Abstract
Stroke patients with spasticity usually require long-lasting care and interventions but frequently report that outpatient and community treatment is limited, reflecting a significant unmet need in health and social care provision. Rehabilitation and spasticity management services are essential for patient recovery, with improvements in both activity and participation reducing the burden on patients, family and society. Current clinical guidance provides scope for improvements in both post-stroke management and spasticity prevention. However, access to specialist services can be limited and the patient journey does not always match national recommendations. Identification of spasticity and its predictors and lack of subsequent referral to rehabilitation or specialist spasticity services are key issues in the management of post-stroke spasticity. Implementation of a traffic light classification system prioritises patients at an increased risk of spasticity and promotes early and consistent management across the spectrum of primary and secondary care. The proposed system is based on clinical evidence, expert consensus and recent clinical guidelines. It provides simple and straightforward criteria for management, multidisciplinary consultation and referral to specialist spasticity services, with patients allocated by monitoring requirements and a low (green/periodic monitoring), medium (amber/routine referral) or high risk (red/urgent referral) of spasticity.
Keywords: stroke, spasticity, guidelines, consensus, recommendation
Introduction
Stroke is the second most common cause of death and a leading cause of long-term disability in Europe and the World.1-3 Stroke survivors present with a high incidence of long-term disabilities and complications,4 with up to a third remaining physically dependent,1 and approximately half of all patients presenting with neurological impairment at 6 months.5 Although age-standardised rates of stroke mortality have decreased, the absolute annual incidence and burden is increasing.6
Spasticity is a motor and sensory disorder characterised by an intermittent or sustained increase in tonic stretch reflexes with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex.7,8 It is common after stroke, with the prevalence of motor impairments estimated at up to 80% in stroke survivors.3,9-13
Spasticity is estimated to occur in almost 25% of patients within 2 weeks post-stroke.14,15 However, after 12 months, the overall prevalence of spasticity increases to 38% in patients surviving a first stroke and 44% for those with recurrent stroke admissions.16 Severe or disabling spasticity has been reported in approximately 15% of post-stroke patients.10,17
Although a distinct problem, spasticity is also implicated in the formation of muscle contractures: loss of motion over time due to abnormal shortening of the soft tissue structures, restricting joint mobility and leading to pain and stiffness. Spasticity and muscle contractures are distinct issues, although spasticity is implicated in contracture formation and contractures may actually potentiate spasticity in some patients.18
While the natural history of spasticity and contracture development is highly variable, permanent loss of joint range is reported to occur within 2–6 weeks post-stroke11,12 peaking at 1-3 months;12 impairments may continue to develop over 3–6 months.3,12 Electromyography studies suggest that reflex-mediated increase in muscle spasticity reaches its maximum between 1 and 3 months after stroke.11
Spasticity is observed frequently in the upper extremities, developing commonly in the elbow (79%), wrist (66%) and shoulder (58%).12 The most frequent predictors of spasticity and contracture include weakness, reduced motor control and functional impairment.3,15 Risk of pain is often associated with reduced sensation, shoulder subluxation, weakness and stroke severity.3 In lower limbs, the prevalence of increased muscle tone and spasticity is significantly lower, potentially less severe and involves fewer muscles.15,19,20 Common lower limb involvement includes ankle plantar flexors, followed by hip adductors, knee extensors, knee flexors and hip internal rotators.19
Limitations in mobility immediately after stroke predict the incidence of pain at 3 months; pain is correlated with impaired upper extremity movement and function.21 The occurrence of shoulder pain is reported in up to 40% of patients but may be observed in as many as 90% of post-stroke patients with hemiplegia or those receiving rehabilitation.3 Impairment of daily functioning and early leg weakness have been identified as significant predictors of lower limb spasticity at 12 months post-stroke.22
Clinical study outcomes suggest that the highest incidence of pain appears within the first 6 months post-stroke, although it may initially occur as early as 1 week or at up to 16 months.3 One analysis reports that a majority of patients with shoulder pain at 4 months continued to report pain at 16 months.23 Post-stroke pain and spasticity are often observed as interlinked predictors of each other, with increases in pain associated with spasticity, and vice versa.
Spasticity in stroke is a substantial burden on patients, who often experience reductions in both their ability to perform daily living activities (active function), personal care tasks (passive function) and in health-related quality of life,17,24 and for carers, who exhibit poorer physical and emotional health compared with the general population.17 Furthermore, access to specialist spasticity services is often limited or non-existent, with patients waiting over 6 months for services and a majority discharged from secondary care without recommended therapy.1 Early identification and treatment of at-risk patients will increase their quality of care and help improve function, increase independence and avoid long-term complications.1,12,25
Intervention
Effective and timely intervention aims to increase functional abilities, improve personal care and impact on quality of life.12,26 Goals for intervention have been explored and evaluated extensively, and 6 categories have been identified through a series of studies. Goal categories can be assigned consistently into 2 domains, each subdivided into 3 key goal areas (Table 1).27-29
Table 1.
