Dear Editor,
Intrinsic features of the humoral immune response including antigen specificity, antibody glycosylation, and subclass of immunoglobulin (Ig) G have been shown to influence the progression of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection and Coronavirus-19 disease (COVID-19) outcomes [1], [2], [3], [4]. Specifically, a poor COVID-19 outcome reportedly depends on an imbalanced humoral response whereby impaired viral neutralization translates into excessive systemic inflammation [2]. Severe COVID-19, for instance, has been associated with higher serum concentration of pro-inflammatory spike-specific IgG3 and afucosylated IgG1 antibodies capable of triggering exaggerated macrophage activation [2], [3], [4]. Similarly, anti type I interferon (IFN) autoantibodies have been demonstrated in critical COVID-19 patients, phenocopying inborn errors of type I IFN immunity and leading to impaired innate and intrinsic antiviral immune responses [1]. On the other hand, anti-spike monoclonal neutralizing antibodies of IgG1 subclass from the plasma of convalescent COVID-19 patients have been shown to prevent life-threatening disease and are currently under evaluation in clinical trials [5].
In view of their different inflammatory properties, we aimed to assess a possible association between IgG subclasses and COVID-19 related mortality. One hundred twenty-eight consecutive patients (41 females, 31.3 %) referred to the Emergency Department of San Raffaele Hospital (Milan, Italy) for COVID-19 between June and December 2020 were included and prospectively followed-up with daily data collection into an electronic case report form (COVID-BioB Study, Ethical Committee approval no. 34/int/2020, ClinicalTrials.gov NCT04318366) (Table 1 ). All patients tested positive for SARS-CoV-2 nasopharyngeal swab and were treated with local standard of care (6 mg/day intravenous dexamethasone for 10 days and 4000 units/day subcutaneous enoxaparin) in addition to antibiotic and antipyretic therapy. IgG subclasses were measured before the institution of glucocorticoid treatment.
Table 1.
Clinical features of the patients’ cohort and predictors of mortality. Data describing clinical and serological features of the patients’ cohort are reported as median (interquartile range). Data describing univariate and multivariate analyses are reported as odds ratio. P value (significant difference < 0.05) considers the comparison between non-survivors and survivors.
| Total (N=128) | Survivors (n=98) | Non-survivors (n=30) | p value | OR | Univariate Analysis (p value) | OR | Multivariate Analysis (p value) | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 69 (57-81) | 64 (55-77) | 82.5 (73-86) | 0.0001 | 1.06 | 0.0001 | 1.05 | 0.02 |
| Sex (M) | 87 (67%) | 64 (65%) | 23 (23%) | 0.27 | 0.68 | 0.36 | ||
| Diabetes n (%) | 28 (21.9%) | 14 (14%) | 14 (47%) | 0.007 | 1.4 | 0.38 | ||
| Hypertension n (%) | 67 (52%) | 44 (45%) | 23 (77%) | 0.003 | 2.99 | 0.01 | 0.41 | 0.08 |
| PaO2/FiO2 (mmHg) | 275 (204-325) | 285 (260-335) | 204 (98-300) | 0.002 | 0.99 | 0.015 | 0.99 | 0.62 |
| ESR (< 20 mm/hr) | 69 (46-92) | 65 (42-77) | 95 (50-110) | 0.17 | 1.02 | 0.27 | ||
| CRP (< 6 mg/L) | 80 (42-150) | 70 (33-125) | 120 (62-195) | 0.004 | 1.01 | 0.002 | 1.01 | 0.001 |
| IL-6 (<10 pg/mL) | 37.35 (16-87.3) | 30.4 (10.4-75) | 67.3 (36.4-224) | 0.007 | 1 | 0.96 | ||
| IgG1 (3824-9286 mg/L) | 5294 (3961-6345) | 5294 (4007-6403) | 5338 (3872-6425) | 0.88 | 1 | 0.68 | ||
| IgG2 (2418-7003 mg/L) | 3625 (2702-4759) | 3639 (2675-4633) | 3481 (2824-5039) | 0.67 | 1 | 0.3 | ||
| IgG3 (218-760 mg/L) | 749 (502-1038) | 756 (491-1217) | 687 (487-840) | 0.34 | 0.99 | 0.19 | ||
| IgG4 (39-864 mg/L) | 352 (207-558) | 340 (195-471) | 426 (269-757) | 0.04 | 1 | 0.002 | NA | |
| IgG (8400-16000 mg/L) | 10026 (8837-12197) | 9014 (8156-11920) | 10032 (7611-13088) | 0.78 | 1 | 0.73 | ||
| IgG4/IgG | 0.03 (0.02-0.05) | 0.03 (0.02-0.05) | 0.05 (0.03-0.08) | 0.007 | 1.01 | 0.04 | NA | |
| IgG4/IgG1 | 0.06 (0.04-0.11) | 0.06 (0.04-1) | 0.07 (0.05-0.17) | 0.012 | 2406 | 0.0001 | 292.5 | 0.01 |
Abbreviations: erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); interleukin-6 (IL-6); Odds ratio (OR).
