Abstract
Background: Mucin 5AC (MUC5AC) belongs to the glycoprotein family of secreted gel-forming mucins and is physiologically expressed in some epithelial cells. Studies have shown that MUC5AC is also expressed in several cancer types suggesting a potential utility for the distinction of tumor types and subtypes. Methods: To systematically determine MUC5AC expression in normal and cancerous tissues, a tissue microarray containing 10 399 samples from 111 different tumor types and subtypes as well as 608 samples of 76 different normal tissue types was analyzed by immunohistochemistry. Results: MUC5AC was expressed in normal mucus-producing cells of various organs. At least weak MUC5AC positivity was seen in 44 of 111 (40%) tumor entities. Of these 44 tumor entities, 28 included also tumors with strong positivity. MUC5AC immunostaining was most commonly seen in esophageal adenocarcinoma (72%), colon adenoma (62%), ductal adenocarcinoma of the pancreas (64%), mucinous carcinoma of the ovary (46%), diffuse gastric adenocarcinoma (44%), pancreatic ampullary adenocarcinoma (41%), intestinal gastric adenocarcinoma (39%), and bronchioloalveolar carcinoma (33%). Clinically relevant tumors with complete or almost complete absence of MUC5AC staining included small cell carcinoma of the lung (0% of 17), clear cell renal cell carcinoma (0% of 507), papillary thyroid carcinoma (0% of 359), breast cancer (2% of 1097), prostate cancer (2% of 228), soft tissue tumors (0.1% of 968), and hematological neoplasias (0% of 111). Conclusion: The highly standardized analysis of a broad range of cancers identified a ranking order of tumors according to their relative prevalence of MUC5AC expression.
Keywords: MUC5AC, multitumor tissue microarray, immunohistochemistry
Introduction
Mucin 5AC (MUC5AC) belongs to the subset of 5 secreted gel-forming mucins (MUC2, -5B, -5AC, -6, and -19) which are encoded by a gene cluster located at chromosome 11p15. MUC5AC is a glycoprotein with multiple cysteine-rich domains in both N- and C-terminal regions that are responsible for the formation of polymers, a critical feature for gel forming.1 The mucin layer protects the epithelial surfaces from chemical and mechanical damage as well as microbial pathogens, which are bound and subsequently removed by the mucociliary system.2,3 MUC5AC is normally expressed in epithelia of the upper and lower respiratory tract, stomach, and endocervix ,4–7 but it can be aberrantly expressed in a variety of cancers and their precursor lesions, including tumors arising from epithelia that are physiologically MUC5AC negative.8–14 There is growing evidence that MUC5AC expression in cancer cells may actively contribute to tumor aggressiveness. For example, it has been suggested that MUC5AC may interact with integrin β4 to facilitate lung cancer metastasis,15 enhance colorectal cancer tumorigenesis by deregulation of p53 and ß-catenin16 and repress apoptosis and cadherin-dependent cell adhesion in pancreatic cancer cells.17,18
Several previous studies have, moreover, shown that MUC5AC immunostaining could offer additional diagnostic information in various tumor types. For example, aberrant MUC5AC expression has been found in intraductal papillary mucinous neoplasia and pancreatic cancers, suggesting that MUC5AC can aid in assuring the diagnosis of pancreatic carcinomas,19 classifications of gastric polyps and Barrett metaplasia,20 or as a diagnostic tool in the dermapathology.21 It is also suggested that MUC5AC expression is helpful to distinguish between sessile serrated adenomas/polyps and hyperplastic polyps.22 Studies analyzing MUC5AC expression in cancers by immunohistochemistry (IHC) have described highly variable data. For example, the described frequency of positive MUC5AC immunostaining ranged from 29% to 100% in nonsmall-cell lung cancer,23,24 15% to 100% in lung adenocarcinoma,13,25 25% to 85% in gastric cancer,26,27 0% to 100% in ovarian carcinoma,9,28 28% to 80% of the ampulla Vater,29,30 or 14% to 54% in cervical carcinomas.12,31 Technical factors, such as staining protocols and antibodies used, different definitions of thresholds to determine positivity, as well as possible selection bias with respect to the analyzed tumors may have caused these discrepancies. To better understand the relative importance of MUC5AC expression in different cancer types and normal tissues, a comprehensive study analyzing many cancerous and noncancerous tissues under highly standardized conditions is desirable.
This study was thus designed to collect comparable data on the rate of MUC5AC expression in a broad range of different tissues. For this purpose, more than 10 000 tissue samples from 111 different tumor types and subtypes, and 76 nonneoplastic tissues were evaluated by IHC in a tissue microarray (TMA) format.
Material and Methods
Tissue Microarrays
To study MUC5AC expression in normal and neoplastic human tissues, we used a preexisting TMA containing 10 399 primary tumors from 111 tumor types and subtypes as well as 608 samples of 76 different normal tissues. All samples were derived from the archives of the Institute of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and the Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. TMA tissue spot diameter was 0.6 mm. The usage of archived diagnostic leftover tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Immunohistochemistry
Freshly cut TMA sections were immunostained on 1 day and in 1 experiment. Slides were deparaffinized and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in pH 7.8 Dako Target Retrieval Solution buffer (Dako). A primary antibody specific against MUC5AC protein (mouse monoclonal, MSVA-109, MS Validated Antibodies) was applied at 37 °C for 60 min at a dilution of 1:200. Bound antibody was then visualized using the EnVision Kit (Dako) according to the manufacturer's directions. For tumor tissues, the percentage of positive neoplastic cells was estimated, and the staining intensity was semiquantitatively recorded (0, 1+, 2+, 3+). For statistical analyses, the staining results were categorized into 4 groups. Tumors without any staining were considered to be negative. Tumors with 1+ staining intensity in ≤70% of cells or 2+ intensity in ≤30% of cells were considered weakly positive. Tumors with 1+ staining intensity in >70% of cells, 2+ intensity in 31% to 70%, or 3+ intensity in ≤30% were considered moderately positive. Tumors with 2+ intensity in >70% or 3+ intensity in >30% of cells were considered strongly positive.
Statistics
No statistical analysis was performed in this study. The list of tumor types and the fraction of MUC5AC positive samples per tumor type was generated using JMP 14 software (SAS Institute Inc.).
Results
Technical Issues
A total of 8028 (77.2%) of 10 399 tumor samples and >500 normal samples were interpretable in our TMA analysis. Noninterpretable samples demonstrated lack of unequivocal tumor cells.