Intervention goal categories.9
| Domain 1: Symptom/impairment |
|---|
| A. Pain/discomfort |
| B. Involuntary movements |
| C. Range of movement/contracture prevention |
| Domain 2: Activities/function |
| A. Passive function (ease of caring for the affected limb) |
| B. Active function (using the affected limb in active tasks) |
| C. Mobility |
Rehabilitation is essential in facilitating patient recovery post-stroke (including management of spasticity), with the potential to make significant improvements in both activity and participation. Such improvements reduce the burden on patients, family and society, while also making significant long-term cost savings.2 However, decisions on rehabilitation requirements, including type, intensity and location, are usually made by multidisciplinary teams who must also manage resource pressures and the availability of community support and rehabilitation facilities.2 Stroke patients with spasticity usually require long-lasting care and interventions but frequently report that outpatient and community treatment is limited, reflecting a significant perceived unmet need in health and social care provision.24
Current treatment options for spasticity may include physical treatments such as long-duration stretch (such as limb casting or splinting to prevent the consequences of spasticity), positioning, exercise and pharmaceutical intervention including oral spasticity medication and focal treatments. Traditionally, spasticity has been managed in a sequential fashion.12 However, most clinicians in the United Kingdom currently adhere to recommendations from the Royal College of Physicians (RCP), applying a more systematic and tailored approach to reducing spasticity and combining physical treatments supported by pharmacotherapy.30
Oral spasticity medications generally have evidence for benefit, but individual response to treatment can be variable and is associated with systemic side effects.12 Regional treatments, such as intrathecal baclofen, have demonstrated efficacy in appropriately selected cases. A recent study in stroke patients with severe spasticity (Ashworth score ≥3 in ≥2 affected lower extremity muscle groups) demonstrated that intrathecal baclofen provided statistically significant treatment effects in pain and patient quality of life compared with oral antispastic therapy.31 Focal spasticity has demonstrated significant benefits following intramuscular injections of botulinum toxin therapy.30,32-34
The lack of high-quality research into pharmacological interventions to treat spasticity following stroke was clearly highlighted in a recent Cochrane review.35 With the exception of botulinum toxin injections, there was insufficient high-quality evidence to make conclusions about the effect of pharmacological interventions on post-stroke spasticity. Also, evidence suggests there is a significantly increased risk of adverse effects in patients receiving antispasmodics when compared with placebo, and particular care should be taken to monitor patients receiving such regimens.35
The ASPIRE (Adult Spasticity International Registry) study is a prospective, international observational study of spasticity patients treated with focal botulinum toxin injections.33 An interim, 1-year follow-up analysis suggests that 85% of patients (n = 731) and 93% of clinicians expressed extreme satisfaction/satisfaction that botulinum toxin injections was beneficial for relieving spasticity. Patients (91%) and physicians (98%) indicated they would definitely/probably continue botulinum toxin treatment for spasticity.36 Botulinum toxin injections have been associated with weakness, extremity pain, injection site reactions, localised pain, irritation and haemorrhage.37 Recent meta-analyses of stroke patients with spasticity suggest the frequency of adverse events is not significantly different to that observed with placebo.38,39
A recent literature review of botulinum toxin in combination with other treatment modalities suggests that extracorporeal shock wave therapy (single sonic pulses characterised by high peak pressure [100 MPa], fast pressure rise [< 10 ns] and short duration [10 ms]) provides improved post-injection outcomes compared with electrical stimulation, including spasticity and pain; electrical stimulation of injected muscles may boost the efficacy of botulinum toxin, although the most appropriate stimulation protocol has yet to be defined.40
Both adhesive taping and casting may improve the effect of botulinum toxin on limb spasticity, with casting providing better outcomes for spasticity, range of motion and gait. However, there is little current consensus over appropriate timing, duration, target or appropriate casting material.40
Guidelines
Implementing current evidence and guidance provides scope for improvements in both post-stroke management and secondary prevention.24 The RCP Stroke guidelines state that the whole stroke pathway should be incorporated when commissioning organisations generate a care portfolio.41 The whole stroke pathway includes hyperacute and acute care, secondary prevention and community or long-term rehabilitation. Stroke rehabilitation services should provide an inpatient stroke unit that delivers rehabilitation for all admitted stroke patients, specialist-supported early discharge for at-home or tertiary care rehabilitation and a service capable of delivering specialist rehabilitation in outpatient and community settings.41
Nonetheless, despite the availability of clinical guidelines directing post-stroke management and rehabilitation,30,42-44 the patient journey does not always match such national recommendations. Access to specialist services is limited: the National UK stroke audit showed that only 34% of post-acute stroke services offer specialist spasticity management.45 Over half of patients enrolled in the ASPIRE-S (Action on Secondary Prevention Interventions and Rehabilitation in Stroke) study experienced a delay in care before receiving community rehabilitation, with some waiting over 6 months for services; almost 60% did not receive the therapy recommended at discharge. The complex nature of stroke care results in patients having contact with many different services, providers and healthcare professionals at varying stages of stroke recovery and rehabilitation.1 Early identification and treatment of patients at risk of developing spasticity should improve physical function and increase independence while also increasing their quality of care.1,12 With regular assessments, patients benefit from earlier treatment and avoid long-term complications; this is especially pertinent for the most impaired patients and those with reduced access to specialist care.25
When stroke and rehabilitation services are not adequately resourced, patient transition between services and care providers is hampered, presenting a significant challenge to professionals, patients and their families/carers.1
Early assessment and management of post-stroke spasticity are critical for avoiding long-term complications. The SALGOT study, examining post-stroke upper limb spasticity, concluded that reduced sensorimotor function was the most important predictor for both spasticity and severe spasticity at 12 months post-stroke and that spasticity could be predicted with high sensitivity at 10 days post-stroke.25
Clinical experience suggests that implementation of recommended standard care varies widely across centres and services. To elucidate best care in spasticity management and rehabilitation, a panel of expert specialists met on several occasions in an Advisory Board capacity and subsequently created a consensus for recommended clinical practice.
