Abstract
Diabetic skeletal muscles show reduced contractile force and increased fatigability. Hands are a target for several diabetes‐induced complications. Therefore, reduced handgrip strength often occurs as a consequence of diabetes. The aim of this study was to examine whether long‐term exercise can prevent reduction of grip strength in type 2 diabetes mellitus (T2DM) model OLETF rats, and to explore the mechanisms underlying diabetes‐induced grip strength reduction. Ten 5‐week‐old OLETF rats were used as experimental animals, and five non‐diabetic LETO rats as controls of OLETF rats. Half OLETF rats performed daily voluntary wheel‐running for 17 months (OLETF + EXE), and the rest of OLETF and LETO rats were sedentary. Grip strength was higher in OLETF + EXE and LETO groups than in OLETF group. OLETF group with hyperglycemia showed an increase in HbA1c, serum TNF‐α, and muscle SERCA activity, but a decrease in circulating insulin. Each fiber area, total fiber area, and % total fiber area in type IIb fibers of extensor digitorum longus muscles were larger in OLETF + EXE and LETO groups than in OLETF group. There was a positive correlation between grip strength and the above three parameters concerning type IIb fiber area. Therefore, type IIb fiber atrophy may be the major direct cause of grip strength reduction in OLETF group, although there seems multiple etiological mechanisms. Long‐term wheel‐running may have blocked the diabetes‐induced reduction of grip strength by preventing type IIb fiber atrophy. Regular exercise may be a potent modality for preventing not only the progression of diabetes but muscle dysfunction in T2DM patients.
Keywords: grip strength, OLETF rat, type 2 diabetes mellitus, wheel‐running exercise
Two types of hanging test were performed to evaluate not only grip ability but also muscle fatigue resistance in diabetic OLETF rats.
1. INTRODUCTION
The global prevalence of diabetes in adults has been increasing over recent decades (Cho et al., 2018; World Health Organization, 2009). The International Diabetes Federation estimated that there were 451 million people with diabetes worldwide in 2017, and the number of diabetic people was predicted to increase to 693 million by 2045 (Cho et al., 2018). Diabetes showing manifestation of chronic hyperglycemia is well‐known to increase the risk of disabling metabolic disorders including renal failure, cardiovascular disease, peripheral vascular disease, and retinopathy (World Health Organization, 2009). Moreover, diabetic skeletal muscles showed the reduced contractile force in humans (Andersen et al., 1996, 1997, 1998; Andreassen et al., 2009; Leenders et al., 2013) and rodents (Cotter et al., 1989; Fahim et al., 1998; Safwat et al., 2013; Sanchez et al., 2005; Stephenson et al., 1994), and also the increased fatigability in humans (Fritschi & Quinn, 2010) and mice (Chiu et al., 2016). Hands are a target for several diabetes‐induced complications. Diabetic patients showed a significantly higher incidence of hand abnormalities, such as Dupuytren's contracture, carpal tunnel syndrome, limited joint mobility syndrome, and flexor tenosynovitis (trigger finger), compared with non‐diabetic adults (Chammas et al., 1995; Redmond et al., 2009). Therefore, reduced handgrip strength was associated with hand disability in diabetes (Redmond et al., 2009), and was reported in patients with type 2 diabetes mellitus (T2DM) (Gundmi et al., 2018). More than 90% of the diabetic patients are T2DM, of which the onset could be prevented by physical activity (Bassuk & Manson, 2005; Grøntved et al., 2014) or healthy diet plus physical activity (The Diabetes Prevention Program Research Group, 2002).
Otsuka Long‐Evans Tokushima Fatty (OLETF) rat with the homozygously disrupted cholecystokinin type‐A receptor gene (Takiguchi et al., 1997) is a polygenic model of T2DM, which is characterized by late onset of hyperglycemia, a chronic course of disease, mild obesity, inheritance by males, hyperplastic foci of pancreatic islets, and renal complication (Kawano et al., 1992). In OLETF rats, daily voluntary wheel‐running exercise was found to have preventive effects against the development of T2DM (Mikus et al., 2010; Shima et al., 1993). In nondiabetic young OLETF rats without pancreatectomy, moreover, such a wheel‐running exercise resulted in beneficial effects on the pancreas as reflected by an increased growth of the pancreas, accompanied by increases in B‐cell mass and insulin content (Shima et al., 1997). In addition, we reported that long‐term wheel‐running exercise can prevent not only the development of T2DM but deterioration of bone properties in OLETF rats (Minematsu et al., 2017). The aims of this study were to examine whether diabetic OLETF rats show the reduced grip strength like T2DM patients, and also to examine the effects of daily physical activity on grip strength in OLETF rats. Moreover, the mechanisms underlying diabetes‐induced reduction of grip strength were explored in terms of blood biochemical and skeletal muscle histochemical properties. In this study, therefore, the voluntary wheel‐running exercise started at 1 month of age and ended at 18 months of age.
2. METHODS
2.1. Protocol approval
This study was approved by the Committee of Research Facilities of Laboratory Animal Science, Kio University and was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85–23, revised in 1996).
2.2. Animals and experimental design
Ten 5‐week‐old male OLETF rats were used as experimental animals and five five‐week‐old male Long‐Evans Tokushima Otsuka (LETO) rats as controls of OLETF rats (Japan SLC Inc.). Both OLETF and LETO rats were established from the same colony of Long‐Evans rats (Kawano et al., 1992). The OLETF rats have been often used as an animal model of spontaneous T2DM, while the LETO rats with no manifestation of T2DM have been generally used as control animals of the experimental OLETF rats (Kawano et al., 1992). They were divided into three groups as follows; LETO, OLETF, and OLETF + EXE groups (n = 5 each). The OLETF + EXE rats were housed individually in a cage equipped with an exercise wheel (116 cm‐circumference, 10 cm‐inside width; Shinano), that allowed free access to the wheel, for 17 months. The number of revolutions was recorded weekly. The LETO and OLETF rats, which were housed individually in a standard cage, were regarded as a sedentary animal. Both cages were set in an animal facility where the room temperature and lighting were controlled (temperature, 23 ± 2℃; lighting, a fixed 12:12‐h light‐dark cycle). All the rats were fed a standard rodent chow (CE‐2; Clea Japan Inc.) and water ad libitum throughout the experiment. Grip strength test and hanging tests were performed within 1 month before death. At the end of 17 month‐experimental periods, the collections of blood, tibias, and skeletal muscles were performed.
2.3. Grip strength test
In each rat, grip strength of both fore limb and hind limb was assessed using a grip strength meter (model MK‐380 M, Muromachi Kikai Co.) as described elsewhere (McMahon et al., 2014). This meter has a wire mesh grid attached to a force transducer. Each test animal was placed carefully on the metallic mesh grid and allowed to grasp the mesh grid through all paws. The animal was then pulled back gently by holding the tail with increasing force until its grip was lost. It took 4–5 seconds to complete each measurement. This test provided the maximum grip strength force attained. Each measurement was repeated twice by the same person, and the higher force value was recorded. In the second measurement, one rat of the OLETF group disliked grasping the mesh grid, resulting in a failure in the grip strength test.
2.4. Mesh‐hanging tests
Two types of hanging test, that is, a reverse hanging test (Figure 4a) and a vertical hanging test (Figure 4b), were performed to evaluate not only grip ability but muscle fatigue resistance. In both hanging tests, a wire mesh grid (dimensions, 80 × 50 cm; wire mesh spacing, 12 mm; wire thickness, 1.5 mm) was used. Each test animal was allowed to grasp the mesh grid through all paws in a face‐up position in the reverse hanging test and also in a standing position in the vertical hanging test. Then we measured a grip‐holding time until the grip was released. In both hanging tests, each measurement was repeated twice by the same person, and the longer hanging time was recorded. In the second measurement, one rat of the OLETF group disliked grasping the mesh grid, resulting in a failure in both mesh‐hanging tests.
FIGURE 4.
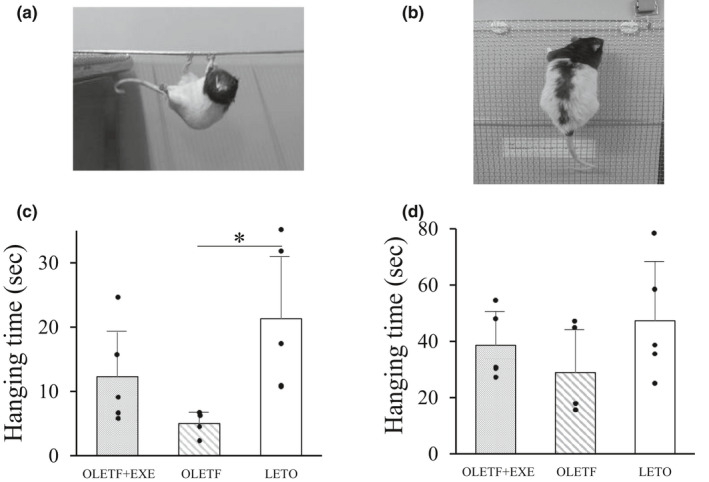
Photograph of animal in the reverse (a) and vertical (b) hanging tests, and hanging time in the reverse (c) and vertical (d) hanging tests of three groups. Values are means + SD. Statistical comparisons between the groups were assessed by one‐way ANOVA followed up with Fisher's LSD post hoc analysis. *Significant difference (p < 0.05). Symbols represent data from individual animals. In both hanging tests, n = 4 in the OLETF group, and n = 5 in the OLETF + EXE and LETO groups. Effect sizes; reverse hanging test, 0.426; vertical hanging test, 0.200
