h after the final treatment. TPA treatment was found to induce epidermal hyperplasia in both transgenic and wild-type mice (Fig. 6B and 7). However, only in the epidermis of the K5 E2F1 transgenic mice did TPA treatment provoke an apoptotic response over background levels (Fig. 6A and 7). Although the total number of apoptotic cells in the TPA-treated epidermis from K5 E2F1 mice may appear low, this is likely a consequence of performing this analysis on animal tissue. In vivo, apoptotic cells are rapidly removed through phagocytosis by macrophages and other phagocytes (6).
FIG. 6.
Response of K5 E2F1 transgenic mice to DMBA and TPA treatment. Line 1.1 mice and wild-type (wt) siblings were either left untreated or treated with 10 nM DMBA one time or 1.0 μg of TPA four times over 2 weeks. Skin sections were taken 24 h after treatment, and a TUNEL assay was performed. Each group contained three mice, and the average number of apoptotic cells per 10 mm of skin is presented (A). The average thickness of the epidermis from the TPA-treated mice and untreated controls from the experiment whose results are shown in panel A was calculated (B).
FIG. 7.
Induction of apoptosis by TPA in the epidermis of K5 E2F1 transgenic mice. Wild-type mice (A, C, and E) and K5 E2F1 line 1.1 mice (B, D, and F) were treated with 1.0 μg of TPA four times over 2 weeks. Skin sections were used for staining with hematoxylin and eosin (A and B), immunostaining for E2F1 protein (C and D), and performance of TUNEL assays (E and F). TUNEL-positive cells in panel F are marked by arrows.
DISCUSSION
There is much indirect evidence that the activation of E2F transcription factors, via alterations in the p16INK4a-cyclin D-Rb pathway, is a key event in the development of most human cancers. Findings obtained with K5 E2F1 transgenic mice now demonstrate directly that increased E2F1 activity can contribute to tumor development. Tumors arise in the skin, as well as a few other K5-expressing tissues, including the odontogenic epithelium, forestomach, and vagina. Even though the transgene is expressed in other K5-expressing tissues, such as the thymus, bladder, and esophagus, we have not observed tumors in these tissues. A previous study analyzing the effect of p53 deficiency on the phenotype of K5 E2F1 transgenic mice also found that the vast majority of tumors arose in the skin, with only one other tumor of the odontogenic epithelium (16). These findings suggest that some tissues are more sensitive to the oncogenic effects of increased E2F1 activity than are others.
As in human cancers, recent studies suggest that upregulation of E2F transcriptional activity occurs during mouse skin carcinogenesis. Overexpression of cyclin D1 occurs in 100% of mouse skin papillomas and squamous cell carcinomas obtained by the DMBA-TPA treatment protocol (2, 19). We and others have also found increased expression of other G1 phase regulators, such as cyclin D2 and cyclin E, in early papillomas and/or squamous cell carcinomas of the mouse skin (20, 26). Moreover, the levels of several E2F family members, including E2F1, are elevated in mouse skin papillomas (20, 26). Based on these findings, together with our previous data demonstrating that the K5 E2F1 transgene can promote skin tumor development, one might have expected that K5 E2F1 transgenic mice would be more sensitive to two-stage carcinogenesis. Instead, we found that K5 E2F1 transgenic mice are resistant to tumor development in this model system. This demonstrates that increased E2F1 activity can either promote or suppress tumorigenesis in the same tissue, dependent upon the experimental conditions.