Consensus Recommendations
Screening and Identification
The Expert Consensus panel agreed that identification of spasticity and its predictors and lack of subsequent referral to rehabilitation or specialist spasticity services are key issues, especially in stroke teams. It has been demonstrated that many post-stroke complications are either preventable or may be potentially ameliorated with treatment. While it may be argued (although with ongoing debate in this area) that stroke recovery attains a plateau after a certain time, long-term and late therapy can increase the functional status of patients. Thus, a coordinated and long-term management approach to post-stroke complications may provide benefits to some patients.24
Acute stroke teams may not commonly focus on spasticity signs or management. As such, post-stroke patients with symptoms of spasticity such as muscle stiffness are often managed by physiotherapists in the post-acute setting. There is usually a delay between identification of spasticity by physiotherapy clinicians and the process of obtaining an accompanying sign-off for spasticity referral.
Recommendation: if a post-stroke screening assessment indicates a risk of spasticity (e.g. if the results of an assessment show marked increase muscle tone, loss of range, a functional or care problem or the Modified Ashworth Scale (MAS) score46 is ≥2) the patient should be monitored and then immediately referred at the first sign of spasticity. Direct referral by physiotherapy clinicians is recommended to facilitate this process.
Before any intervention is undertaken to manage spasticity, it is important to assess its impact and severity.26 A number of grading scales have been used to quantify spasticity as a positive feature of upper motor neurone impairment, including the MAS and modified Tardieu scale (MTS). The MAS has acknowledged limitations in its measurement properties, particularly reliability and validity, but is used widely as it is relatively easy and quick to complete.46
Other measures of spasticity are used widely, such as flexor muscle stretch velocity,47 resistance to passive movement,48,49 range of movement50 and reflex threshold and measurement by electromyography (EMG).51,52 However, reliability and agreement between clinical scales varies widely by assessment and assessor,53 and there are often limitations in their utility within the realities of clinical practice.
A recent observational cohort study of stroke patients suggested that severe functional impairment (as suggested by high NIHSS, MRS and low MMSE, BMRC and BI scores) was associated with a high risk of developing PSS. Although many studies recommended a variety of clinical predictors for PSS, as yet there is no confirmation over which offer the most accurate prediction.15
The MTS considers both the range of motion measured during slow passive stretch (R2) and the angle of muscle reaction during fast passive stretch (R1); large and small differences between R2 and R1 indicate spasticity and muscle contracture, respectively. The MTS can be time-consuming to complete, but has tested, more robust validity, although possesses some similar challenges to MAS regarding reliability.54,55
It is also important to recognise that not every ‘tight’ muscle is spastic or may lead to spasticity or cause problems with activity or care even when spasticity is present. A clinically detectable increase in resistance to passive stretch may be due to spasticity, rigidity, muscle stiffness or a fixed muscle contracture.26 The Arm activity measure (ArmA) and Leg activity measure (LegA) have been developed for this purpose.56-60 ArmA and LegA help identify when spasticity is impacting on care (passive function), activity performance (active function) and, for the leg using the LegA, spasticity-related quality of life. They are patient- or carer-reported and quick to complete in busy clinical environments. Both measures were systematically developed with users and clinical experts and have undergone rigorous psychometric testing with robust validity and reliability outcomes.
Recommendation: a standardised method for early identification, such as the Arm and Leg activity measures, and assessing spasticity should be embedded in standard clinical routines.
Earlier assessment and prediction of post-stroke spasticity requires early identification of at-risk patients by healthcare professionals involved in the care of stroke patients.61 Patients with post-stroke complications tend to reside in secondary care for longer, presenting a significant opportunity for rehabilitation. Unfortunately, referral rates from both sub-acute care and rehabilitation services are generally low due to a paucity of understanding, low prioritisation of post-stroke spasticity and limitations in service provision. Understanding the predictive factors for spasticity is beneficial for patients requiring clinical intervention and is associated with improved long-term outcomes.62
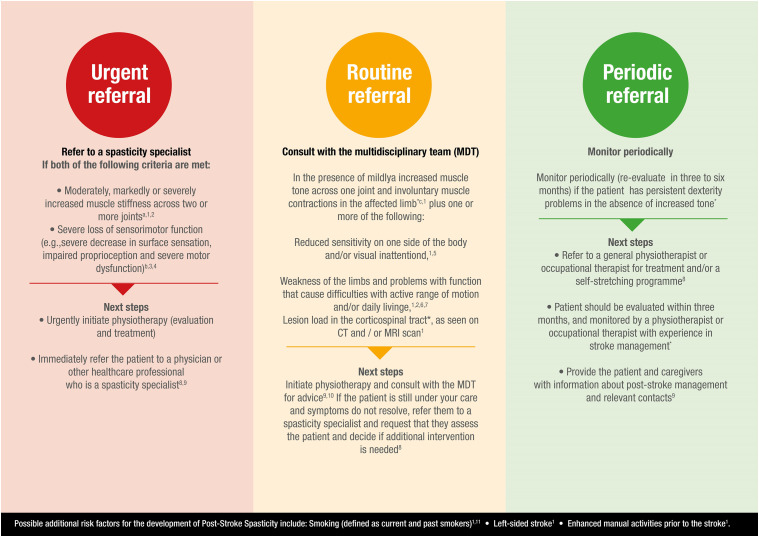
A system to aid this has been developed, categorising individuals into 3 levels of risk. These levels of risk are termed ‘traffic light’ categories and are graded low, medium and high risk (Figure 1).63
Figure 1.