2.5. Collections of blood, tibias, and skeletal muscles
After 5–6 h of fasting, the rats were anesthetized with inhaled 2% (volume/volume) isoflurane on a mechanical ventilator at 12:00 p.m.–03:00 p.m. The chest was opened through a median sternotomy, and more than 10 ml of blood was obtained from the left ventricle using a 20‐guage needle. At the end of the experiment, all animals were euthanized by exsanguination under deep anesthesia with inhalation of 3% isoflurane. Serum samples, obtained from blood centrifugation at 3500 rpm for 10 min, were stored at −80℃ until biochemical analyses and enzyme‐linked immunosorbent assays (ELISA). Bilateral skeletal muscles (extensor digitorum longus (EDL), soleus (SOL), rectus femoris (RF), and biceps brachii (BB)) were obtained from the carcasses after blood collection and were weighed using a digital scale. The EDL muscles were frozen in liquid nitrogen‐cooled isopentane and stored at −80℃ until histochemical analyses. The RF muscles were frozen in liquid nitrogen and stored at −80℃ until measurements of sarcoplasmic reticulum Ca2+‐ATPase (SERCA) activity. The length of bilateral tibias was measured with digital caliper in order to obtain the ratio of muscle weight to tibial length (TL). When body mass differs between animals as shown by somewhat large standard deviation (SD) of body mass in OLETF group (Table 1), the ratio of muscle mass to TL is an applicable parameter to normalize muscle weight (Yin et al., 1982). Therefore, in addition to muscle mass, we herein present muscle mass normalized to TL as reported in AGE‐enriched diet‐fed mice (Egawa et al., 2017) and exercised DM rats (Farrell et al., 1999).
TABLE 1.
Body mass, tibial length, blood glucose, and serum biochemical data
| OLETF + EXE | OLETF | LETO | Effect size | |
|---|---|---|---|---|
| Body mass (g) | 529 ± 43 | 496 ± 88 | 576 ± 18 | 0.286 |
| Tibial length (mm) | 45.7 ± 0.3 | 45.0 ± 0.8 | 45.8 ± 0.4 | 0.320 |
| Blood glucose (mg/dl) | 69 ± 26 | 234 ± 94* | 71 ± 12 | 0.651 |
| Total protein (g/dl) | 5.2 ± 0.3** | 5.3 ± 0.5 | 5.7 ± 0.2 | 0.229 |
| Total cholesterol (mg/dl) | 150 ± 15*** | 255 ± 11** | 24 ± 6 | 0.962 |
Values are expressed as mean ± SD. N = 5 in each group.
Statistical comparisons between the groups were assessed by one‐way ANOVA followed by Fisher's LSD post hoc test.
Significantly different from the OLETF + EXE and LETO groups (p < 0.01)
Significantly different from the LETO group (p < 0.01)
Significantly different from the OLETF and LETO groups (p < 0.01).
2.6. Biochemical analyses and ELISA
Blood samples were analyzed for blood glucose concentrations and hemoglobin A1c (HbA1c) levels. Serum samples were analyzed for total protein and total cholesterol, and also with commercially available ultrasensitive ELISA kit for rat insulin (Mercodia) and ELISA strip for rat tumor necrosis factor‐α (TNF‐α; Signosis Inc.), using a microplate photometer (Multiskan FC; Thermo Fisher Scientific).
2.7. Histochemical analyses of EDL muscles
Histochemical analyses were performed on the frozen EDL muscle samples. Serial transverse sections (10 μm) were cut from each EDL muscle using a cryostat (CM1850, Leica microsystems) maintained at −20℃. Two serial sections were brought to room temperature and air‐dried for 30 min. One section underwent a myosin ATPase‐staining and the other a succinic dehydrogenase (SDH)‐staining, as previously reported by us (Imagita et al., 2014). Fiber type classification was performed on the basis of the staining intensities. Fiber types based on the ATPase activity were distinguished as slow‐twitch‐oxidative (Type I), fast‐twitch‐oxidative‐glycolytic (Type IIa) and fast‐twitch‐glycolytic (Type IIb), according to the classification of Peter et al. (Peter et al., 1972). To further confirm the classification of fiber types, another section was stained for the SDH activity (Nachlas et al., 1957). The cross‐sectional area (CSA) of each fiber was measured on the digitized image (1 mm2) of ATPase‐stained sections using a computer‐assisted image processing system (Image J version 1.46, NIH).
2.8. Preparations of RF muscle homogenates
The RF muscle pieces were homogenized in nine volumes of ice‐cold buffer solution (pH 7.4) consisting of 300 mM sucrose, 20 mM MOPS‐KOH, 0.0014 mM pepstatin, 0.83 mM benzamidine, 0.0022 mM leupeptin, and 0.2 mM phenylmethanesulphonyl fluoride, using a hand‐held glass homogenizer. The homogenates were then centrifuged at 2000 g for 20 min (2℃). The supernatant thus obtained was quickly frozen in liquid nitrogen and stored at −80℃ for the measurements of SERCA activity. The protein concentrations were determined by the method of Bradford (Bradford, 1976) using bovine serum albumin as a standard.
2.9. Measurements of SERCA activity in RF muscles
The SERCA activity was spectrophotometrically measured in triplicate at 37℃ as previously reported (Yamada et al., 2004). Briefly, the assay mixture (pH 7.5) contained 20 mM HEPES, 1 mM EGTA, 200 mM KCl, 15 mM MgCl2, 0.8 mM CaCl2, 10 mM sodium azide, 0.4 mM NADH, 10 mM phosphoenolpyruvate, 18 U/ml pyruvate kinase, 18 U/ml lactate dehydrogenase, and 0.005% Triton X‐100. The assay mixture was incubated for 3 min after the addition of a 20 μl aliquot of the homogenate. The reaction was started by adding Mg‐ATP to give a final concentration of 4 mM. Finally, the CaCl2 concentration was increased to 20 mM in order to selectively inhibit SERCA activity. The remaining activity was defined as background ATPase activity. The activity of SERCA was obtained by subtracting the background ATPase activity from total ATPase activity.
2.10. Statistical analyses
Values were expressed as mean ± SD. Statistical comparisons between the groups were assessed by one‐way ANOVA followed up with Fisher's LSD post hoc analysis. Moreover, effect sizes are presented as η2 in ANOVA of all groups. In changes in weekly running distance with aging, significant differences between the first week value and the latter values were determined using repeated‐measures ANOVA followed up with Sidak post hoc analysis. In addition, Pearson's correlation coefficients were determined to examine the relationship between grip strength and other measured parameters. All statistical analyses were performed using the Excel Statistics software (Excel 2012 version 1.08 for Windows; Social Survey Research Information Co., Ltd.,). A P value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Final body mass, final TL, weekly running distance, diabetes indices, and serum biochemical data
At the end of the experiment, there was no statistically significant difference in final body mass and TL between the groups (Table 1). In the OLETF + EXE rats, there was a considerable individual difference in changes of weekly running distance with aging (Figure 1a). As shown in Figure 1b, the mean weekly running distance sharply increased from age 5–6 weeks (18.9 ± 1.8 km/week) through age 9–10 weeks (56.9 ± 21.8 km/week), and thereafter gradually declined to 18.0 ± 4.7 km/week at age 32–33 weeks, after which the running distance was similar to that of the first week for the running (age 5–6 weeks). The mean total running distance during the experimental period of 17 months was 1667 ± 375 km, with a wide range from 1171 to 2219 km. Diabetes indices, that is, blood glucose and HbA1c levels, were much lower in the OLETF + EXE and LETO groups than in the OLETF group (Table 1 and Figure 2a). Thus the OLETF + EXE and LETO rats showed similar diabetes indices. Serum concentration of total protein was lower in the OLETF + EXE group than in the LETO group (Table 1). Although the OLETF group showed the highest levels of total cholesterol in the three groups, the OLETF + EXE group showed the intermediate total cholesterol levels between the LETO group's and OLETF group's levels (Table 1). Circulating insulin levels were significantly higher in the OLETF + EXE group than in the OLETF and LETO groups (Figure 2b). The OLETF group indicated the highest levels of TNF‐α among the three groups (Figure 2c).
FIGURE 1.
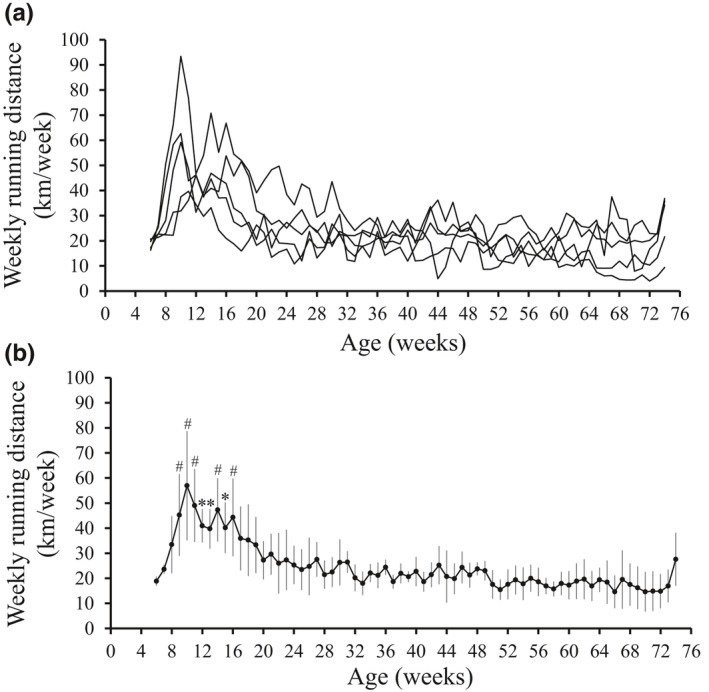
Weekly running distance. Five OLETF rats performed daily voluntary wheel‐running for a 17‐month period from age 5 weeks through age 74 weeks. (a) Individual changes in weekly running distance with aging (n = 5). (b) Changes in the mean weekly running distance with aging. Values are means ± SD (n = 5). Significant differences between the first week value and the latter values were determined using repeated‐measures ANOVA followed up with Sidak post hoc analysis. The overall p value obtained by repeated‐measures ANOVA is below 0.001. *Significantly different from the first week value (p < 0.01). #Significantly different from the first week value (p < 0.001)
FIGURE 2.
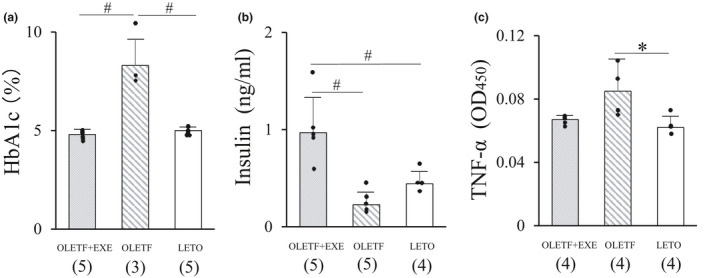
