Abstract
Objective
To characterize the long-term effects of intermittent parathyroid hormone (I-PTH) on the mandibular condylar cartilage (MCC) and subchondral bone of the temporomandibular joint, in vivo and in vitro.
Materials and Methods
For the in vivo experiments, sixteen 10-week-old mice were divided into 2 groups: (1) I-PTH (n = 8)—subcutaneous daily injection of PTH; (2) control group (n = 8)—subcutaneous daily injection of saline solution. Experiments were carried out for 4 weeks. Mice were injected with calcein, alizarin complexone, and cell proliferation marker before euthanasia. For the in vitro experiments, primary chondrocyte cultures from the MCC of eight 10-week-old mice were treated with I-PTH for 14 days.
Results
There was a significant increase in bone volume, tissue density, mineral deposition, osteoclastic activity, cell proliferation in the cartilage, and cartilage thickness in the I-PTH-treated mice when compared with the control group. In addition, immunohistochemistry in cartilage revealed that I-PTH administration led to an increase in expression of vascular endothelial growth factor and to a decreased expression of sclerostin, matrix metallopeptidase 13, and aggreganase-1 (ADAM-TS4). Quantitative polymerase chain reaction analysis of the I-PTH-treated chondrocytes revealed significantly decreased relative expression of collagen type X (Col10a1), alkaline phosphatase (Alp), and Indian Hedgehog (Ihh) and remarkable increased expression of Sox9, fibroblast growth factor 2 (Fgf2), and proteoglycan 4 (Prg4).
Conclusion
I-PTH administration causes anabolic effects at the subchondral region of the mandibular condyle while triggers anabolic and protective effects at the MCC.
Keywords: mandibular condylar cartilage, temporomandibular joint, parathyroid hormone
Introduction
Parathyroid hormone (PTH) is one of the key hormones that regulate bone and cartilage growth.1 It is known that PTH produces both anabolic and catabolic effects in bone depending on the mode of administration. Continuous treatment of PTH stimulates catabolic modeling of bone, whereas intermittent PTH (I-PTH) increases anabolic bone modeling.2,3 The action of PTH is mediated by the parathyroid hormone receptor 1 (PTH1R), which is expressed in the chondrocytes of the mandibular condylar cartilage (MCC).4
Previous studies have demonstrated that I-PTH not only prevents the degeneration of the articular cartilage but also retains the ultrastructure of the subchondral bone.5,6 Despite its established mechanisms whereby PTH stimulates anabolic bone formation, the molecular mechanism, which leads to anabolic role of PTH in the MCC, is not fully understood. Our short-term studies on the effects of I-PTH administration in growing and adult mice suggested an anabolic effect at the mandibular condyle, characterized by an increase in cartilage thickness and enhanced mineralization in the subchondral region.7,8 However, the effects of longer I-PTH administration are not known.
The aim of this study was to characterize the long-term effects of I-PTH on the MCC and the subchondral bone of the temporomandibular joint (TMJ) of mice and also to investigate the molecular mechanisms by which I-PTH exerts it effects, in vitro.
Materials and Methods
Ethical Statement
The Institutional Animal Care Committee of the University of Connecticut Health Center approved the experimental protocol involving the mice in this study. The mice were obtained from Jackson Labs (Bar Harbor, ME, USA). Mice were group housed in individually ventilated cages (Thoren Caging, Hazleton, PA, USA) with a photoperiod of 12:12. The room temperature and humidity were maintained at 22°C and 30% to 70%, respectively.
In Vivo Studies
Study Design
We used 10-week-old male C57BL/6J mice for this study. The treatment group received daily subcutaneous injections of PTH [1-34] for 4 weeks, while control animals were injected with saline. The mandibular condyles were assessed by micro–computed tomography (micro-CT), histomorphometric analysis, and immunostaining analysis.
