Abstract
Background:
Lumacaftor/ivacaftor (LUM/IVA) has shown modest benefits in previous research, but the exact effects in the cystic fibrosis (CF) lung remain unclear. This study aims to offer novel information on the mode of action of the cystic fibrosis transmembrane conductance regulator (CFTR)-modulating drug by assessing lung structure and function using functional respiratory imaging (FRI).
Methods:
CF patients aged ⩾12 years homozygous for F508del were recruited in an open-label study. Before and after 12 weeks of treatment with LUM/IVA, FRI was used to visualize regional information, such as air trapping, lobar volume and airway wall volume. Secondary outcomes included the CF-CT scoring system, spirometry, the Cystic Fibrosis Questionnaire–Revised (CFQ-R) questionnaire, exercise tolerance and nutritional status.
Results:
Of the 12 patients enrolled in the study, 11 completed all study visits. Concerning the FRI parameters, hyperinflation of the lung decreased, indicated by a reduction in air trapping and lobar volume at expiration. Also, a decrease in airway wall volume and a redistribution of pulmonary blood volume were noted, which might be related to a decrease in mucus impaction. Airway resistance, airway volume, internal airflow distribution and aerosol deposition pattern did not show significant changes. No significant improvements were found in any of the CF-CT scores or in the spirometric parameters. Other secondary outcomes showed similar results compared with previous research. Correlations at baseline were found between FRI and conventional outcomes, including physical functioning, spirometry and CF-CT scores.
Conclusions:
LUM/IVA decreased lung hyperinflation in combination with a potential decrease in mucus impaction, which can be related to an improved mucociliary transport. These results indicate that several FRI parameters, reflecting regional and distal lung structures, are more sensitive to changes caused by LUM/IVA than conventional respiratory outcomes.
Keywords: CFTR modulator, computational fluid dynamics, cystic fibrosis, functional respiratory imaging
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Deficient or dysfunctional CFTR protein results in a disturbed chloride and bicarbonate transport affecting multiple organs, including the respiratory system, gastrointestinal tract, and exocrine organs.1 F508del is the most prevalent mutation with more than 40% of patients with CF in Europe being homozygous for this allele.2 This mutation is associated with defective protein folding, which has an impact on the amount of CFTR protein reaching the epithelial membrane.3 In addition, ion transport is limited due to impaired functionality of the limited quantity of protein that does reach the cell surface.4 Orkambi® was developed by Vertex Pharmaceuticals (Boston, Massachusetts, USA) as a CFTR-modulating drug for patients homozygous for F508del and is composed of two active agents, ivacaftor and lumacaftor. Lumacaftor is a CFTR corrector that improves the processing and the stability of the protein, thus increasing the trafficking of mature protein to the epithelial membrane. The potentiator ivacaftor further increases CFTR-mediated chloride transport by extending the time the ion channels are open.5 Previous large multi-centre randomized controlled trials have shown modest benefits of lumacaftor-ivacaftor (LUM/IVA). The primary outcome, the percentage of predicted Forced Expiratory Volume in one second (ppFEV1), showed a mean absolute increase of approximately 3% from baseline. In addition, LUM/IVA was associated with clinically meaningful reductions in pulmonary exacerbations.5,6 In a long-term extension study, a 42% slower rate of ppFEV1 decline was found compared with matched controls.7 Although these findings are promising, the clinical response is highly variable and the exact effects of LUM/IVA in the CF lung remain unclear.8
Functional respiratory imaging (FRI) is a quantifiable method to measure biological responses to therapeutic interventions using high-resolution computed tomography (HRCT) and computational fluid dynamics (CFD). A 3D reconstruction of the respiratory system combined with flow simulations can be used to determine parameters such as patient-specific airflow distribution, airway resistance and volume, lobar volume, and air trapping. This functional and structural evaluation of the lungs allows for an in-depth description of respiratory health and post-treatment effects.9 Previous studies have demonstrated the validity of the lobar ventilation models and CFD simulations by comparing the results to 3He magnetic resonance imaging (MRI) ventilation measurements and combined single photon emission computed tomography and CT (SPECT/CT) in patients with asthma.10,11 Besides a detailed report of characteristics on a lobar level, this method has shown to be a more sensitive technique than conventional lung function tests in various obstructive airway diseases. In chronic obstructive pulmonary disease (COPD) and asthma, several studies assessing the effects of bronchodilators and anti-inflammatory compounds showed significant improvements in FRI parameters, while these changes were less pronounced in the results of spirometry and body plethysmography.12–14 In the CF population, FRI has demonstrated its value by establishing patient-specific airway models to predict the deposition of inhaled antibiotics.15 Given these previous findings, we hypothesize that FRI parameters related to hyperinflation, airway volume and airway wall thickness could provide valuable insights into the effects of LUM/IVA as imaging biomarkers on lung structure and functionality. This study aims to offer additional and novel information about the mode of action of the CFTR-modulating drug by assessing lung structure and function across multiple FRI parameters.
Methods
Study design
An open-label, single-arm study was conducted to investigate the effects of LUM/IVA over an intervention period of 12 weeks. The investigational product, Orkambi®, was administered as two tablets (lumacaftor 200 mg/ivacaftor 125 mg) twice a day in compliance with the prescribing information and the European Medicines Agency (EMA) approval. Treatment was started after the completion of all baseline measurements. Participants visited the hospital every 4 weeks during the 12-week period at which following study assessments were carried out: physical examination and spirometry. Other assessments were only performed at baseline and at 12 weeks: CT chest imaging, nitrogen multiple breath washout (N2MBW) test, Six-Minute Walk Test (6MWT), sweat chloride test and the Cystic Fibrosis Questionnaire–Revised (CFQ-R). Adverse events and adverse drug reactions were monitored throughout the study. The study was approved by the Ethics committee of the Antwerp University Hospital (EudraCT 2018-001573-24).