Post-stroke spasticity risk classification system.63 ∗ Based on the clinical expertise of Dr Rhoda Allison, Dr Ganesh Bavikatte, Professor Philippe Marque, Associate Professor Barry Rawicki, Dr Maria Matilde de Mello Sposito, Dr Paul Winston and Professor Jörg Wissel. (a) Mildly increased muscle stiffness is a Modified Ashworth Scale (MAS) 1 or +1, while moderately is MAS 2, markedly is MAS 3 and severe is MAS 4∗ (cf Bohannon RW, et al 1987 for more information).46 (b) Measured using the Fugl-Meyer Upper Extremity Scale37 (cf Fugl-Meyer AR, et al 1975 for more information).73 (c) Muscle contractions may occur due to spasms, disturbed reciprocal inhibition or spastic dystonia and should be differentiated from contractures. (d) Visual inattention includes hemianopsia, scotoma or visual neglect. (e) Can be measured with the Barthel Index (low score) and EQ-5D (low score).61
Low risk patients should be monitored periodically if they have persistent dexterity problems in the absence of increased tone and re-evaluated after 3–6 months.
Medium risk patients present with moderately increased muscle stiffness at 1–14 days post-stroke (MAS score ≥ 146), left-sided weakness or paresis, involuntary muscle contractions, reduced sensitivity on one side of the body at 1–5 days post-stroke, left-sided visual inattention or extensive lesions in various areas, as seen on CT and/or MRI scan.61,64-68 Younger age at time of a stroke may also be considered a medium risk factor for spasticity development.25 Medium risk patients should be referred to the multidisciplinary team for assessment. If the symptoms do not resolve or are not possible to manage, they should be referred to a spasticity specialist to determine whether intervention is required.34
Patients at high risk of spasticity present with weakness, especially of the upper limb; problems with dexterity that cause difficulties with daily living, particularly issues with finger dexterity; markedly increased muscle stiffness in 1 joint (MAS score ≥ 246) or moderately increased muscle stiffness in ≥2 joints at 4–12 weeks post-stroke or reduced sensorimotor function at 3 days, 10 days and 4 weeks post-stroke.25,61 High risk patients with muscle stiffness should be directly referred to a spasticity specialist and immediately assessed for physiotherapy assessment.
Recommendation: patients should be monitored according to their risk of spasticity; a post-stroke spasticity ‘traffic light’ risk system has been developed to aid this (Figure 1).63
For the purposes of the traffic light model, low or ‘green’ risk patients have a lower-ranked association with spasticity development but may still be at some risk – they are not at a reduced risk of spasticity compared with patients without risk factors. A patient’s risk level is determined by their highest ranked risk factor.
Patients residing in the community or tertiary care centres are often the most vulnerable, with reduced access to spasticity clinics and physiotherapists for long-term monitoring. Such patients are at high risk of non-management.
Recommendation: use of a tool for assessing and identifying post-stroke spasticity. For example, the 11-point post-stroke quality of life checklist developed by the World Stroke Organization (WSO) is an aid to help stroke patients and healthcare professionals identify changes and problems in long-term community care patients (Table 2); it is designed to enable patient discussions with clinicians as part of a regular follow-up procedure.69
Table 2.
WSO post-stroke checklist.69
| 1. Secondary stroke prevention |
| 2. Activities of daily living |
| 3. Mobility |
| 4. Spasticity |
| 5. Pain |
| 6. Incontinence |
| 7. Communication |
| 8. Relationship with family |
| 9. Mood |
| 10. Cognition/thinking |
| 11. Life after stroke |
The missing 12th point from the WSO checklist is the impact of stroke on carers and family. Healthcare professionals should ensure carers have access to services that will help them provide the support required for the patient.69
Both patient and carer well-being are interdependent, with patient anxiety, depressive symptoms, cognitive impairment and quality of life being significant predictors of carer anxiety and depressive symptoms at 6 months post-stroke. Thus, early interventions, including increased training and support programmes that involve patient carers, may lower the risk or impact of negative quality of life components.70
Referral and Treatment
Early recognition of risk factors and immediate action is critical for spasticity identification and treatment. The traffic light classification system is a straightforward tool to prioritise patients at an increased risk of developing spasticity (Figure 1).63 A patient’s risk level is determined by the highest ranked risk factor, which should then determine the next steps for referral. The traffic light risk classification comprises recommendations from clinical evidence and expertise and recent clinical guidelines.
