Abstract
Introduction
We assessed the potential relationship between COVID-19 and laboratory-confirmed cutaneous leishmaniasis (CL)-registered cases with a history of scarring, compared with volunteer participants without history of CL.
Methods
This case-control retrospective study was conducted in southeastern Iran with a high anthroponotic cutaneous leishmaniasis (ACL) burden.
Results
Overall, n=1010 CL cases (n=479 male, n=531 female) were evaluated for infection with SARS-CoV-2. In the CL case group, 2 men and 1 woman (0.3% in total) had a mild form of COVID-19 disease; none were hospitalized or died. In contrast, of n=2020 participants without history of CL, n=57 (2.9%) contracted laboratory-confirmed COVID-19, including mild (66.7%), hospitalized (26.3%), critical (3.5%) and fatal (3.5%). There was a strong negative association between CL infection and COVID-19. The burden of COVID-19 in CL-cured participants significantly reduced the morbidity (odds ratio: 0.12; CI: 0.03–0.30; P <0.001) and mortality (percentile: -4.10, -0.02).
Conclusion
Participants with a history of CL scar had significantly reduced incidence of COVID-19 morbidity and mortality. The cross-protection mediated by CL may retard COVID-19 in endemic countries. However, further longitudinal studies are needed to explore the potential profile and duration of this protection offered by CL against COVID-19.
Keywords: COVID-19, cutaneous leishmaniasis, coinfection, prophylactic effect
Introduction
Leishmaniasis is a neglected disease caused by protozoan parasites belonging to over 20 human parasitic species of Leishmania. This complex disease is manifested in several clinical and epidemiological presentations and is extensively distributed among one billion at-risk populations in over 100 countries, primarily in the developing world (Bailey et al., 2017; World Health Organization, 2017). Cutaneous leishmaniasis (CL) is the most abundant form; it causes skin lesions associated with extensive public health problems (Alvar et al., 2012; Bailey et al., 2019; Du et al., 2016). Healed cutaneous lesions cause permanent scars and disfigurement, with psychological, social and economic consequences (Aflatoonian et al., 2019; Bamorovat et al., 2018a). Leishmania tropica and Leishmania major are the most common causes of CL in semi-arid tropics and subtropics, notably in the Eastern Mediterranean Region, where 74% of total cases have been reported (Akhoundi et al., 2016; Bailey et al., 2017; Bamorovat et al., 2018a; World Health Organization, 2017).
Both innate and adaptive immune systems contribute to the induction of anti-Leishmania immunity, but effector T helper (Th) 1 cell-mediated immunity plays a more critical role in protection (Jafarzadeh et al., 2019; Scott and Novais, 2016). Generally, solid immunity is associated with a cell-mediated immune response, while in the absence of T cell-mediated immunity, non-protective reactions are expressed as a robust humoral response (Jafarzadeh et al., 2019; Scott and Novais, 2016).
Cytokines such as interleukin (IL)-12 and interferon (IFN)-γ play a fundamental role in protection against leishmaniasis through inducing the polarization of Th1 cells. Th1 cell-derived cytokines, especially IFN-γ and TNF-α, promote macrophage activation leading to Leishmania clearance via nitric oxide and other reactive oxygen species (Jafarzadeh et al., 2019; Scott and Novais, 2016). Th2 cell differentiation is driven by IL-4, promoting vulnerability through macrophage inhibition and abrogation of IL-12 expression (Jafarzadeh et al., 2019; Scott and Novais, 2016). Leishmaniasis progression is related to dominant Th2 and Treg cell-related responses and high IL-4, IL-5, transforming growth factor (TGF)-β, and IL-10 production (Jafarzadeh et al., 2019; Saha et al., 2020; Scott and Novais, 2016).
Both lines (Th1 and Th2) have a similar signaling pathway utilizing IL-4 receptor alpha, IL-13, and share several features with IL-4 (Matthews et al., 2000). IL-13 has disease-promoting properties and operates independently of IL-4 (Belkaid et al., 2001), suggesting that IL-13 and IL-4 have additive effects. Prevention of IL-12 generation by macrophages and high levels of IL-13 inhibit Th1 cell-mediated responses, causing skewing of harmful Th2 cells. The clinical outcome of infection with Leishmania correlates with cytokine development profile and timing. Many immune cells are expressed, predominantly by CD4+ T cells (such as Th1 and Th2 cells), but also by CD8+ T cells and CD4-CD8 double-negative T cells (Liese et al., 2008), including dendritic cells, macrophages, natural killer (NK) cells (Ronet et al., 2010), and B cells (Gessner et al., 1993).