Participants
Participants aged 12 years or older were eligible for inclusion if they had a confirmed diagnosis of CF, homozygous for the F508del mutation, a ppFEV1 ⩾50%, and if they were clinically stable. A full description of the eligibility criteria is provided in the Supplementary Material. Patients were recruited from July 2018 to October 2019 through referral from three CF centres: Antwerp University Hospital, GZA hospital campus Sint-Vincentius, and Ghent University Hospital. All study assessments were performed at the Antwerp University Hospital.
Study assessments
Functional respiratory imaging
HRCT scans were taken with a GE VCT LightSpeed 64-slice scanner (GE Healthcare, Chicago, Illinois, USA) at two breathing levels, total lung capacity (TLC) and functional residual capacity (FRC) monitored with a pneumotachograph. A description of specific CT settings can be found in the Supplementary Material. Images were imported into a medical image processing software package, Mimics (Materialize, Leuven, Belgium), for segmentation and 3D reconstruction of the airways and lung lobes. All segmentation and postprocessing steps were automated, except for the identification of the fissures to separate the lung lobes, which were manually adjusted. The computed models were used to determine structural parameters, such as lobar volume, airway volume, and air trapping. After segmentation and postprocessing, the models were used for CFD simulations by solving Reynolds-averaged Navier-Stokes equations, to calculate regional airway resistance and to predict the deposition pattern of inhaled drugs. Pulmonary vasculature was segmented using algorithms based on shape analysis to recognize tubular structures. The cross-sectional area of each identified blood vessel was determined to compute pulmonary blood distribution. Although Orkambi® is not delivered by aerosol, simulations were performed to evaluate whether the treatment would affect the lung deposition of one of the most frequently inhaled drugs by CF patients, dornase alfa. All subjects were coupled with the Pari eFlow Nebulizer (Starnberg, Germany) and simulated using a tidal breathing profile. The particle characteristics of dornase alfa were taken from literature.16 Details of the analysis can be found in the Supplementary Material and previously published work.11,17 The following FRI parameters were evaluated in this study: air trapping, lobar volume, airway volume, airway wall volume, airway resistance, pulmonary blood distribution, ventilation/perfusion matching, internal airflow distribution and aerosol deposition.
Secondary outcomes
Structural abnormalities on inspiratory and expiratory chest CTs were visually scored using the CF-CT scoring system, a validated upgraded version of the Brody II score.18,19 The scans were scored by two independent observers blinded to subject ID and study visit, of whom one experienced chest radiologist and one certified researcher trained in CF-CT scoring. The presence and extent of the following abnormalities were evaluated: bronchiectasis, mucus plugging, parenchymal abnormalities and air trapping. All scores were expressed as a percentage of the maximum score. Data recorded during the monthly physical examination included body weight and length, peripheral oxygen saturation (SpO2), pulse rate and blood pressure. Pulmonary function was measured by spirometry and N2MBW. Exercise tolerance was evaluated by the Six-Minute Walk Distance (6MWD). During the exercise test, pulse oximetry was used to monitor SpO2. Also, the Borg score for dyspnoea and fatigue were questioned before and immediately after the 6MWT. Health-related quality of life (QoL) was evaluated using the CFQ-R. Sweat chloride concentration was measured by pilocarpine iontophoresis. A more detailed description of the study assessments can be found in the Supplementary Material.
Statistical analyses
An initial sample size of 14 subjects was set to reach a power of 80% with a significance level of 0.05. Since no pilot data were available, the calculation was based on the change in airway volume (Cohen’s effect size of 0.82) reported in a previous FRI study including patients with COPD.12
The analysis of the FRI parameters was based on a linear mixed-effects model, with visit, lobe and their interaction as fixed effects and lung lobe within each subject as a random effect. The heterogeneity across lobes (within subject) was modelled using an unstructured variance-covariance matrix, with independence assumed between subjects. For spirometry, anthropometrics and vital signs a similar approach was taken, excluding the lobar component. Changes in CF-CT scores, exercise tolerance, CFQ-R domain scores, lung clearance index and sweat chloride concentration were explored with the paired samples t test or the Wilcoxon signed-rank test, depending on the distribution of the variable. Additional correlation analyses were performed to determine the association between FRI parameters and secondary endpoints using the Pearson or Spearman correlation, depending on the distribution of the data. The distribution of the data was evaluated by QQ plots and the Shapiro-Wilk test. Normally distributed data are represented as mean ± standard deviation, and non-normal data as median [range]. Intraclass correlation coefficients were calculated using a two-way mixed-effects model for average measures to evaluate the interobserver reliability of the CF-CT score.20 For all analyses, a p value < 0.05 was considered statistically significant.
Results
Participants
Twelve participants were enrolled in the study, of whom one discontinued treatment after 10 weeks due to respiratory complaints, including cough and increased sputum. Therefore, the latter subject was excluded in further analyses determining the effects of LUM/IVA. Besides the discontinuation of the study at the patient’s own initiative, no serious adverse events were reported throughout the study. Baseline characteristics are presented in Table 1. The intended sample size of 14 subjects was not reached, since no other participants could be motivated to enter the trial. The main reason was the relatively short intervention period in combination with the fact that patients were not able to continue taking the drug after the trial, as Orkambi® was not reimbursed in Belgium at that time.
Table 1.
Baseline characteristics (n = 12).
| Sex (M/F) | 11/1 |
| Age (years) | 23.0 [17;46] |
| BMI (kg/m2) | 22.3 ±3.5 |
| Sweat chloride (mmol/L) | 92.5 ±14.1 |
| ppFEV1 (%) | 73.3 ±23.0 |
BMI, body mass index.
