Abstract
Background
The WHO declared COVID-19 a pandemic on March 11th, 2020. This serious outbreak and the precipitously increasing numbers of deaths worldwide necessitated the urgent need to develop an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. The development of COVID-19 vaccines has moved quickly. In this study, we assessed the efficacy, safety, and immunogenicity of an inactivated (SARS-CoV-2) vaccine.
Methods
We conducted a randomized, double-blind, placebo-controlled trial to evaluate the efficacy, immunogenicity, and safety of an inactivated SARS-CoV-2 vaccine and its lot-to-lot consistency. A total of 1620 healthy adults aged 18–59 years were randomly assigned to receive 2 injections of the trial vaccine or placebo on a day 0 and 14 schedule. This article was based on an interim report completed within 3 months following the last dose of study vaccine. The interim analysis includes safety and immunogenicity data for 540 participants in the immunogenicity subset and an efficacy analysis of the 1620 subjects. For the safety evaluation, solicited and unsolicited adverse events were collected after the first and second vaccination within 14 and 28 days, respectively. Blood samples were collected for an antibody assay before and 14 days following the second dose.
Results
Most of the adverse reactions were in the solicited category and were mild in severity. Pain at the injection site was the most frequently reported symptom. Antibody IgG titer determined by enzyme-linked immunosorbent assay was 97.48% for the seroconversion rate. Using a neutralization assay, the seroconversion rate was 87.15%. The efficacy in preventing symptomatic confirmed cases of COVID-19 occurring at least 14 days after the second dose of vaccine using an incidence rate was 65.30%.
Conclusions
From the 3-month interim analysis, the vaccine exhibited a 65.30% efficacy at preventing COVID-19 illness with favorable safety and immunogenicity profiles.
Keywords: Adults, Efficacy, Safety, Immunogenicity, SARS-CoV-2 Inactivated Vaccine
Abbreviations: COVID-19, Coronavirus Disease 2019; ELISA, Enzyme Link Immunoassay; GMT, Geometric Mean Titer; IgG, Immunoglobulin G; rRT-PCR, Real-time Reverse Transcriptase-PCR; SARS, Severe Acute Respiratory Syndrome; WHO, World Health Organization
1. Introduction
The coronavirus disease 2019 (COVID-19) has inflicted catastrophic damage to public health, economic, and social stability worldwide [1]. In December 2019, a series of pneumonia cases of unknown origin emerged in Wuhan, Hubei, China, with clinical a presentation resembling viral pneumonia. The outbreak began in early November or December and the number of cases quickly rose. As of May 2020, >80,000 cases were confirmed in China, including healthcare workers, which resulted in>4,000 deaths [2], [3], [4], [5]. The virus is airborne, highly transmissible between humans, and has a long and insidious incubation period. The outbreak rapidly escalated out of China and throughout the world, pushing the World Health Organization (WHO) to declare a pandemic on March 11th, 2020 [6]. As of December 20th, 2020, the number of COVID-19 cases was>75 million with over 1.6 million deaths occurring globally [7]. Based on a WHO report, by January 20th, 2021, there were 939,948 confirmed cases of COVID-19 with 26,857 deaths in Indonesia [8].
Currently, there is no effective treatment available for coronavirus infection. Vaccination is crucial for blocking the rapid spread of deadly infectious diseases, such as the highly contagious COVID-19, especially when effective treatments or cures are not available [9]. Significant efforts have been focused on the development of vaccines and therapeutic drugs. Over the past decade, the scientific community and the vaccine industry have been asked to respond urgently to epidemics including H1N1 influenza, Ebola, Zika, and most recently, SARS-CoV-2 [10]. The WHO is currently preparing a comprehensive analysis of vaccine and therapeutic drug candidates that may be effective against SARS-CoV-2 and will use an evidence-based framework to transparently select the most promising therapeutic and vaccine candidates to evaluate in the clinic [11]. Multiple SARS-CoV-2 vaccines types, such as DNA-based and RNA-based formulations, recombinant subunit-containing viral epitopes, adenovirus-based vectors, and purified inactivated virus are under development. Purified inactivated viruses have been traditionally used for vaccine development and have been found to be safe and effective for preventing many viral diseases including influenza and polio [12], [13], [14].
As of January 25th, 2021, there are 64 vaccines in human clinical trials and 20 have reached the final stages of testing. At least 173 preclinical vaccines are under active investigation in animals [15].The preclinical study results of inactivated SARS-CoV-2 Vaccine (Vero Cell), developed by Sinovac Life Sciences Co. Ltd. indicate that the vaccine provided partial or complete protection in macaques from severe interstitial pneumonia after a SARS-CoV-2 challenge without observable antibody dependent enhancement [16]. A phase I/II clinical trial has been conducted in China since April 2020. The preliminary results indicate a favorable safety and immunogenicity profile with a two-dose vaccine schedule. No significant changes in inflammatory factors were observed indicating a small risk of immunopathology induced by the SARS-CoV-2 vaccine [17].
In this article, we report the efficacy of inactivated SARS-CoV-2 vaccine in preventing COVID-19 including safety and immunogenicity data based on the phase III trial collected during a 3-month period after the second injection in 18–59 year-old subjects in Indonesia. This data set and trial results form the basis of an application for emergency use authorization in Indonesia.