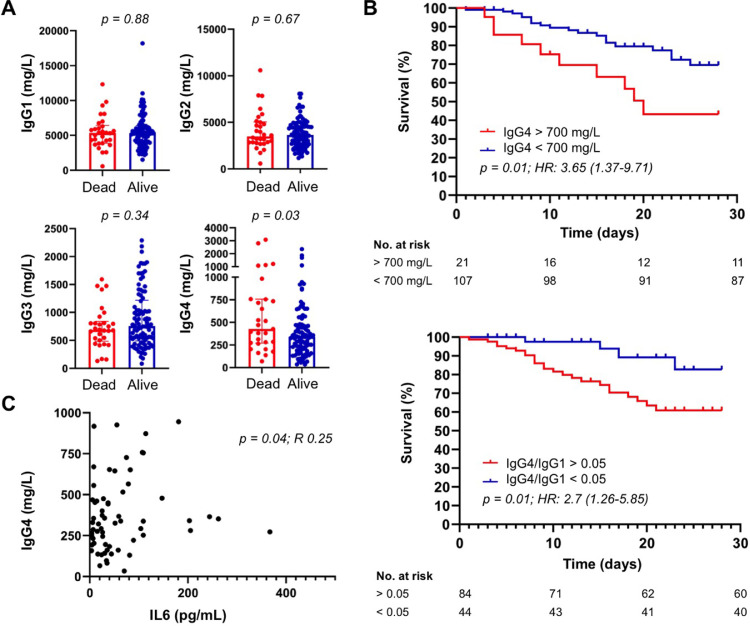
At baseline, IgG1, IgG2, IgG3, IgG4 subclasses were elevated in 8 (6%), 6 (5%), 6 (5%), and 13 (10%) patients, respectively. Thirty patients (23%) died at 30-days follow-up. As shown in Table 1 and Fig. 1 A, age, C-reactive protein (CRP), interleukin (IL)-6 serum IgG4 level, IgG4/IgG ratio, and IgG4/IgG1 ratio were significantly higher in non-survivors, while the PaO2/FiO2 ratio was significantly lower in survivors. Receiving operating curves (ROC) curves of statistically significant variables were created to predict mortality at 30 days. The AUC for age, serum IgG4, IgG4/IgG and IgG4/IgG1 ratio was 0.78 (95%CI 0.69-0.97), 0.63 (95%CI 0.51-0.75), 0.66 (95%CI 0.55-0.76), and 0.65 (95%CI 0.53-0.77), respectively (p<0.05 for all the analyses). At logistic regression analysis, all variables were significantly associated with a poor outcome but only age, CRP, and the IgG4/IgG1 ratio represented independent predictors of 30-days mortality at multivariate analysis (Table 1). Specifically, a concentration of serum IgG4 > 700 mg/dl and an IgG4/IgG1 ratio > 0.05 were associated with a significantly increased mortality at 30-days (Fig. 1B). Of note, a significantly positive correlation was found between serum IgG4 and IL-6 level, an established predictor of COVID-19 related mortality (Fig. 1C) [8], [9], [10].
Fig. 1.
Serum IgG4 level predicts COVID-19 related mortality. (A) Serum IgG4 concentration, but not IgG1, IgG2, and IgG3, is significantly higher in COVID-19 non-survivors compared to survivors (p value < 0.05). (B) Increased serum IgG4 level (> 700 mg/L) and IgG4/IgG1 ratio (> 0.05) are associated with increased mortality in COVID-19 patients (p value < 0.05 for both survival analyses). (C) Serum IgG4 concentration positively correlates with serum IL-6 levels (p value < 0.05).
In this prospective study we found that serum IgG4 level predicts a poor COVID-19 outcome. Based on the available literature, IgG4 antibodies may contribute to COVID-19 progression via at least two possible mechanisms, yet to be verified. Because anti-spike IgG4 have shown poor in vitro neutralizing capacity compared to IgG1, IgG2, and IgG3 antibodies, a first possibility is that hosts with prominent IgG4 immune responses might be more permissive to SARS-CoV-2 infection [6]. On the other hand, as neutralizing anti-IFNγ autoantibodies observed in adult patients with multiple opportunistic infections are predominantly of IgG4 subclass, it is tempting to speculate that anti-IFN antibodies associated with impaired anti-SARS-CoV-2 immunity and life-threatening COVID-19 pneumonia might also be IgG4 [7]. Despite intrinsic limitations mainly related to the limited number of patients enrolled, our study identifies IgG4 antibodies as a possible additional overlooked variable of the humoral immune response against SARS-CoV-2 associated with COVID-19 progression.
Contributorship
ML and EDT equally contributed to design of the work. All authors contributed to acquisition, analysis and interpretation of data. All authors revised the work critically for important intellectual content and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval information
This study was approved by the San Raffaele Hospital Ethical Committee (no. 34/int/2020).
Data sharing statement
The authors agree to share the data generated by the present research and to make them openly and publicly available upon publication.
Declaration of Competing Interest
The authors have not received any financial support or other benefits from commercial sources for the work reported in the manuscript, or any other financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
References
- 1.Bastard P, Rosen LB, Zhang Q. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. .e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty S, Gonzalez J, Edwards K. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2020;22:67–73. doi: 10.1038/s41590-020-00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates JL, Ehrbar DJ, Hunt DT. Serological analysis reveals an imbalanced IgG subclass composition associated with COVID-19 disease severity. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suthar MS, Zimmerman MG, Kauffman RC. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne SK, Burbelo PD, Chetchotisakd P. Adult-onset immunodeficiency in Thailand and Taiwan. NEJM. 2012;367:725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della-Torre E, Lanzillotta M, Campochiaro C. Respiratory impairment predicts response to IL-1 and IL-6 blockade in COVID-19 patients with severe pneumonia and hyper-inflammation. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.675678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Della-Torre E, Campochiaro C, Cavalli G. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della-Torre E, Della-Torre F, Kusanovic M. Treating COVID-19 with colchicine in community healthcare setting. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108490. [DOI] [PMC free article] [PubMed] [Google Scholar]