Low risk, ‘green’ patients should receive information about post-stroke spasticity, including symptoms that may develop; inclusion of relatives and carers in consultations often proves valuable. They should be provided with contact information in case development of spasticity is suspected71 and potentially be referred to a community physiotherapist for appropriate training.34,71
Recommendation: in low risk patients, ensure the patient receives periodic monitoring within the community. If community-based monitoring is not possible, enable patients and carers to monitor themselves and self-refer, if required.
Medium risk, ‘amber’ patients should be referred for a spasticity specialist consultation, ensuring the patient receives a follow-up appointment once a year with a specialist rehabilitation service.34 Provision of once-monthly treatment with a community physiotherapist is the target for clinical care,34 although this is rarely, if ever, achieved. Thus, patient or carer self-referral and identification may be a more pragmatic approach.
Recommendation: arrange regular primary care follow-up to monitor for spasticity development;72 patients should be reviewed at 6 weeks and then regular follow-up should occur within the first 6 months post-stroke. Physiotherapy should be initiated and multidisciplinary team consulted for advice.
High risk, ‘red’ patients should be referred for prompt evaluation by a spasticity assessor or a rehabilitation centre specialising in spasticity.71 The rehabilitation centre should have access to a multidisciplinary team and all available first-line treatments.34,71
Recommendation: liaise with the spasticity specialist to discuss a community-based support programme (e.g. weekly stretching under physiotherapist supervision).34
In practical terms, the traffic light classification system is suggested for use in both primary and secondary care by clinicians and physiotherapists. It is proposed for identifying spasticity risk in patients who have experienced a stroke, ideally within 12 weeks post-stroke, and should be used at regular follow-up visits.
Considerations
Spasticity is a physiological consequence of neurological injury, potentially causing profound disability in combination with other features of an upper motor neurone syndrome and significantly affecting the process of rehabilitation. Spasticity is often associated with restrictions in activity and care.26
Optimum management is dependent on an awareness of the natural history of spasticity, an appreciation of the impact on patients and a comprehensive approach to minimise that impact.26
Local commissioning organisations should ensure that the whole stroke pathway is delineated. A joined-up approach to the pathway is recommended to ensure continuity of patient care, which incorporates management of spasticity. Early identification of spasticity predictors is essential within the acute post-stroke care setting to enable early rehabilitation, supported discharge and secondary prevention referrals. Nonetheless, even when patients are identified and managed early, spasticity requires long-term community-based care and review, for both the patient and carer.
Patients will encounter various healthcare professionals during their care and rehabilitation, with varying levels of expertise and understanding of the needs of spasticity. Thus, we consider that a standardised method of assessing spasticity is highly desirable, ensuring a consistent level of assessment, referral and follow-up. Currently, spasticity management and continuity of care has little clinical ownership, lending to a general lack of accountability for any delay in action or treatment.
Management of patients with post-stroke spasticity is more effective when performed in a multidisciplinary setting, especially when there is clear communication and education between patients, carers and healthcare providers. It should be highlighted that spasticity responds well to treatment, dependent on the form and therapy applied.
Oral antispasmodic medications such as baclofen, tizanidine and dantrolene provide a systemic effect for generalised spasticity. They are most useful for more widespread spasticity of modest severity, but maximal efficacy may be limited by sedation, muscle weakness or liver toxicity.30 Regional spasticity responds well to intrathecal medication, while focal spasticity has demonstrated benefits following intramuscular injections of botulinum toxin therapy.32
Conclusions
In conclusion, early prediction of post-stroke spasticity will prioritise at-risk patients for assessment and management. Early identification and treatment should be embedded in clinical routines. The proposed traffic light system for identifying patients at an increased risk of spasticity will enable early and consistent management while offering simple and straightforward criteria for referral to specialist spasticity services.
Acknowledgements
Writing and editorial assistance was provided to the authors by Dr Allan Johnson of Jango Communications Limited, Bracknell, Berkshire, United Kingdom.