The findings that E2F1 inhibits tumor promotion by TPA and that TPA treatment induces epidermal apoptosis in K5 E2F1 transgenic mice suggest that the mechanism by which E2F1 suppresses tumorigenesis involves the induction of apoptosis. This hypothesis is consistent with our finding that impairment of E2F1-mediated apoptosis, through loss of p53 function, results in spontaneous skin tumor development in K5 E2F1 transgenic mice (16). It is also consistent with recent studies using E2F1 knockout mice which suggest that the ability of E2F1 to induce apoptosis underlies its tumor suppressor function (13). Although the level of apoptosis observed in TPA-treated K5 E2F1 epidermis is significant, the level is not sufficient to offset TPA-induced hyperplasia. In fact, K5 E2F1 transgenic mice have an enhanced hyperplastic response to TPA compared to wild-type sibling controls (Fig. 4B). If apoptosis is the mechanism by which E2F1 suppresses tumor promotion, then it must occur preferentially in a subset of critical cells, perhaps the initiated cells.
The nature of the signal that TPA treatment generates to enhance E2F1-mediated apoptosis is unclear. The major activity of TPA with regard to tumor promotion is the activation of members of the protein kinase C (PKC) family (3). TPA directly binds PKC, causing an increase in PKC’s affinity for calcium and the stimulation of enzymatic activity. It is believed that a key substrate for PKC is the raf kinase (12). Phosphorylation of raf by PKC leads to stimulation of the mitogen-activated protein kinase signaling pathway and cell proliferation. However, activation of the raf pathway has also been shown to promote apoptosis under some circumstances (10). Another substrate for PKC is the p53 tumor suppressor protein (8, 23). Phosphorylation of p53 by PKC converts p53 from its latent state to its high-affinity DNA-binding state. It is possible that activation of raf and/or induction of p53 by PKC, when combined with E2F1 overexpression, leads to apoptosis of initiated cells that otherwise would develop into a papilloma.
The ability of increased E2F1 activity to inhibit tumor development under some conditions may have valuable applications in cancer therapy. Studies using recombinant adenoviruses have shown that E2F1 can kill human tumor cells both in vitro and in nude mice (5, 7). With further study, it may be possible to design cancer therapies that would enhance the tumor-suppressive activity of E2F1 while inhibiting its oncogenic activity. Since upregulation of E2F1 appears to be a very common event in human cancers, such a treatment could be useful for a broad range of tumor types.
ACKNOWLEDGMENTS
We thank Jennifer Smith for technical assistance; Becky Brooks and Shawnda Sanders for preparation of the manuscript; Dale Weiss, Lezlee Coghlan, and coworkers for animal care; and Judy Ing and Chris Yone for artwork.
K5 E2F1 transgenic mice were generated at the NICHD Transgenic Mice Development Facility (NTMDF) at the University of Alabama at Birmingham (contract N01-HD-5-3229). This work was funded by grants from the American Cancer Society (CN-152 to D.G.J.) and the National Institutes of Health (CA 79648 to D.G.J., CA 42157 to C.J.C., NIEHS Center grant ES007784, and CA 16672).
REFERENCES
- 1.Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Bianchi A B, Fischer S M, Robles A I, Rinchik E M, Conti C J. Overexpression of cyclin D1 in mouse skin carcinogenesis. Oncogene. 1993;8:1127–1133. [PubMed] [Google Scholar]
- 3.Conti C J. The mouse skin as a model for chemical carcinogenesis. In: Sundberg J P, editor. Handbook of mouse mutations with skin and hair abnormalities. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 39–46. [Google Scholar]
- 4.DeGregori J, Leone G, Miron A, Jakoi L, Nevins J R. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fueyo J, Gomez-Manzano C, Yung W K A, Liu T J, Alemany R, McDonnell T J, Shi X, Rao J S, Levin V A, Kyritsis A P. Overexpression of E2F-1 in glioma triggers apoptosis and suppresses tumor growth in vitro and in vivo. Nat Med. 1998;4:685–690. doi: 10.1038/nm0698-685. [DOI] [PubMed] [Google Scholar]