2. Materials and methods
2.1. Study design and population
This study was an observer-blinded, randomized, placebo-controlled two arm with parallel groups, prospective intervention, phase III study that began in August 2020 in Bandung, Indonesia to evaluate the efficacy, immunogenicity, and safety of an inactivated SARS-CoV-2 vaccine and its lot-to-lot consistency. The main exclusion criteria included evolving mild, moderate, or severe illness, especially infectious disease or fever (body temperature ≥ 37.5℃), patients with serious chronic diseases, positive result from a nasopharyngeal swab RT-PCR test, reactive IgG and IgM for SARS-CoV-2, women who are lactating, pregnant or planning to become pregnant during the study period, serious chronic diseases (serious cardiovascular disease, uncontrolled hypertension and diabetes, liver and kidney disease, malignant tumors, or any condition which according to the investigator may interfere with the assessment of the trial objectives), uncontrolled coagulopathy or blood disorders, history of asthma, history of allergy to vaccines or vaccine ingredients, history of confirmed or suspected immunosuppressive or immunodeficient state, or received in the previous 4 weeks a treatment likely to alter the immune response [intravenous immunoglobulins, blood-derived products, or long-term corticosteroid therapy (>2 weeks)], history of uncontrolled epilepsy or other progressive neurological disorders, and having received any vaccination within 1 month before or after administration of the study vaccine.
After being informed about the study and signing an informed consent form, the medical history of the subjects was evaluated, and they were provided a physical exam. The blinded investigator team evaluated the inclusion and exclusion criteria. Eligible subjects were randomly assigned at a ratio of 1:1 into two study arms to receive either 3 μg/0.5 mL dose of inactivated SARS-CoV-2 vaccine or placebo on day 0 and 14. The randomization list was generated automatically using the website, www.sealedenvelope.com, and the vaccinated arms were grouped into three different batch numbers (batch 1/batch 2/batch 3) of SARS-CoV-2 vaccine. The subjects were randomized and vaccinated per treatment group by an unblinded team. The alphabetical code remained confidential and maintained by the unblinded team and was not to be opened until the end of the study.
The study protocol, subject information sheet and consent forms, and the subject’s diary card was approved by the Research Ethics Committee of Universitas Padjadjaran (Ethical Approval No. 669/UN6.KEP/EC/2020) and Indonesian Regulatory Authorities. This trial was conducted in accordance with ICH Good Clinical Practice guidelines, the Declaration of Helsinki, and local regulatory requirements. The clinical trial was registered at clinicaltrials.gov with entry number NCT04508075 and in the Indonesian Clinical Research Registry (INA-WXFM0YX).
2.2. Study vaccine
The study vaccine, developed by Sinovac Life Sciences Co., Ltd., was an inactivated SARS-CoV-2 whole virion vaccine with aluminum hydroxide as an adjuvant. The study vaccine was manufactured by inoculating novel coronavirus (CZ02 Strain) into African green monkey kidney cells (Vero Cell). The virus was successfully incubated, harvested, inactivated using β-propiolactone, concentrated, purified, and adsorbed by aluminum hydroxide. The bulk vaccine was then formulated with phosphate-buffered saline and sodium chloride as the inactivated final product. A dosage of 3 μg/0.5 mL was selected for this study. Three batches of study vaccine were used (20200308, 20200412, and 20200419). The placebo contained water for injection packaged in ampoules (0.5 mL/dose) and manufactured by PT Bio Farma. The study vaccine was administered intramuscularly into the left deltoid region by an unblinded investigator. The vaccine was stored at + 2℃ to + 8℃.
2.3. Surveillance for COVID-19 and efficacy assessment
The primary outcome of the study was to assess the efficacy of two doses of the inactivated SARS-CoV-2 vaccine in preventing COVID-19 cases compared with placebo. The primary efficacy endpoint was incidence of laboratory confirmed-symptomatic COVID-19 cases starting at 14 days following the second dose. COVID-19 case defined according to the case definition of the national guidelines for the diagnosis and treatment of COVID-19 in Indonesia [18]. Subjects were surveilled for COVID-19 disease after the first dose of vaccine by a combination of active and passive surveillance. The surveillance team performed monthly contact (by phone or text message) to actively collect information from subjects whether they have any symptoms suggesting COVID-19 disease or admitted to hospital for any reason. Any subject who has at least one specific symptoms (cough, taste or smell disorders, or dyspnea) or has two or more non-specific symptoms (fever, chills, sore throat, fatigue, nasal congestion or runny nose, body pain, muscle pain, headache, nausea, vomiting, or diarrhea) for at least two consecutive days was scheduled to have nasopharyngeal swab sample taken for SARS-CoV-2 rRT-PCR test. Subjects were also regularly reminded to report if they have any of the above symptoms.
The rRT-PCR was performed by the Central Laboratory of Universitas Padjadjaran. Nasopharyngeal samples were processed in a dedicated BSL-2 laboratory with BSL-3 practices under a certified Class II Biological Safety Cabinet. Once a clinical sample was treated with lysis buffer for RNA extraction, the samples then moved to a less restrictive environment to complete the RNA extraction and real-time RT-PCR. A 140 μl aliquot of the specimen was added to 560 μl of lysis buffer (Qiagen Viral Mini kit). RNA extraction was done based on the manufacturer’s protocol and immediately processed for RT-PCR. The remaining nucleic acid was stored at –80℃ for sequence analysis.