Footnotes
Declarations of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Conflicting interests: GB has received educational support and honoraria from Allergan, Ipsen and Merz. GS has received Speaker fees from Bayer, BI, BMS, Covedien, Grunenthal, Medtronic, MSD and Pfizer. Advisory board fees from Allergan, Amgen, Bayer, BI and Pfizer. Travel expenses from, Bayer, BI, Medtronic, Merc, MSD and Pfizer. He has no personal financial interest in any of the material mentioned in this article. SA has a specific interest in outcomes evaluation and has published on the use of Goal Attainment Scaling in this context, as well as a number of the other standardised measures such as the Arm and Leg activity measures. All of these tools are freely available. He has received educational support and honoraria from Allergan, Ipsen and Mertz, who manufacture botulinum toxin products and has received research grants from Ipsen. He has no personal financial interest in any of the material mentioned in this article. RA has received educational support and honoraria from Allergan, and educational support from Ipsen and Mertz, who all manufacture botulinum toxin products. She has no personal financial interest in any of the material mentioned in this article. DH has contributed to Allergan, Ipsen and Merz educational materials.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Allergan UK (now Abbvie Ltd) as part of an Educational Grant. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Guarantor: GB
Contributorship: All authors were involved in conception and development of the manuscript. GB produced the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Hall P, Williams D, Hickey A, et al. Access to rehabilitation at six months post stroke: A Profile from the action on secondary prevention interventions and rehabilitation in stroke (ASPIRE-S) study. Cerebrovasc Dis 2016;42:247-254. [DOI] [PubMed] [Google Scholar]
- 2.Enderby P, Pandyan A, Bowen A, et al. Accessing rehabilitation after stroke – a guessing game? Disabil Rehabil, 2017;39(7):709-713. [DOI] [PubMed] [Google Scholar]
- 3.Allison R, Shenton L, Bamforth K, et al. Incidence, time course and predictors of impairments relating to caring for the profoundly affected arm after stroke: A systematic review. Physiother Res Int 2016;21:210-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol 2010;9:105-118. [DOI] [PubMed] [Google Scholar]
- 5.Rohde D, Williams D, Gaynor E, et al. Secondary prevention and cognitive function after stroke: A study protocol for a 5-year follow-up of the ASPIRE-S cohort. BMJ Open 2017;7:e014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer L, Mellon L, Hall P, et al. Secondary prevention after ischaemic stroke: the ASPIRE-S study. BMC Neurol. 2015;15:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lance JW. Symposium synopsis. In: Feldman RG, Young PR, Koella WP, eds. Spasticity: Disordered Motor Control. Chicago, IL, USA: Year Book Medical Publishers, 1980: 485-494. [Google Scholar]
- 8.Pandyan AD, Gregoric M, Barnes MP, et al. Spasticity: Clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005;27:2-6. [DOI] [PubMed] [Google Scholar]
- 9.Sommerfeld DK, Gripenstedt U, Welmer A-K. Spasticity after stroke: An overview of prevalence, test instruments, and treatments. Am J Phys Med Rehabil 2012;91:814-820. [DOI] [PubMed] [Google Scholar]
- 10.Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology 2013;80:S13-S19. [DOI] [PubMed] [Google Scholar]
- 11.Sommerfeld DK, Eek E, Svensson A-K, et al. Spasticity after stroke. Its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134-140. [DOI] [PubMed] [Google Scholar]
- 12.Kuo C-L, Hu G-C. Post-stroke spasticity: A review of epidemiology, pathophysiology, and treatments. Int J Gerontol 2018;12:280-284. [Google Scholar]
- 13.Urban PP, Wolf T, Uebele M, et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41:2016-2020. [DOI] [PubMed] [Google Scholar]
- 14.Wissel J, Schelosky LD, Scott J, et al. Early development of spasticity following stroke: A prospective, observational trial. J Neurol 2010;257:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaess-Leistner S, Ri SJ, Audebert HJ, et al. Early clinical predictors of post-stroke spasticity. Top Stroke Rehabil 2020:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Watkins CL, Leathley MJ, Gregson JM, et al. Prevalence of spasticity post stroke. Clin Rehabil 2002;16:515-522. [DOI] [PubMed] [Google Scholar]
- 17.Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: Sequelae and burden on stroke survivors and caregivers. Neurology 2013;80:S45-S52. [DOI] [PubMed] [Google Scholar]
- 18.O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119(Pt 5):1737-1749. [DOI] [PubMed] [Google Scholar]
- 19.Spundik S, McCabe J, Skelly M, et al. Association of spasticity and motor dysfunction in chronic stroke. Annal Phys Rehab Med 2019;62:397-402. [DOI] [PubMed] [Google Scholar]
- 20.Katoozian L, Tahan N, Zoghi M, et al. The onset and frequency of spasticity after first ever stroke. J Natl Med Assoc 2018;110(6):547-552. [DOI] [PubMed] [Google Scholar]
- 21.Sommerfeld DK, Welmer A.-K.Pain following stroke, initially and at 3 and 18 months after stroke, and its association with other disabilities. Eur J Neurol. 2012;19(10):1325-1330. [DOI] [PubMed] [Google Scholar]