Data are presented as mean ± standard deviation or median [range].
FRI parameters
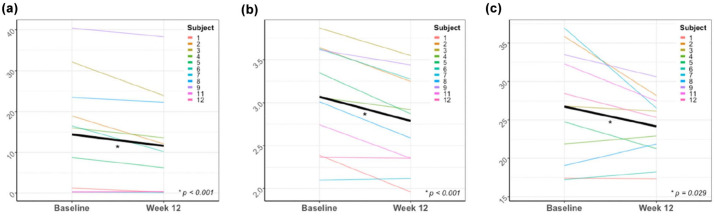
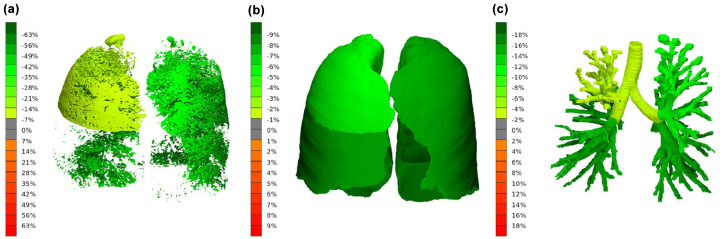
The FRI analysis showed a decrease in air trapping (16.12 ± 19.13% to 13.14 ± 17.55%, p < 0.001), lobar volume after normal expiration (0.63 ± 0.30 L to 0.57 ± 0.27 L, p < 0.001) and airway wall volume (22.89 ± 16.14 mL/L to 21.32 ± 12.51 mL/L, p = 0.03) per lung lobe. The change in total air trapping, lobar volume at FRC and airway wall volume (i.e. the sum of all five lobes) for each individual have been plotted in Figure 1, and an illustration of the 3D reconstruction of these parameters is shown in Figure 2. Also, a significant improvement of the ventilation/perfusion matching was found (12.92 ± 8.65% to 14.66 ± 7.43%, p < 0.001). After treatment, a higher proportion of pulmonary blood was found in blood vessels smaller than 5 mm2 (BV5%) and a lower proportion in vessels larger than 10 mm2 (BV10%). However, when considering the absolute volumes, no changes were observed in BV5, while the volume of blood vessels between 5 and 10 mm2 (BV5_10) and BV10 decreased. No significant changes were found for lobar volume after maximal inspiration, airway volume, airway resistance, internal airflow distribution, intrathoracic and peripheral deposition of dornase alpha and the central/peripheral deposition ratio. The results for airway wall volume, airway volume and airway resistance were corrected for lung volume at lobar level. An overview of the results of the FRI analysis can be found in Table 2.
Figure 1.
Mean change and individual changes of total (a) air trapping (%), (b) lobar volume at FRC (L), and (c) airway wall volume (mL/L).
Figure 2.
Treatment response in subject 5. The average change per lung lobe from baseline in (a) air trapping, (b) lobar volume at FRC, and (c) airway wall volume.
Table 2.
Results FRI (n = 11).
| FRI parameter | Baseline | Week 12 | p value |
|---|---|---|---|
| AT at FRC (%) | 16.12 ± 19.13 | 13.14 ± 17.55 | <0.001* |
| iVlobe at FRC (L) | 0.63 ± 0.30 | 0.57 ± 0.27 | <0.001* |
| iVlobe at TLC (L) | 1.22 ± 0.62 | 1.21 ± 0.59 | 0.678 |
| siVaw at TLC (mL/L) | 6.42 ± 4.87 | 6.58 ± 4.78 | 0.173 |
| siVaww at TLC (mL/L) | 22.89 ± 16.14 | 21.32 ± 12.51 | 0.029* |
| siRaw at TLC (kPa*s/L) | 0.06 ± 0.04 | 0.06 ± 0.03 | 0.470 |
| BV5 (mL) | 35.13 ± 17.68 | 34.82 ± 17.22 | 0.618 |
| BV5% | 58.05 ± 9.45 | 61.04 ± 6.84 | 0.002* |
| BV5_10 (mL) | 11.14 ± 5.05 | 10.40 ± 4.70 | <0.001* |
| BV5_10% | 19.08 ± 2.61 | 18.85 ± 2.60 | 0.254 |
| BV10 (mL) | 12.80 ± 6.49 | 11.08 ± 5.83 | 0.002* |
| BV10% | 22.86 ± 8.09 | 20.11 ± 5.62 | 0.002* |
| V/Q matching | 12.92 ± 8.65 | 14.66 ± 7.43 | <0.001* |
| IAD to upper lobes (%) | 11.19 ± 6.43 | 11.36 ± 6.15 | 0.946 |
| TLD (% delivered dose) | 35.06 ± 13.93 | 35.01 ± 11.58 | 0.992 |
| PLD (% delivered dose) | 18.34 ± 6.37 | 19.21 ± 6.17 | 0.748 |
| C/P ratio | 0.90 ± 0.29 | 0.84 ± 0.26 | 0.595 |
AT, air trapping; BV5, blood volume of vessels smaller than 5 mm2; BV5%, BV5 in relation to total blood volume; BV5_10, blood volume of vessels between 5 and 10 mm2; BV5_10%, BV5_10 in relation to total blood volume; BV10, blood volume of vessels larger than 10 mm2; BV10%, BV10 in relation to total blood volume; C/P ratio, central-to-peripheral deposition; FRI, functional respiratory imaging; IAD, internal airflow distribution; iVlobe, lobar volume; PLD, peripheral lung deposition; siRaw, specific airway resistance; siVaw, specific airway volume; siVaww, specific airway wall volume; TLD, total lung deposition; V/Q, ventilation/perfusion.
Data are presented as mean ± standard deviation.
Statistically significant (P < 0.05).