The real-time reverse transcriptase-PCR (rRT-PCR) reagent kit from ABT (Beijing Applied Bioscience Technology) and the Multiple Real-Time PCR Kit for Detection of 2019-nCoV were used. The results were analyzed by software provided by the manufacturer of the Light Cycler (Roche). Comparative viral load was calculated using the CT (Cycle Threshold) values of consecutive specimens. The incidence of suspected COVID-19 cases within 14 days to 6 months after the second dose of immunization was analyzed to determine efficacy.
2.4. Immunogenicity assessment
To assess the immune response, 4 mL blood samples were collected from 540 subjects before the first injection (Day 0) and 14 days after the second injection. The ability of the antibodies present in the blood sample to bind to the receptor binding domain (RBD) of SARS-CoV-2 was assessed blindly using an enzyme-linked immunosorbent assay (ELISA) at the Clinical Trial Laboratory of Bio Farma. The ELISA titers were determined by end point dilution and calculated using GraphPad Prism version 8.4.3 software [19], [20], [21]. The antibody increment and GMT 14 days post-last immunization were evaluated. ELISA seropositive antibody IgG titer was defined as titer > 200 and seroconversion was defined as a four-fold increase of anti-RBD antibody IgG titer (ELISA) at 14 days after two doses of vaccine compared with the baseline. The neutralization of antibody (NAb) assay was also conducted at the National Intitute of Health Reasearch & Development. A four-fold increase in antibody titer compared with the baseline value was considered as the measure of seroconversion. Seropositivity was defined as detected antibody ≥ 1:4. The immunogenicity data were analyzed in the per protocol population using SPSS software. Pre-vaccination titer levels for subjects with zero titer were assigned a value of 200 for ELISA and 2 to enable GMT and titer increment calculations.
2.5. Safety assessment
Subjects were given diary cards to record solicited adverse events (local pain, redness, swelling, induration, fever, myalgia, and malaise) and unsolicited adverse events occurring within 30 min, 7 days, and 8–28 days following each dose. Pain was graded as mild (pain at injection site when touched), moderate (pain with movements), and severe (significant pain at rest). Redness, induration, and swelling intensity were measured using a plastic bangle and categorized as mild (<5 cm), moderate (5–10 cm), and severe (>10 cm). Fever was graded as mild (38.0–38.4°C), moderate (38.5–38.9°C), and severe (≥39.0°C). Fatigue, myalgia, and unsolicited events were graded as mild (no interference with activity), moderate (some interference with activity not requiring medical intervention), and severe (prevents daily activity, requires medical intervention).
Any serious adverse events were reported up to 6 months after the second dose. Diary card was reviewed by the blinded investigator at 14 days following the first injection, 14, and 28 days after the second injection. The safety data were reviewed by a Data Safety Monitoring Board (DSMB) and analyzed in the intention-to-treat population using SPSS software.
2.6. Sample size determination and statistical analysis
The study was powered for efficacy analysis. Sample size was determined based on 95% confidence interval and 80% power. Assuming that 2% of the population would develop COVID-19 infection in the placebo arm, a minimum of 810 subjects in each vaccinated and placebo group would provide 80% power to reject the null hypothesis of no difference if the true efficacy was 60% with a 5% dropout rate. In this study, the total cohort was 1620 subjects with 810 subjects in the vaccinated group and 810 subjects in the placebo group.
Vaccine efficacy (VE) will be estimated by (1 - RR) × 100, where RR (relative risk) is calculated as the incidence in the vaccinated group divided by the incidence in the placebo group per person-years.
To analyze the immunogenicity, GMTs comparation between vaccine and placebo group was calculated after logarithmic transformation using t-test or ANOVA (F-test). Serum immune response proportions (seropositive rate, seroconversion) and vaccine lot-to-lot comparison was calculated using Chi-square test. The incidence rates of solicited and unsolicited adverse events between both groups were analyzed using Chi-square test. A p-value of<0.05 was considered to be significant.
3. Results
3.1. Study population
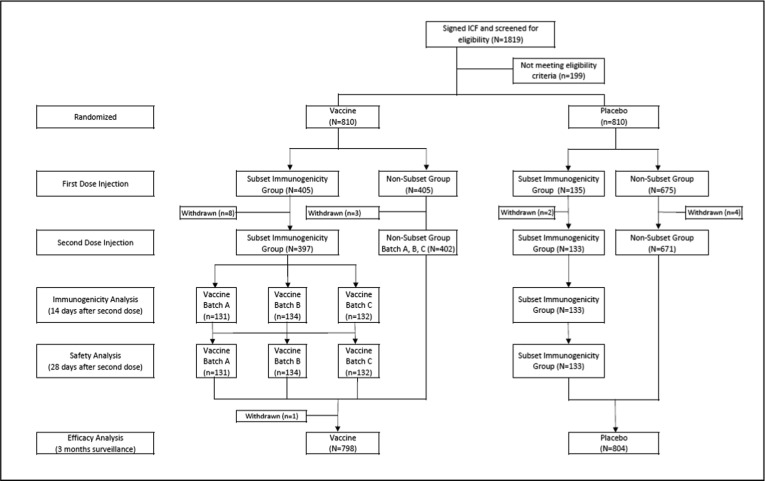
Between August 11, 2020, and October 21, 2020, a total of 1819 participants were screened and 199 subjects were excluded due to not meeting the inclusion criteria or meeting one of the exclusion criteria. From 1620 subjects randomized in the study, there were 17 subjects that withdrawn from the study prior to the second dose [Fig. 1 ]. The first 540 participants were included in the immunogenicity subset group.