All animal experiments were approved by the Institutional Animal Care and Use Committee at University of Connecticut Health Center. The mice were randomly divided into 2 groups: (1) I-PTH group (n = 8)—80 μg/kg of the body weight PTH [1-34] (Prospec-Tany TechnoGene Ltd., Ness Ziona, Israel) was injected subcutaneously daily for 4 weeks and (2) control group (n = 8)—saline was injected subcutaneously daily for 4 weeks. The animals were fed a standard diet over the entire experimental period. All mice were injected with alizarin complexone (2 μg/kg body weight) on the 24th day and calcein (2 μg/kg body weight) on the 27th day. Furthermore, mice were injected with 5-ethnyl-2′-deoxyuridine (EdU, Life Technologies, Grand Island, NY, USA), in a concentration of 30 mg/kg per body weight, 48 and 24 hours before euthanization. Mice were euthanized 24 hours after the last injection of PTH or saline.
Micro-CT
We further evaluated the microstructure of the subchondral bone and calcified cartilage were scanned (SCANCO Medical AG, Brüttisellen, Switzerland) in 70% ethanol and serial tomographic projections were acquired at 55 kV and 145 µA, with a voxel size of 6 µm and 1,000 projections per rotation collected at 300,000 µs. To distinguish calcified tissue from noncalcified tissue, an automated algorithm using local threshold segmented the reconstructed gray scale images. Our region of interest was the mushroom shaped head of the condyle and within the region of interest we recorded the bone volume and tissue volume to calculate the bone volume fraction (BVF), tissue density, trabecular spacing, and trabecular thickness.
Histomorphometry
Mandibular condyles were fixed for 24 hours in 10% formalin and placed in 30% sucrose overnight before embedding in cryomedium (Thermo Shandon, Pittsburgh, PA, USA) using disposable molds (Thermo Shandon, Pittsburgh, PA, USA). The medial surfaces of the samples were embedded against the base of the mold, parallel to the floor of the mold. Specimens were stored at −20°C before sectioning. Histological sections (5-7 μm thickness) were performed using a Leica Cryostat (Nussloch, Germany). Sections were transferred to slides using a tape transfer method. Sequential sections were mounted using 50% glycerol buffered in phosphate buffered saline (PBS) and were stored in the dark at 4°C. Sections were examined with an observer ZI fluorescent microscope (Carl Zeiss, Thornwood, NY, USA) using appropriate filters (Chroma Technology, Bellow Falls, VT, USA).
Histological Staining
Histological sections were stained following a previously described protocol.9 The 5- to 7-μm MCC sections remain adherent to glass slides through all of the process of staining and imaging. The first step was to image the bone labels alizarin complexone (red) and calcein (green). Baseline imaging of the sections was performed with the observer ZI fluorescent microscope (Carl Zeiss, Thornwood, NY, USA) using appropriate fluorescent protein filters. Subsequently, coverslips were removed by soaking slides in PBS, and sections were stained for tartrate-resistant acid phosphatase (TRAP) using the ELF97 substrate (Life Technologies, Grand Island, NY). After imaging for TRAP, coverslips were removed again and sections were stained for EdU (ClickiT EdU Alexa Fluor 555HCS kit, Life Technologies, Grand Island, NY, USA) and DAPI (Thermo Fisher Scientific, Waltham, MA, USA) and reimaged.
Additional slides were used for Safranin O staining (IHC WORLD, LLC; Ellicott, MD, USA) and immunostaining for sclerostin (SOST, R&D Systems, Minneapolis, MN, USA), vascular endothelial growth factor (VEGF), matrix metallopeptidase 13 (MMP13), and aggreganase-1 (ADAM-TS4) (ABCAM, Cambridge, MA, USA).
Histological Analysis and Quantification
We examined mineralization and TRAP activity in the subchondral bone by counting the number of red (alizarin complexone), green (calcein), and yellow (TRAP) pixels and dividing it by the total number of pixels in the subchondral region. Cellular proliferation was quantified by counting EdU- and DAPI-positive pixels in the proliferative zone of the MCC and calculating the percentage of EdU-positive pixels over DAPI-positive pixels. Distance mapping (cartilage thickness) in Safranin O–stained sections was analyzed using Digimizer Image software (MedCalc Software, Ostend, Belgium) and measurements were performed from the outer cellular layer of MCC to the tidemark (in 3 different locations in the entire MCC).