Serum levels of hemoglobin A1c (a), insulin (b) and tumor necrosis factor‐α (c) in three groups. Values are means + SD. Statistical comparisons between the groups were assessed by one‐way ANOVA followed up with Fisher's LSD post hoc analysis. *Significant difference (p < 0.05). #Significant difference (p < 0.01). Symbols represent data from individual animals, and the number in the parentheses represents the number of examined animals. Effect sizes; HbA1c, 0.840; insulin, 0.699; TNF‐α, 0.374. HbA1c, hemoglobin A1c; TNF‐α, tumor necrosis factor‐α
3.2. Grip strength and hanging capacity
The grip strength was significantly higher in the OLETF + EXE and LETO groups than in the OLETF group (Figure 3). A similar pattern was observed in both the reverse and vertical hanging tests (Figure 4c,d). The OLETF group showed the shortest hanging time among the three groups.
FIGURE 3.
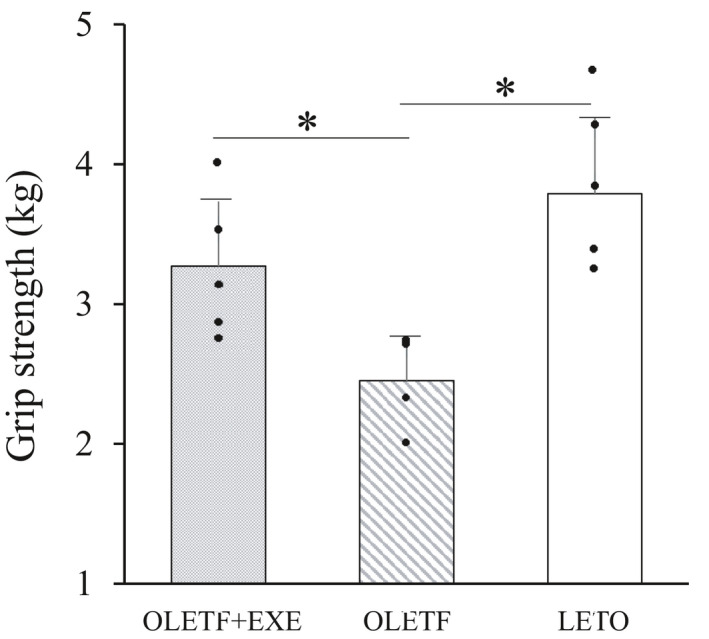
Grip strength in three groups. Values are means + SD. Statistical comparisons between the groups were assessed by one‐way ANOVA followed up with Fisher's LSD post hoc analysis. *Significant difference (p < 0.05). Symbols represent data from individual animals. N = 4 in the OLETF group, and n = 5 in the OLETF+EXE and LETO groups. Effect size is 0.702
3.3. Muscle mass and muscle mass/TL ratio
Table 2 indicates the muscle mass and muscle mass/TL ratio in the EDL, SOL, BB and RF muscles. Although the OLETF + EXE and OLETF rats had similar muscle mass and muscle mass/TL ratio in four kinds of muscles, the LETO rats had the increased muscle mass and muscle mass/TL ratio in the EDL, SOL, and BB muscles, compared with the OLETF + EXE and OLETF rats.
TABLE 2.
Muscle mass and the ratio of muscle weight to tibial length
| OLETF + EXE | OLETF | LETO | Effect size | |
|---|---|---|---|---|
| EDL mass (mg) | 220.5 ± 6.7** | 216.9 ± 28.8** | 257.9 ± 8.8 | 0.577 |
| EDL mass/TL (mg/mm) | 4.83 ± 0.15** | 4.82 ± 0.50** | 5.63 ± 0.19 | 0.585 |
| SOL mass (mg) | 188.9 ± 20.4* | 180.3 ± 34.6** | 233.6 ± 15.0 | 0.527 |
| SOL mass/TL (mg/mm) | 4.14 ± 0.42* | 4.00 ± 0.62** | 5.10 ± 0.31 | 0.522 |
| BB mass (mg) | 283.5 ± 8.7* | 268.9 ± 33.7** | 322.9 ± 21.4 | 0.539 |
| BB mass/TL (mg/mm) | 6.21 ± 0.15* | 5.97 ± 0.58** | 7.04 ± 0.38 | 0.560 |
| RF mass (mg) | 1480.2 ± 84.7 | 1440.0 ± 180.5 | 1630.2 ± 133.8 | 0.303 |
| RF mass/TL (mg/mm) | 32.4 ± 1.7 | 32.0 ± 3.1 | 35.6 ± 2.8 | 0.273 |
Values are expressed as mean ± SD. N = 5 in each group.
Statistical comparisons between the groups were assessed by one‐way ANOVA followed by Fisher's LSD post hoc test.
Abbreviations: BB, biceps brachii; EDL, extensor digitorum longus; RF, rectus femoris; SOL, soleus; TL, tibial length.
Significantly different from the LETO group (p < 0.05)
Significantly different from the LETO group (p < 0.01).
3.4. Characterization of EDL muscle fibers
In three groups, we identified the fiber type of EDL muscle associated with grip of hind limb (Figure 5). In the type I fibers, the OLETF group showed an increase in % fiber number (Figure 5a), total fiber CSA (Figure 5c) and % total fiber CSA (Figure 5d) compared with the LETO group. To the contrary, in the type IIb fibers, the OLETF and OLETF + EXE groups showed a decrease in % fiber number (Figure 5a) compared with the LETO group, and also the OLETF group showed a decrease in each fiber CSA (Figure 5b), total fiber CSA (Figure 5c) and % total fiber CSA (Figure 5d) compared with the OLETF + EXE and LETO groups. Moreover, in the type IIa fibers, the OLETF and OLETF + EXE groups exhibited a decrease in each fiber CSA compared with the LETO group (Figure 5b). As shown in Figure 5b,d, there was no significant difference in each fiber CSA and % total fiber CSA of the type IIb fibers between the OLETF + EXE and LETO groups.
FIGURE 5.
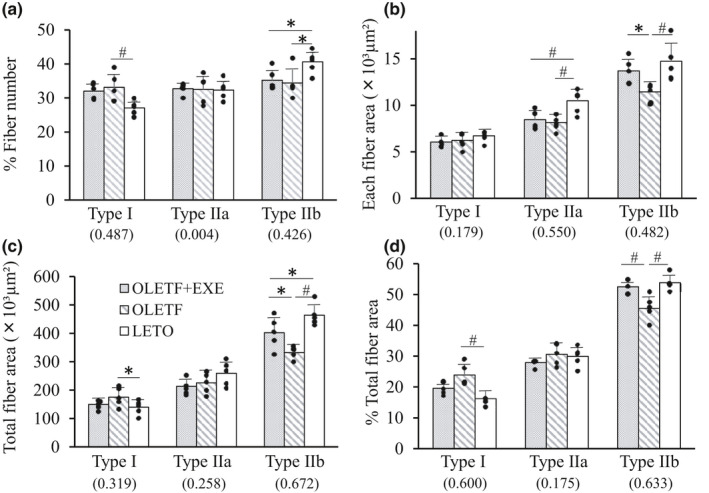
Characterization of extensor digitorum longus muscle fibers in the three groups. Percent fiber number (a), each fiber area (b), total fiber area (c), and percent total fiber area (d) in the type I, type IIa, and type IIb fibers. Grey bars, the OLETF + EXE group; striped bars, the OLETF group; white bars, the LETO group. Values are means + SD. Statistical comparisons between the groups were assessed by one‐way ANOVA followed up with Fisher's LSD post hoc analysis. *Significant difference (p < 0.05). #Significant difference (p < 0.01). Symbols represent data from individual animals, and the value in the parentheses represents effect size. N = 5 in each group
3.5. SERCA activity in RF muscles
In the RF muscles, the SERCA activity was significantly higher in the OLETF group than in the OLETF + EXE and LETO groups (Figure 6). The mean SERCA activity in the OLETF + EXE group was similar to that of the LETO group.
FIGURE 6.
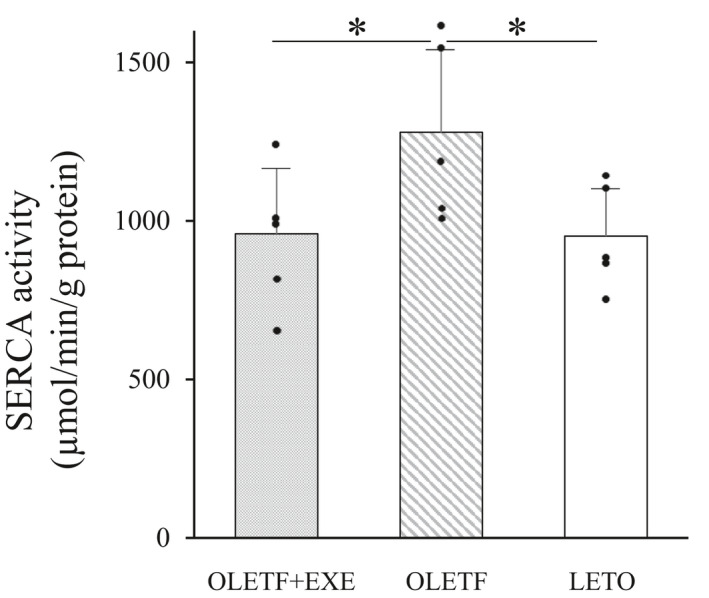
Sarcoplasmic reticulum Ca2+‐ATPase activity in the rectus femoris muscles from three groups. Values are means + SD. Statistical comparisons between the groups were assessed by one‐way ANOVA followed up with Fisher's LSD post hoc analysis. *Significant difference (p < 0.05). Symbols represent data from individual animals. N = 5 in each group. SERCA, sarcoplasmic reticulum Ca2+‐ATPase. Effect size is 0.351
3.6. Correlations between grip strength and various parameters
We examined correlations between grip strength and various measured parameters, using the combined data from the three groups (Table 3). There was a good negative correlation between grip strength and blood glucose, HbA1c, total cholesterol, TNF‐α, % fiber number of type I, or % total fiber CSA of type I and also a negative, but not significant (p = 0.075), correlation between grip strength and SERCA activity. To the contrary, there was a good positive correlation between grip strength and muscle mass (EDL, SOL and BB), each fiber CSA of type IIa or IIb fibers, total fiber CSA of type IIa or IIb fibers, or % total fiber CSA of type IIb fibers.
TABLE 3.
Correlations between grip strength and various parameters
| r | p | |
|---|---|---|
| Body mass | 0.448 | 0.123 |
| Blood glucose | −0.819 | 0.001 |
| Hb A1c | −0.695 | 0.012 |
| Total protein | 0.001 | 0.998 |
| Total cholesterol | −0.775 | 0.002 |
| Insulin | 0.215 | 0.502 |
| TNF‐α | −0.684 | 0.020 |
| SERCA activity | −0.510 | 0.075 |
| EDL mass | 0.633 | 0.020 |
| SOL mass | 0.663 | 0.013 |
| BB mass | 0.600 | 0.030 |
| RF mass | 0.466 | 0.109 |
| %FN‐I | −0.636 | 0.019 |
| %FN‐IIa | 0.483 | 0.095 |
| %FN‐IIb | 0.285 | 0.345 |
| FA‐I | 0.379 | 0.201 |
| FA‐IIa | 0.566 | 0.044 |
| FA‐IIb | 0.773 | 0.002 |
| TFA‐I | −0.408 | 0.167 |
| TFA‐IIa | 0.560 | 0.047 |
| TFA‐IIb | 0.752 | 0.003 |
| %TFA‐I | −0.707 | 0.007 |
| %TFA‐IIa | 0.169 | 0.582 |
| %TFA‐IIb | 0.568 | 0.043 |
Pearson's correlation coefficients were calculated to determine the relation between grip strength and other measured parameters.
Abbreviations: %TFA, percent total fiber area; BB, biceps brachii; EDL, extensor digitorum longus; FA, fiber area; FN, fiber number; Hb A1c, hemoglobin A1c; RF, rectus femoris; SERCA, sarcoplasmic reticulum Ca2+‐ATPase; SOL, soleus; TFA, total fiber area; TNF‐α, tumor necrosis factor‐α.
4. DISCUSSION
In this study, we have examined the effects of long‐term wheel‐running exercise not only on grip strength/hanging capacity but on blood biochemical/muscle histochemical properties in the OLETF rats. Table 4 summarizes the comparison between the OLETF + EXE rats and the sedentary OLETF or LETO rats in terms of various parameters. Our findings are as follows: (a) Grip strength as well as hanging capacity was higher in the OLETF + EXE and LETO rats than in the OLETF rats. (b) Blood glucose, HbA1c, total cholesterol, and TNF‐α levels were lower in the OLETF + EXE and LETO rats than in the OLETF rats. To the contrary, serum insulin levels were higher in the OLETF + EXE rats than in the OLETF and LETO rats. (c) Muscle mass and muscle mass/TL ratio were smaller in the OLETF + EXE and OLETF rats than in the LETO rats. (d) Each fiber CSA, total fiber CSA and % total fiber CSA in the type IIb fibers of EDL muscles were larger in the OLETF + EXE and LETO rats than in the OLETF rats. (e) SERCA activity in the muscles was lower in the OLETF + EXE and LETO rats than in the OLETF rats. (f) Grip strength was correlated negatively with blood glucose, HbA1c, total cholesterol, TNF‐α, % fiber number of type I fibers, % total fiber CSA of type I fibers and SERCA activity and also positively with muscle mass, each fiber CSA of type IIa or IIb fibers, total fiber CSA of type IIa or IIb fibers and % total fiber CSA of type IIb fibers. Thus, in the OLETF rats, long‐term wheel‐running could prevent not only the development of T2DM but the reduction of grip strength. To the best of our knowledge, this is the first study to report not only the effects of long‐term exercise on grip strength/hanging capacity but the correlations between grip strength and blood biochemical/muscle histochemical properties in the OLETF rats. There seems to be several possible mechanisms underlying diabetes‐induced reduction of grip strength in the OLETF rats as described below.
TABLE 4.
OLETF + EXE group is compared with the OLETF and LETO groups in terms of various parameters
| Measurements | Vs. OLETF group | Vs. LETO group |
|---|---|---|
| Body size parameters | ||
| Body mass |