Fig. 1.
Participant Disposition.
There were 1046 male participants (64.57%) and 574 female participants (35.43%). The participants were come from various age distribution from 18 to 59 years with average 35.5 ± 11.2 years old. Among the subset immunogenicity subjects, there were 314 male participants (58.15%) and 226 female participants (41.85%) with an average age of 35.82 years ± 11.4 years old. The details of the demographic data are provided in Table 1 .
Table 1.
Demographic Data.
| Parameter | Vaccine (N = 811) |
Placebo (N = 809) |
Total (N = 1620) |
|---|---|---|---|
| Mean age [years] (SD) | 35.6 (11.3) | 35.4 (11.0) | 35.5 (11.2) |
| Mean height [m] (SD) | 1.63 (0.09) | 1.63 (0.09) | 1.63 (0.09) |
| Mean weight [kg] (SD) | 65.6 (13.5) | 64.8 (13.6) | 65.2 (13.5) |
| BMI (kg/m2) | 24.8 (4.4) | 24.5 (4.5) | 24.6 (4.5) |
| Sex n(%) | |||
| Male | 505 (62.3) | 541 (66.8) | 1046 (64.57) |
| Female | 305 (37.7) | 269 (33.2) | 574 (35.43) |
| Demographic Data in the Immunogenicity Subset Group | |||
| Parameter | Vaccine (N = 405) |
Placebo (N = 135) |
Total (N = 540) |
| Mean age [years] (SD) | 36.0 (11.5) | 35.3 (10.9) | 35.82 (11.4) |
| Mean height [m] (SD) | 161.8 (8.9) | 161.7 (9.8) | 161.8 (9.2) |
| Mean weight [kg] (SD) | 64.6 (13.2) | 65.9 (13.6) | 64.9 (13.3) |
| BMI (kg/m2) | 24.6 (4.3) | 25.2 (4.7) | 24.75 (4.4) |
| Sex n(%) | |||
| Male | 229 (56.5) | 85 (63.0) | 314 (58.15) |
| Female | 176 (43.5) | 50 (37.0) | 226 (41.85) |
Abbreviations: N = number of participants, SD = Standard deviation.
All study vaccines were administered according to the randomization list. Treatment compliance was defined as receiving both doses of vaccine/placebo within the specified time period. For the 540 participants in the immunogenicity subset, 10 subjects withdrew prior to the second dose vaccination and not included in the immunogenicity analysis. Meanwhile, 1 subject withdrew after the second dose of the study vaccine. These dropout subjects included 9 from the vaccinated group and 2 from the placebo group. The details for treatment compliance in the subset immunogenicity group are presented in Table 2 . Early withdrawal resulted from consent withdrawal by the subject or the subject met the contraindication criteria for the second vaccination (not in healthy condition during the second vaccination schedule). The study results presented in this article are based on a preliminary immunogenicity and safety data analysis of 540 subjects in the immunogenicity subset group, whereas the efficacy results are based on preliminary efficacy data from 1620 subjects with median ∼ 2.5 months of surveillance period.
Table 2.
Treatment Compliance in Immunogenicity Subset Group.
| Vaccine n (%) |
Placebo n (%) |
Total N (%) |
|
|---|---|---|---|
| Subjects screened for RT-PCR test | 405 | 135 | 540 |
| Subjects screened for IgM/IgG test | 405 | 135 | 540 |
| Subjects enrolled | 405 | 135 | 540 |
| First vaccination completed | 405 | 135 | 540 |
| Second vaccination completed | 397 | 133 | 530 |
| Intention-to-treat population (for safety and efficacy analysis) | 405 | 135 | 540 |
| Per-protocol population (for immunogenicity analysis 14 days after last injection) | 397 | 133 | 530 |
3.2. Efficacy
During the surveillance period, 320 COVID-19 suspect cases and 49 laboratory confirmed COVID-19 cases were collected. From these 49 confirmed COVID-19 cases, 25 cases (7 cases in the vaccine group and 18 cases in the placebo group) were symptomatic and occurred from 14 days following the second dose up to 3 months. There were no severe, critical, or deaths of laboratory confirmed COVID-19 cases observed [Table 3 ].
Table 3.
Summary of Primary Efficacy Endpoint.
| Vaccine |
Placebo |
||||||
|---|---|---|---|---|---|---|---|
| Endpoint | No. of cases | Mean follow-up days | Incidence rate (per 100 person years) |
No. of cases | Mean follow-up days | Incidence rate (per 100person years) |
Vaccine Efficacy (%) |
| Symptomatic confirmed laboratory cases COVID-19 starting 14 days after second injection | 7 | 80.78 |
3.904 |
18 | 72.08 |
11.25 |
65.30% |
| Severe | 0 | 0 | 0 | 0 |
– |
||
| Critical | 0 | 0 | 0 | 0 | |||
| Death | 0 | 0 | 0 | 0 | |||
Vaccine efficacy was defined as percentage reduction in relative risk using the ratio of incidence rate in the vaccine group and placebo group. Incidence rate was calculated by the number of subjects with laboratory-confirmed COVID-19 divided by the total number of subjects at risk adjusted by time (person years). The vaccine showed 65.3% efficacy in preventing symptomatic COVID-19.