Secondary outcomes
The interobserver variability analysis of the CF-CT scoring method showed a moderate to excellent agreement between the two observers (Table 3). None of the CF-CT components nor the total score changed significantly after treatment.
Table 3.
Interobserver variability of the CF-CT scoring method presented as ICC [95% CI].
| Total score | 0.84 [0.62; 0.93] |
| Bronchiectasis | 0.86 [0.68; 0.94] |
| Mucus plugging | 0.81 [0.56; 0.92] |
| Bronchial wall thickening | 0.48 [−0.20; 0.78] |
| Parenchyma | 0.93 [0.83; 0.97] |
| Air trapping | 0.67 [0.23; 0.86] |
CF, cystic fibrosis; CI, confidence interval; CT, computed tomography; ICC, intraclass correlation coefficient.
ppFEV1 increased by 2.0 ± 4.0% (p = 0.3), but this result was not significant. No other significant changes were found in other spirometric parameters. For the N2MBW, the results of only five subjects were retained after evaluating the quality of the tests. The mean LCI2.5 did not significantly decrease from 11.98 [9.44; 20.32] to 11.53 [8.54; 15.52] (p = 0.3).
The median 6MWD increased from 650 m [512; 790] to 725 m [525; 839] (p = 0.02). Although exercise tolerance increased, no significant changes were found in Borg score for dyspnoea and fatigue. The response of oxygen saturation to exercise was also similar between visits.
The CFQ-R questionnaire showed a trend towards an improvement of the physical domain score as the majority of the patients scored higher after treatment (88% (17; 100) to 88% (58; 100), p = 0.05). No other domain scores reached statistical significance.
Body mass index (BMI) increased steadily during 12 weeks of treatment (22.2 ±–3.7 kg/m2 to 22.9 ± 4.0 kg/m2, p = 0.04). Also, systolic (118.5 ± 9.5 mmHg to 129.1 ± 12.8 mmHg, p = 0.047) and diastolic blood pressure (69.9 ± 12.1 mmHg to 78.7 ± 6.3 mmHg, p = 0.04) increased after treatment. All other vital signs remained stable.
Finally, sweat chloride concentration tended to decrease after 12 weeks (91.6 ± 14.5 mmol/L to 81.6 ± 14.7 mmol/L, p = 0.07). A summary of the results of the secondary outcomes can be found in Table 4.
Table 4.
Results secondary outcomes (n = 11).
| Parameter | Baseline | Week 12 | p value |
|---|---|---|---|
| CF-CT Total score (%) | 23.71 [12.81; 34.10] | 23.46 [7.46; 36.68] | 0.4648 |
| CF-CT Bronchiectasis (%) | 27.43 [14.76; 37.50] | 24.31 [3.65; 43.23] | 0.9658 |
| CF-CT Mucus (%) | 30.56 [8.33; 55.56] | 30.56 [1.39; 43.06] | 0.1139 |
| CF-CT Bronchial wall thickening (%) | 29.17 [20.37; 47.45] | 30.56 [15.74; 40.97] | 0.5935 |
| CF-CT Parenchyma (%) | 9.26 [0.00; 21.30] | 6.48 [0.00; 25.93] | 0.3411 |
| CF-CT Air trapping (%) | 33.33 [22.22; 67.59] | 31.48 [3.70; 64.82] | 0.6835 |
| ppFEV1 (%) | 74.64 ±23.56 | 76.64 ±23.56 | 0.278 |
| ppFVC (%) | 86.09 ±15.34 | 86.82 ±14.82 | 0.361 |
| ppMEF25 (%) | 62.55 ±69.53 | 64.82 ±78.04 | 0.238 |
| ppMEF25-75 (%) | 56.91 ±42.22 | 62.09 ±49.32 | 0.201 |
| LCI2.5 (n = 5) | 11.98 [9.34; 20.32] | 11.53 [8.54; 15.52] | 0.313 |
| Sweat chloride (mmol/L) | 91.64 ±14.45 | 81.64 ±14.67 | 0.070 |
| BMI (kg/m2) | 22.22 ±3.67 | 22.87 ±4.01 | 0.044* |
| CFQ-R physical (%) | 88 [17; 100] | 88 [58; 100] | 0.052 |
| CFQ-R respiratory (%) | 78 [44; 89] | 83 [61; 94] | 0.139 |
| 6MWD (m) | 650 [512; 790] | 725 [525; 839] | 0.021* |
BMI, body mass index; CF, cystic fibrosis; CFQ-R, Cystic Fibrosis Questionnaire–Revised; CT, computed tomography; 6MWD, Six-Minute Walk Distance.
Data are presented as mean ± standard deviation or median [range].
Statistically significant (p < 0.05).
Correlations FRI and secondary outcomes
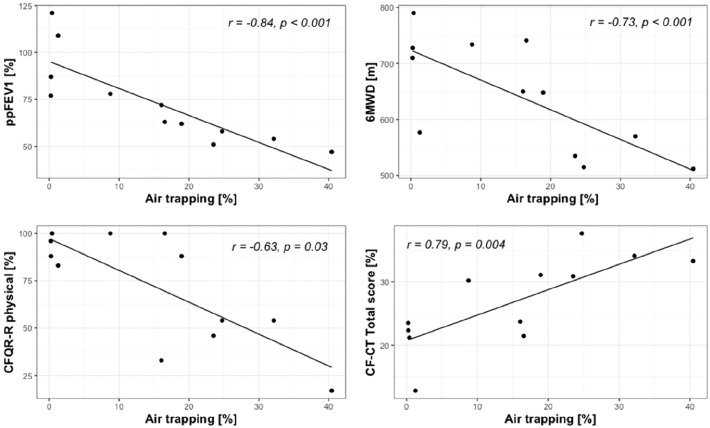
Significant correlations were found at baseline between air trapping and ppFEV1 (r = −0.84, p < 0.001), 6MWD (r = −0.73, p = 0.007) and CFQ-R physical domain (r = −0.63, p = 0.03); and between lobar volume after normal expiration and ppFEV1 (r = −0.68, p = 0.01). Multiple correlations were found between FRI parameters and CF-CT scores at baseline. The total CF-CT score correlated with air trapping (r = 0.79, p = 0.004), lobar volume at FRC (r = 0.73, p = 0.01), BV10% (r = 0.72, p = 0.01) and BV5% (−0.69, p = 0.02). Interestingly, the CF-CT subscore for mucus plugging correlated with the absolute volumes of BV5_10 (r = 0.74, p = 0.006) and BV10 (r = 0.75, p = 0.005). No significant correlations could be found between the relative change in FRI parameters and secondary outcomes. Baseline correlations between air trapping measured by FRI and secondary outcomes are shown in Figure 3.