MUC5AC in Normal Tissues
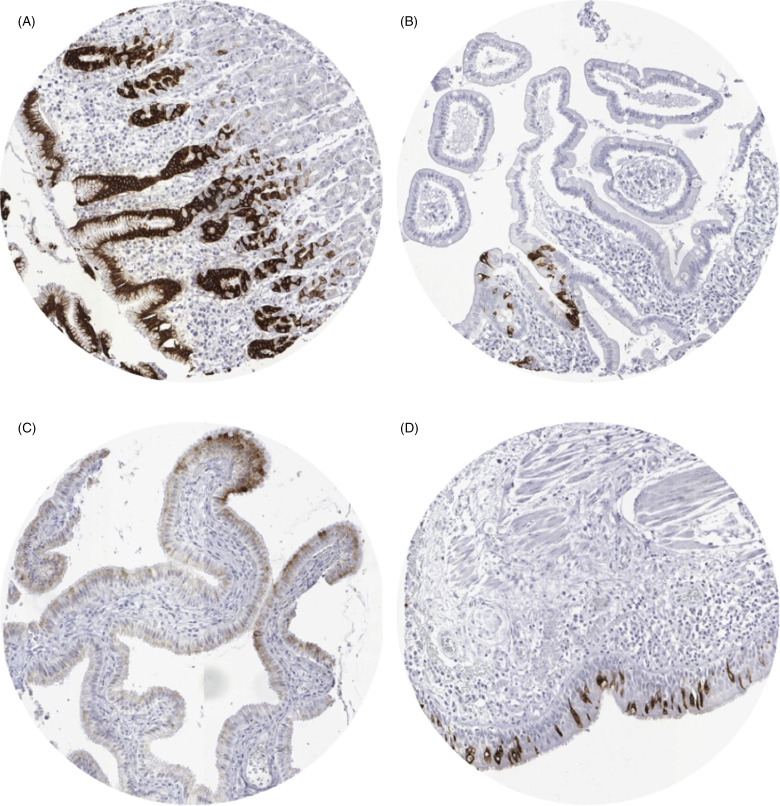
A strong (3+) cytoplasmic MUC5AC staining was found in 100% of the columnar cells of the stomach surface epithelium (Figure 1A), a small fraction of surface epithelial cells of the duodenum (Figure 1B), small intestine, appendix, and colon, columnar surface cells of the transitional epithelium of the anal canal, columnar cells of the gallbladder surface epithelium (2+, Figure 1C), and in goblet cells of the bronchial system (Figure 1D) and the paranasal sinuses (3+). In addition, moderate to strong MUC5AC staining was seen in normal appearing endocervical glands from 2 of 7 donors (2+). MUC5AC staining was absent in aorta/intima, aorta/media, heart (left ventricle), skeletal muscle, skeletal muscle/tongue, myometrium, muscular wall of the gastrointestinal (GI)-tract (appendix, esophagus, stomach, ileum, and colon descendens), muscular wall of the renal pelvis and bladder, glans of the penis, ovarian stroma, keratinocytes of the epidermis, sebaceous glands, lip, oral cavity, surface of the tonsil, epidermis of the anal canal, mucosa and submucosa of the esophagus, squamous epithelium of the ectocervix, urothelial cells of the bladder and renal pelvis, lamina propria of the bladder and renal pelvis, amnion/chorion, lymph nodes, spleen, thymus, tonsil, lamina propria of the duodenum, ileum, colon descendens, rectum, appendix and gallbladder, placental trophoblastic cells (cytotrophoblast and syncytiotrophoblast), decidua, gastric epithelial cells, enterocytes of the small and large intestine including appendix, hepatocytes, Kupffer cells, bile duct epithelium of the liver, pancreas, renal cortex and medulla, prostate, epididymis, mucosa and submucosa of the lung bronchi (except globlet cells), mucinous and/or serous epithelium as well as ductal cells of the salivatory glands (parotis, glandular submandibularis and glandular sublingualis), thyroid, sertoli cells, leydig cells, testicular germ cells, submucosal bronchial glands, bronchus epithelium, pneumocytes, epithelium of the paranasal sinus (except goblet cells), glandular and ductal epithelium of the breast, endometrium, fallopian tube, ovary (corpus luteum and follicular cyst), parathyroid gland, cortical cells of the adrenal gland, adrenal medullary cells, neuronal and glial cells of the cerebrum and cerebellum, cells of the neurohypophysis and adenohypophysis.
Figure 1.
Mucin 5AC (MUC5AC) staining in normal tissues. (A) Stomach, corpus, (B) duodenum (C) gallblader, (D) bronchial mucosa.
MUC5AC in Tumor Tissues
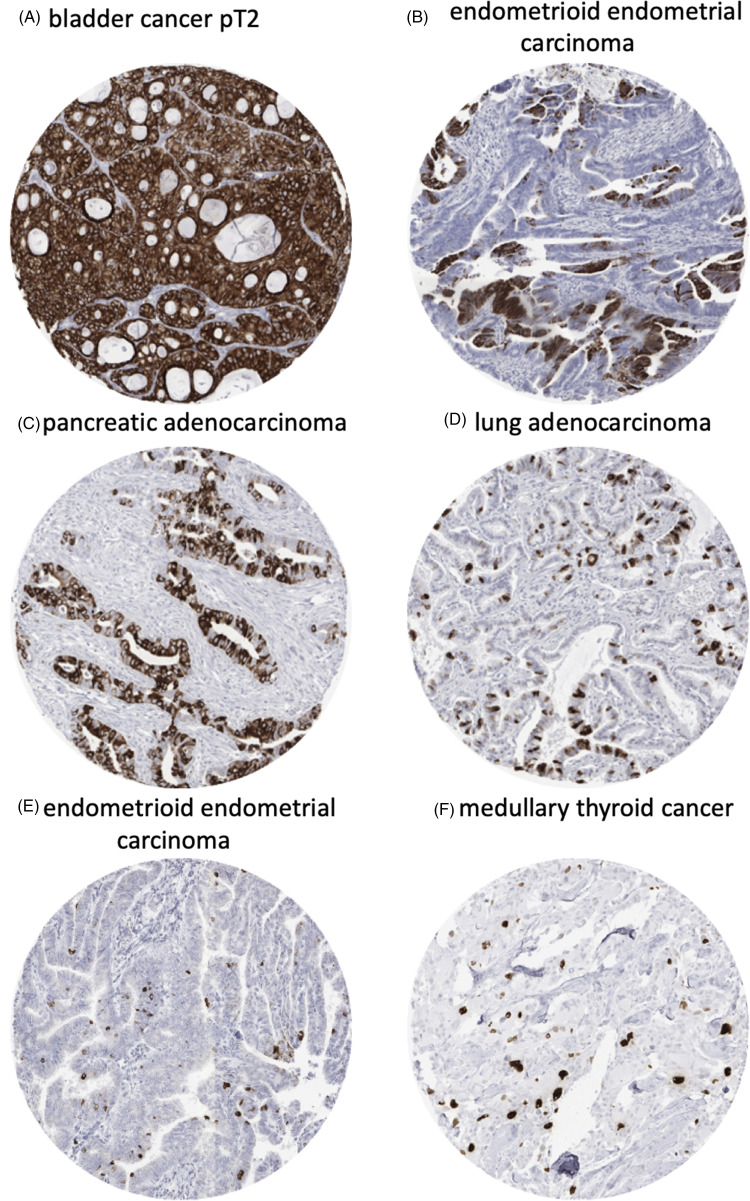
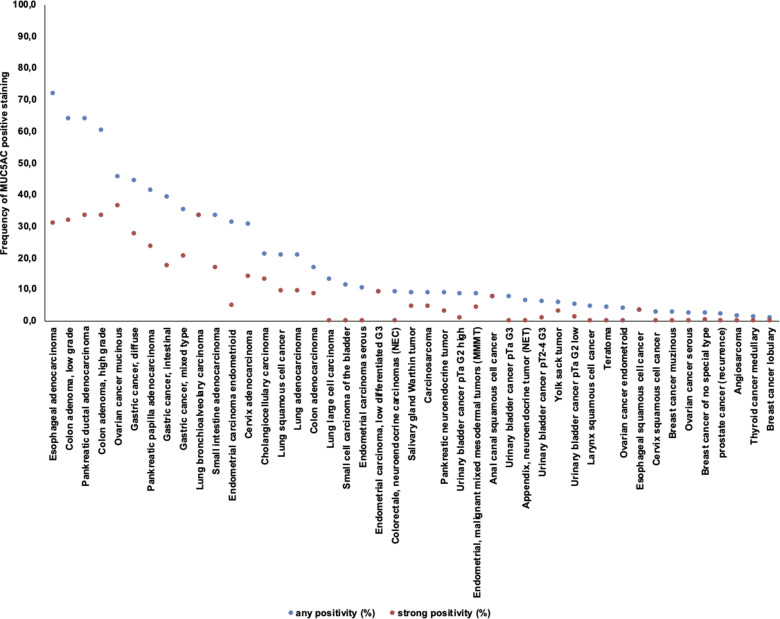
MUC5AC immunostaining was cytoplasmic and sometimes showed a tendency towards particular strong staining at the apical pole of tumor cells. Some tumors showed intense diffuse staining involving all cells while others showed patchy staining of either group of cells or even scattered individual cells. Representative images are given in Figure 2. In total, 540 (6.7%) of 8028 analyzable tumors showed positive immunostaining for MUC5AC (Table 1). MUC5AC immunostaining was considered weak in 152 (1.9%), moderate in 185 (2.3%), and strong in 203 (2.5%) of tumors. Overall, 44 of 111 (39.6%) tumor categories showed a detectable MUC5AC expression with 28 (25.2%) categories showing at least in a small proportion strong positivity. The categories with the highest rate of positive staining (33%-72%) included adenocarcinomas of various types, primarily from the GI tract, the female genital tract, and the lung. These tumor types also showed the highest fractions of tumors with strong positivity (17%-31%). Important tumor types with low or absent MUC5AC immunostaining included sarcomas, lymphomas, endocrine tumors, renal cancer, breast cancer, prostate cancer, and various skin tumors. A graphical representation of ranking order of MUC5AC positive and strongly positive cancers is given in Figure 3.
Figure 2.
Mucin 5AC (MUC5AC) staining in different tumor types and subtypes. (A) Bladder cancer pT2, (B) endometrioid endometrial carcinoma, (C) pancreatic adenocarcinoma, (D) lung adenocarcinoma, (E) endometrioid endometrial carcinoma, (F) medullary thyroid cancer.
Table 1.
MUC5AC Immunostaining in Human Tumors.
| Tumor entity | n on TMA | MUC5AC immunostaining | ||||||
|---|---|---|---|---|---|---|---|---|
| n analyzable | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | Positive (%) | |||