- 6.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt K K, Deng J, Liu T-J, Wilson-Heiner M, Swisher S G, Clayman G, Hung M-C. Adenovirus-mediated overexpression of the transcription factor E2F-1 induces apoptosis in human breast and ovarian carcinoma cell lines and does not require p53. Cancer Res. 1997;57:4722–4726. [PubMed] [Google Scholar]
- 8.Hupp T R, Lane D P. Two distinct signaling pathways activate the latent DNA binding function of p53 in a casein kinase II-independent manner. J Biol Chem. 1995;270:18165–18174. doi: 10.1074/jbc.270.30.18165. [DOI] [PubMed] [Google Scholar]
- 9.Johnson D G, Cress D, Jakoi L, Nevins J R. Oncogenic capacity of the E2F1 gene. Proc Natl Acad Sci USA. 1994;91:12823–12827. doi: 10.1073/pnas.91.26.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 11.Leder A, Kuo A, Cardiff R D, Sinn E, Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci USA. 1990;87:9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquardt B, Fritch D, Stabel S. Signalling from TPA to MAP kinase requires protein kinase C, raf and MEK: reconstitution of the signalling pathway in vitro. Oncogene. 1994;9:3213–3218. [PubMed] [Google Scholar]
- 13.Pan H, Yin C, Dyson N J, Harlow E, Yamasaki L, Dyke T V. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol Cell. 1998;2:283–292. doi: 10.1016/s1097-2765(00)80273-7. [DOI] [PubMed] [Google Scholar]
- 14.Pena J C, Rudin C M, Thompson C B. A Bcl-XL transgene promotes malignant conversion of chemically initiated skin papillomas. Cancer Res. 1998;58:2111–2116. [PubMed] [Google Scholar]
- 15.Pierce A M, Fischer S M, Conti C J, Johnson D G. Deregulated expression of E2F1 induces hyperplasia and cooperates with ras in skin tumor development. Oncogene. 1998;16:1267–1276. doi: 10.1038/sj.onc.1201666. [DOI] [PubMed] [Google Scholar]
- 16.Pierce A M, Gimenez-Conti I B, Schneider-Broussard R, Martinez L A, Conti C J, Johnson D G. Increased E2F1 activity induces skin tumors in mice heterozygous and nullizygous for p53. Proc Natl Acad Sci USA. 1998;95:8858–8863. doi: 10.1073/pnas.95.15.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez A, Bravo A, Jorcano J L, Vida M. Sequences 5′ of the bovine keratin 5 gene direct tissue-and-cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- 19.Robles A I, Conti C J. Early overexpression of cyclin D1 in mouse skin carcinogenesis. Carcinogenesis. 1995;16:781–786. doi: 10.1093/carcin/16.4.781. [DOI] [PubMed] [Google Scholar]
- 20.Rodriquez-Puebla M L, LaCava M, Gimenez-Conti I B, Johnson D J, Conti C J. Deregulated expression of cell-cycle proteins during premalignant progression in SENCAR mouse skin. Oncogene. 1998;17:2251–2258. doi: 10.1038/sj.onc.1202131. [DOI] [PubMed] [Google Scholar]
- 21.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 22.Singh P, Wong S, Hong W. Overexpression of E2F-1 in rat embryo fibroblasts leads to neoplastic transformation. EMBO J. 1994;13:3329–3338. doi: 10.1002/j.1460-2075.1994.tb06635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka I, Morin F, Seizinger B R, Kley N. Regulation of the sequence-specific DNA binding function of p53 by protein kinase C and protein phosphatases. J Biol Chem. 1995;270:5405–5411. doi: 10.1074/jbc.270.10.5405. [DOI] [PubMed] [Google Scholar]
- 24.Xu G, Livingston D M, Krek W. Multiple members of the E2F transcription factor family are the products of oncogenes. Proc Natl Acad Sci USA. 1995;92:1357–1361. doi: 10.1073/pnas.92.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson N J. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S-Y, Liu S-C, Goodrow T, Morris R, Klein-Szanto A J P. Increased expression of G1 cyclins and cyclin-dependent kinases during tumor progression of chemically induced mouse skin neoplasms. Mol Carcinog. 1997;18:142–152. [PubMed] [Google Scholar]