Figure 3.
Baseline correlations between air trapping measured by FRI and ppFEV1, 6MWD, CFQ-R physical and CF-CT total score.
Between secondary outcomes, ppFEV1 was correlated with CF-CT total score (r = −0.82, p = 0.002), 6MWD (r = 0.76, p = 0.007) and CFQ-R physical domain (r = 0.66, p = 0.02) at baseline. In addition, significant correlations were found between 6MWD and CF-CT total score (r = −0.71, p = 0.01) and CFQ-R physical domain (r = 0.86, p < 0.001). When comparing relative changes over time, only a change in ppFEV1 was associated with a change in CFQ-R physical domain (r = 0.78, p = 0.005).
Discussion
This study aimed to gain more insight into the short-term effects of LUM/IVA by evaluating the patient’s lung structure and function across multiple FRI parameters. The results showed a decrease in lung hyperinflation indicated by a significant decrease in air trapping and in lobar volume after normal expiration. Furthermore, airway wall volume decreased and the proportion of pulmonary blood volume shifted from larger to smaller blood vessels. The larger proportion of blood in smaller blood vessels would indicate an average decrease in pulmonary vascular resistance downstream that resulted from an improved regional ventilation. The absolute volume of BV5, however, did not significantly change. We hypothesize that both the redistribution of pulmonary blood and the decrease in airway wall volume could be related to a reduction in mucus impaction, due to the similar attenuation properties of mucus, airway walls and blood vessels in the CT images causing bias. Consequently, the calculation of the total airway wall thickness includes mucus adhering to the airway wall. Second, mucus plugs obstructing small airways could have been recognized by the applied algorithms as larger blood vessels due to the corresponding shape and attenuation values. This could explain the significant correlation between the CF-CT subscore for mucus plugging and the absolute values of BV5_10 and BV10 at baseline. Although these assumptions indicate improved mucociliary clearance, the CF-CT score for mucus plugging did not show any differences after treatment. This could be related to the insensitivity of the CF-CT score to detect subtle changes, as the smallest increment of the score represents an increase in the extent of one-third of a lobe.21 The same reasoning can be applied to explain the lack of change in other CF-CT scores. More particularly, differences in air trapping and airway wall volume determined by FRI were not reflected in CF-CT scores for air trapping and bronchial wall thickening, respectively. In contrast to our study, a recent retrospective study evaluating structural changes using the modified Brody score after the initiation of LUM/IVA therapy showed a significant improvement in the CT total score, which was mostly related to a decrease in mucus plugging.22 The higher number of participants and longer treatment period compared with our study could explain these significant differences. In addition, Sheikh et al.23 reported significant improvements on HRCT imaging using the Brody scoring system after 1 year of ivacaftor treatment in patients with a G551D mutation. Similar to the retrospective study by Arnaud et al., these significant results could be related to a longer treatment period, but more importantly, ivacaftor has been associated with a much higher effectiveness in patients with a G551D mutation compared with LUM/IVA in patients homozygous for F508del.24
Another important finding of this study is that the changes in several FRI parameters were also not reflected in any of the spirometric parameters in this small group of patients. The limitations of spirometry to measure therapeutic responses have been widely recognized: they are considered too insensitive to detect treatment effects and the heterogeneity of the pathology cannot be captured.25 Nevertheless, FEV1 is still considered one of the most important primary endpoints by the EMA for research in CF, since FEV1 is an established marker for disease progression and a strong predictor of survival.26,27 Over the past decade, alternative endpoints for clinical research in CF have been proposed, of which the LCI, CT scores and magnetic resonance imaging (MRI) are regarded to be the most promising.28,29 LCI, CT scores as well as MRI outcomes have shown to be more sensitive to detect early lung disease, which is an important advantage since the progression of CF lung disease is slowing down and the FEV1 remains within the normal range at older age.25,30,31 Two previous observational studies showed that LUM/IVA therapy was associated with a decrease in LCI, while no significant changes were observed in ppFEV1.32,33 Unfortunately, a recent real-world study by Reix et al.34 could not confirm these results in a heterogenous group of patients monitored during the first year of LUM/IVA treatment. LCI nor FEV1 changed significantly, and discordant results between FEV1 and LCI were observed on an individual level. While LCI monitoring has shown to be valuable for the follow-up of patients with mild lung disease, the authors indicate that the application of this biomarker in a clinical setting remains less clear in patients with moderate to severe lung disease. The MBW has previously been associated with poor reproducibility in patients with more advanced lung disease and mucus plugging, which has shown to be a considerable limitation in our study.35 Another disadvantage of the LCI is that this measure cannot distinguish between regional characteristics, as the respiratory system is regarded as a single unit.35 On the other hand, CT scores, such as the CF-CT scoring system, provide more regional information in terms of semiquantitative measures per lung lobe, but they are dependent on subjective observations.36 The role of CT scores in previous CFTR modulator studies have been discussed earlier. A more objective CT measure is the airway-artery ratio for the quantification of bronchiectasis,37 but to our knowledge this method has not been used to assess the effects of CFTR modulator therapy to date. Finally, MRI is increasingly used in CF to evaluate structural as well as functional characteristics of the respiratory system.29 Graeber et al.32 recently showed that the MRI morphology score and perfusion score significantly improved after 8 to 16 weeks of LUM/IVA therapy in a comparable patient cohort. The MRI score is a visual semiquantitative scoring method using conventional proton MRI combined with contrast-enhanced perfusion MRI.38 Similar to our findings, they reported that LUM/IVA may result in improved ventilation and in a decrease in mucus impaction in the small airways as indicated by a decrease in perfusion deficits reflecting hypoxic pulmonary vasoconstriction. Other more sophisticated MRI techniques are emerging as well, such as hyperpolarized gas MRI, which can directly visualize ventilation defects, but these techniques require expensive specialized equipment available in only a few centres worldwide.29 Their role in the assessment of CFTR modulator therapy is yet to be determined.