- 22.Martin A, Abogunrin S, Kurth H, et al. Epidemiological, humanistic, and economic burden of illness of lower limb spasticity in adults: a systematic review. Neuropsychiatric Dis Treat 2014:10;111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindgren I, Jonsson AC, Norrving B, et al. Shoulder pain after stroke: a prospective population-based study. Stroke 2007;38:343-348. [DOI] [PubMed] [Google Scholar]
- 24.Hotter B, Padberg I, Liebenau A, et al. Identifying unmet needs in long-term stroke care using in-depth assessment and the post-stroke checklist – the managing aftercare for stroke (MAS-I) study. Euro Stroke J 2018;3(3):237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opheim A, Danielsson A, Alt Murphy M, et al. Early prediction of long-term upper limb spasticity after stroke. Part of the SALGOT study. Neurology. 2015;85(10):873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavikatte G, Gaber T. Approach to spasticity in general practice. Br J Med Pract. 2009;2(3):29-34. [Google Scholar]
- 27.Ashford S, Siegert RJ, Williams H, et al. Categorisation of goals set using goal attainment scaling for treatment of leg spasticity: A multicentre analysis. Disabil Rehabil 2019;41(16):1925-1930. [DOI] [PubMed] [Google Scholar]
- 28.Ashford S, Fheodoroff K, Jacinto J, et al. Common goal areas in the treatment of upper limb spasticity: A multicentre analysis. Clin Rehabil 2016;30(6) 617-622. [DOI] [PubMed] [Google Scholar]
- 29.Turner-Stokes L, Ashford S, Jacinto J, et al. Impact of integrated upper limb spasticity management including botulinum toxin A on patient-centred goal attainment: Rationale and protocol for an international, prospective, longitudinal cohort study (ULIS III). BMJ Open 2016;6(6):e011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royal College of Physicians . Spasticity in adults: Management using botulinum toxin. National guidelines. London: RCP, 2018. Available at: rcplondon.ac.uk/guidelines-policy/spasticity-adults-management-using-botulinum-toxin. Accessed November 19, 2019. [Google Scholar]
- 31.Creamer M, Cloud G, Peter Kossmehl P, et al. Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. A randomized trial (SISTERS). Stroke. 2018;49:2129-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esquenazi A, Albanese A, Chancellor MB, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013;67:115-128. [DOI] [PubMed] [Google Scholar]
- 33.Bavikatte G, Francisco G, Bandari D, et al. The adult spasticity international registry (ASPIRE) study: Real-world treatment utilization and effectiveness of onabotulinumtoxin A in post-stroke patients treated for spasticity. Neurology 2018;90(15 Suppl). Abstract P5.048. [Google Scholar]
- 34.Wissel J, Ward AB, Erztgaard P, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med 2009;41:13-25. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay C, Kouzouna A, Simcox C, et al. Pharmacological interventions other than botulinum toxin for spasticity after stroke. Cochrane Database Syst Rev 2016;10(10):CD010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francisco G, Bandari D, Bavikatte G, et al. The adult spasticity international registry (ASPIRE) study: 1-year results (P4.034). Neurology 2017;88(16 suppl l):P4.034. [Google Scholar]
- 37.BOTOX® (botulinum toxin type A) summary of product characteristics. Available at: ema.europa.eu/en/documents/referral/botox-article-29-referral-annex-i-ii-iii_en.pdf. Accessed April 14, 2021.
- 38.Dong Y, Wu T, Hu X, et al. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: a systematic review with meta-analysis and trial sequential analysis. Eur J Phys Rehabil Med 2017;53(2):256-267. [DOI] [PubMed] [Google Scholar]
- 39.Sun LC, Chen R, Fu C, et al. Efficacy and safety of botulinum toxin type A for limb spasticity after stroke: A meta-analysis of randomized controlled trials. BioMed Res Int 2019;2019:8329306. doi: 10.1155/2019/8329306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picelli A, Santamato A, Chemello E, et al. Adjuvant treatments associated with botulinum toxin injection for managing spasticity: An overview of the literature. Annal Phys Rehab Med 2019;62:291-296. [DOI] [PubMed] [Google Scholar]
- 41.Royal College of Physicians Intercollegiate Working Party for Stroke guidelines. 5th ed, 2016.
- 42.National Institute for Health and Care Excellence . Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. NICE guideline ng128, 1 May 2019. Available at: nice.org.uk/guidance/ng128. Accessed October 8, 2019. [PubMed]
- 43.National Institute for Health and Care Excellence . Stroke rehabilitation in adults. NICE guideline ng162, 12 June 2013. Available at: nice.org.uk/guidance/cg162. Accessed October 8, 2019. [PubMed]
- 44.Scottish Intercollegiate Guidelines Network . Management of patients with stroke: Rehabilitation, prevention and management of complications, and discharge planning. Clinical guideline sign118, June 2010. Available at: sign.ac.uk/assets/sign118.pdf. Accessed October 08, 2019.
- 45.Sentinel Stroke National Audit Programme . Phase 2 – Post-acute stroke service provider audit. Available at: https://www.strokeaudit.org/results/PostAcute/National.aspx[accessed 06Feb2020].