In Vitro Studies
Primary Chondrocyte Micro Mass Culture
Eight C57BL/6J male mice were euthanized at 10 weeks of age by CO2 asphyxiation. Mandibles were dissected and the MCC (outer layer of mandibular condyle) was removed from the subchondral region by carefully cutting it using surgical blades. Isolated MCCs were placed in PBS with 50 U/mL penicillin-streptomycin (P/S; Thermo Fisher Scientific, Waltham, MA, USA). To retrieve chondrocytes, MCCs were incubated at 37° C in the P/S solution with collagenase D at 3 mg/mL (Sigma-Aldrich, St. Louis, MO, USA) and dispase at 2 mg/mL (Thermo Fisher Scientific, Waltham, MA, USA). Cells released from the tissue were transferred to media (Dulbecco’s modified Eagle medium [DMEM] with high glucose and l-glutamine) (Thermo Fisher Scientific, Waltham, MA, USA), 50 U/mL P/S, and 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and kept on ice. Cells were centrifuged for 5 minutes at 1,200 rpm, at 4°C. Cells were then resuspended in media and counted using a hemocytometer. Cells were plated (50 μL) for micro mass culture, adding 50,000 cells per well, using 6-well polystyrene flat bottom microplates (Fisher Scientific, Hampton, NH, USA). Three milliliters of media were added to each well 2.5 hours after plating cells. Media was changed daily and cells were treated for 14 days. Cells were kept in a 5% oxygen incubator. There were 3 biological replicates for each group. Human PTH [1-34] (Prospec-Tany TechnoGene Ltd., Ness Ziona, Israel) dissolved in 1 mg/mL in 4 mM HCl with 0.1% BSA was stored at minus 20°C. The stock PTH was diluted in PBS to make a working concentration of 25 µg/mL. The final concentration of PTH used in media was 50 µg/mL, and PBS was used for control cells.
RNA Isolation and Gene Expression
RNA was isolated using Trizol (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol. For quantitative polymerase chain reaction (qPCR) analysis, 1 µg of RNA was used from each sample for reverse transcription using Superscript II (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer instructions, with oligo dT 12-18 primer and RNase OUT RNase inhibitor. Sequences of primers (IDT) used in qPCR are found in Supplementary Table S1 (available in the online version of the article). qPCR was carried out using a BioRad CFX instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). We used the Sybr Select Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) with a 20 µL reaction volume. The qPCR protocol used was as follows: 50°C for 2 minutes, followed by a 95°C step for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A dissociation curve was then run and the temperature cycled from 95°C to 65°C and back to 95°C. Melting curves were examined to determine product specificity. We used 96-well plates and samples were run in technical duplicate and averaged. The delta-delta Ct method was used for analysis. GAPDH was used to normalize the expression values. Data are expressed as fold-change versus the control ± the standard error of the mean (SEM). The primers for the genes analyzed are in Table 1.
Table 1.
Primers for the Genes Analyzed.
|
Col10a1 Alp Sox9 Fgf2 Ihh Prg4 |
Statistical Analysis
Descriptive statistics were used to examine the distribution of bone volume fraction, tissue density, trabecular thickness, trabecular spacing, histological analysis and gene expression. A 1-sample Kolmogorov-Smirnov test was used to examine the normality of data distribution. Outcomes were compared between the I-PTH and control groups. Statistically significant differences among means were determined by unpaired t test (Student t test). All statistical tests were 2 sided and a P value of <0.05 was deemed to be statistically significant. Statistical analyses were computed using GraphPad Prism (San Diego, CA, USA)
Results
In Vivo Results
Micro-CT Analysis
Our goal was to compare the calcified tissue mass after long-term treatment with I-PTH (Fig. 1A). We observed there was a significant increase in the BVF in the experimental group (24.08%) when compared with the control group (Fig. 1B). Similarly, there was significant increase in the tissue density (6.13%; Fig 1C) and trabecular thickness (9.75%; Fig. 1D) in the experimental group when compared with the control group. However, there was a significant decrease (14.71%; Fig. 1E) in trabecular spacing in the experimental group when compared with the control group. These results suggest that I-PTH treatment can trigger an anabolic effect, increasing mineralization at the MCC and subchondral region.