|

|
| Tibial length |

|

|
| Diabetic parameters | ||
| Blood glucose |
|

|
| HbA1c |
|

|
| Serum biochemical parameters | ||
| Insulin |
|
|
| TNF‐α |
|

|
| Total protein |

|
|
| Total cholesterol |
|
|
| Grip strength and hanging capacity | ||
| Grip strength |
|

|
| Reverse hanging capacity |
|

|
| Vertical hanging capacity |
|

|
| Muscle mass and SERCA activity | ||
| Muscle mass (EDL, SOL, BB) |

|
|
| Muscle mass/TL (EDL, SOL, BB) |

|
|
| SERCA activity (RF) |
|

|
| EDL muscle fiber properties | ||
| %FN‐I |

|
|
| %FN‐IIa |

|

|
| %FN‐IIb |

|
|
| FA‐I |

|

|
| FA‐IIa |

|
|
| FA‐IIb |
|

|
| TFA‐I |

|

|
| TFA‐IIa |

|
|
| TFA‐IIb |
|
|
| %TFA‐I |
|
|
| %TFA‐IIa |

|

|
| %TFA‐IIb |
|

|
HbA1c, hemoglobin A1c; TNF‐α, tumor necrosis factor‐α; SERCA, sarcoplasmic reticulum Ca2+‐ATPase; EDL, extensor digitorum longus; SOL, soleus; BB, biceps brachii; RF, rectus femoris; TL, tibial length; FN, fiber number; FA, fiber area; TFA, total fiber area.
 , no difference;
, no difference;  , significant increase;
, significant increase; 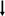 , significant decrease;
, significant decrease; 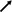 , increase without statistical significance;
, increase without statistical significance; 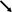 , decrease without statistical significance.
, decrease without statistical significance.
First, the grip strength appears to depend primarily upon the wet muscle mass because there was a positive correlation between the grip strength and the muscle mass (Table 3). Likewise, in a magnetic resonance imaging study on DM patients, the maximal isokinetic muscle strength of ankle dorsal/plantar flexors was related to the muscle volume (Andersen et al., 1997). Moreover, in streptozotocin (STZ)‐induced DM rats with the decreased EDL muscle mass, the isometric contractile force of EDL (Cotter et al., 1989) or of single EDL fibers (Stephenson et al., 1994) was significantly lower than that of non‐DM rats.
The present OLETF group showed the decreased levels of circulating insulin, which is well known to regulate muscle protein synthesis/proteolysis. In alloxan‐induced DM rats, the protein synthesis of fast‐type muscles (gastrocnemius (GA) and tibialis anterior (TA)) was impaired by the block of peptide‐chain initiation, which was caused by the insulin deficiency (Flaim et al., 1980). STZ‐induced DM rats with reduced plasma insulin levels showed the enhanced degradation of myofibrillar protein (Smith et al., 1989). Both the impaired muscle protein synthesis and enhanced myofibrillar protein degradation in DM state could be restored by the exogenous insulin treatment (Flaim et al., 1980; Smith et al., 1989). Therefore, in our OLETF group, the decreased muscle mass may have been due to the insulin deficiency.
Circulating insulin‐like growth factor I (IGF‐I), which stimulates muscle protein synthesis like insulin, also declined in DM rats (Derakhshanian et al., 2017; Farrell et al., 1999). In fasted mice, administration of IGF‐I or insulin induced a 52%–65% increase in protein synthesis of fast‐type muscles (GA and plantaris), but only a 11%–25% increase in that of slow‐type SOL muscle (Bark et al., 1998). Furthermore, IGF‐I overexpression in the transgenic murine muscle led to myofiber hypertrophy (Coleman et al., 1995), and local IGF‐I infusion into the skeletal muscles resulted in muscle hypertrophy (Adams & McCue, 1998). Insulin and IGF‐I are thought to promote protein synthesis via an activated PI3K/Akt/mTOR signaling pathway, resulting in myofiber/muscle hypertrophy (Bassel‐Duby & Olson, 2006). On the other hand, in STZ‐induced DM rats, the decline in circulating IGF‐I induced the gene expression of atrogin‐1/MAFbx, a muscle‐specific ubiquitin‐ligase required for muscle atrophy, resulting in the loss of muscle mass (Dehoux et al., 2004). In DM rats, chronic resistance exercise induced the increase in both plasma IGF‐I levels and muscle protein synthesis, leading to a rise in the muscle mass/TL ratio (Farrell et al., 1999). In the current study, however, wheel‐running exercise failed in increasing the muscle mass, although it led to the increase in serum insulin concentrations. At present, the cause of such a discrepancy concerning the exercise effect on DM muscle mass remains to be resolved.
Second, the grip strength also appears to depend primarily upon type IIb fiber CSA in EDL muscles, because the grip strength correlated fairly well with each fiber CSA, total fiber CSA and % total fiber CSA of type IIb fibers. Similarly, a positive correlation was found between type II fiber CSA in the vastus lateralis (VL) muscle and the maximum extension strength of leg muscles in T2DM patients (Leenders et al., 2013). The present OLETF group showed the reduction in both grip strength and EDL type IIb fiber CSA. Likewise, forelimb grip strength and fiber CSA of fast glycolytic TA muscle were reduced in db/db mice, of which fast‐type muscle mass was decreased (Ostler et al., 2014). In STZ‐induced DM rats, moreover, EDL muscles with 44% reduction in total type IIb fiber CSA indicated a decrease in the maximum isometric twitch tension (Cotter et al., 1989). In addition, in insulin‐treated DM rats, the decrease in peak force of single type IIb fiber from EDL muscles was due to type IIb fiber atrophy (Sanchez et al., 2005). In view of the above‐mentioned observations, it seems most likely that the reduced grip strength in the present OLETF group is mainly ascribable to fast‐type muscle atrophy accompanied by type IIb fiber atrophy.
Circulating corticosterone levels were elevated in STZ‐induced DM rats (Smith et al., 1989) and db/db mice (Ostler et al., 2014). Glucocorticoid treatment resulted in a 25% reduction in fast‐type muscle mass by the decreased protein synthesis, but did not affect the slow‐type SOL mass in normal rats (Rannels & Jefferson, 1980). Moreover, type IIb fiber CSA in the corticosteroid‐treated rat diaphragm was reduced by 51%, whereas type I and IIa fiber CSAs were unaffected (Dekhuijzen et al., 1995). Overexpression of IGF‐I in TA muscle prevented muscle/myofiber atrophy in glucocorticoid‐treated rats (Schakman et al., 2005). Therefore, glucocorticoid‐induced muscle/myofiber atrophy appears to be caused by downregulating the IGF‐I‐signaling pathways (Schakman et al., 2009). Exercise could induce not only the increase in circulating IGF‐I levels (Farrell et al., 1999) but the decrease in diabetes‐induced serum corticosterone levels (Hwang et al., 2011) in DM rats, resulting in the prevention of muscle atrophy (Farrell et al., 1999). Although the present wheel‐running exercise caused the decrease in % type IIb fiber number and the increase, but not significant, in % type I fiber number in agreement with the observation that 45‐day wheel‐running exercise resulted in reduced proportions of type IIb fibers in EDL muscles of normal rats (Kriketos et al., 1995), it prevented the decrease in type IIb fiber CSA in EDL muscles. Lifelong (21 months) wheel‐running exercise combined with mild caloric restriction resulted in a significant increase in IGF‐I protein levels and fiber CSA in rat fast‐type plantaris muscles (Kim et al., 2008). In view of these results, our long‐term (17 months) wheel‐running exercise may have prevented type IIb fiber atrophy by the enhanced IGF‐I expression of EDL muscles, increased circulating insulin, and decreased circulating corticosterone levels.
Third, DM‐induced reduction of grip strength may be attributable to inflammation, because the serum TNF‐α concentrations were increased in the present OLETF group and were correlated negatively with grip strength. Likewise, circulating levels of pro‐inflammatory cytokines, TNF‐α and interleukin (IL)‐6, were increased in older T2DM patients with excessive loss of skeletal muscle mass (Park et al., 2009), and such higher TNF‐α/IL‐6 levels were associated with lower grip strength in elderly persons (Visser et al., 2002). The TNF‐α caused skeletal muscle atrophy via the IKKβ/ NF‐κB/MURF1 signaling pathway (Cai et al., 2004). In 26‐month‐old rats, moreover, the superficial VL muscles composed predominantly of type II fast fibers exhibited an increase in TNF‐α expression and TNF‐α‐induced apoptosis compared with the SOL muscles composed predominantly of type I slow fibers, suggesting TNF‐α signal transduction specific to type II fast fibers (Phillips & Leeuwenburgh, 2005). Thus, TNF‐α appears to be a powerful fast‐type fiber‐wasting cytokine.
In this study, the mean concentration of serum TNF‐α was lower in the OLETF+EXE group than in the OLETF group. Similarly, in patients with T2DM and the metabolic syndrome, aerobic plus resistance‐combined physical exercise for 1 year decreased serum levels of pro‐inflammatory cytokines TNF‐α, IL‐1β, IL‐6, and interferon‐γ, but reversely increased serum levels of anti‐inflammatory cytokines IL‐4 and IL‐10, thus indicating that exercise has a full anti‐inflammatory effect in T2DM (Balducci et al., 2010). In addition, 8 weeks of aerobic exercise increased the protein content of NF‐κB‐inhibitor IκB and reduced TNF‐α protein content in skeletal muscle of T2DM patients, suggesting that NF‐κB signaling is downregulated by exercise (Sriwijitkamol et al., 2006). Thus, exercise may prevent diabetes‐induced muscle atrophy by downregulating NF‐κB signaling in the potential inflammatory pathway.