Although FRI as described in this study is a new tool in the field of CF research, previous studies in other obstructive lung diseases, such as COPD and asthma, have shown that FRI is able to overcome several of the shortcomings or challenges associated with other methods as described earlier.12,39 Although FRI is based on CT imaging, this imaging modality currently remains the gold standard to evaluate structural abnormalities of the respiratory system, due to its higher spatial resolution compared with MRI. FRI biomarkers obtain regional information of both structural and functional characteristics using objective image processing techniques. Therefore, FRI has the potential to provide valuable contributions to future interventional studies targeting CF lung disease. Similar to studies in COPD and asthma patients, our results confirm that FRI is more sensitive than conventional outcomes, such as spirometry and CF-CT scores, to pick up local changes in the respiratory system. Consequently, a smaller sample size is required and the intervention period of a clinical trial can be reduced. This is an important benefit since disease progression in CF is slowing down as mentioned earlier, which would imply larger clinical studies of a longer duration to demonstrate significant changes in conventional outcomes.40
In this study, important correlations were found between FRI and other respiratory outcomes, including physical functioning, spirometry, and structural abnormalities on chest CT. This indicates that improvements in specific FRI parameters could be related to an improved clinical outcome in the long term. Correlations between CT findings and lung function in CF have been reported in previous research, showing a higher correlation between LCI and CT than FEV1 and CT.41,42 Therefore, it would have been interesting to compare the LCI with FRI parameters, but unfortunately the number of successful MBW tests was insufficient to perform such analysis.
Although the result of ppFEV1 in this study was not significant, the mean change from baseline was comparable to the findings of the pivotal clinical trials.6 Changes in most secondary outcomes were also similar to those reported in previous research, including nutritional status,7 exercise tolerance,43 QoL6,44 and arterial blood pressure.7
The study is subject to several limitations. First, the obtained results could not be compared to a control group. A high variability was found between subjects across several FRI parameters as well as secondary outcomes, which can be related to the varying levels of disease severity and wide age range representing a heterogeneous group of patients. No previous FRI studies have been performed in the CF population to determine which changes can be expected over time and what treatment effects can be considered clinically important.
Second, only 12 participants were enrolled instead of the calculated sample size of 14. Although this is relatively small sample size, it should be stressed that all FRI parameters were evaluated at a lobar level, which implies five data points per subject. This partially explains why significant results could be detected by FRI in contrast to secondary outcomes. In retrospect, it would have been more appropriate for a study including CF patients to perform the sample size calculation using an FRI parameter other than airway volume, such as air trapping. Due to the presence of structural airway damage, in particular bronchiectasis, LUM/IVA is not expected to affect airway volume considerably during a short intervention period. The choice of airway volume to estimate the effect size was based on a previous study including COPD patients, since no other FRI studies with CF patients have been performed to date.
Over the past few years, new CFTR modulators have been proposed for the treatment of CF patients homozygous for F508del. The most recent triple combination therapy, elexacaftor plus tezacaftor plus ivacaftor, has shown substantially larger effects on lung function, sweat chloride concentration, QoL and pulmonary exacerbations than those of LUM/IVA.45–47 Therefore, it is expected that LUM/IVA will become less relevant in future CF management, since this treatment provides only modest health benefits and is associated with a very high cost.5 Nonetheless, this study demonstrated the added value of FRI analysis to assess the effectiveness of CFTR modulators. This method revealed a more detailed outline of the mode of action of the drug, allowing a better understanding of post-treatment effects. With that in mind, it would be highly interesting to conduct similar future studies to assess the effects of new CFTR modulator therapies in CF patients.
Conclusion
This study is the first to perform FRI to evaluate the effects of CFTR-modulating drugs on lung functionality. The analysis showed that LUM/IVA decreased lung hyperinflation in combination with a potential decrease in mucus impaction, which can be related to an improved mucociliary transport. These FRI parameters, reflecting regional and distal lung structures, appear to be more sensitive to changes caused by LUM/IVA than conventional outcomes, such as spirometry and subjective CT scores. The objective and quantitative FRI methodology could play an important role in acquiring profound knowledge about the local effects in the respiratory system of new CFTR modulators in future research.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666211046774 for The short-term effects of ORKAMBI (lumacaftor/ivacaftor) on regional and distal lung structures using functional respiratory imaging by Eline Lauwers, Dennis Belmans, Benjamin Mignot, Kris Ides, Kim Van Hoorenbeeck, Annemiek Snoeckx, Cedric Van Holsbeke, Vicky Nowé, Eva Van Braeckel, Wilfried De Backer, Jan De Backer and Stijn Verhulst in Therapeutic Advances in Respiratory Disease
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vertex Pharmaceuticals supported the research financially, but they were not involved in data collection, analyses, interpretation of data or writing of the manuscript. Co-authors DB, BM, CVH, WDB and JDB are employees of Fluidda NV, a company that develops and markets the FRI technology described in this article. The other authors have no financial relationships with this organization and received no direct funding from Fluidda NV.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This is an investigator-initiated study supported by Vertex Pharmaceuticals.