| Tumors of the skin | Pilomatrixoma | 35 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Basal cell carcinoma | 48 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Benign nevus | 29 | 22 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the skin | 50 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Malignant melanoma | 48 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Merkel cell carcinoma | 46 | 42 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 50 | 43 | 95.3 | 0.0 | 4.7 | 0.0 | 4.7 |
| Oral squamous cell carcinoma (floor of the mouth) | 50 | 42 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pleomorphic adenoma of the parotid gland | 50 | 42 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Warthin tumor of the parotid gland | 49 | 45 | 91.1 | 2.2 | 2.2 | 4.4 | 8.9 | |
| Basal cell adenoma of the salivary gland | 15 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the lung, pleura and thymus | Squamous-cell carcinoma of the lung | 250 | 192 | 79.2 | 2.6 | 8.9 | 9.4 | 20.8 |
| Large-cell carcinoma of the lung | 31 | 23 | 87.0 | 13.0 | 0.0 | 0.0 | 13.0 | |
| Adenocarcinoma of the lung | 250 | 192 | 79.2 | 2.6 | 8.9 | 9.4 | 20.8 | |
| Bronchioloalveolar carcinoma | 6 | 6 | 66.7 | 16.7 | 0.0 | 16.7 | 33.3 | |
| Small-cell carcinoma of the lung | 20 | 17 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Malignant mesothelioma | 39 | 39 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mesothelioma, other types | 76 | 65 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Thymoma | 29 | 27 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 48 | 37 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Squamous cell carcinoma of the vulva | 50 | 33 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the cervix | 50 | 38 | 97.4 | 2.6 | 0.0 | 0.0 | 2.6 | |
| Adenocarcinoma of the cervix uteri | 50 | 36 | 69.4 | 13.9 | 2.8 | 13.9 | 30.6 | |
| Endometrioid endometrial carcinoma | 236 | 210 | 69.0 | 11.9 | 14.3 | 4.8 | 31.0 | |
| Endometrial serous carcinoma | 82 | 58 | 89.7 | 8.6 | 1.7 | 0.0 | 10.3 | |
| Endometrial MMMT | 28 | 24 | 91.7 | 4.2 | 0.0 | 4.2 | 8.3 | |
| Endometrial carcinoma, low differentiated G3 | 13 | 11 | 90.9 | 9.1 | 0.0 | 0.0 | 9.1 | |
| Endometrial clear cell carcinoma | 8 | 4 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Endometrial stromal sarcoma | 12 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Endometrioid carcinoma of the ovary | 37 | 26 | 96.2 | 3.8 | 0.0 | 0.0 | 3.8 | |
| Serous carcinoma of the ovary | 50 | 41 | 97.6 | 0.0 | 2.4 | 0.0 | 2.4 | |
| Mucinous carcinoma of the ovary | 26 | 22 | 54.5 | 4.5 | 4.5 | 36.4 | 45.5 | |
| Brenner tumor | 9 | 7 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 1311 | 854 | 97.7 | 0.2 | 2.0 | 0.1 | 2.3 |
| Lobular carcinoma of the breast | 214 | 131 | 99.2 | 0.0 | 0.8 | 0.0 | 0.8 | |
| Medullary carcinoma of the breast | 26 | 22 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tubular carcinoma of the breast | 27 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mucinous carcinoma of the breast | 58 | 39 | 97.4 | 0.0 | 2.6 | 0.0 | 2.6 | |
| Phyllodes tumor of the breast | 50 | 36 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 22 | 36.4 | 18.2 | 13.6 | 31.8 | 63.6 |
| Adenomatous polyp, high-grade dysplasia | 50 | 30 | 40.0 | 16.7 | 10.0 | 33.3 | 60.0 | |
| Adenocarcinoma of the colon | 50 | 36 | 83.3 | 0.0 | 8.3 | 8.3 | 16.7 | |
| Adenocarcinoma of the small intestine | 10 | 6 | 66.7 | 0.0 | 0.0 | 33.3 | 33.3 | |
| Gastric adenocarcinoma, diffuse type | 146 | 113 | 55.8 | 8.0 | 8.8 | 27.4 | 44.2 | |
| Gastric adenocarcinoma, intestinal type | 144 | 115 | 60.9 | 16.5 | 5.2 | 17.4 | 39.1 | |
| Gastric adenocarcinoma, mixed type | 62 | 54 | 64.8 | 9.3 | 5.6 | 20.4 | 35.2 | |
| Adenocarcinoma of the esophagus | 50 | 39 | 28.2 | 23.1 | 17.9 | 30.8 | 71.8 | |
| Squamous cell carcinoma of the esophagus | 49 | 31 | 96.8 | 0.0 | 0.0 | 3.2 | 3.2 | |
| Squamous cell carcinoma of the anal canal | 50 | 26 | 92.3 | 0.0 | 0.0 | 7.7 | 7.7 | |
| Cholangiocarcinoma | 120 | 100 | 79.0 | 1.0 | 7.0 | 13.0 | 21.0 | |
| Hepatocellular carcinoma | 50 | 48 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Ductal adenocarcinoma of the pancreas | 50 | 33 | 36.4 | 24.2 | 6.1 | 33.3 | 63.6 | |
| Pancreatic/Ampullary adenocarcinoma | 30 | 17 | 58.8 | 11.8 | 5.9 | 23.5 | 41.2 | |
| GIST | 50 | 34 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the urinary system | Noninvasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 177 | 94.9 | 2.3 | 1.7 | 1.1 | 5.1 |
| Noninvasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 141 | 91.5 | 3.5 | 4.3 | 0.7 | 8.5 | |
| Noninvasive papillary urothelial carcinoma, pTa G3 | 187 | 187 | 92.5 | 2.1 | 5.3 | 0.0 | 7.5 | |