Fourth, non‐enzymatic glycosylation (glycation) of grip‐associated proteins may cause the attenuation of grip strength, because the glycosylated hemoglobin, that is, HbA1c, were elevated in the present OLETF group and correlated negatively with grip strength. Such a negative correlation between HbA1c and grip strength was also found in patients with T2DM (Leenders et al., 2013). Skeletal muscle myosin from DM patients was more glycosylated and had lower Ca2+‐ATPase activity compared with control myosin of healthy subjects (Syrovỳ & Hodnỳ, 1992). In rat myofibrillar preparations, as myofibrillar proteins were glycated by incubation with ribose, Mg2+‐activated ATPase activity of myofibrils was lowered (Syrovỳ & Hodnỳ, 1993). Similarly, in single fibers from rat skinned EDL muscles exposed to glucose‐6‐phosphate, the maximum Ca2+‐activated force per CSA and Mg2+‐activated ATPase activity were significantly reduced (Patterson et al., 2000). Moreover, rat skeletal muscle myosin was incubated with glucose and subsequently analyzed for structural and functional modifications by matrix‐assisted laser desorption/ionization (MALDI) mass spectrometry and a single‐fiber in vitro motility assay (Ramamurthy et al., 2001). Glycation‐related structural alterations, revealed by MALDI spectra, were paralleled by a significant reduction in the in vitro motility speed, suggesting a structural‐related decline in myosin mechanics in response to glucose exposure (Ramamurthy et al., 2001).
In type 1 DM (T1DM) patients, skin collagen was glycated with the duration of diabetes, and there was a positive correlation between glycated collagen levels and finger‐joint stiffness (Monnier et al., 1986). Moreover, the glycated collagen matrix exhibited a decreased elasticity and increased toughness compared with the non‐glycated collagen matrix (Liao et al., 2009). In addition, fibroblast‐mediated contraction of the glycated collagen matrix was reduced as compared with that of the non‐glycated collagen matrix (Liao et al., 2009). Thus, the glycated collagen, too, may contribute to reduced grip strength in DM.
Advanced glycation end‐products (AGEs) accumulated in skin correlated negatively with grip strength in adult men (Momma et al., 2011). AGEs, accumulated in rat EDL myofibers with aging, selectively modified actin and several metabolic enzymes involved in energy production, like creatine kinase (CK) and β‐enolase (Snow et al., 2007). Therefore, such AGE‐induced posttranslational modifications of contraction‐associated proteins are considered as one of the causes of muscle dysfunction. In fact, mice fed an AGE‐enriched diet for 16 weeks exhibited skeletal muscle dysfunction, including the reduction in grip strength, fatigue resistance and in vitro muscle force production (Egawa et al., 2017). In addition, the AGEs accumulation in the muscles of STZ‐induced DM mice and a DM patient was associated with muscle atrophy and muscle dysfunction via a putative mechanism of the RAGE‐mediated, AMPK‐downregulated, Akt signaling pathway (Chiu et al., 2016).
Fifth, grip strength weakness in DM may be associated with the downregulation of SERCA gene expression, because vitamin D receptor knockout mice and diet‐induced vitamin D deficient mice showed the reduction in both grip strength and SERCA gene expression (Girgis et al., 2015). Skeletal muscle contraction is tightly associated with excitation‐contraction coupling in myofibers. The sarcoplasmic reticulum (SR), which releases Ca2+ via the ryanodine receptor during contraction and, during relaxation, takes it up by the SERCA pump, plays a major role in intracellular Ca2+ handling. Thus, muscle contraction/relaxation function is critically dependent on effective Ca2+ handling. For instance, in DM rats 8 weeks after the STZ‐injection, SERCA activity and isometric contractile force of the skeletal muscles are increased (Ganguly et al., 1986). To the contrary, high‐fat diet‐fed T2DM rats 12 weeks after the STZ‐injection showed the decrease in both SERCA gene expression and contractile performance of GA muscles (Safwat et al., 2013). In these rats, however, adiponectin gene therapy and/or swimming exercise induced the marked SERCA gene expression, and consequently improved muscle contractility (Safwat et al., 2013). Such a muscle dysfunction in T2DM appears to be mediated via abnormal Ca2+ handling due to impaired SERCA expression. In our OLETF group, grip strength was much reduced as compared with two other groups, but, contrary to our expectation, SERCA activity in the skeletal muscle was enhanced as reported in STZ‐induced DM rats (Ganguly et al., 1986) and T1DM patients (Harmer et al., 2014). Unphosphorylated phospholamban (PLB) inhibits the SERCA activity by lowering its apparent Ca2+ affinity. Phosphorylation of PLB by Ca2+/calmodulin kinase II relieves this inhibition and elicits Ca2+ uptake activity of SERCA (Vangheluwe et al., 2005). Calmodulin levels were elevated in the GA muscle from STZ‐induced DM mice and diabetic db/db mice (Morley et al., 1982). Therefore, it seems likely that the enhanced SERCA activity reported herein is ascribable to PLB phosphorylation induced by increased Ca2+/calmodulin kinase II. Similar to the reverse relation between SERCA activity and grip strength in our OLETF rats, hindlimb unweighting caused dramatic increases in SERCA1a activity in rat skeletal muscle, of which contractile performance deteriorated (Schulte et al., 1993). Such an enhanced SERCA activity may be a compensative response to the reduced muscle contractility.
Sixth, the reduced grip strength in DM state may be caused by failure in neuromuscular signal transmission, since the muscle‐nerve preparations of T2DM db/db mice displayed reduced axonal excitability and force deficit with indirect stimulation via the nerve compared with the control preparations (Bayley et al., 2016). This is supported by a negative correlation between isokinetic muscle strength at the ankle or knee and the neuropathy rank‐sum score in T1DM patients (Andersen et al., 1996). Such a muscle strength may be impaired by incomplete re‐innervation following axonal loss in DM patients with neuropathy (Andersen et al., 1998). Moreover, in DM patients, the gene expression of neurotrophin‐3 was reduced in striated muscles and was related to muscle weakness and neuropathy, suggesting that incomplete re‐innervation in DM state is caused by neurotrophin‐3 deficiency (Andreassen et al., 2009). On the other hand, muscles of STZ‐induced DM mice showed the desensitization of nicotinic acetylcholine receptor channels (Nojima et al., 1995). Moreover, such DM mice exhibited neuromuscular ultrastructure changes including severe loss of synaptic vesicles and degeneration of mitochondria at neuromuscular junctions, and, at the muscle level, swollen mitochondria with disorganization of their cristae and disruption of the t‐tubules (Fahim et al., 1998).
Last, the impaired grip strength in our OLETF group may be attributed to the defective energy transduction and/or the shortage of energy reserves in diabetic muscles. The cytosolic CK plays a pivotal role in anaerobic energy transduction. The cytosolic ATP concentration is maintained relatively constant primarily via a high‐energy phosphate transfer by CK to form ATP, that is, the rapid enzymatic transfer of a phosphate from phosphocreatine (PCr) to ADP to form ATP is catalyzed by CK. Thus, PCr can serve as a high‐energy reservoir to sustain the muscle contraction, especially in anaerobic quick intensive exercise such as grip strength test. In STZ‐induced DM rats, CK activity was decreased in SOL (Su et al., 1992) and EDL muscles (Xin et al., 1999), and, consequently, the intracellular ATP levels were reduced in diabetic muscle (Moore et al., 1983). Indeed, single myofibers from CK‐deficient mice displayed the reduced tetanic force during initial unfatigued period of electrical stimulation (Dahlstedt et al., 2000). A study using MRI spectroscopy demonstrated that the intramuscular PCr/inorganic phosphate (Pi) ratio, which is considered as an index of muscle energy reserve state, is lower in neuropathic DM patients than in the healthy subjects (Dinh et al., 2009). In fact, PCr‐depleted skeletal muscles exhibited the isometric contractile dysfunction in rats fed a diet containing the creatine analogue, β‐guanidinopropionic acid (Petrofsky & Fitch, 1980).
In view of these facts, the reduced grip strength in our OLETF group may have been caused by the above‐mentioned multiple mechanisms derived from DM‐related disorder or diabetic complications, though type IIb fiber atrophy may be the major direct cause of reduced grip strength.