- 46.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67(2):206-207. [DOI] [PubMed] [Google Scholar]
- 47.Pandyan AD, Van Wick FMJ, Stark S, et al. The construct validity of a spasticity measurement device for clinical practice: An alternative to the ashworth scales. Disabil Rehabil 2006;28(9):579-585. [DOI] [PubMed] [Google Scholar]
- 48.Pandyan AD, Price CIM, Rodgers H, et al. Biomechanical examination of a commonly used measure of spasticity. Clin BioMech 2001;16;859-865. [DOI] [PubMed] [Google Scholar]
- 49.Levin MF. On the nature and measurement of spasticity. Clin Neurophysiol 2005;116:1754-1755. [DOI] [PubMed] [Google Scholar]
- 50.Levin MF, Hiengkaew V, Nilanont Y, et al. Relationship between clinical measures of upper limb movement quality and activity poststroke. Neurorehabilitation Neural Repair 2019,33(6):432-441. [DOI] [PubMed] [Google Scholar]
- 51.Levin MF, Feldman AG, Mullick AA, et al. A new standard in objective measurement of spasticity. J Med Dev Trans ASME 2013;7(3):030909. [Google Scholar]
- 52.Levin MF, Solomon JM, Shah A, et al. Activation of elbow extensors during passive stretch of flexors in patients with post-stroke spasticity. Clin Neurophysiol 2018;129(10):2065-2074. [DOI] [PubMed] [Google Scholar]
- 53.Bhimani RH, Anderson LC, Henly SJ, et al. Clinical measurement of limb spasticity in adults: state of the science. J Neurosci Nurs. 2011;43(2):104-115. [DOI] [PubMed] [Google Scholar]
- 54.Morris S.Ashworth and Tardieu scales: Their clinical relevance for measuring spasticity in adult and pediatric neurological populations. Phys Ther Rev 2002;7:53-62. [Google Scholar]
- 55.Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol 1999;6(suppl 4):S23-S35. [Google Scholar]
- 56.Ashford S, Alexandrescu R, Siegert RJ. The arm activity measure (ArmA) passive function sub-scale conjoint measurement and ordinal scaling. Clin Rehabil 2016;30(7):714-718. [DOI] [PubMed] [Google Scholar]
- 57.Ashford S, Slade M, Siegert RJ, et al. Initial psychometric evaluation of the arm activity measure (ArmA): A measure of activity in the hemiparetic arm. Clin Rehabil. 2013;27(8):728-740. [DOI] [PubMed] [Google Scholar]
- 58.Ashford S, Slade M, Turner-Stokes L. Conceptualisation and development of the arm activity measure (ArmA) for assessment of activity in the hemiparetic arm. Disabil Rehabil. 2013;35(18):1513-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashford S, Siegert RJ, Williams H, et al. The leg activity measure (LegA), a measure of passive and active function and impact on quality of life in acquired brain injury for outcome evaluation in leg spasticity. Disabil Rehabil. 2019; Epubdoi/org/10.1080/09638288.2019.1643933. [Google Scholar]
- 60.Ashford S, Jackson D, Mahaffey P, et al. Conceptualisation and development of the leg activity measure (LegA) for patient and carer reported assessment of activity (function) in the paretic leg in people with acquired brain injury, Physiotherapy Reasearch International. 2017;22(2):e1660. [DOI] [PubMed] [Google Scholar]
- 61.Wissel J, Verrier M, Simpson DM, et al. Post-stroke spasticity: predictors of early development and considerations for therapeutic intervention. PMR. 2015;7(1):60-67. [DOI] [PubMed] [Google Scholar]
- 62.Sunnerhagen KS. Predictors of spasticity after stroke. Curr Phys Med Rehabil Rep 2016;4:182-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wissel J, Bavikatte G, de Mello Sposito M, et al. Development of an early identification tool in post-stroke spasticity (PSS): The PSS risk classification system. Arch Phys Med Rehabil 2020-11;101(11):e35. [Google Scholar]
- 64.Leathley MJ, Gregson JM, Moore AP, et al. Predicting spasticity after stroke in those surviving to 12 months. Clin Rehabil 2004;18:438-443. [DOI] [PubMed] [Google Scholar]
- 65.Wilkinson D, Sakel M, Camp SJ, et al. Patients with hemispatial neglect are more prone to limb spasticity, but this does not prolong their hospital stay. Arch Phys Med Rehabil 2012;93:1191-1195. [DOI] [PubMed] [Google Scholar]
- 66.Moura R, Fukujima MM, Aguiar AS, et al. Predictive factors for spasticity among ischemic stroke patients. Arq Neuropsiquiatr 2009;67:1029-1036. [DOI] [PubMed] [Google Scholar]
- 67.Picelli A, Tamburin S, Gajofatto F, et al. Association between severe upper limb spasticity and brain lesion location in stroke patients. BioMed Res Int 2014;2014:162754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung DK, Climans SA, Black SE, et al. Lesions characteristics of individuals with upper limb spasticity after stroke. Neurorehabil Neural Repair 2016;30:63-70. [DOI] [PubMed] [Google Scholar]
- 69.World Stroke Organization . The Post Stroke Checklist (PSC): Improving Life After Stroke. Available at: worldstrokecampaign.org/learn/the-post-stroke-checklist-psc-improving-life-after-stroke.html. Accessed September 19, 2019].
- 70.Atteih S, Mellon L, Hall P, et al. Implications of stroke for caregiver outcomes: findings from the ASPIRE-S study. Int J Stroke. 2015;10(6):918-923. [DOI] [PubMed] [Google Scholar]
- 71.NICE Pathways: Stroke overview, available at: pathways.nice.org.uk/pathways/stroke#path=view%3A/pathways/stroke/managing-movement-difficulties-after-a-stroke.xml&content=view-node%3Anodes-physiotherapy. Accessed October 2019.
- 72.Duncan PW, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke 2005;36:e100-e143. [DOI] [PubMed] [Google Scholar]
- 73.Fugl-Meyer AR, Jääskö L, Leyman Iet al. The post-stroke hemiplegic patient. Scand J Med 1975;7:13-31. [PubMed] [Google Scholar]