Figure 1.
Increased bone volume and density at the mandibular condyle after long-term intermittent parathyroid hormone (I-PTH) administration. Coronal micro–computed tomography (micro-CT) images of condyles of control (CTRL) and I-PTH-injected mice (A). Quantification of bone parameters: (B) BVF = bone volume fraction, (C) tissue density, (D) trabecular thickness, and (E) trabecular spacing. Histograms (B-E) represent means ± standard deviation (SD) for n = 8 per group. Statistically significant difference between groups: *P < 0.05. Region of interest is illustrated by dotted lines in (A). Scale bar = 500 µm.
Histological Findings
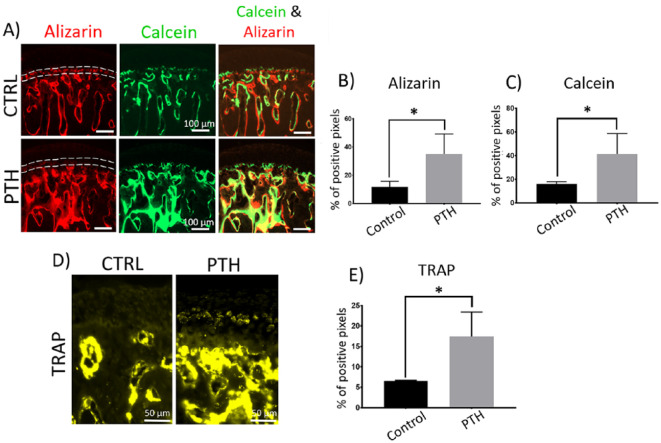
In general, histological analysis showed that the MCC had a smooth surface and normal cellularity with no cellular or matrix abnormalities in both I-PTH and control groups. The mineralization label that separates the calcified and noncalcified cartilage (Tidemark) was present in both the I-PTH and the control group (Fig. 2A). However, the quantity of mineral deposition was significantly higher in the I-PTH group, as represented by an increased uptake of fluorochrome markers into the mineralizing matrix, when compared to the control group. The amount of alizarin complexone in the subchondral bone was approximately 242% higher in the I-PTH group, whereas the amount of calcein was approximately 160% higher in the I-PTH group when compared with control (Fig. 2B and C). Additionally, we noticed there was a faint line of alizarin complexone in the tidemark of the in I-PTH group, whereas the control group had higher intensity of alizarin complexone in that region (Fig. 2A). This finding suggests an increased dynamic remodeling in the I-PTH group, given that alizarin complexone was the first bone label injected. In addition, I-PTH stimulated the catabolic remodeling of the subchondral bone as observed by a significant increase in the TRAP stained region (127% increase) in the subchondral bone (Fig. 2D and E). We also observed TRAP positive cells in the hypertrophic zone of the MCC in the I-PTH group, but none in the control group (Fig. 2D).
Figure 2.
Increased mineralization and bone remodeling at the subchondral region of mandibular condyle after long-term intermittent parathyroid hormone (I-PTH) administration. Sagittal sections of mandibular condyles of control (CTRL) and I-PTH-injected mice illustrating alizarin complexone and calcein labeling (A). Dotted lines in (A) in the alizarin complexone images in CTRL and PTH images represent the tidemark: faint line of alizarin complexone is observed in the PTH image. Quantification of alizarin complexone (red, B) and calcein (green, C) percentage of positive pixels over the subchondral bone area. Sagittal sections of mandibular condyles of control (CTRL) and I-PTH-injected mice stained for tartrate-resistant acid phosphatase (TRAP) (D). Quantification of percentage of TRAP-positive pixels (yellow, E) in the subchondral bone area. Histograms (B, C, and E) represent means ± standard deviation (SD) for n = 5 per group. Statistically significant difference between groups: *P < 0.05. Scale bar = 100 µm (A) and 50 µm (D).