3.3. Immunogenicity
3.3.1. Antibody IgG titer by ELISA
The seropositive rate of SARS-CoV-2 IgG antibody in the vaccine group at 14 days after the second injection was 99.74%. The seropositive rate in the vaccine group increased significantly compared with the placebo group. The seroconversion rate at 14 days after the second injection in the vaccine group was 97.48% which was significantly different compared with a 0.75% seroconversion rate in the placebo group. There was a 23.5-fold increase of IgG antibody GMT at 14 days after the second injection in the vaccine group, whereas there was no significant increase of GMT in the placebo group. The results of the IgG analysis using ELISA are presented in Table 4 .
Table 4.
Antibody Titer between the Vaccine and Placebo Groups.
| Antibody Titer | Time Point | Parameter | Group |
p-value | |
|---|---|---|---|---|---|
| Vaccine (N = 397) | Placebo (N = 133) | ||||
| IgG (ELISA) | V1 | Seropositive rate n(%) (95% CI) |
44 (11.08) (8.36–14.55) |
14 (10.53) (6.37–16.89) |
0.859**) |
| GMT*) (95% CI) Median |
220.27 (212.87–227.93) 200.00 |
220.37 (206.45–235.24) 200.00 |
0.990***) | ||
| V3 | Seropositive rate n(%) (95 %CI) Seroconversion n(%) (95% CI) GMT*) (95% CI) Median |
396 (99.74) (99.26–100) 387 (97.48) (95.43–98.63) 5181.19 (4746.13–5656.14) 5333.35 |
7 (5.29) (1.47–9.06) 1 (0.75) (0.13–4.14) 223.61 (209.08–239.47) 200.00 |
<0.001**) < 0.001**) < 0.001***) |
|
| Neutralization Antibody | V1 | Seropositive rate n(%) (95% CI) GMT*) (95% CI) Median |
0 (0–0.96) 2.00 (−) – |
0 (0–2.81) 2.00 (−) – |
– – |
| V3 | Seropositive rate n(%) (95% CI) Seroconversion n (%) (95% CI) GMT*) (95% CI) Median |
380 (95.72) (93.25–97.31) 346 (87.15) (83.50–90.09) 15.76 (14.57–17.04) 16 |
1 (0.75) (0.13–4.14) 0 (0.00) (0–2.81) 2.02 (1.98–2.05) 2 |
<0.001**) < 0.001**) < 0.001***) |
|
*) The comparison results after logarithmic transformation. **) Chi-square test; ***) t-test.
V1 = before injection;
V3 = 14 days after second injection;
IgG seropositive = titer > 200; seroconversion = four-fold increasing anti-RBD antibody IgG titer compare to baseline 14 days after the second dose.
Antibody neutralization seropositive = titer ≥ 1:4; seroconversion = a change from seronegative (titer < 1:8) to seropositive (titer ≥ 1:8); or a 4-fold increase from baseline titers if titer at baseline ≥ 1:8.
3.3.2. Neutralization antibody
Neutralization antibody seropositive was defined as a titer ≥ 1:4 and seroconversion was defined as a change from a titer < 1:8 to a titer ≥ 1:8; or a 4-fold increase from baseline if the titer at baseline ≥ 1:8. After the full schedule of vaccine administration, the seropositive rate of SARS-CoV-2 antibody using the neutralization assay in the vaccine group at 14 days was significantly different compared with that of the placebo group. The seroconversion rate 14 days after the second injection in the vaccine group was 87.15% with no seroconversion in the placebo group. There was a 7.88-fold increase of antibody neutralization GMT at 14 days after the second injection. The neutralization antibody results are presented in Table 4.
3.3.3. Lot-to-lot consistency
Another objective of the study was to evaluate the consistency of 3 batches of inactivated SARS-CoV-2 vaccine. The IgG antibody seropositive rate for the three batches of vaccine (batch numbers 20200308, 20200412, and 20200419) were 100%, 99.25%, and 100%, respectively, whereas the seroconversion rates were 96.18%, 97.76%, and 98.48%, respectively for the 14 day time point after the second vaccination. The GMT of the three batches was 5093.78, 5421.63, and 5032.34, respectively, for the 14 day time point after the second injection.
We compared the proportion of participants with seropositive and seroconversion between the 3 batches of SARS-CoV-2 vaccine. The results indicated that there was no significantly different proportion between the 3 vaccine batches as shown in Table 5 .
Table 5.
Comparison of Antibody Titer in Different Vaccine Batches.
|
Antibody |
Time Point |
Parameter |
Batch |
p-value** |
||
|---|---|---|---|---|---|---|
| Batch 20200308 (n = 131) |
Batch 20200412 (n = 134) |
Batch 20200419 (n = 132) |
||||
| IgG (ELISA) | V1 | Seropositive rate n(%) (95% CI) |
14 (10.70) (6.47–17.14) |
16 (11.94) (7.48–18.52) |
14 (10.61) (6.42–17.02) |
0.927**) |
| GMT*) (95% CI) Median |
215.16 (205.70–225.05) 200.00 |
223.40 (208.36–239.52) 200.00 |
222.26 (209.08–236.27) 200.00 |
0.384***) |
||
| V3 | Seropositive rate n (%) (95% CI) Seroconversion n (%) (95% CI) GMT*) (95% CI) Median |
131 (1 0 0) (97.15–100) 126 (96.18) (92.38–98.36) 5093.78 (4369.78–5937.59) 5105.05 |
133 (99.25) (95.89–99.87) 131 (97.76) (93.62–99.24) 5421.63 (4656.29–6312.77) 5787.62 |
132 (1 0 0) (97.17–100) 130 (98.48) (94.64–99.58) 5032.34 (4314.30–5869.76) 5302.40 |
0.374**) 0.476**) 0.898***) |
|
| Neutralization Antibody | V1 | Seropositive rate n(%) (95% CI) |
0 (0–2.85) |
0 (0–2.94) |
0 (0–2.91) |
– |
| GMT*) (95% CI) Median |
2.00 – – |
2.00 – – |
2.00 – – |
– | ||
| V3 | Seropositive rate n (%) (95% CI) Seroconversion n (%) (95% CI) GMT*) (95% CI) Median |
126 (96.18) (91.38–98.36) 118 (90.08) (83.76–94.11) 15.97 (14.03–18.18) 16.00 |
127 (94.78) (89.61–97.45) 119 (88.81) (82.35–93.10) 16.59 (14.47–19.02) 16.00 |
127 (96.21) (91.44–98.37) 109 (82.58) (75.21–88.10) 14.75 (12.78–17.02) 16.00 |
0.803**) 0.150**) 0.470***) |
|
*) The comparison results after logarithmic transformation. **) Chi-square test; ***) ANOVA (F-test).