| Urothelial carcinoma, pT2 to 4 G3 | 940 | 727 | 93.9 | 2.3 | 2.9 | 0.8 | 6.1 | |
| Small-cell neuroendocrine carcinoma of the bladder | 18 | 18 | 88.9 | 0.0 | 11.1 | 0.0 | 11.1 | |
| Sarcomatoid urothelial carcinoma | 25 | 25 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Clear cell renal cell carcinoma | 858 | 507 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Papillary renal cell carcinoma | 255 | 152 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 10 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Chromophobe renal cell carcinoma | 131 | 94 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Oncocytoma | 177 | 109 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate | 49 | 46 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Adenocarcinoma of the prostate (recurrence) | 330 | 182 | 97.8 | 1.1 | 1.1 | 0.0 | 2.2 | |
| Small-cell neuroendocrine carcinoma of the prostate | 17 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Seminoma | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Embryonal carcinoma of the testis | 50 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Yolk sack tumor | 50 | 35 | 94.3 | 0.0 | 2.9 | 2.9 | 5.7 | |
| Teratoma | 50 | 24 | 95.8 | 4.2 | 0.0 | 0.0 | 4.2 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 114 | 106 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Papillary thyroid carcinoma | 392 | 359 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Follicular thyroid carcinoma | 158 | 148 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Medullary thyroid carcinoma | 107 | 83 | 98.8 | 0.0 | 1.2 | 0.0 | 1.2 | |
| Anaplastic thyroid carcinoma | 45 | 37 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical adenoma | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Adrenal cortical carcinoma | 26 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Phaeochromocytoma | 50 | 40 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Appendix, NET | 22 | 16 | 93.8 | 0.0 | 6.3 | 0.0 | 6.3 | |
| Ileum, NET | 49 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Lung, NET | 19 | 19 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pancreas, NET | 102 | 85 | 96.5 | 0.0 | 2.4 | 1.2 | 3.5 | |
| Colorectal, NET | 10 | 10 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| gallbladder, NET | 4 | 4 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pancreas, NEC | 13 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Colorectal, NEC | 11 | 11 | 90.9 | 0.0 | 0.0 | 9.1 | 9.1 | |
| Tumors of haemotopoetic and lymphoid tissues | Hodgkin lymphoma | 45 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Non-Hodgkin lymphoma | 48 | 41 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of soft tissue and bone | Tenosynovial giant cell tumor | 45 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Leiomyoma | 50 | 44 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyosarcoma | 87 | 83 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Liposarcoma | 132 | 117 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Angiosarcoma | 73 | 64 | 98.4 | 0.0 | 1.6 | 0.0 | 1.6 | |
| Angiomyolipoma | 91 | 91 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Dermatofibrosarcoma protuberans | 21 | 21 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 14 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Granular cell tumor | 53 | 48 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 8 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| MPNST | 13 | 13 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Myofibrosarcoma | 26 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Neurofibroma | 117 | 117 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Sarcoma, NOS | 75 | 75 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Paraganglioma | 41 | 41 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| PNET | 23 | 23 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 7 | 7 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Schwannoma | 121 | 121 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Synovial sarcoma | 12 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Osteosarcoma | 43 | 27 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Osteosarcoma | 39 | 33 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
Abbreviations: MUC5AC, mucin 5AC; MMMT, malignant mixed Müllerian tumor; GIST, gastrointestinal stromal tumor; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; MPNST, malignant peripheral nerve sheath tumor; NOS, not otherwise specified; PNET, primitive neuroectodermal tumor.