Regarding the nonmineralized portion of the cartilage, we observed that I-PTH induced a significant increase in thickness compared with the control group (Fig. 3A). The cartilage distance mapping was measured on Safranin O–stained sections and our measurements revealed that the I-PTH group presented with a 21.4% increase in cartilage thickness when compared with the control group (Fig. 3B). Furthermore, we observed that there was a significant increase in cellular proliferation in the I-PTH group (Fig. 3C) as evidenced by an increase in EdU-positive cells (126% more EdU-positive pixels when compared with the control group; Fig. 3D).
Figure 3.
Increased cartilage thickness and chondrocyte proliferation at the mandibular condylar cartilage (MCC) after long-term intermittent parathyroid hormone (I-PTH) administration. Sagittal sections of mandibular condyles of control (CTRL) and I-PTH-injected mice stained for Safranin O (A). MCC and subchondral bone area (Sub. Bone) are labeled. Quantification of cartilage thickness (B). Sagittal sections stained for 5-ethnyl-2′-deoxyuridine (EdU) (C). Quantification of EdU-positive pixels (yellow), representing the amount of cellular proliferation, over DAPI-positive pixels (blue) at the proliferative zone (D). Histograms (B and D) represent means ± standard deviation (SD) for n = 5 per group. Statistically significant difference between groups: *P < 0.05. Scale bar = 50 µm.
To further understand the in vivo changes in the mandibular condyle correlated with I-PTH, we performed immunohistochemistry assays for SOST, VEGF, MMP13, and ADAM-TS4 after I-PTH treatment (Fig. 4).
Figure 4.
Decreased expression of sclerostin (SOST), matrix metallopeptidase 13 (MMP13), and aggreganase-1 (ADAM-TS4) and increased expression of vascular endothelial growth factor (VEGF) at the mandibular condyle of intermittent parathyroid hormone (I-PTH)–injected mice. Immunohistochemistry for SOST (A), VEGF (B), MMP13 (C), and ADAM-TS4 (D) in sagittal sections of condyles. Scale bar = 50 µm.
I-PTH decreased the expression of SOST, a negative regulator of mineralization, at the hypertrophic region of the MCC (Fig. 4A). In addition, we observed an increase in the expression of the angiogenesis promotor VEGF, in both MCC and subchondral region of the I-PTH group (Fig. 4B). Furthermore, the expression of MMP13, a collagenase involved in extracellular matrix breakdown, was decreased in the MCC of I-PTH-treated mice (Fig. 4C). Similarly, I-PTH decreased the expression of ADAM-TS4, a major proteinase that degrades proteoglycans (Fig. 4D).
In Vitro Results
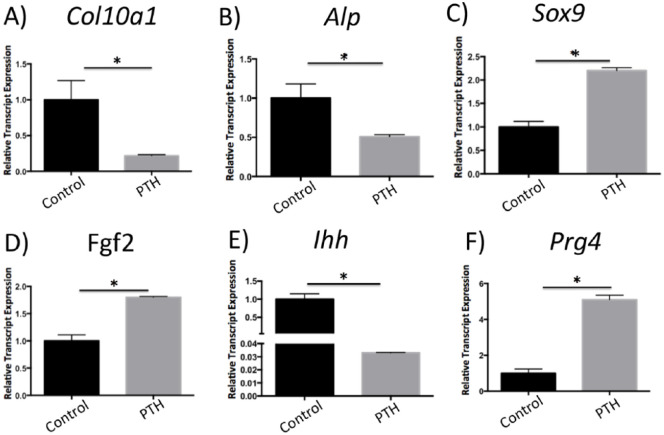
We examined the RNA expression of genes relevant to chondrogenesis and endochondral ossification in primary chondrocyte micro mass cultures treated with I-PTH by qPCR. There was no significant difference between I-PTH-treated chondrocytes and control groups when the expression of Sost, Bmp2, Noggin, Col2a1, Opn, and Runx2 were analyzed by qPCR. However, we found a remarkable decrease in Col10a1 (Fig. 5A) and Alp (Fig. 5B), important markers for cartilage mineralization. In addition, we observed a significant increase in Sox9 (Fig. 5C) and Fgf2 (Fig. 5D) and a significant decrease in Ihh (Fig. 5E), suggesting that I-PTH affects chondrocyte proliferation and differentiation. Interestingly, we observed a substantial increase (more than 5-fold) in Prg4 (Fig. 5F), a novel finding suggesting that I-PTH may improve lubrication and chondrocyte survival in the MCC.