ORCID iD: Eline Lauwers  https://orcid.org/0000-0002-0494-0206
https://orcid.org/0000-0002-0494-0206
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Eline Lauwers, Laboratory of Experimental Medicine and Pediatrics, Faculty of Medicine and Health Sciences, University of Antwerp, Universiteitsplein 1, Wilrijk, 2160 Antwerp, Belgium; Infla-Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium.
Dennis Belmans, FLUIDDA NV, Kontich, Belgium.
Benjamin Mignot, FLUIDDA NV, Kontich, Belgium.
Kris Ides, Laboratory of Experimental Medicine and Pediatrics, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Infla-Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium; Department of Pediatrics, Antwerp University Hospital, Edegem, Belgium; CoSys Research Lab, Faculty of Applied Engineering, University of Antwerp, Antwerp, Belgium; Flanders Make Strategic Research Center, Lommel, Belgium.
Kim Van Hoorenbeeck, Laboratory of Experimental Medicine and Pediatrics, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Infla-Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium; Department of Pediatrics, Antwerp University Hospital, Edegem, Belgium.
Annemiek Snoeckx, Department of Radiology, Antwerp University Hospital, Edegem, Belgium; Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
Cedric Van Holsbeke, FLUIDDA NV, Kontich, Belgium.
Vicky Nowé, Department of Pulmonology, GZA Hospital, Antwerp, Belgium.
Eva Van Braeckel, Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium; Department of Internal Medicine and Pediatrics, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium.
Wilfried De Backer, FLUIDDA NV, Kontich, Belgium; Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium.
Jan De Backer, FLUIDDA NV, Kontich, Belgium.
Stijn Verhulst, Laboratory of Experimental Medicine and Pediatrics, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Infla-Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium; Department of Pediatrics, Antwerp University Hospital, Edegem, Belgium.
References
- 1.Ratjen F, Bell SC, Rowe SM, et al. Cystic fibrosis. Nat Rev Dis Primers 2015; 1: 15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zolin A, Orenti A, Naehrlich L, et al. ECFS Patient Registry annual data report 2017, https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports (accessed 5 May 2020).
- 3.Lukacs GL, Mohamed A, Kartner N, et al. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J 1994; 13: 6076–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Goor F, Straley KS, Cao D, et al. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol 2006; 290: L1117–L1130. [DOI] [PubMed] [Google Scholar]
- 5.Deeks ED. Lumacaftor/ivacaftor: a review in cystic fibrosis. Drugs 2016; 76: 1191–1201. [DOI] [PubMed] [Google Scholar]
- 6.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N Engl J Med 2015; 373: 220–231. [DOI] [PubMed] [Google Scholar]
- 7.Konstan MW, McKone EF, Moss RB, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017; 5: 107–118. [DOI] [PubMed] [Google Scholar]
- 8.Masson A, Schneider-Futschik EK, Baatallah N, et al. Predictive factors for lumacaftor/ivacaftor clinical response. J Cyst Fibros 2019; 18: 368–374. [DOI] [PubMed] [Google Scholar]
- 9.de Backer JW, Vos W, Vinchurkar S, et al. Validation of computational fluid dynamics in CT-based airway models with SPECT/CT. Radiology 2010; 257: 854–862. [DOI] [PubMed] [Google Scholar]
- 10.Tahir BA, Van Holsbeke C, Ireland RH, et al. Comparison of CT-based lobar ventilation with 3He MR imaging ventilation measurements. Radiology 2016; 278: 585–592. [DOI] [PubMed] [Google Scholar]
- 11.De Backer JW, Vos WG, Gorlé CD, et al. Flow analyses in the lower airways: patient-specific model and boundary conditions. Med Eng Phys 2008; 30: 872–879. [DOI] [PubMed] [Google Scholar]
- 12.De Backer LA, Vos W, De Backer J, et al. The acute effect of budesonide/formoterol in COPD: a multi-slice computed tomography and lung function study. Eur Respir J 2012; 40: 298–305. [DOI] [PubMed] [Google Scholar]
- 13.De Backer LA, Vos WG, Salgado R, et al. Functional imaging using computer methods to compare the effect of salbutamol and ipratropium bromide in patient-specific airway models of COPD. Int J Chron Obstruct Pulmon Dis 2011; 6: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos W, De Backer J, Poli G, et al. Novel functional imaging of changes in small airways of patients treated with extrafine beclomethasone/formoterol. Respiration 2013; 86: 393–401. [DOI] [PubMed] [Google Scholar]
- 15.Bos AC, Van Holsbeke C, De Backer JW, et al. Patient-specific modeling of regional antibiotic concentration levels in airways of patients with cystic fibrosis: are we dosing high enough? PLoS ONE 2015; 10: e0118454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemmer A, Krämer I, Kamin W. Physicochemical compatibility and stability of nebulizable drug admixtures containing dornase alfa and tobramycin. Pulm Pharmacol Ther 2014; 28: 53–59. [DOI] [PubMed] [Google Scholar]
- 17.Lins M, Vandevenne J, Thillai M, et al. Assessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Acad Radiol 2020; 27: 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging 2006; 21: 14–21. [DOI] [PubMed] [Google Scholar]
- 19.Wainwright CE, Vidmar S, Armstrong DS, et al. Effect of bronchoalveolar lavage-directed therapy on Pseudomonas aeruginosa infection and structural lung injury in children with cystic fibrosis: a randomized trial. JAMA 2011; 306: 163–171. [DOI] [PubMed] [Google Scholar]