The recent emergence and rapid global spread of SARS-CoV-2 and subsequent COVID-19 disease is an ongoing health pandemic with devastating consequences. In addition to non-structural proteins, SARS-CoV-2 has several types of structural proteins (Shang et al., 2020). A wide range of mechanisms such as hyper-inflammatory reactions, immune imbalances, cytokine storm, massive replication of SARS-CoV-2, extensive cell death, lymphopenia, and coagulopathy contribute to COVID-19 pathogenesis leading to multi-organ failure, especially acute respiratory distress syndrome (Jafarzadeh et al., 2020a; Li et al., 2020; Lingeswaran et al., 2020; Zou et al., 2020).
Innate immunity IFNs provide the first line of defense against viral infections as timely production of adequate IFNs can limit a viral infection (Jafarzadeh et al., 2020b). During the incubation period or early phases of COVID-19 infection, appropriate local type III and type I IFN responses can eliminate SARS-CoV-2 or limit its replication, preventing disease progression to moderate and severe stages (Jafarzadeh et al., 2020b). However, if the early IFN responses fail to control SARS-CoV-2, the virus replicates in the lungs, enters the circulation, and leads to massive tissue destruction as its receptor is expressed by many cell types (Jafarzadeh et al., 2020b).
In adaptive immunity, effector Th1 cell-derived cytokines, especially IL-2 and IFN-γ, activate CD8+ cytotoxic T lymphocytes and NK cells to reduce viral load through killing virus-infected-cells (Frank and Paust, 2020; Miyauchi, 2017). Moreover, Th2 cell-derived cytokines induce B cells to secrete anti-viral antibodies. The specific antibodies against surface viral antigens bind to the free virus to prevent virus attachment to target cells (Jafarzadeh et al., 2008).
We assessed the potential relationship between SARS-CoV-2 infection and laboratory-confirmed CL-registered cases with a history of scar in Kerman County, compared with volunteer participants without CL history. We hypothesized that the immunological memory cells generated by some infectious diseases, such as CL, could reduce the incidence and severity of COVID-19 disease. To date, there is no epidemiological study to indicate that CL may offer protection against SARS-COV-2. To our knowledge, this longitudinal observation is the first evidence-based field assessment to suggest that the patients with a history of CL may be associated with a lower risk of COVID-19.
Methods
Ethical consideration
The current study was given ethical approval by the Joint Ethics Committees of the Kerman University of Medical Sciences and Kerman Leishmaniasis Research Center (Ethics no. IR.KMU.REC. 1399.210, contract no. 99000242).
Design and study site
This study was carried out between July 2020 and December 2020 as a case-control retrospective study in areas high-risk and endemic for ACL caused by L. tropica in Kerman county, southeastern Iran. In the southeast and ∼1000 km away from Tehran, Kerman is the largest province in Iran. This province falls into the hot and dry zones and suffers from water shortages. The average annual precipitation is low (140–150 mm), and maximum rainfalls are in winter. Kerman county is a well-known ACL focal point in Iran (Aflatoonian et al., 2019; Bamorovat et al., 2019b, 2019a; Bamorovat et al., 2018b, 2018a; Sharifi et al., 2015) (Figure 1 ).
Figure 1.
Map of Kerman county, southeastern Iran, where the study was carried out.
The current study was conducted at the Dadbin Health Center, the primary referral clinic for CL care and control operations. The clinic is directly connected to the Kerman Leishmaniasis Research Center in Kerman and the Afzalipour School of Medicine. The clinic is responsible for CL patients that have been referred from various localities within the county. Each CL patient has a case report form recording demographic and clinical status and underlying diseases.