Figure 5.
Gene expression changes in chondrocyte micro mass cultures treated with intermittent parathyroid hormone (I-PTH). Histograms represent relative gene expression tested by quantitative polymerase chain reaction (qPCR) 14 days after I-PTH treatment. The relative expression of Col10a1 (A), Alp (B), and Ihh (E) was markedly decreased, while the expression of Sox9 (C), Fgf2 (D), and Prg4 (F) was significantly increased. Statistically significant difference between groups: *P < 0.05.
Discussion
To the best of our knowledge, this is the first study to demonstrate the long-term effects of I-PTH on the MCC and the subchondral bone of the TMJ. This study chiefly demonstrates that although I-PTH showed increased mineralization of the calcified cartilage and the subchondral bone but the cartilage thickness and metabolism of cartilage were better preserved by I-PTH.
The anabolic action of I-PTH in bone has been studied for many years, yet the molecular mechanisms underlying its anabolic action in the MCC of the TMJ are still incompletely elucidated.
Our in vivo results have shown increased mineralization and turnover in the calcified cartilage and the subchondral bone region, accompanied by increased cellular proliferation and cartilage thickness, as a result of 4 weeks of daily I-PTH injection. These results are consistent with our previous reports in younger and older mice when I-PTH was administrated for shorter periods of time.7,8 In addition, we found decreased expression of SOST and increased expression of VEGF at the MCC of I-PTH-injected mice. SOST is a mineralization inhibitor and a classic target of PTH in bone,3,10 while VEGF is known to promote the endochondral vascularization11 and has been linked to the I-PTH anabolic effects in long bones of rats.12 The altered expression of these proteins correlates with the increased bone volume, tissue (mineral) density, and mineralization after I-PTH administration. Our data are consistent with the literature on the anabolic effects of SOST and VEGF on bone after I-PTH. However, can this enhanced mineralization of the subchondral bone and the calcified cartilage lead to degeneration of the unmineralized portion of the cartilage and subsequently result in osteoarthritis? This question prompted us to analyze, if in addition to increase the mineralization at the hypertrophic and subchondral bone regions, I-PTH treatment could cause the destruction of the extracellular matrix. Our immunostaining suggested a reduced expression of MMP13, a collagenase involved in extracellular matrix breakdown and osteoarthritis.13,14 Furthermore, I-PTH administration induced an inhibition of ADAMTS4, a proteoglycan-degrading enzyme (aggrecanase-1) correlated with degradation.14 This is also consistent with the increased cartilage thickness, proteoglycan distribution and chondrocyte proliferation observed in our in vivo results. Taken together, these results suggest that I-PTH seems to impair the extracellular matrix catabolism and treatment with I-PTH seems to induce a protective effect against extracellular matrix degradation at the MCC, despite the enhanced anabolic mineralization in the calcified cartilage and the subchondral bone region of the TMJ. Similar findings in vertebral disc of rats have been reported by Zhou et al.,15 who tested the effects of intermittent treatment of PTH in ovariectomized rats. The authors found not only an improvement of bone volume and density at vertebral body but also an enhancement of disk extracellular matrix and a decrease in the expression of MMP13 and ADAM-TS4.15 The dual effect of I-PTH in cartilage and bone have also been described in articular cartilage studies; I-PTH has been shown to prevent and repair osteochondral defects by stimulating both articular cartilage and subchondral bone regeneration.5,16 Furthermore, a reduction of SOST and MMP13 in the articular cartilage of an osteoarthritis model after treatment with PTH has been reported,16 which is consistent with our present results.