V1 = before injection.
V3 = 14 days after second injection.
IgG seropositive = titer > 200; seroconversion = four-fold increasing anti-RBD antibody IgG titer compare to baseline 14 days after the second dose.
Antibody neutralization seropositive = titer ≥ 1:4; seroconversion = a change from titer < 1:8 to titer ≥ 1:8; or a 4-fold increase from baseline titers if titer ≥ 1:8 14 days after the second dose.
After the full schedule of vaccine, the seropositive rate of SARS-CoV-2 antibody as determined by the neutralization assay for batch numbers 20200308, 20200412, and 20,200,419 at 14 days after the second injection was above 94%. The seroconversion rate for each vaccine batch at 14 days after the second injection was 90.08%, 88.81%, and 82.58%, respectively. There was an increase of 7 to 8-fold for neutralization antibody GMT in all batches at 14 days following the second injection.
3.4. Safety
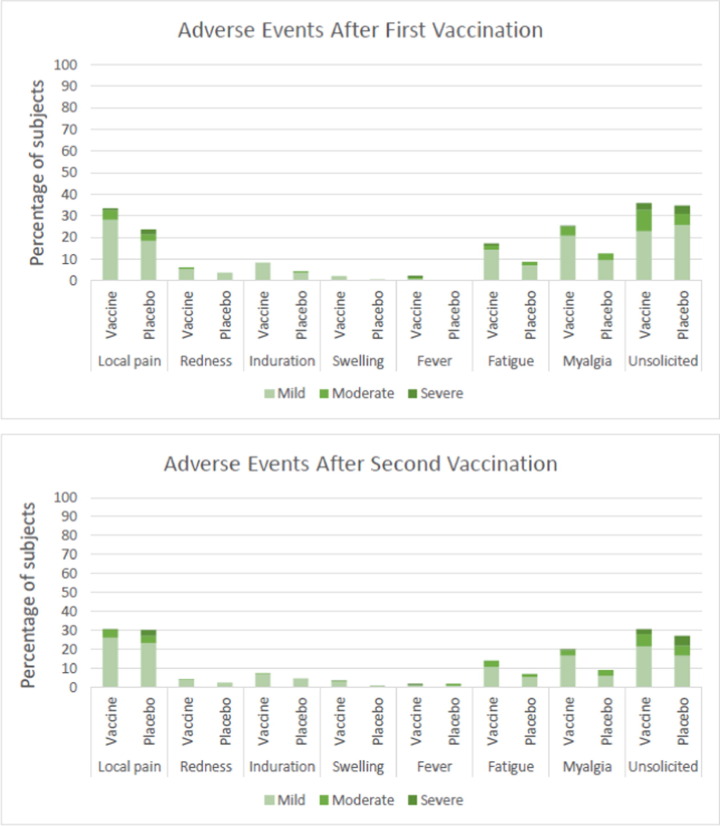
Within the immunogenicity subset group (n = 540), the majority of the reported local reactions was local pain, whereas the most common systemic event was myalgia. In the vaccine group, local pain was reported by 33.5% and 30.5% of the subjects after the first and second injection, respectively [Fig. 2 ]. In the placebo group, local pain was reported by 23.7% and 30.1% of the subjects after the first and second injection, respectively. In the vaccine group, myalgia was reported by 25.6% and 19.9% of the subjects after the first and second injection, respectively. In the placebo group, myalgia was reported by 12.6% and 9.0% of the subjects after the first and second injection, respectively. Based on the system organ class, majority of the unsolicited adverse event was categorized in the nervous system diseases category, specifically headache [Table S1].
Fig. 2.
Adverse Events occurring after the First and Second Vaccine Injection.
The intensity of the adverse events was mostly mild in the vaccine and placebo groups. After the first injection, the percentage of mild adverse events in the vaccine and placebo groups was 54.3% and 46.7%, respectively. After the second injection, the percentage of mild adverse events in the vaccine and placebo groups were 47.9% and 42.9%, respectively. There was a significant difference in the distribution of severe adverse reactions after the second dose between the vaccine and placebo groups, with a higher proportion in the placebo group. Moderate adverse reactions after the first dose in the vaccine groups were significantly higher than the placebo group.