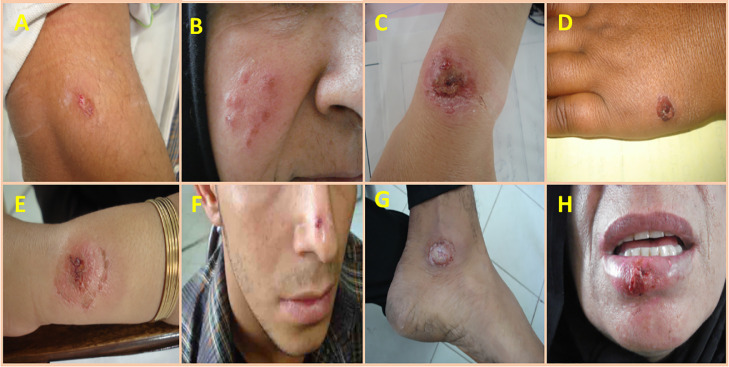
In the case group (CL-cured participants with a history of a scar), the subjects or their guardians were fully informed about the study purpose. Before the study began, several face-to-face meetings and interviews were held with the participants and community health authorities to describe the purpose, procedure, and possible benefits. Throughout the interview, the evaluators warranted that the questions were well-understood by participants or their guardians. CL-cured patients participated voluntarily. A written informed consent form was completed for each patient or guardian for child patients. All data were kept confidential. Figure 2 shows CL patients in the case group with lesions in different body locations. The written informed consent of the cases and the verbal witnessed form of the control group to participate in this study were obtained after detailed information has been given. The patients were allowed to ask questions. The case group consent was documented in their case report forms (CRF) at the Dadbin registry health clinic. While in the control group the patients were not requested to sign a form, but after a piece of thorough information has been made available to them, were witnessed by a relative, friend, or a health professional, with technical knowledge in the field of investigation. The obtainment of the verbal consent was documented on a separate page of the CRF, by the name and signature of the witness.
Figure 2.
Representative images of various lesions from cutaneous leishmaniasis patients from Kerman county, southeastern Iran.
Case-definition
Case group
All CL cases who participated in the present study had a history of the previous scar and were laboratory-confirmed by standard Giemsa and polymerase chain reaction (PCR) (Aflatoonian et al., 2019; Bamorovat et al., 2018a). Patients suspected of being infected with CL referred from various health clinics within Kerman county were diagnosed by direct microscopic examination at the registry center (Dadbin Heath Clinic diagnostic laboratory). Tissue smear preparations were obtained from the edge of the lesions, dried, fixed, stained by routine Giemsa, and visualized under an optical microscope for the presence of Leishman bodies (amastigotes). CL-cured subjects were prospectively evaluated for COVID-19. The case group participants tested for COVID-19 by Multiple One-Step quantitative real-time PCR methods using the COVITECH kit (The Academic Center for Education, Culture and Research (ACECR), Tehran, Iran). This approach is a standard universal detecting test provided by the country's Center for Diseases Control nationally.
Control group
After the selection of the case group (previous CL cases with a history of scar), we used the sequential sampling method to pick the control group. In fact, individuals with exposed COVID-19 and CL in this area (Kerman) were sequentially selected between July 2020 and December 2020. In this regard, suspected COVID-19 patients referred to the Kerman University of Medical Sciences hospitals were identified by a multiple one-step quantitative real-time PCR method using the COVITECH kit. The demographical and clinical data for the control group were obtained in collaboration with the Statistics Center of Kerman University of Medical Sciences. The composition of both groups (controls and cases) was closely related in age, gender and socioeconomic background, and in both groups, patients were selected from the same CL-endemic communities within Kerman county; therefore, they are similarly exposed to CL infection.
Statistical analyses
The statistical software R (version 4.0.2, License GPLv2) was used to analyze data. Firth's bias-reduced penalized-likelihood logistic regression was used to affect the CL and COVID-19 variables in the studied dataset. This model is the Bayesian logistic regression with non-informative Jeffrey's Prior. In this model, instead of using the maximum likelihood method to minimize the bias caused by the rare event in the dataset, the penalized maximum likelihood approach was used to estimate the parameters and regression coefficients. Therefore, accurate coefficient estimation is not a concern in this model, despite the rare event data in the studied dataset. In addition to the P-value, the regression coefficient and odds ratio (OR) were also assessed.
Bayesian inference in ordinal logistic regression was used to analyze the severity of COVID-19 in the case and the control groups. Due to the incidence of the event being relatively low at some levels, standard statistical methods to analyze this dataset may have undesirable and inaccurate outcomes. Normal distribution was used as the prior distribution. The arm and logistic packages in R were used for this analysis.