Our in vitro results only partially replicated the in vivo findings. The primary chondrocyte micro mass cultures consisted of cells extracted from the MCC only, which could explain the different behavior in response to I-PTH. Although we found increased expression of markers for chondrocyte proliferation in vitro (upregulation of Fgf2), we observed decreased markers for mineralization (reduced Col10a1 and Alp), which contradicts our in vivo findings.
We found increased relative gene expression of Sox9 in I-PTH-treated chondrocytes. SOX9 is a transcription factor with important roles for chondrocyte survival and inhibiting of differentiation17,18 and its phosphorylation has been associated with activation of the PTH receptor.19 Furthermore, we observed significant suppressed expression of Ihh in our I-PTH treated chondrocytes in in vitro experiments. IHH is another essential player in chondrogenic differentiation, which has also been implicated with activation of the PTH receptor 19. These results suggest that I-PTH increases mandibular cartilage thickness by increasing chondrocyte proliferation (by increasing Fgf2), delaying chondrocyte differentiation (by increasing Sox9) and inhibiting chondrocyte differentiation (by decreasing Ihh). Finally, the decrease in chondrocyte terminal differentiation leads to a decrease in Col10a1 and Alp in the MCC.
An interesting novel finding was that the relative gene expression of proteoglycan 4 (Prg4) was substantially increased in the in vitro I-PTH-treated chondrocytes. Prg4, which is highly expressed in bone and articular joints and induce a protective and anti-inflammatory effect at the articular joints,20-22 and has been identified as a new target of PTH for skeletal anabolism.23 We suggest that I-PTH may induce a protective effect at the mandibular condyle by stimulating Prg4. One of the limitations of our study is that the experiments were only done in male mice. However, our future studies are focusing on both male and female mice. Furthermore, we are studying the effects of I-PTH in repair and regeneration of the cartilage in an injury model (partial discectomy).
Conclusions
In summary, the present research showed that long-term administration of I-PTH leads to anabolic bone formation at the mandibular condyle, while maintains the integrity of the unmineralized portion of the MCC and increases the thickness of the cartilage. In addition, I-PTH seems to induce a chondroprotective effect at the MCC. Moreover, long-term I-PTH exhibited better performance in balancing the anabolic and catabolic metabolism of the extracellular matrix. Our future directions include using a mouse model with degeneration of the TMJ to investigate whether I-PTH could be used as a therapeutic treatment to improve this condition.
Supplemental Material
Supplemental material, Supplementary_Table_S1 for Intermittent Parathyroid Hormone [1-34] Augments Chondrogenesis of the Mandibular Condylar Cartilage of the Temporomandibular Joint by Eliane H. Dutra, Mara H. O’Brien, Po-Jung Chen, Mei Wei and Sumit Yadav in CARTILAGE
Footnotes
Acknowledgments and Funding: We would like to thank Li Chen for her help with the images. The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institute of Health under Award Number KO8DE025914 to SY, by the Connecticut Institute for Clinical and Translational Science Award to EHD, and by the American Association of Orthodontic Foundation provided to SY and EHD.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Ethical Approval: The Institutional Animal Care Committee of the University of Connecticut Health Center approved the experimental protocol involving the mice in this study.
ORCID iD: Sumit Yadav  https://orcid.org/0000-0002-2434-7995
https://orcid.org/0000-0002-2434-7995
Supplemental Material: The supplementary material for this article is available online.