In an attempt to evaluate muscle fatigue resistance, two types of mesh‐hanging test were carried out in this study. The diabetic OLETF group, as well as dietary AGE‐fed mice in the wire‐hanging test (Egawa et al., 2017), showed the shortest hanging time, indicating the reduced fatigue resistance. The rise in Pi resulting from the breakdown of PCr is thought to play a central role in skeletal muscle fatigue, of which the underlying mechanisms are as follows: increasing myoplasmic Pi reduces active crossbridge force, myofibrillar Ca2+ sensitivity, and SR Ca2+ release, leading to reduced force production (Allen & Westerblad, 2001). In the OLETF rats under the present hanging tests, this PCr breakdown in myoplasm would occur quickly, and, consequently, increasing myoplasmic Pi would cause the earlier grip‐release. Microinjection of CK into CK‐deficient mouse muscle fibers markedly restored both tetanic force and free myoplasmic Ca2+ concentration during a period of high‐intensity electrical stimulation, indicating that CK is important for preventing fatigue during high‐intensity stimulation (Dahlstedt et al., 2003). As mentioned above, CK activity was decreased in diabetic skeletal muscles (Su et al., 1992; Xin et al., 1999). Therefore, the increased muscle fatigability in our diabetic OLETF rats may have been due to decreased CK activity in muscle fibers.
This study has some limitations. First, sample size was small because the Kio University restricted the number of experimental animals based on 3 R principles (reduction, replacement, and refinement). Generalizing our data may be limited because of this modest sample size. Therefore, not only p values but effect sizes are presented herein to confirm whether the difference between groups is significant or not. The statistical analyses seem valid based on the values of effect size. However, studies with a larger sample size are required to further confirm our findings. Second, this study had neither baseline data of blood biomarkers and muscle morphological characteristics in young rats nor data of in vivo muscle function examined at regular intervals throughout the experimental period. These data will bring forth an optimal exercise "dose" for improving muscle function. Third, examination of the isometric contractile force in isolated EDL muscles and single EDL myofibers will further support the present positive effects of exercise on in vivo muscle function. Fourth, we have not measured muscle/myofiber atrophy‐related factors (IGF‐I and corticosterone), quick energy production‐related substances (CK and PCr), muscle force production‐related substances (AGEs and Pi), and neuropathy‐related neurotrophin‐3. The measurements of these substances are needed to better understand the overall picture of the mechanisms underlying reduced grip strength in DM state. Fifth, similar studies using other DM animal models, for example, STZ‐induced DM rodents, T2DM db/db mice, etc., are required for further confirming the data presented in this study. Sixth, rearing the rats individually for the long period of 17 months may have put the rats under some stress, because the rat is a sociable animal. Although the circulating adrenocorticotropic hormone and cortisol levels have not been measured in this study, all rats showed normal behavior and no hair loss, suggesting that they may have suffered from little or no stress. Finally, further studies, in which adult diabetic OLETF rats are subjected to exercise, are needed to examine improvable effects, but not preventive effects herein, of exercise on DM‐induced attenuation of grip strength.
5. CONCLUSION
The present results indicate that the long‐term wheel‐running can prevent the reduction of grip strength in the T2DM model OLETF rats. This exercise could also prevent the type IIb fiber atrophy, caused by diabetes, in EDL muscles. In addition, there was a good positive correlation between grip strength and CSA, total CSA or % total CSA in type IIb fibers. Therefore, type IIb fiber atrophy may be the major direct cause of grip strength reduction in diabetic OLETF rats, though there seems several other etiological mechanisms involving AGE‐induced protein modifications, potential inflammatory signaling pathway, abnormal Ca2+ handling, incomplete neuromuscular signal transmission and impaired energy transduction/reserves. Long‐term wheel‐running may have blocked the diabetes‐induced reduction of grip strength by preventing type IIb fiber atrophy. Regular physical exercise may be a potent modality for preventing not only the progression of diabetes but muscle dysfunction, like grip strength reduction, in T2DM patients.
CONFLICT OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS' CONTRIBUTIONS
Y.T., T.H., H.I., A.M., and S.S. conceived and designed research. Y.T., T.H., T. Yasui, D.T., M.A., S.K., T. Yamakami, K.O., H.W., S.O., A.M., T. Yamada, A.N., and S.S. performed experiments. Y.T., T. Yamada, A.N., and S.S. analyzed data. Y.T., H.I., T.N., S.T., T. Yamada, A.N., and S.S. interpreted results of experiments. Y.T. and S.S. prepared figures. Y.T. and S.S. drafted manuscript. Y.T., T.H., H.I., T. Yasui, D.T., M.A., S.K., T. Yamakami, K.O., H.W., S.O., A.M.,T.N., S.T., T. Yamada, A.N., and S.S. approved the final version of the manuscript.
ACKNOWLEDGMENT
The authors thank Dr. Y. Nishii in the Kio University for technical assistance.
Takada, Y., Hanaoka, T., Imagita, H., Yasui, T., Takeshita, D., Abe, M., Kawata, S., Yamakami, T., Okada, K., Washio, H., Okuda, S., Minematsu, A., Nakamura, T., Terada, S., Yamada, T., Nakatani, A., & Sakata, S. (2021). Long‐term wheel‐running prevents reduction of grip strength in type 2 diabetic rats. Physiological Reports, 9, e15046. 10.14814/phy2.15046
Funding information
This study was supported in part by Grant‐in‐Aid for Scientific Research (C) from Japan Society for the Promotion of Science (20590215 and 24590290) and also in part by a grant from the Kao Research Council for the Study of Health Science (A‐11007).
REFERENCES
- Adams, G. R., & McCue, S. (1998). Localized infusion of IGF‐I results in skeletal muscle hypertrophy in rats. Journal of Applied Physiology, 84, 1716–1722. 10.1152/jappl.1998.84.5.1716 [DOI] [PubMed] [Google Scholar]
- Allen, D. G., & Westerblad, H. (2001). Role of phosphate and calcium stores in muscle fatigue. Journal of Physiology, 536, 657–665. 10.1111/j.1469-7793.2001.t01-1-00657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, H., Gadeberg, P. C., Brock, B., & Jakobsen, J. (1997). Muscular atrophy in diabetic neuropathy: A stereological magnetic resonance imaging study. Diabetologia, 40, 1062–1069. 10.1007/s001250050788 [DOI] [PubMed] [Google Scholar]
- Andersen, H., Poulsen, P. L., Mogensen, C. E., & Jakobsen, J. (1996). Isokinetic muscle strength in long‐term IDDM patients in relation to diabetic complications. Diabetes, 45, 440–445. 10.2337/diab.45.4.440 [DOI] [PubMed] [Google Scholar]
- Andersen, H., Stålberg, E., Gjerstad, M. D., & Jakobsen, J. (1998). Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle and Nerve, 21, 1647–1654. [DOI] [PubMed] [Google Scholar]
- Andreassen, C. S., Jakobsen, J., Flyvbjerg, A., & Andersen, H. (2009). Expression of neurotrophic factors in diabetic muscle—relation to neuropathy and muscle strength. Brain, 132, 2724–2733. 10.1093/brain/awp208 [DOI] [PubMed] [Google Scholar]
- Balducci, S., Zanuso, S., Nicolucci, A., Fernando, F., Cavallo, S., Cardelli, P., Fallucca, S., Alessi, E., Letizia, C., Jimenez, A., Fallucca, F., & Pugliese, G. (2010). Anti‐inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutrition, Metabolism and Cardiovascular Diseases, 20, 608–617. 10.1016/j.numecd.2009.04.015 [DOI] [PubMed] [Google Scholar]
- Bark, T. H., McNurlan, M. A., Lang, C. H., & Garlick, P. J. (1998). Increased protein synthesis after acute IGF‐I or insulin infusion is localized to muscle in mice. American Journal of Physiology‐Endocrinology and Metabolism, 275(1), E118–E123. 10.1152/ajpendo.1998.275.1.E118 [DOI] [PubMed] [Google Scholar]
- Bassel‐Duby, R., & Olson, E. N. (2006). Signaling pathways in skeletal muscle remodeling. Annual Review of Biochemistry, 75, 19–37. 10.1146/annurev.biochem.75.103004.142622 [DOI] [PubMed] [Google Scholar]
- Bassuk, S. S., & Manson, J. E. (2005). Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. Journal of Applied Physiology, 99, 1193–1204. 10.1152/japplphysiol.00160.2005 [DOI] [PubMed] [Google Scholar]
- Bayley, J. S., Pedersen, T. H., & Nielsen, O. B. (2016). Skeletal muscle dysfunction in the db/db mouse model of type 2 diabetes. Muscle and Nerve, 54, 460–468. 10.1002/mus.25064 [DOI] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Cai, D., Frantz, J. D., Tawa, N. E., Melendez, P. A., Oh, B.‐C., Lidov, H. G. W., Hasselgren, P.‐O., Frontera, W. R., Lee, J., Glass, D. J., & Shoelson, S. E. (2004). IKKβ/NF‐κB activation causes severe muscle wasting in mice. Cell, 119, 285–298 [DOI] [PubMed] [Google Scholar]
- Chammas, M., Bousquet, P., Renard, E., Poirier, J.‐L., Jaffiol, C., & Allieu, Y. (1995). Dupuytren's disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. Journal of Hand Surgery, 20A, 109–114. 10.1016/S0363-5023(05)80068- [DOI] [PubMed] [Google Scholar]
- Chiu, C.‐Y., Yang, R.‐S., Sheu, M.‐L., Chan, D., Yang, T.‐H., Tsai, K.‐S., Chiang, C.‐K., & Liu, S.‐H. (2016). Advanced glycation end‐products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE‐mediated, AMPK‐down‐regulated, Akt pathway. Journal of Pathology, 238, 470–482. 10.1002/path.4674 [DOI] [PubMed] [Google Scholar]
- Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., & Malanda, B. (2018). IDF atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Reseach and Clinical Practice, 138, 271–281. [DOI] [PubMed] [Google Scholar]
- Coleman, M. E., DeMayo, F., Yin, K. C., Lee, H. M., Geske, R., Montgomery, C., & Schwartz, R. J. (1995). Myogenic vector expression of insulin‐like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. Journal of Biological Chemistry, 270, 12109–12116. 10.1074/jbc.270.20.12109 [DOI] [PubMed] [Google Scholar]
- Cotter, M., Cameron, N. E., Lean, D. R., & Robertson, S. (1989). Effects of long‐term streptozotocin diabetes on the contractile and histochemical properties of rat muscles. Quarterly Journal of Experimental Physiology, 74, 65–74. 10.1113/expphysiol.1989.sp003240 [DOI] [PubMed] [Google Scholar]
- Dahlstedt, A. J., Katz, A., Tavi, P., & Westerblad, H. (2003). Creatine kinase injection restores contractile function in creatine‐kinase‐deficient mouse skeletal muscle fibres. Journal of Physiology, 547, 395–403. 10.1113/jphysiol.2002.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt, A. J., Katz, A., Wieringa, B., & Westerblad, H. (2000). Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB Journal, 14, 982–990. 10.1096/fasebj.14.7.982 [DOI] [PubMed] [Google Scholar]
- Dehoux, M., van Beneden, R., Pasko, N., Lause, P., Verniers, J., Underwood, L., Ketelslegers, J.‐M., & Thissen, J.‐P. (2004). Role of the insulin‐like growth factor I decline in the induction of atrogin‐1/MAFbx during fasting and diabetes. Endocrinology, 145, 4806–4812. 10.1210/en.2004-0406 [DOI] [PubMed] [Google Scholar]
- Dekhuijzen, P. N. R., Gayan‐Ramirez, G., Bisschop, A., Bock, V. D., Dom, R., & Decramer, M. (1995). Corticosteroid treatment and nutritional deprivation cause a different pattern of atrophy in rat diaphragm. Journal of Applied Physiology, 78, 629–637. 10.1152/jappl.1995.78.2.629 [DOI] [PubMed] [Google Scholar]
- Derakhshanian, H., Javanbakht, M. H., Zarei, M., Djalali, E., & Djalali, M. (2017). Vitamin D increases IGF‐I and insulin levels in experimental diabetic rats. Growth Hormone and IGF Research, 36, 57–59. 10.1016/j.ghir.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Dinh, T., Doupis, J., Lyons, T. E., Kuchibhotla, S., Julliard, W., Gnardellis, C., Rosenblum, B. I., Wang, X., Giurini, J. M., Greenman, R. L., & Veves, A. (2009). Foot muscle energy reserves in diabetic patients without and with clinical peripheral neuropathy. Diabetes Care, 32, 1521–1524. 10.2337/dc09-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa, T., Tsuda, S., Goto, A., Ohno, Y., Yokoyama, S., Goto, K., & Hayashi, T. (2017). Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. British Journal of Nutrition, 117, 21–29. 10.1017/S0007114516004591 [DOI] [PubMed] [Google Scholar]
- Fahim, M. A., El‐Sabban, F., & Davidson, N. (1998). Muscle contractility decrement and correlated morphology during the pathogenesis of streptozotocin‐diabetic mice. Anatomical Record, 251, 240–244. [DOI] [PubMed] [Google Scholar]
- Farrell, P. A., Fedele, M. J., Hernandez, J., Fluckey, J. D., Miller, J. L.III, Lang, C. H., Vary, T. C., Kimball, S. R., & Jefferson, L. S. (1999). Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. Journal of Applied Physiology, 87, 1075–1082. 10.1152/jappl.1999.87.3.1075 [DOI] [PubMed] [Google Scholar]
- Flaim, K. E., Copenhaver, M. E., & Jefferson, L. S. (1980). Effects of diabetes on protein synthesis in fast‐ and slow‐twitch rat skeletal muscle. American Journal of Physiology‐Endocrinology and Metabolism, 239(1), E88–E95. 10.1152/ajpendo.1980.239.1.E88 [DOI] [PubMed] [Google Scholar]
- Fritschi, C., & Quinn, L. (2010). Fatigue in patients with diabetes: A review. Journal of Psychosomatic Research, 69, 33–41. 10.1016/j.jpsychores.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly, P. K., Mathur, S., Gupta, M. P., Beamish, R. E., & Dhalla, N. S. (1986). Calcium pump activity of sarcoplasmic reticulum in diabetic rat skeletal muscle. American Journal of Physiology‐Endocrinology and Metabolism, 251(5), E515–E523. 10.1152/ajpendo.1986.251.5.E515 [DOI] [PubMed] [Google Scholar]
- Girgis, C. M., Cha, K. M., Houweling, P. J., Rao, R., Mokbel, N., Lin, M., Clifton‐Bligh, R. J., & Gunton, J. E. (2015). Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcified Tissue International, 97, 602–610. 10.1007/s00223-015-0054-x [DOI] [PubMed] [Google Scholar]
- Grøntved, A., Pan, A., Mekary, R. A., Stampfer, M., Willett, W. C., Manson, J. E., & Hu, F. B. (2014). Muscle–strengthening and conditioning activities and risk of type 2 diabetes: A prospective study in two cohorts of US women. PLoS Med, 11, e1001587. 10.1371/journal.pmed.1001587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundmi, S., Maiya, A. G., Bhat, A. K., Ravishankar, N., Hande, M. H., & Rajagopal, K. V. (2018). Hand dysfunction in type 2 diabetes mellitus: Systematic review with meta‐analysis. Annals of Physical and Rehabilitation Medicine, 61, 99–104. 10.1016/j.rehab.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Harmer, A. R., Ruell, P. A., Hunter, S. K., McKenna, M. J., Thom, J. M., Chisholm, D. J., & Flack, J. R. (2014). Effects of type 1 diabetes, sprint training and sex on skeletal muscle sarcoplasmic reticulum Ca2+ uptake and Ca2+‐ATPase activity. Journal of Physiology, 592, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I. K., Yi, S. S., Yoo, K. Y., Park, O. K., Yan, B., Song, W., Won, M. H., Yoon, Y. S., & Seong, J. K. (2011). Effect of treadmill exercise on blood glucose, serum corticosterone levels and glucocorticoid receptor immunoreactivity in the hippocampus in chronic diabetic rats. Neurochemical Research, 36, 281–287. 10.1007/s11064-010-0315-z [DOI] [PubMed] [Google Scholar]
- Imagita, H., Sakata, S., Minematsu, A., Kanemura, N., Moriyama, H., Takemoto, H., & Mita, T. (2014). Effects of exercise on fiber properties in the denervated rodent diaphragm. American Journal of Biomedical and Life Sciences, 2, 141–145. 10.11648/j.ajbls.20140206.11 [DOI] [Google Scholar]
- Kawano, K., Hirashima, T., Mori, S., Saitoh, Y., Kurosumi, M., & Natori, T. (1992). Spontaneous long‐term hyperglycemic rat with diabetic complications. Otsuka long‐evans tokushima fatty (OLETF) strain. Diabetes, 41, 1422–1428. 10.2337/diab.41.11.1422 [DOI] [PubMed] [Google Scholar]
- Kim, J.‐H., Kwak, H.‐B., Leeuwenburgh, C., & Lawler, J. M. (2008). Lifelong exercise and mild (8%) caloric restriction attenuate age‐induced alterations in plantaris muscle morphology, oxidative stress and IGF‐1 in the Fischer‐344 rat. Experimental Gerontology, 43, 317–329. 10.1016/j.exger.2007.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriketos, A. D., Pan, D. A., Sutton, J. R., Hoh, J. F., Baur, L. A., Cooney, G. J., Jenkins, A. B., & Storlien, L. H. (1995). Relationships between muscle membrane lipids, fiber type, and enzyme activities in sedentary and exercised rats. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 269(5), R1154–R1162. 10.1152/ajpregu.1995.269.5.R1154 [DOI] [PubMed] [Google Scholar]
- Leenders, M., Verdijk, L. B., van der Hoeven, L., Adam, J. J., van Kranenburg, J., Nilwik, R., & van Loon, L. J. C. (2013). Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. Journal of American Medical Directors Association, 14, 585–592. 10.1016/j.jamda.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Liao, H., Zakhaleva, J., & Chen, W. (2009). Cells and tissue interactions with glycated collagen and their relevance to delayed diabetic wound healing. Biomaterials, 30, 1689–1696. 10.1016/j.biomaterials.2008.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, C. D., Chai, R., Radley‐Crabb, H. G., Watson, T., Matthews, K. G., Sheard, P. W., Soffe, Z., Grounds, M. D., & Shavlakadze, T. (2014). Lifelong exercise and locally produced insulin‐like growth factor‐1 (IGF‐1) have a modest influence on reducing age‐related muscle wasting in mice. Scandinavian Journal of Medicine and Science in Sports, 24, e423–e435. 10.1111/sms.12200 [DOI] [PubMed] [Google Scholar]
- Mikus, C. R., Rector, R. S., Arce‐Esquivel, A. A., Libla, J. L., Booth, F. W., Ibdah, J. A., Laughlin, M. H., & Thyfault, J. P. (2010). Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long‐Evans Tokushima fatty rats. Journal of Applied Physiology, 109, 1203–1210. 10.1152/japplphysiol.00064.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minematsu, A., Hanaoka, T., Takeshita, D., Takada, Y., Okuda, S., Imagita, H., & Sakata, S. (2017). Long‐term wheel‐running can prevent deterioration of bone properties in diabetes mellitus model rats. Journal of Musculoskeletal and Neuronal Interactions, 17, 433–443. [PMC free article] [PubMed] [Google Scholar]
- Momma, H., Niu, K., Kobayashi, Y., Guan, L., Sato, M., Guo, H., Chujo, M., Otomo, A., Yufei, C., Tadaura, H., Saito, T., Mori, T., Miyata, T., & Nagatomi, R. (2011). Skin advanced glycation end product accumulation and muscle strength among adult men. European Journal of Applied Physiology, 111, 1545–1552. 10.1007/s00421-010-1779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier, V. M., Vishwanath, V., Frank, K. E., Elmets, C. A., Dauchot, P., & Kohn, R. R. (1986). Relation between complications of type 1 diabetes mellitus and collagen‐linked fluorescence. New England Journal of Medicine, 314, 403–408. [DOI] [PubMed] [Google Scholar]