Results
Overall, n=1010 laboratory-confirmed cases with a history of CL scar (n=479 male, n=531 female) were explored and evaluated for COVID-19 infection. In the case group, 2 men and 1 woman (0.3% in total) were infected with a mild form of COVID-19; none were hospitalized or died. In contrast, in the n=2020 participants selected as the control group (n=998 male, n=1022 female), n=57 (2.9%, n=28 male, n=29 female) had laboratory-confirmed COVID-19, including mild (66.7%), hospitalized (26.3%), critical (3.5%) and fatal (3.5%). The findings indicated that there was a strong negative association between CL-cured infection and COVID-19 incidence. Firth's bias-reduced logistic regression analysis indicated that the burden of COVID-19 disease in CL-cured participants was significantly reduced in terms of morbidity (OR=0.12, CI: 0.03–0.30 and P <0.001) and mortality (percentile: -4.10, -0.02) compared with the non-CL control group (Tables 1 and 2 ).
Table 1.
A Bayesian logistic regression model with non-informative Jeffrey's Prior analysis of COVID-19 infections in the cutaneous leishmaniasis (CL)-cured participants compared with the non-CL control group.
| Variable | COVID-19 | SE | OR | 95% CI for OR | P-value | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| CL | |||||||
| Yes | 3 | 1007 | -2.13 | 0.55 | 0.12 | 0.03 - 0.30 | <0.001 |
| No | 57 | 1963 | 0 | 1 | |||
: Regression Coefficient, SE: Standard Error, OR: Odds Ratio.
Table 2.
Bayesian ordinal logistic regression model for the prophylactic effect of cutaneous leishmaniasis (CL) against the severity of COVID-19.
| Variable | COVID-19 | Mean | Std. Deviation | Percentile | |||||
|---|---|---|---|---|---|---|---|---|---|
| Non | Mild | Hospitalized | Critical | Fatal | 2.5 | 97.5 | |||
| CL | |||||||||
| Yes | 1007 | 3 | 0 | 0 | 0 | -2.06 | 0.52 | -4.10 | -0.02 |
| No | 1963 | 38 | 15 | 2 | 2 | 0 | |||
As shown in Table 2, converge probability did not include the value of zero, which showed a significant relationship between the two variables (percentile = -4.10, -0.02). On the other hand, considering the negative numbers of the estimated parameter, it can be argued that the chance of severity of COVID-19 in participants with a history of CL was lower than that in patients with no history of CL.
Discussion
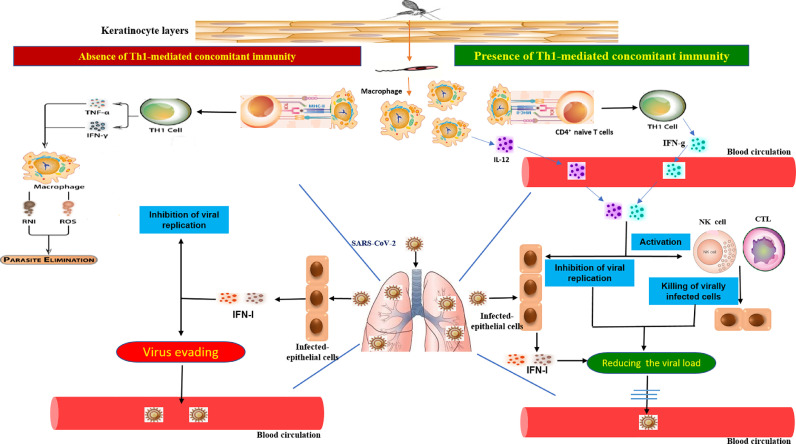
Our study showed that participants with a history of CL had a significantly reduced incidence of COVID-19 with lower severity of the disease than the non-CL control group. This observation does not demonstrate whether any protection against COVID-19 is temporary or long-lasting. The cured CL cases recruited in this study had infected between 2013 and 2020. Clinical records show that the severity of patients with COVID-19 was significantly lower in healed CL cases than in non-CL participants. This observation indicates that the protection exists in cured CL participants with previous scars, suggesting that the cross-protective effect of CL can be long-lasting. Our understanding of the extensive immune protection facilitated via memory cells and the current epidemiological evidence from COVID-19 following CL infection offer a rational immunological basis for CL's potential prophylactic effect against severe COVID-19. Figure 3 illustrates the proposed model for the role of leishmanial-mediated concomitant immunity in promoting anti-SARS-CoV-2 immune responses.