Figure 3.
Ranking order of Mucin 5AC (MUC5AC) immunostaining in tumors. Both the frequency of positive cases (blue dots) and the frequency of strongly positive cases (orange dots) are shown.
Discussion
Our comprehensive analysis of >100 tumor entities identified 44 tumor types and subtypes that at least occasionally express MUC5AC. The highest rates of positivity were seen for colonic adenomas (62%), adenocarcinomas of the esophagus (72%), pancreas (64%) and the stomach (44%) as well for mucinous carcinoma of the ovary (46%). Most other cancer types with glandular differentiation, such as endometrium cancer, adenocarcinoma of the cervix uteri, adenocarcinoma of the lung and cholangiocarcinoma were also MUC5AC positive in a fraction of cases. In principle, these findings are concordant with the literature, as several earlier reports have already described most of these tumor entities to express MUC5AC.9,10,13,14,19,23,25,28,32–37 However, the large number of analyzed tumor entities in our study allowed us to identify 22 tumor categories that can express MUC5AC but were never described to do so earlier. These included for example small-cell carcinomas of the urinary bladder, neuroendocrine tumor of the pancreas, appendix and colon as well as squamous cell carcinomas of the anal canal and the larynx. MUC5AC expression was also seen in 1 case each of angiosarcoma and medullary thyroid carcinoma.
The vast majority of MUC5AC positive cancers were derived from organs where at least some cells normally express MUC5AC. This particularly applies to carcinomas arising from the stomach, lung, and cervix uteri, where the physiologic expression of MUC5AC is well known.2,8,12 MUC5AC expression is also seen in few scattered epithelial cells of the normal colon as well as in columnar cells that can cover the anal transitional epithelium and the urothelium. It is possible that the same mechanisms that drive MUC5AC expression in these specialized cells also apply for MUC5AC expression in some tumors derived from these organs. It is of note that—irrespective of a cancer origin—patterns of MUC5AC expression often contain a limited number of strongly positive cells being interspersed between negative cancer cells in a fairly regular way (mosaic pattern). MUC5AC expression is also seen in carcinomas arising from tissues that completely lack positive MUC5AC immunostaining under physiological circumstances. In these tissues, MUC5AC expression may serve as a useful surrogate marker of neoplastic transformation. For example, the nonneoplastic pancreas does not express MUC5AC, while MUC5AC expression can be detected in most pancreatic adenocarcinomas and even in its precursor lesions as early as low-grade PanINs.38 The same applies to ovarian mucinous carcinomas.28 Studies have shown that the putative underlying mechanisms of MUC5AC neo-expression include promotor hypomethylation.11 MUC5AC transcription is also directly and indirectly regulated by various cancer-associated transcription factors (eg AP-1, SP-1, NF-kB, HNF-5a-alpha) and pathways (eg MAPK, Akt/PI3K)39–41 as well as by several mediators of inflammation (IL-1-beta, IL-6, TNF-alpha, IL-13).41–44
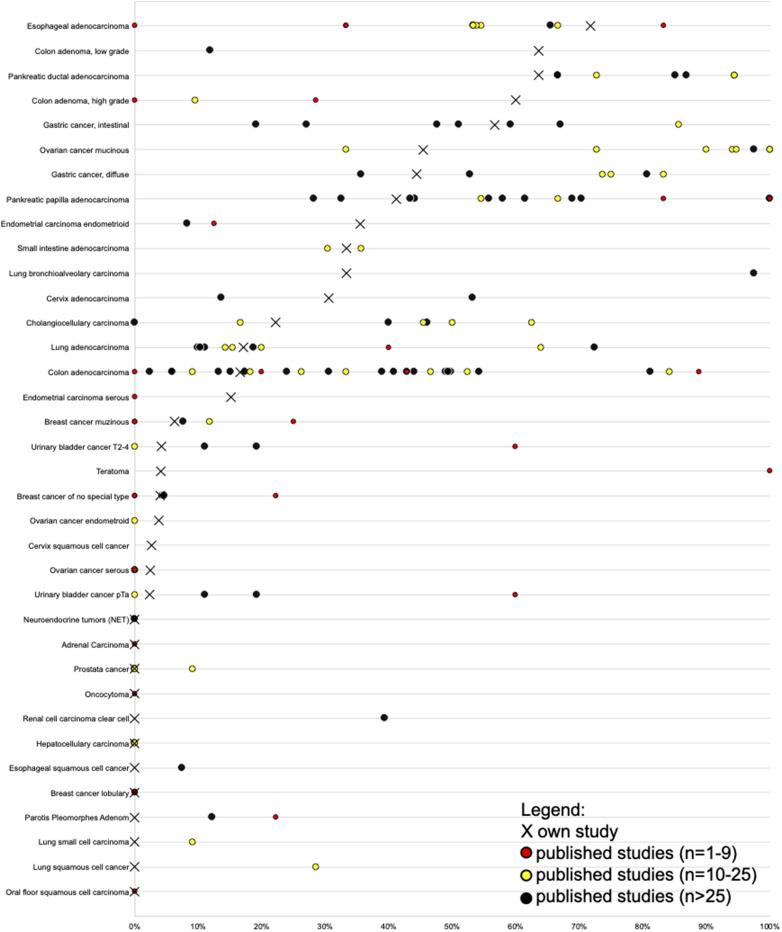
Tumor types that are always MUC5AC negative are of diagnostic interest, because a positive MUC5AC immunostaining will virtually exclude such entities from diagnostic considerations. In the present analysis, 67 of 111 (60%) analyzed tumor types and subtypes did never show MUC5AC immunostaining. These tumor categories are the least likely to be the cause of a MUC5AC positive metastasis. Clinically relevant tumors with complete or almost complete absence of MUC5AC staining included small cell carcinoma of the lung (0% of 17), papillary thyroid carcinoma (0% of 359), breast cancer (2% of 1097), prostate cancer (2% of 228), soft tissue tumors (0.1% of 968), hematological neoplasias (0% of 111), and clear cell renal cell carcinoma (0% of 507). That the only earlier study analyzing MUC5AC by IHC in clear cell renal cell carcinoma had identified 39% positive cases45 illustrates the generally low concordance rate between IHC studies performed in different laboratories. A summary of published IHC data on MUC5AC expression is depicted in Figure 4 for all cancer types.