- 20.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenow T, Oudraad MCJ, Murray CP, et al. PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med 2015; 191: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 22.Arnaud F, Stremler-Le Bel N, Reynaud-Gaubert M, et al. Computed tomographic changes in patients with cystic fibrosis treated by combination therapy with lumacaftor and ivacaftor. J Clin Med 2021; 10: 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh SI, Long FR, McCoy KS, et al. Computed tomography correlates with improvement with ivacaftor in cystic fibrosis patients with G551D mutation. J Cyst Fibros 2015; 14: 84–89. [DOI] [PubMed] [Google Scholar]
- 24.Gramegna A, Contarini M, Aliberti S, et al. From ivacaftor to triple combination: a systematic review of efficacy and safety of CFTR modulators in people with cystic fibrosis. Int J Mol Sci 2020; 21: 5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petousi N, Talbot NP, Pavord I, et al. Measuring lung function in airways diseases: current and emerging techniques. Thorax 2019; 74: 797–805. [DOI] [PubMed] [Google Scholar]
- 26.Aaron SD, Stephenson AL, Cameron DW, et al. A statistical model to predict one-year risk of death in patients with cystic fibrosis. J Clin Epidemiol 2015; 68: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 27.Liou TG, Adler FR, Fitzsimmons SC, et al. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001; 153: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiddens HAWM, Puderbach M, Venegas JG, et al. Novel outcome measures for clinical trials in cystic fibrosis. Pediatr Pulmonol 2015; 50: 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goralski JL, Stewart NJ, Woods JC. Novel imaging techniques for cystic fibrosis lung disease. Pediatr Pulmonol 2021; 56(Suppl. 1): S40–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent L, Reix P, Innes JA, et al. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros 2014; 13: 123–138. [DOI] [PubMed] [Google Scholar]
- 31.Stahl M, Wielpütz MO, Graeber SY, et al. Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2017; 195: 349–359. [DOI] [PubMed] [Google Scholar]
- 32.Graeber SY, Boutin S, Wielpütz MO, et al. Effects of lumacaftor-ivacaftor on lung clearance index, magnetic resonance imaging and airway microbiome in Phe508del homozygous patients with cystic fibrosis. Ann Am Thorac Soc 2021; 18: 971–980. [DOI] [PubMed] [Google Scholar]
- 33.Shaw M, Khan U, Clancy JP, et al. Changes in LCI in F508del/F508del patients treated with lumacaftor/ivacaftor: results from the prospect study. J Cyst Fibros 2020; 19: 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reix P, Tatopoulos A, Ioan I, et al. Real-world assessment of LCI following lumacaftor-ivacaftor initiation in adolescents and adults with cystic fibrosis. J Cyst Fibros. Epub ahead of print 25 June 2021. DOI: 10.1016/j.jcf.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Horsley A, Wild JM. Ventilation heterogeneity and the benefits and challenges of multiple breath washout testing in patients with cystic fibrosis. Paediatr Respir Rev 2015; 16(Suppl. 1): 15–18. [DOI] [PubMed] [Google Scholar]
- 36.Calder AD, Bush A, Brody AS, et al. Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr Radiol 2014; 44: 1496–1506. [DOI] [PubMed] [Google Scholar]
- 37.Kuo W, de Bruijne M, Petersen J, et al. Diagnosis of bronchiectasis and airway wall thickening in children with cystic fibrosis: objective airway-artery quantification. Eur Radiol 2017; 27: 4680–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichinger M, Optazaite DE, Kopp-Schneider A, et al. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol 2012; 81: 1321–1329. [DOI] [PubMed] [Google Scholar]
- 39.De Backer J, Vos W, Vinchurkar S, et al. The effects of extrafine beclometasone/formoterol (BDP/F) on lung function, dyspnea, hyperinflation, and airway geometry in COPD patients: novel insight using functional respiratory imaging. J Aerosol Med Pulm Drug Deliv 2015; 28: 88–99. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery AB, Abuan T, Yeager MA. Regulatory aspects of phase 3 endpoints for new inhaled antibiotics for cystic fibrosis patients with chronic pseudomonas aeruginosa infections. J Aerosol Med Pulm Drug Deliv 2012; 25: 198–203. [DOI] [PubMed] [Google Scholar]
- 41.Owens CM, Aurora P, Stanojevic S, et al. Lung clearance index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax 2011; 66: 481–488. [DOI] [PubMed] [Google Scholar]
- 42.Gustafsson PM, De Jong PA, Tiddens HA, et al. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 2008; 63: 129–134. [DOI] [PubMed] [Google Scholar]
- 43.Wark PAB, Cookson K, Thiruchelvam T, et al. Lumacaftor/ ivacaftor improves exercise tolerance in patients with Cystic Fibrosis and severe airflow obstruction. BMC Pulm Med 2019; 19: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017; 5: 557–567. [DOI] [PubMed] [Google Scholar]
- 45.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griese M, Costa S, Linnemann RW, et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with cystic fibrosis and one or more F508del alleles: interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med 2021; 203: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666211046774 for The short-term effects of ORKAMBI (lumacaftor/ivacaftor) on regional and distal lung structures using functional respiratory imaging by Eline Lauwers, Dennis Belmans, Benjamin Mignot, Kris Ides, Kim Van Hoorenbeeck, Annemiek Snoeckx, Cedric Van Holsbeke, Vicky Nowé, Eva Van Braeckel, Wilfried De Backer, Jan De Backer and Stijn Verhulst in Therapeutic Advances in Respiratory Disease