Figure 3.
A proposed model of immune response.
Leishmania-mediated T helper (Th)1 cell concomitant immunity can induce the production of Th1 type cytokines such as interleukin (IL)-12 and interferon (IFN)-γ, which enter the blood from the infected skin and then are diffused into the SARS-CoV-2-infected lungs. IL-12 and IFN-γ can potentiate the anti-viral immune response and prevent viral spreading into the blood. Th1 type cytokines inhibit viral replication and activate local natural killer cells and CD8+ cytotoxic T lymphocytes, eliminating virus-infected cells to limit viral load in the lungs (Right). In the absence of Leishmania-mediated Th1 cell concomitant immunity, SARS-CoV-2-infected epithelial cells can produce type I IFN-γ, which can control COVID-19 replication. If SARS-CoV-2 evades the type I IFN-γ-mediated response, it then enters the blood and causes blood viremia and subsequent consequences.
The cross-protection arbitrated by CL would significantly advantage developing countries, notably the Mediterranean region, where approximately 74% of the global CL cases are found. A lower burden of COVID-19 incidence and case-fatality during the pandemic has been repeatedly documented in tropical and subtropical areas (World Health Organization, 2020a). The above finding on CL is in addition to the speculative partial protective effect of a robust BCG vaccination policy routinely implemented in these countries and further contributes to lowering the morbidity and mortality profiles of COVID-19. At present, no direct evidence exists to demonstrate that the BCG vaccine protects people against COVID-19. Two clinical trials addressing this issue are proceeding, and the World Health Organization will assess the results when available (World Health Organization, 2020b). However, since the emergence of SARS-CoV-2, a growing body of evidence has been compiled indicating the BCG vaccine's ability to induce a range of general immunological effects harmful to other conditions (Curtis et al., 2020; Escobar et al., 2020). It appears that CL may have the potential to train the innate and adaptive immune systems to produce active memory cells and associated components against COVID-19, a process called trained immunity (Arts et al., 2018; Kleinnijenhuis et al., 2014).
There are many interactions between parasitic and microbial infections. Concomitant infections are a common phenomenon in nature and are frequently associated with parasites. Many examples of these interactions resulting in susceptibility or resistance among parasitic, viral and bacterial infections have been described (Cox, 2001; Griffiths et al., 2014). In concomitant infections, several modalities of parasites' burden on the immune system are well documented, including immunosuppression and immunopotentiation (Cox, 2001; Griffiths et al., 2014). T lymphocytes' polarization towards cell-mediated or antibody-mediated responses depends on the quantity and quality of antigens produced by the parasite. Essential in these interactions is that the cytokines and the effector molecules act non-specifically; therefore, any microbial organisms can be caught up in the cytokine network concurrently or subsequently.
Concomitant agents are characterized as being inherently heterologous, a mechanism that is not yet well-documented. One reason that limited consideration has been given to concomitant infections is that the interactions involved are difficult and complex to understand (Cox, 2001; Shen et al., 2019; Wait et al., 2020). The best-studied molecules are generated by trypanosomes that produce lymphocyte activating factors to produce IFN-γ and a cascade of immunological molecules to stimulate macrophage activity and IL-12 production (Cox, 2001). The exact role of these components and effector cells induced by the leishmanial agents is not clear, but significantly Leishmania-derived molecules can interact with the other elements of the immune system and may be involved in a long-lasting enhancement in immune surveillance to COVID-19.
In addition to concomitant immunity, the antigenic similarities between some Leishmania species and SARS-CoV-2 need more investigation. If antigenic similarities are proven, then Leishmania-mediated cross-immunity may protect CL-cured individuals from SARS-CoV-2 due to the induction of long-lasting memory cells. Therefore, vaccination with whole Leishmania vaccines may confer protection against COVID-19.
CL typically produces skin ulcers on the arms, legs and, particularly, face (World Health Organization, 2014). Within 3 to 18 months, over 90% of cases of CL recover spontaneously (Davies et al., 2003). At the sandfly bite site, the disease starts as a slight red swelling (papule). The papule improves in size and becomes a nodule that ulcerates and finally crusts over. Eventually, most CL sores heal naturally, although it is impossible to predict the length of this phase in an individual case. For primary sores, topical or systemic treatment approaches such as chemicals and freezing are used (Ejov and Dagne, 2014). Meglumine antimoniate (Aflatoonian et al., 2019; Karamian et al., 2015; World Health Organization, 2014) is the conventional treatment for CL. Individuals are usually resistant to reinfection from the same species after healing, and a lifelong immunity will often be produced.