Figure 4.
Graphical comparison of Mucin 5AC (MUC5AC) data from this study (x) in comparison with the previous literature. Red dots are used for studies involving 1 to 9 cases, yellow dots are used for studies involving 10 to 25 cases, and black dots are used for studies involving >25 cases.
Our highly standardized analysis of 111 human tumor types and subtypes resulted in a ranking order according to the prevalence of MUC5AC expression (Figure 3). These data also show that most cancer types with infrequent MUC5AC positivity were considered “low expressors.” As in medullary thyroid carcinoma (Figure 2F), this tumor often shows few scattered MUC5AC (highly) positive cancer cells in an otherwise clearly negative tumor. Importantly, all absolute numbers and prevalences described in this study are specific to the reagents and the protocol used in our laboratory. It appears certain that the discrepant results between different studies describing MUC5AC IHC data are mainly due to the use of different commercially available antibodies, staining protocols and criteria for interpretation of staining. It is well known that different antibodies designed for the same target protein will vary markedly in their binding properties.46–53 This also applies to very common and important targets. For example, we have earlier shown the impact of different antibodies targeting epidermal growth factor receptor (EGFR) gene on the outcome EGFR analysis across human tumor types.54 Moreover, PD-L1 expression is routinely tested by IHC in cancers that are being considered for immune checkpoint-inhibitor therapy. The utilized PD-L1 antibodies are highly characterized but vary considerably in their binding properties. This results in a significant staining variability for PD-L1 antibodies both within tumor cells and nonneoplastic immune and stroma cells.55
That MUC5AC is broadly expressed across different tumor entities limits its use in the distinction of cancers of different origins. The occurrence of relevant subgroups of MUC5AC positive and negative tumors within many clinically relevant cancer types raises, however, the question of a possible prognostic or predictive role of MUC5AC expression. Several studies have indeed suggested a possible link of MUC5AC positivity to patient outcome in clear cell renal cell cancer,45 colorectal cancer,14 adenocarcinoma of the cervix,31 intrahepatic cholangiocarcinoma,56 gastric cancer,32 and nonsmall-cell lung cancer.57 Other established or proposed diagnostic applications include the distinction of serrated adenomas from hyperplastic polyps in the colon,22 the classification of gastric polyps and Barrett metaplasia20 as well as a parameter for malignancy in pancreatic biopsies.19 Moreover, high serum levels of MUC5AC have earlier been reported for patients with MUC5AC positive pancreatic adenocarcinomas, colorectal cancer, cholangiocarcinoma, gastric cancer, and biliary tract cancer.58–63 Serum MUC5AC measurement of patients with MUC5AC positive cancers could be utilized for detection of recurrence and measuring response to therapy. A limitation of the study could be the missing evaluability of about 22% of the tumor samples. However, a statistical bias, which could potentially result from the exclusion of noninterpretable samples, is highly unlikely in our study as noninterpretable samples were evenly distributed across all pathological diagnoses.
Conclusions
In summary, our data provide a systematic overview of MUC5AC expression in human cancers using a standardized approach across all tissues and demonstrate potential diagnostic applications. Given a positivity rate of >60% in pancreatic cancer and a complete absence of MUC5AC in normal pancreatic tissue, MUC5AC IHC may be most useful for supporting a diagnosis of pancreatic cancer.
Acknowledgments
The authors are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for their excellent technical assistance.
Footnotes
Authors’ Notes: SDR, TK, AHM, RS, and GS designed the study. MM, FB, DD, AML, AH, DH, CMK, CF, KM, AM, CB, PL, TSC, RU, WW, SS, SM, and EB performed the immunohistochemical analyses and/or contributed to the pathological validation of the tumors, the TMA construction, and data collection. MK, CHM, and RS carried out the data analyses. GS, RS, SDR, TK, AHM, and MK wrote the first draft of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to the published, and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Institute of Pathology of the UKE receives royalties on the sale of MUC5AC clone MSVA-109 from MS Validated Antibodies GmbH (owned by a family member of GS).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The usage of archived diagnostic leftover tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
ORCID iDs: Katharina Möller https://orcid.org/0000-0002-9739-4274
Ronald Simon https://orcid.org/0000-0003-0158-4258
References
- 1.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70(1):459-486. doi: 10.1146/annurev.physiol.70.113006.100702 [DOI] [PubMed] [Google Scholar]
- 2.Van de Bovenkamp JH, Mahdavi J, Korteland-Van Male AM, et al. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter. 2003;8(5):521-532. doi: 10.1046/j.1523-5378.2003.00173.x [DOI] [PubMed] [Google Scholar]
- 3.Perez-Vilar J, Randell SH, Boucher RC. C-Mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology. 2004;14(4):325-337. doi: 10.1093/glycob/cwh041 [DOI] [PubMed] [Google Scholar]
- 4.Jumblatt MM, McKenzie RW, Jumblatt JE. MUC5AC Mucin is a component of the human precorneal tear film. Invest Ophthalmol Vis Sci. 1999;40(1):43-49. [PubMed] [Google Scholar]
- 5.Moniaux N, Escande F, Porchet N, et al. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6(1):D1192-D1206. doi: 10.2741/moniaux [DOI] [PubMed] [Google Scholar]
- 6.Porchet N, Pigny P, Buisine MP, et al. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem Soc Trans. 1995;23(4):800-805. doi: 10.1042/bst0230800 [DOI] [PubMed] [Google Scholar]
- 7.Severn TL, Hutton DA, Sama A, et al. Expression of MUC2 and MUC5AC by human nasal mucosa. Biochem Soc Trans. 1997;25(1):2S. doi: 10.1042/bst025002s [DOI] [PubMed] [Google Scholar]