Of the 1620 subjects enrolled to the study, there were nine serious adverse events (SAE) that occurred in all subjects with a classification not related to vaccine products (five SAEs). One SAE was very unlikely and three SAEs were reported as less likely to be related to the vaccine product as assessed by the DSMB.
4. Discussion
The efficacy of 2 doses of SARS-CoV-2 vaccine at preventing COVID-19 was evaluated up to 6 months after the second dose of injection. However, this interim report consisted of an efficacy analysis of 1620 participants within 3 months following the final dose of study vaccine. The efficacy analysis was performed based on the primary endpoint for all enrolled subjects with a data cut-off date of January 9th, 2021. The efficacy in preventing symptomatic confirmed cases of COVID-19 occurring at least 14 days after the second dose of vaccine was 65.30% (person years) with 7 COVID-19 cases occurring in the vaccine group and 18 COVID-19 cases occurring in the placebo group. There were no severe, critical, or incidents of death from laboratory confirmed COVID-19 infection.
A phase III study for the study vaccine was also conducted in Brazil, Turkey, and Chile. Each country has a specific study design depending on its pandemic situation, but the main design is similar. Efficacy data from other countries may support the registration in each country. Based on the interim result, vaccine efficacy in Brazil and Turkey was 50.65% and 83.5%, respectively [22], [23]. Vaccine effectiveness study was conducted in Chile with result of 65.9% [24]. The variability of efficacy result between the countries may reflect variance in study characteristics such as population, testing rate/capture of milder case, and force of infection [22].
The efficacy results in this study were higher compared with that of the same study in Brazil. The Brazilian study showed that after 14 days following vaccination with 2 doses of vaccine using a 0 and 14 day schedule, the efficacy rate against COVID-19 was 50.65% for all cases, 83.70% for cases requiring medical treatment, and 100.00% for hospitalized, severe, and fatal cases. This may be the result of Brazil having a high-risk population, particularly health care workers, thus leading to a higher COVID-19 infection rate. In contrast, the Indonesian study used the general population with a smaller occupational exposure to COVID-19 infection [22], [25].
Efficacy is one of the key indices to evaluate a vaccine. It measures the effect of vaccination by calculating the proportionate reduction in cases among vaccinated subjects in a double-blind placebo-controlled randomized clinical trial. VE is measured by calculating the risk of disease among vaccinated and unvaccinated subjects and determining the percent reduction in risk of disease relative to the unvaccinated group. The greater the percent reduction of illness in the vaccinated group, the higher the VE [26], [27], [28].
In this study, the most common adverse events were pain at the site of injection and myalgia which were reported in vaccine and placebo recipients and with a significantly higher proportion of participants in the vaccinated group compared with the placebo group. Most adverse events were mild or moderate in severity. In the vaccine group, fever was reported in 2.5% of the participants after the first dose and 1.8% after the second dose of vaccine. No significant differences in proportion between the vaccine and placebo group were observed. Overall, reactogenicity events were mild and resolved within a couple of days after onset. These results indicate that the vaccine was well-tolerated. The occurrence of fever following vaccination with SARS-CoV-2 inactivated vaccine was lower compared with other COVID-19 vaccine candidates, such as the novel chimpanzee adenovirus vector vaccine, ChAdOx1 nCoV-19 viral-vector vaccines (18% in participants without paracetamol), or RNA vaccines (16% in younger vaccine recipients and by 11% of older recipients reported after the second dose) [29], [30].
The immune response based on the seropositive and seroconversion rate of SARS-CoV-2 antibody IgG titer using ELISA at 14 days after the second injection were 99.74% and 97.48%, respectively. The IgG antibody GMT before injection and 14 days after the second injection were 220.27 and 5181.19, respectively. The seroconversion rate of RBD-specific IgG in this study were similar to that of the phase II study which was 97% [GMT 1094.3 (95% CI 936.7–1278.4)] at 14 days following the second dose [17].
The immune response based on the seropositive and seroconversion rate of SARS-CoV-2 neutralizing antibody using the neutralization assay in the vaccine group at 14 days after the second injection were 95.72% and 87.15%, respectively. The neutralization antibody GMT was 15.76 at 14 days after the second injection. The study vaccine phase I/II clinical trials conducted in China in April 2020 to evaluate the safety and immunogenicity of 2 doses of vaccine at intervals of 0 and 14 days (emergency schedule) and 0–28 days (routine schedule). In the phase I/II trials, it was found that immune responses induced by the day 0 and 28 vaccination schedule were larger than those induced from the day 0 and 14 vaccination schedule. In the phase 2 trial, the seroconversion rate of neutralizing antibodies to live SARS-CoV-2 for the same dosage used in this study were 92% with a GMT of 27.6 (95% CI 22.7–33.50) at 14 days after the second dose and 94% with a GMT of 23.8 (95% CI 20.5–27.7) at 28 days after the second dose in the day 0 and 14 vaccination cohort. Meanwhile, the seroconversion rate was 97% with a GMT of 44.1 (95% CI 37.2–52.2) at 28 days after the second dose in the day 0 and 28 vaccination cohort. However, based on the phase I/II clinical trial results, this study used the emergency vaccination schedule (day 0 and 14) which may be suitable for emergency use during the COVID-19 pandemic since antibody responses may be induced within a relatively short period of time [17].
Comparing the three different batches of vaccine (batch number 20200308, 20200412, and 20200419), we observed no significant differences in the proportion of participants with seropositive and seroconversion rates based on ELISA and neutralization assay, which demonstrated good consistency between each batch of the SARS-CoV-2 vaccine. The results of this interim report show the efficacy above the value required by the WHO [31].