One of the few parasitic diseases likely to be controllable by vaccination is leishmaniasis. However, efforts to produce efficacious vaccines have so far been inconclusive, although numerous clinical and field trials of inactivated and live vaccines have been conducted around the world (Khamesipour et al., 2005). One live prophylactic vaccine is a mixture of Uzbekistan-registered live virulent L. major combined with an inactive Leishmania parasite (Khamesipour et al., 2005). In hyper-endemic Asian countries, it is commonly recognized that after recovery from CL, patients are typically protected against reinfection. Therefore, an active lesion exudate has been used to inoculate young children, particularly girls, on their buttocks for centuries. This approach would develop a self-healing lesion and shield the face and other exposed body parts against lesions (Modabber, 1989; Nadim et al., 1983). This practice is known as leishmanization (LZ) inoculation of live virulent Leishmania. Leishmanization has been used in many countries for over 60 years (Khamesipour et al., 2005). The problem with live metacyclic promastigotes is that ∼1–3% of patients develop non-healing forms after inoculation. Nevertheless, a population of over 2 million Iranian people who voluntarily participated in the Iran-Iraq war was vaccinated against zoonotic CL caused by L. major (Khamesipour et al., 2005; Nadim et al., 1983).
The BCG vaccine is generally used in infancy as prevention against tuberculosis (TB). Many studies suggest that the BCG vaccine protects against infant mortality through non-specific heterologous protection to other infectious diseases (Shann, 2013), possibly, through innate immune epigenetic mechanisms (Moorlag et al., 2019; Shann, 2013). Exposure to the BCG vaccine may reduce the severity of COVID-19 and lower mortality; therefore the vaccine could help to develop therapeutic or preventive strategies against SARS-CoV-2 infection (Escobar et al., 2020; Malik et al., 2020; Mohapatra et al., 2020). A vaccine against patients with TB is considered an alternative therapeutic modality. The BCG vaccine is known to induce innate and adaptive immunities, thereby activating both non-specific and cross-reactive immune responses; when combined, these responses could effectively resist other pathogens, including SARS-CoV-2 (Malik et al., 2020).
Iesa et al. show that malaria caused by Plasmodium falciparum in endemic areas substantially lowers the incidence of COVID-19 (Iesa et al., 2020). The authors detected potential common targets for an immune response to SARS-CoV-2 by immune determinants' shared characteristics as conferred by the previous CL patients. Possible cross-reactivity was proposed via HLA-A*02:01 and consequent CD8+ T-cell stimulation. The authors concluded that immunodominant epitope conservation between SARS-CoV-2 and P. falciparum thrombospondin-related anonymous protein might motivate the low COVID-19 incidence in malaria-affected areas by providing immunity in COVID-19 patients previously infected with malaria (Iesa et al., 2020).
As mentioned, COVID-19 is an emerging disease. Other emerging diseases are likely to occur in the future. With the immunological memory cells they create, infectious diseases, such as CL, may retard and prevent the severity of emerging diseases through an immune response similar to the SARS-CoV-2 pathway. Further clinical trials should be conducted to investigate our hypothesis that LZ reduces COVID-19 incidence and severity.
In our retrospective study n=3 (0.3%) CL-cured participants contracted a mild form of COVID-19 compared with n=57 (2.9%) cases in participants without a history of CL who experienced a spectrum of COVID-19 severity, from mild to fatal. In the world's vast CL-endemic countries, COVID-19 may be prevented by various prophylactic vaccines and previous infectious conditions such as CL. However, further longitudinal studies are needed to explore the potential profile and duration of CL's protection against COVID-19. Furthermore, exploring the precise association between all forms of leishmaniasis and COVID-19 in multi-central approaches will help to design more effective prophylactic and therapeutic measures for planning future control strategies.
Funding
This study was funded by the Vice-Chancellor of Research, Kerman University of Medical Sciences Kerman, Iran (grant number 99000242).