- 8.Adler KB, Tuvim MJ, Dickey BF. Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne). 2013;4:129. doi: 10.3389/fendo.2013.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albarracin CT, Jafri J, Montag AG, et al. Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. Hum Pathol. 2000;31(6):672-677. doi: 10.1053/hupa.2000.6799 [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay P, Chakraborty S, Ponnusamy MP, et al. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815(2):224-240. doi: 10.1016/j.bbcan.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pothuraju R, Krishn SR, Gautam SK, et al. Mechanistic and functional shades of mucins and associated glycans in colon cancer. Cancers (Basel). 2020;12(3):649. doi: 10.3390/cancers12030649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riethdorf L, O'Connell JT, Riethdorf S, et al. Differential expression of MUC2 and MUC5AC in benign and malignant glandular lesions of the cervix uteri. Virchows Arch. 2000;437(4):365-371. doi: 10.1007/s004280000273 [DOI] [PubMed] [Google Scholar]
- 13.Shang G, Jin Y, Zheng Q, et al. Histology and oncogenic driver alterations of lung adenocarcinoma in Chinese. Am J Cancer Res. 2019;9(6):1212-1223. [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Zuo D, Liu T, et al. Prognostic and clinicopathological significance of MUC family members in colorectal cancer: a systematic review and meta-analysis. Gastroenterol Res Pract. 2019;2019:2391670. doi: 10.1155/2019/2391670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakshmanan I, Rachagani S, Hauke R, et al. MUC5AC Interactions with integrin beta4 enhances the migration of lung cancer cells through FAK signaling. Oncogene. 2016;35(31):4112-4121. doi: 10.1038/onc.2015.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pothuraju R, Rachagani S, Krishn SR, et al. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol Cancer. 2020;19(1):37. doi: 10.1186/s12943-020-01156-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshi H, Sawada T, Uchida M, et al. MUC5AC Protects pancreatic cancer cells from TRAIL-induced death pathways. Int J Oncol. 2013;42(3):887-893. doi: 10.3892/ijo.2013.1760 [DOI] [PubMed] [Google Scholar]
- 18.Inaguma S, Kasai K, Ikeda H. GLI1 Facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene. 2011;30(6):714-723. doi: 10.1038/onc.2010.459 [DOI] [PubMed] [Google Scholar]
- 19.Narkhede RA, Desai GS, Prasad PP, et al. Diagnosis and management of pancreatic adenocarcinoma in the background of chronic pancreatitis: core issues. Dig Dis. 2019;37(4):315-324. doi: 10.1159/000496507 [DOI] [PubMed] [Google Scholar]
- 20.Niv Y, Fass R. The role of mucin in GERD and its complications. Nat Rev Gastroenterol Hepatol. 2011;9(1):55-59. doi: 10.1038/nrgastro.2011.211 [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43(11):1005-1016. doi: 10.1111/cup.12782 [DOI] [PubMed] [Google Scholar]
- 22.Khaidakov M, Lai KK, Roudachevski D, et al. Gastric proteins MUC5AC and TFF1 as potential diagnostic markers of colonic sessile serrated adenomas/polyps. Am J Clin Pathol. 2016;146(5):530-537. doi: 10.1093/ajcp/aqw142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HK, Kwon MJ, Seo J, et al. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in ALK-positive lung cancer: comparison with EGFR-mutated lung cancer. Pathol Res Pract. 2019;215(3):459-465. doi: 10.1016/j.prp.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 24.Wakata K, Tsuchiya T, Tomoshige K, et al. A favourable prognostic marker for EGFR mutant non-small cell lung cancer: immunohistochemical analysis of MUC5B. BMJ Open. 2015;5(7):e008366. doi: 10.1136/bmjopen-2015-008366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Ferrer A, Curull V, Barranco C, et al. Mucins as differentiation markers in bronchial epithelium. Squamous cell carcinoma and adenocarcinoma display similar expression patterns. Am J Respir Cell Mol Biol. 2001;24(1):22-29. doi: 10.1165/ajrcmb.24.1.4294 [DOI] [PubMed] [Google Scholar]
- 26.Koyama T, Sekine S, Taniguchi H, et al. Hepatocyte nuclear factor 4A expression discriminates gastric involvement by metastatic breast carcinomas from primary gastric adenocarcinomas. Hum Pathol. 2011;42(11):1777-1784. doi: 10.1016/j.humpath.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 27.Silva EM, Begnami MD, Fregnani JH, et al. Cadherin-catenin adhesion system and mucin expression: a comparison between young and older patients with gastric carcinoma. Gastric Cancer. 2008;11(3):149-159. doi: 10.1007/s10120-008-0468-5 [DOI] [PubMed] [Google Scholar]
- 28.Sugai M, Umezu H, Yamamoto T, et al. Expression of hepatocyte nuclear factor 4 alpha in primary ovarian mucinous tumors. Pathol Int. 2008;58(11):681-686. doi: 10.1111/j.1440-1827.2008.02293.x [DOI] [PubMed] [Google Scholar]
- 29.Higashi M, Goto M, Saitou M, et al. Immunohistochemical study of mucin expression in periampullary adenomyoma. J Hepatobiliary Pancreat Sci. 2010;17(3):275-283. doi: 10.1007/s00534-009-0176-5 [DOI] [PubMed] [Google Scholar]
- 30.Lee MJ, Lee HS, Kim WH, et al. Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol. 2003;16(5):403-410. doi: 10.1097/01.MP.0000067683.84284.66 [DOI] [PubMed] [Google Scholar]
- 31.Mitsuhashi A, Yamazawa K, Nagai Y, et al. Correlation between MUC5AC expression and the prognosis of patients with adenocarcinoma of the uterine cervix. Ann Surg Oncol. 2004;11(1):40-44. doi: 10.1007/BF02524344 [DOI] [PubMed] [Google Scholar]
- 32.Zhang CT, He KC, Pan F, et al. Prognostic value of Muc5AC in gastric cancer: a meta-analysis. World J Gastroenterol. 2015;21(36):10453-10460. doi: 10.3748/wjg.v21.i36.10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song S, Byrd JC, Guha S, et al. Induction of MUC5AC mucin by conjugated bile acids in the esophagus involves the phosphatidylinositol 3-kinase/protein kinase C/activator protein-1 pathway. Cancer. 2011;117(11):2386-2397. doi: 10.1002/cncr.25796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Che N, Liu Z, et al. MUC5AC Enhances tumor heterogeneity in lung adenocarcinoma with mucin production and is associated with poor prognosis. Jpn J Clin Oncol. 2020;50(6):701–711. doi: 10.1093/jjco/hyaa016 [DOI] [PubMed] [Google Scholar]
- 35.Sasaki M, Nakanuma Y, Ho SB, et al. Cholangiocarcinomas arising in cirrhosis and combined hepatocellular-cholangiocellular carcinomas share apomucin profiles. Am J Clin Pathol. 1998;109(3):302-308. doi: 10.1093/ajcp/109.3.302 [DOI] [PubMed] [Google Scholar]
- 36.Hebbar V, Damera G, Sachdev GP. Differential expression of MUC genes in endometrial and cervical tissues and tumors. BMC Cancer. 2005;5(1):124. doi: 10.1186/1471-2407-5-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Shin N, Kim GH, et al. Mucin expression in gastric cancer: reappraisal of its clinicopathologic and prognostic significance. Arch Pathol Lab Med. 2013;137(8):1047-1053. doi: 10.5858/arpa.2012-0193-OA [DOI] [PubMed] [Google Scholar]