Currently this study is still on-going to evaluate antibody persistence and efficacy up to 6 months after the second dose of vaccine. One limitation of our study is that it only assesses the efficacy of healthy adults aged 18–59 years with a limited number of subjects. Therefore, it still requires further research to obtain vaccine efficacy, safety, and immunogenicity data in the population aged 60 years of age and over, with or without comorbidities.
5. Conclusion
Based on the interim analysis, the vaccine showed a 65.30% efficacy at preventing COVID-19 illness with a good safety and immunogenicity profile.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank all the subjects who participated in this study, the head of the Bandung District Health Office, the Dean of Faculty of Medicine Universitas Padjadjaran, the head and staff of Puskesmas Garuda, Puskesmas Dago, Puskesmas Ciumbuleuit, Puskesmas Sukapakir, Klinik Kesehatan UNPAD, for their support.
We would also like to thank our Sub-Investigators, Susi Susanah, Prayudi Santoso, Rudi Wicaksana, Yovita Hartantri, Hendarsyah S., Viramitha Kusnandi Rusmil, Reni Ghrahani, Mia Milanti Dewi, Akbar Tirtosudiro, Riyadi, Yudith Ermaya, Fina Meilyana Andriyani for their valuable contribution to the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.09.052.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ophinni Y., Hasibuan A.S., Widhani A., Maria S., Koesnoe S., Yunihastuti E., et al. COVID-19 Vaccines: Current Status and Implication for Use in Indonesia. Acta Med Indones. 2020;52:388–412. [PubMed] [Google Scholar]
- 2.WHO Coronavirus disease (COVID-19) Situation Report-132. 2020 [Google Scholar]
- 3.Gupta S.D. Coronavirus Pandemic: A Serious Threat to Humanity. J Health Manag. 2020;22(1):1–2. doi: 10.1177/0972063420921260. [DOI] [Google Scholar]
- 4.Zhu N.a., Zhang D., Wang W., Li X., Yang B.o., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Basteiro A.L., Chaccour C., Guinovart C., Llupià A., Brew J., Trilla A., et al. Monitoring the COVID-19 epidemic in the context of widespread local transmission. Lancet Respir Med. 2020;8(5):440–442. doi: 10.1016/S2213-2600(20)30162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 n.d. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed January 23, 2021).
- 7.World Health Organization Weekly Epidemiological Update on COVID-19. World Heal Organ. 2020;1:4. [Google Scholar]
- 8.WHO. Situation Report-7 INDONESIA Situation Report 19 Internal for SEARO. 2020.
- 9.Wenjiang Fu1 2*, Jieni Li3 and Paul Scheet 4. Covid-19 Vaccine Efficacy: Accuracy, Uncertainty and Projection of Cases. MedRxiv 2020. 10.1101/2020.12.16.20248359.
- 10.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 11.WHO. WHO R&D Blueprint novel Coronavirus Outline of designs for experimental vaccines and therapeutics. vol. 205. 2020.
- 12.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vellozzi C., Burwen D.R., Dobardzic A., Ball R., Walton K., Haber P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27(15):2114–2120. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 14.Murdin A.D., Barreto L., Plotkin S. Inactivated poliovirus vaccine: Past and present experience. Vaccine. 1996;14(8):735–746. doi: 10.1016/0264-410X(95)00211-I. [DOI] [PubMed] [Google Scholar]
- 15.(WHO) WHO. Draft landscape and tracker of COVID-19 candidate vaccines 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed January 25, 2021).
- 16.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science (80-) 2020;369(6499):77–81. doi: 10.1126/science:abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Articles Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.KEMENKES. Keputusan Menteri Kesehatan Republik Indonesia Nomor HK.01.07/MenKes/413/2020 Tentang Pedoman Pencegahan dan Pengendalian Corona Virus Disease 2019 (Covid-19). MenKes/413/2020 2020;2019:207.
- 19.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S., Hillyer C., Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzelak L., Temmam S., Planchais C., Demeret C., Huon C., Guivel-Benhassine F., et al. SARS-CoV-2 serological analysis of COVID-19 hospitalized patients, pauci-symptomatic individuals and blood donors. MedRxiv. 2020;17:25. doi: 10.1101/2020.04.21.20068858. [DOI] [Google Scholar]
- 22.SAGE (Strategic Advisory Group of Experts). Background document on the inactivated vaccine Sinovac-CoronaVac against COVID-19. 2021.
- 23.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398:213–22. 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed]
- 24.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinovac Announces Phase III Results of Its COVID-19 Vaccine-SINOVAC - Supply Vaccines to Eliminate Human Diseases n.d. http://www.sinovac.com/?optionid=754&auto_id=922 (accessed March 17, 2021).
- 26.CDC. Principles of Epidemiology | Lesson 3 - Section 6 n.d. https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html (accessed February 22, 2021).
- 27.Fda, Cber. Contains Nonbinding Recommendations Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry. 2020.
- 28.Fda, Cber. Contains Nonbinding Recommendations Emergency Use Authorization for Vaccines to Prevent COVID-19 Guidance for Industry Preface Public Comment. 2020.
- 29.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine _ Enhanced Reader.pdf. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(WHO) WHO. CONSIDERATIONS FOR EVALUATION OF COVID19 VACCINES 2020;21:1–14. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed January 25, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.