References
- 1.Hansen S, Hauge EM, Jensen JEB, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28(4):736-45. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici R.T cells, osteoblasts, and osteocytes: interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann N Y Acad Sci. 2016;1364:11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva BC, Bilezikian JP.Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki K, Suda N, Kuroda T.Distribution of parathyroid hormone-related protein (PTHrP) and type I parathyroid hormone (PTH) PTHrP receptor in developing mouse mandibular condylar cartilage. Arch Oral Biol. 1999;44(10):853-60. [DOI] [PubMed] [Google Scholar]
- 5.Orth P, Cucchiarini M, Zurakowski D, Menger MD, Kohn DM, Madry H.Parathyroid hormone [1-34] improves articular cartilage surface architecture and integration and subchondral bone reconstitution in osteochondral defects in vivo. Osteoarthritis Cartilage. 2013;21(4):614-24. [DOI] [PubMed] [Google Scholar]
- 6.Kudo S, Mizuta H, Takagi K, Hiraki Y.Cartilaginous repair of full-thickness articular cartilage defects is induced by the intermittent activation of PTH/PTHrP signaling. Osteoarthritis Cartilage. 2011;19(7):886-94. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien MH, Dutra EH, Lima A, Nanda R, Yadav S.PTH [1-34] induced differentiation and mineralization of mandibular condylar cartilage. Sci Rep. 2017;7(1):3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutra EH, O’Brien MH, Gutierrez T, Lima A, Nanda R, Yadav S.PTH [1-34]-induced alterations predispose the mandibular condylar cartilage to mineralization. Orthod Craniofac Res. 2017;20(Suppl 1):162-6. [DOI] [PubMed] [Google Scholar]
- 9.Dyment NA, Hagiwara Y, Jiang X, Huang J, Adams DJ, Rowe DW.Response of knee fibrocartilage to joint destabilization. Osteoarthritis Cartilage. 2015;23(6):996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer I, Keller H, Leupin O, Kneissel M.Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010;21(4):237-44. [DOI] [PubMed] [Google Scholar]
- 11.Carlevaro MF, Cermelli S, Cancedda R, Cancedda FD.Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113 (Pt 1):59-69. [DOI] [PubMed] [Google Scholar]
- 12.Prisby R, Menezes T, Campbell J.Vasodilation to PTH (1-84) in bone arteries is dependent upon the vascular endothelium and is mediated partially via VEGF signaling. Bone. 2013;54(1):68-75. [DOI] [PubMed] [Google Scholar]
- 13.Bae JW, Takahashi I, Sasano Y, Onodera K, Mitani H, Kagayama M, et al. Age-related changes in gene expression patterns of matrix metalloproteinases and their collagenous substrates in mandibular condylar cartilage in rats. J Anat. 2003;203(2):235-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Liu Y, Ding W, Long T, Shi J.Expression of hypoxia inducible factor-2 alpha in overloaded-stress induced destruction of mandibular condylar chondrocytes. Arch Oral Biol. 2017;77:51-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Tian F-M, Gou Y, Wang P, Zhang H, Song HP, et al. Enhancement of lumbar fusion and alleviation of adjacent segment disc degeneration by intermittent PTH(1-34) in ovariectomized rats. J Bone Miner Res. 2016;31(4):828-38. [DOI] [PubMed] [Google Scholar]
- 16.Yan JY, Tian FM, Wang WY, Cheng Y, Song HP, Zhang YZ, et al. Parathyroid hormone (1-34) prevents cartilage degradation and preserves subchondral bone micro-architecture in guinea pigs with spontaneous osteoarthritis. Osteoarthritis Cartilage. 2014;22(11):1869-77. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B.The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda T, Kawaguchi H, Kamekura S, Ogata N, Mori Y, Nakamura K, et al. Distinct roles of Sox5, Sox6, and Sox9 in different stages of chondrogenic differentiation. J Bone Miner Metab. 2005;23(5):337-40. [DOI] [PubMed] [Google Scholar]
- 19.Kronenberg HM.How PTHrP controls growth plate chondrocytes. IBMS BoneKEy. 2005;2(11):7-15. [Google Scholar]
- 20.Hill A, Duran J, Purcell P.Lubricin protects the temporomandibular joint surfaces from degeneration. PLoS One. 2014;9(9):e106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL.Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56(3):882-91. [DOI] [PubMed] [Google Scholar]
- 22.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novince CM, Michalski MN, Koh AJ, Sinder BP, Entezami P, Eber MR, et al. Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism. J Bone Miner Res. 2012;27(1):11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_S1 for Intermittent Parathyroid Hormone [1-34] Augments Chondrogenesis of the Mandibular Condylar Cartilage of the Temporomandibular Joint by Eliane H. Dutra, Mara H. O’Brien, Po-Jung Chen, Mei Wei and Sumit Yadav in CARTILAGE