- Moore, R. D., Munford, J. W., & Pillsworth, T. J. (1983). Effects of streptozotocin diabetes and fasting on intracellular sodium and adenosine triphosphate in rat soleus muscle. Journal of Physiology, 338, 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley, J. E., Levine, A. S., Brown, D. M., & Handwerger, B. S. (1982). Calmodulin levels in diabetic mice. Biochemical and Biophysical Research Communications, 108, 1418–1423. 10.1016/S0006-291X(82)80065-X [DOI] [PubMed] [Google Scholar]
- Nachlas, M. M., Tsou, K.‐C., Souza, E. D., Cheng, C.‐S., & Seligman, A. M. (1957). Cytochemical demonstration of succinic dehydrogenase by the use of a new p‐nitrophenyl substituted ditetrazole. Journal of Histochemistry and Cytochemistry, 5, 420–436. 10.1177/5.4.420 [DOI] [PubMed] [Google Scholar]
- Nojima, H., Tsuneki, H., Kimura, I., & Kimura, M. (1995). Accelerated desensitization of nicotinic receptor channels and its dependence on extracellular calcium in isolated skeletal muscles of streptozotocin‐diabetic mice. British Journal of Pharmacology, 116, 1680–1684. 10.1111/j.1476-5381.1995.tb16391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostler, J. E., Maurya, S. K., Dials, J., Roof, S. R., Devor, S. T., Ziolo, M. T., & Periasamy, M. (2014). Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. American Journal of Physiology Endocrinology and Metabolism, 306, E592–E605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. W., Goodpaster, B. H., Lee, J. S., Kuller, L. H., Boudreau, R., de Rekeneire, N., Harris, T. B., Kritchevsky, S., Tylavsky, F. A., Nevitt, M., Cho, Y.‐W., & Newman, A. B. (2009). Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care, 32, 1993–1997. 10.2337/dc09-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, M. F., Stephenson, D. G., Kemp, J. G., & Stephenson, G. M. M. (2000). Ca2+‐activation characteristics of single fibres from chemically skinned rat muscle incubated with glucose‐6‐phosphate. Pflügers Archiv ‐ European Journal of Physiology, 439, 845–852. 10.1007/s004249900245 [DOI] [PubMed] [Google Scholar]
- Peter, J. B., Barnard, R. J., Edgerton, V. R., Gillespie, C. A., & Stempel, K. E. (1972). Metabolic profiles of three fiber types of skeletal muscle in guinea pig and rabbits. Biochemistry, 11, 2627–2633. [DOI] [PubMed] [Google Scholar]
- Petrofsky, J. S., & Fitch, C. D. (1980). Contractile characteristics of skeletal muscles depleted of phosphocreatine. Pflügers Archiv‐ European Journal of Physiology, 384, 123–129. 10.1007/BF00584427 [DOI] [PubMed] [Google Scholar]
- Phillips, T., & Leeuwenburgh, C. (2005). Muscle fiber‐specific apoptosis and TNF‐α signaling in sarcopenia are attenuated by life‐long calorie restriction. FASEB Journal, 19, 668–670. 10.1096/fj.04-2870fje [DOI] [PubMed] [Google Scholar]
- Ramamurthy, B., Höök, P., Jones, A. D., & Larsson, L. (2001). Changes in myosin structure and function in response to glycation. FASEB Journal, 15, 2415–2422. 10.1096/fj.01-0183com. [DOI] [PubMed] [Google Scholar]
- Rannels, S. R., & Jefferson, L. S. (1980). Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. American Journal of Physiology‐Endocrinology and Metabolism, 238(6), E564–E572. 10.1152/ajpendo.1980.238.6.E564 [DOI] [PubMed] [Google Scholar]
- Redmond, C. L., Bain, G. I., Laslett, L. L., & McNeil, J. D. (2009). Hand syndromes associated with diabetes: Impairments and obesity predict disability. Journal of Rheumatology, 36, 2766–2771. 10.3899/jrheum.090239 [DOI] [PubMed] [Google Scholar]
- Safwat, Y., Yassin, N., Din, M. G. E., & Kassem, L. (2013). Modulation of skeletal muscle performance and SERCA by exercise and adiponectin gene therapy in insulin‐resistant rat. DNA and Cell Biology, 32, 378–385. 10.1089/dna.2012.1919 [DOI] [PubMed] [Google Scholar]
- Sanchez, O. A., Snow, L. M., Lowe, D. A., Serfass, R. C., & Thompson, L. V. (2005). Effects of endurance exercise‐training on single‐fiber contractile properties of insulin‐treated streptozotocin‐induced diabetic rats. Journal of Applied Physiology, 99, 472–478. 10.1152/japplphysiol.01233.2004 [DOI] [PubMed] [Google Scholar]
- Schakman, O., Gilson, H., de Coninck, V., Lause, P., Verniers, J., Havaux, X., Ketelslegers, J. M., & Thissen, J. P. (2005). Insulin‐like growth factor‐I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid‐treated rats. Endocrinology, 146, 1789–1797. 10.1210/en.2004-1594 [DOI] [PubMed] [Google Scholar]
- Schakman, O., Gilson, H., Kalista, S., & Thissen, J. P. (2009). Mechanisms of muscle atrophy induced by glucocorticoids. Hormone Research, 72(suppl 1), 36–41. 10.1159/000229762 [DOI] [PubMed] [Google Scholar]
- Schulte, L. M., Navarro, J., & Kandarian, S. C. (1993). Regulation of sarcoplasmic reticulum calcium pump gene expression by hindlimb unweighting. American Journal of Physiology‐Cell Physiology. 10.1152/ajpcell.1993.264.5.C1308 [DOI] [PubMed] [Google Scholar]
- Shima, K., Shi, K., Sano, T., Iwami, T., Mizuno, A., & Noma, Y. (1993). Is exercise training effective in preventing diabetes mellitus in the Otsuka‐Long‐Evans‐Tokushima fatty rat, a model of spontaneous non‐insulin‐dependent diabetes mellitus? Metabolism, 42, 971–977. 10.1016/0026-0495(93)90009-D [DOI] [PubMed] [Google Scholar]
- Shima, K., Zhu, M., Noma, Y., Mizuno, A., Murakami, T., Sano, T., & Kuwajima, M. (1997). Exercise training in Otsuka Long‐Evans Tokushima Fatty rat, a model of spontaneous non‐insulin‐dependent diabetes mellitus: Effects on the B‐cell mass, insulin content and fibrosis in the pancreas. Diabetes Research and Clinical Practice, 35, 11–19. 10.1016/S0168-8227(96)01357-5 [DOI] [PubMed] [Google Scholar]
- Smith, O. L. K., Wong, C. Y., & Gelfand, R. A. (1989). Skeletal muscle proteolysis in rats with acute streptozotocin‐induced diabetes. Diabetes, 38, 1117–1122. [DOI] [PubMed] [Google Scholar]
- Snow, L. M., Fugere, N. A., & Thompson, L. V. (2007). Advanced glycation end‐product accumulation and associated protein modification in type II skeletal muscle with aging. Journal of Gerontology: Biological Sciences, 62A, 1204–1210. [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol, A., Christ‐Roberts, C., Berria, R., Eagan, P., Pratipanawatr, T., DeFronzo, R. A., Mandarino, L. J., & Musi, N. (2006). Reduced skeletal muscle inhibitor of κ,Bβ content is associated with insulin resistance in subjects with type 2 diabetes. Diabetes, 55, 760–767. [DOI] [PubMed] [Google Scholar]
- Stephenson, G. M. M., O'Callaghan, A., & Stephenson, D. G. (1994). Single‐fiber study of contractile and biochemical properties of skeletal muscles in streptozotocin‐induced diabetic rats. Diabetes, 43, 622–628. 10.2337/diab.43.5.622. [DOI] [PubMed] [Google Scholar]
- Su, C.‐Y., Payne, M., Strauss, A. W., & Dillmann, W. H. (1992). Selective reduction of creatine kinase subunit mRNAs in striated muscle of diabetic rats. American Journal of Physiology‐Endocrinology and Metabolism, 263(2), E310–E316. 10.1152/ajpendo.1992.263.2.E310 [DOI] [PubMed] [Google Scholar]
- Syrovỳ, I., & Hodnỳ, Z. (1992). Non‐enzymatic glycosylation of myosin: Effects of diabetes and aging. General Physiology and Biophysics, 11, 301–307. [PubMed] [Google Scholar]
- Syrovỳ, I., & Hodnỳ, Z. (1993). In vitro non‐enzymatic glycosylation of myofibrillar proteins. International Journal of Biochemistry, 25, 941–946. 10.1016/0020-711X(93)90251-9 [DOI] [PubMed] [Google Scholar]
- Takiguchi, S., Takata, Y., Funakoshi, A., Miyasaka, K., Kataoka, K., Fujimura, Y., Goto, T., & Kono, A. (1997). Disrupted cholecystokinin type‐A receptor (CCKAR) gene in OLETF rats. Gene, 197, 169–175. 10.1016/S0378-1119(97)00259-X [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangheluwe, P., Raeymaekers, L., Dode, L., & Wuytack, F. (2005). Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: Cell biological implications. Cell Calcium, 38, 291–302. 10.1016/j.ceca.2005.06.033 [DOI] [PubMed] [Google Scholar]
- Visser, M., Pahor, M., Taaffe, D. R., Goodpaster, B. H., Simonsick, E. M., Newman, A. B., Nevitt, M., & Harris, T. B. (2002). Relationship of interleukin‐6 and tumor necrosis factor‐α with muscle mass and muscle strength in elderly men and women: The health ABC study. Journal of Gerontology: Medical Sciences, 57A, M326–M332. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2009). Global health risks: Mortality and burden of disease attributable to selective major risks. World Health Organization. [Google Scholar]
- Xin, Z., Bassirat, M., Zeinab, K., & Helme, R. D. (1999). Effects of diabetes on creatine kinase activity in streptozotocin‐diabetic rats. Chinese Medical Jornal, 112, 1028–1031. [PubMed] [Google Scholar]
- Yamada, T., Inashima, S., Matsunaga, S., Nara, I., Kajihara, H., & Wada, M. (2004). Different time course of changes in sarcoplasmic reticulum and myosin isoforms in rat soleus muscle at early stage of hyperthyroidism. Acta Physiologica Scandinavica, 180, 79–87. 10.1046/j.0001-6772.2003.01220.x [DOI] [PubMed] [Google Scholar]
- Yin, F. C. P., Spurgeon, H. A., Rakusan, K., Weisfeldt, M. L., & Lakatta, E. G. (1982). Use of tibial length to quantify cardiac hypertrophy: Application in the aging rat. American Journal of Physiology‐Heart and Circulatory Physiology, 243(6), H941–H947. 10.1152/ajpheart.1982.243.6.H941 [DOI] [PubMed] [Google Scholar]