- 38.Ohya A, Yamanoi K, Shimojo H, et al. Gastric gland mucin-specific O-glycan expression decreases with tumor progression from precursor lesions to pancreatic cancer. Cancer Sci. 2017;108(9):1897-1902. doi: 10.1111/cas.13317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wulfert S, Schilasky S, Krueger S. Transcriptional and biochemical characterization of cytosolic pyruvate kinases in arabidopsis thaliana. Plants (Basel). 2020;9(3):353. doi: 10.3390/plants9030353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serratrice G, Bille F, Pouget J, et al. Exophthalmos in atrophic polychondritis. 2 cases. Presse Med. 1987;16:968. [PubMed] [Google Scholar]
- 41.Van Seuningen I, Pigny P, Perrais M, et al. Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front Biosci. 2001;6:D1216-D1234. doi: 10.2741/seuning [DOI] [PubMed] [Google Scholar]
- 42.Qin Y, Jiang Y, Sheikh AS, et al. Interleukin-13 stimulates MUC5AC expression via a STAT6-TMEM16A-ERK1/2 pathway in human airway epithelial cells. Int Immunopharmacol. 2016;40:106-114. doi: 10.1016/j.intimp.2016.08.033 [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Garvin LM, Nickola TJ, et al. IL-1beta induction of MUC5AC gene expression is mediated by CREB and NF-kappaB and repressed by dexamethasone. Am J Physiol Lung Cell Mol Physiol. 2014;306:L797-L807. doi: 10.1152/ajplung.00347.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CH, Kim KE, Yoon JH, et al. Upregulation of MUC5AC gene expression by IL-4 through CREB in human airway epithelial cells. J Cell Biochem. 2009;108:974-981. doi: 10.1002/jcb.22330 [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Liu Y, Xie H, et al. High mucin 5AC expression predicts adverse postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Oncotarget. 2017;8:59777-59790. doi: 10.18632/oncotarget.15894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acs G, Acs P, Beckwith SM, et al. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561-3565. [PubMed] [Google Scholar]
- 47.Andersson S, Sundberg M, Pristovsek N, et al. Corrigendum: insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun. 2017;8:16164. doi: 10.1038/ncomms16164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott S, Swift S, Busse L, et al. Epo receptors are not detectable in primary human tumor tissue samples. PLoS One. 2013;8:e68083. doi: 10.1371/journal.pone.0068083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laflamme C, McKeever PM, Kumar R, et al. Implementation of an antibody characterization procedure and application to the major ALS/FTD disease gene C9ORF72. Elife. 2019;8:e48363. doi: 10.7554/eLife.48363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinclair AM, Todd MD, Forsythe K, et al. Expression and function of erythropoietin receptors in tumors: implications for the use of erythropoiesis-stimulating agents in cancer patients. Cancer. 2007;110:477-488. doi: 10.1002/cncr.22832 [DOI] [PubMed] [Google Scholar]
- 51.Trincavelli ML, Da Pozzo E, Ciampi O, et al. Regulation of erythropoietin receptor activity in endothelial cells by different erythropoietin (EPO) derivatives: an in vitro study. Int J Mol Sci. 2013;14:2258-2281. doi: 10.3390/ijms14022258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Au NH, Gown AM, Cheang M, et al. P63 expression in lung carcinoma: a tissue microarray study of 408 cases. Appl Immunohistochem Mol Morphol. 2004;12:240-247. doi: 10.1097/00129039-200409000-00010 [DOI] [PubMed] [Google Scholar]
- 53.Saper CB. A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem. 2009;57:1-5. doi: 10.1369/jhc.2008.952770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sauter G, Simon R, Hillan K. Tissue microarrays in drug discovery. Nat Rev Drug Discov. 2003;2:962-972. doi: 10.1038/nrd1254 [DOI] [PubMed] [Google Scholar]
- 55.Schats KA, Van Vre EA, De Schepper S, et al. Validated programmed cell death ligand 1 immunohistochemistry assays (E1L3N and SP142) reveal similar immune cell staining patterns in melanoma when using the same sensitive detection system. Histopathology. 2017;70:253-263. doi: 10.1111/his.13056 [DOI] [PubMed] [Google Scholar]
- 56.Abe T, Amano H, Shimamoto F, et al. Prognostic evaluation of mucin-5AC expression in intrahepatic cholangiocarcinoma, mass-forming type, following hepatectomy. Eur J Surg Oncol. 2015;41:1515-1521. doi: 10.1016/j.ejso.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 57.Yu CJ, Shih JY, Lee YC, et al. Sialyl Lewis antigens: association with MUC5AC protein and correlation with post-operative recurrence of non-small cell lung cancer. Lung Cancer. 2005;47:59-67. doi: 10.1016/j.lungcan.2004.05.018 [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Wang Y, Zhao T, et al. Evaluation of serum MUC5AC in combination with CA19-9 for the diagnosis of pancreatic cancer. World J Surg Oncol. 2020;18:31. doi: 10.1186/s12957-020-1809-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duan B, Bai J, Qiu J, et al. Histone-lysine N-methyltransferase SETD7 is a potential serum biomarker for colorectal cancer patients. EBioMedicine. 2018;37:134-143. doi: 10.1016/j.ebiom.2018.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuenco J, Wehnert N, Blyuss O, et al. Identification of a serum biomarker panel for the differential diagnosis of cholangiocarcinoma and primary sclerosing cholangitis. Oncotarget. 2018;9:17430-17442. doi: 10.18632/oncotarget.24732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pabalan N, Sukcharoensin S, Butthongkomvong K, et al. Expression and serum levels of mucin 5AC (MUC5AC) as a biomarker for cholangiocarcinoma: a meta-analysis. J Gastrointest Cancer. 2019;50:54-61. doi: 10.1007/s12029-017-0032-9 [DOI] [PubMed] [Google Scholar]
- 62.Ruzzenente A, Iacono C, Conci S, et al. A novel serum marker for biliary tract cancer: diagnostic and prognostic values of quantitative evaluation of serum mucin 5AC (MUC5AC). Surgery. 2014;155:633-639. doi: 10.1016/j.surg.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Zhang L, Hu G. Potential application of alternatively glycosylated serum MUC1 and MUC5AC in gastric cancer diagnosis. Biologicals. 2009;37:18-25. doi: 10.1016/j.biologicals.2008.08.002 [DOI] [PubMed] [Google Scholar]