Conflict of interest
We declare no conflict of interest.
Ethics approval
The current study was given ethical approval by the Joint Ethics Committees of the Kerman University of Medical Sciences and Kerman Leishmaniasis Research Center (Ethics no. IR.KMU.REC. 1399.210, contract no. 99000242).
Consent to participate
All participants received written and oral information on the study and signed a non-opposition statement.
Consent for publication
All participants signed a non-opposition statement. Data were anonymised for publication.
Availability of data and material
Data are available to any reader directly upon reasonable request.
Code availability
Not applicable.
Acknowledgments
We would like to thank the patients for their cooperation and the health personnel and staff at Dadbin Health Clinic, County Health Surveillance Systems, to perform this study.
References
- Aflatoonian MR, Sharifi I, Aflatoonian B, Bamorovat M, Heshmatkhah A, Babaei Z, et al. Associated-risk determinants for anthroponotic cutaneous leishmaniasis treated with meglumine antimoniate: A cohort study in Iran. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts RJW, Moorlag SJ, Novakovic B, Li Y, Wang S-Y, Oosting M, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Bailey F, Mondragon-Shem K, Haines LR, Olabi A, Alorfi A, Ruiz-Postigo JA, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: A systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey F, Mondragon-Shem K, Hotez P, Ruiz-Postigo JA, Al-Salem W, Acosta-Serrano Á, et al. A new perspective on cutaneous leishmaniasis—Implications for global prevalence and burden of disease estimates. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamorovat M, Sharifi I, Aflatoonian MR, Sadeghi B, Shafiian A, Oliaee RT, et al. Host's immune response in unresponsive and responsive patients with anthroponotic cutaneous leishmaniasis treated by meglumine antimoniate: A case-control study of Th1 and Th2 pathways. Int Immunopharmacol. 2019;69:321–327. doi: 10.1016/j.intimp.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Bamorovat M, Sharifi I, Aflatoonian MR, Sharifi H, Karamoozian A, Sharifi F, et al. Risk factors for anthroponotic cutaneous leishmaniasis in unresponsive and responsive patients in a major focus, southeast of Iran. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamorovat M, Sharifi I, Fekri A, Keyhani A, Aflatoonian MR, Heshmatkhah A, et al. A single-group trial of end-stage patients with anthroponotic cutaneous leishmaniasis: Levamisole in combination with Glucantime in field and laboratory models. Microb Pathog. 2019;128:162–170. doi: 10.1016/j.micpath.2018.12.040. [DOI] [PubMed] [Google Scholar]
- Bamorovat M, Sharifi I, Mohammadi MA, Eybpoosh S, Nasibi S, Aflatoonian MR, et al. Leishmania tropica isolates from non-healed and healed patients in Iran: A molecular typing and phylogenetic analysis. Microb Pathog. 2018;116:124–129. doi: 10.1016/j.micpath.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti–IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox FEG. Concomitant infections, parasites and immune responses. Parasitology-Cambridge. 2001;122:23–38. doi: 10.1017/s003118200001698x. [DOI] [PubMed] [Google Scholar]
- Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395:1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. Bmj. 2003;326:377–382. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Hotez PJ, Al-Salem WS, Acosta-Serrano A. Old world cutaneous leishmaniasis and refugee crises in the Middle East and North Africa. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejov M, Dagne D. Strategic framework for leishmaniasis control in the WHO European Region 2014–2020. Copenhagen World Heal Organ Reg Off Eur. 2014 [Google Scholar]
- Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci. 2020;117:17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K, Paust S. Dynamic Natural Killer Cell and T Cell Responses to Influenza Infection. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A, Vieth M, Will A, Schröppel K, Röllinghoff M. Interleukin-7 enhances antimicrobial activity against Leishmania major in murine macrophages. Infect Immun. 1993;61:4008–4012. doi: 10.1128/iai.61.9.4008-4012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EC, Pedersen AB, Fenton A, Petchey OL. Analysis of a summary network of co-infection in humans reveals that parasites interact most via shared resources. Proc R Soc B Biol Sci. 2014;281 doi: 10.1098/rspb.2013.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iesa MAM, Osman MEM, Hassan MA, Dirar AIA, Abuzeid N, Mancuso JJ, et al. SARS-CoV-2 and Plasmodium falciparum common immunodominant regions may explain low COVID-19 incidence in the malaria-endemic belt. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh A, Nemati M, Saha B, Bansode YD, Jafarzadeh S. Protective Potentials of Type III Interferons in COVID-19 Patients: Lessons from Differential Properties of Type I-and III Interferons. Viral Immunol. 2020 doi: 10.1089/vim.2020.0076. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A, Nemati M, Sharifi I, Nair A, Shukla D, Chauhan P, et al. Leishmania species-dependent functional duality of toll-like receptor 2. IUBMB Life. 2019;71:1685–1700. doi: 10.1002/iub.2129. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A, Zarei S, Shokri F. Low dose revaccination induces robust protective anti-HBs antibody response in the majority of healthy non-responder neonates. Vaccine. 2008;26:269–276. doi: 10.1016/j.vaccine.2007.10.044. [DOI] [PubMed] [Google Scholar]
- Karamian M, Bojd F, Salehabadi A, Hemmati M, Barati DA. Effectiveness of meglumine antimoniate against L. tropica in a recently emerged focus of cutaneous leishmaniasis in Birjand, eastern Islamic Republic of Iran. EMHJ-Eastern Mediterr Heal J. 2015;21:280–286. doi: 10.26719/2015.21.4.280. [DOI] [PubMed] [Google Scholar]
- Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, Noazin S, et al. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23:3642–3648. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LAB, Jacobs C, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:1–7. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese J, Schleicher U, Bogdan C. The innate immune response against Leishmania parasites. Immunobiology. 2008;213:377–387. doi: 10.1016/j.imbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Lingeswaran M, Goyal T, Ghosh R, Suri S, Mitra P, Misra S, et al. Inflammation, Immunity and Immunogenetics in COVID-19: A Narrative Review. Indian J Clin Biochem. 2020:1. doi: 10.1007/s12291-020-00897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik YS, Ansari MI, Ganesh B, Sircar S, Bhat S, Pande T, et al. BCG vaccine: a hope to control COVID-19 pandemic amid crisis. Hum Vaccin Immunother. 2020;16:2954–2962. doi: 10.1080/21645515.2020.1818522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie ANJ. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–1462. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- Miyauchi K. Helper T cell responses to respiratory viruses in the lung: development, virus suppression, and pathogenesis. Viral Immunol. 2017;30:421–430. doi: 10.1089/vim.2017.0018. [DOI] [PubMed] [Google Scholar]
- Modabber F. Experiences with vaccines against cutaneous leishmaniasis: of men and mice. Parasitology. 1989;98:S49–S60. doi: 10.1017/s0031182000072243. [DOI] [PubMed] [Google Scholar]
- Mohapatra PR, Mishra B, Behera B. BCG vaccination induced protection from COVID-19. Indian J Tuberc. 2020 doi: 10.1016/j.ijtb.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorlag S, Arts RJW, Van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1983;76:377–383. [PubMed] [Google Scholar]
- Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- Saha B, Jafarzadeh A, Nemati M, Chauhan P, Patidar A, Sarkar A, et al. Interleukin-27 Functional Duality Balances Leishmania Infectivity and Pathogenesis. Front Immunol. 2020;11:1573. doi: 10.3389/fimmu.2020.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P, Novais FO. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. 2016;16:581. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther. 2013;35:109–114. doi: 10.1016/j.clinthera.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Aflatoonian M, Fekri A. A comprehensive review of cutaneous leishmaniasis in Kerman Province, Southeastern Iran-narrative review article. Iran J. 2015;44:299. [PMC free article] [PubMed] [Google Scholar]
- Shen S-S, Qu X-Y, Zhang W-Z, Li J, Lv Z-Y. Infection against infection: parasite antagonism against parasites, viruses and bacteria. Infect Dis Poverty. 2019;8:49. doi: 10.1186/s40249-019-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wait LF, Dobson AP, Graham AL. Do parasite infections interfere with immunisation? A review and meta-analysis. Vaccine. 2020 doi: 10.1016/j.vaccine.2020.06.064. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Health Observatory data repository: Number of cases of cutaneous leishmaniasis reported. 2017.
- World Health Organization . World Health Organization; 2020. Responding to community spread of COVID-19: interim guidance, 7 March 2020. [Google Scholar]
- World Health Organization . World Health Organization; 2020. Bacille Calmette-Guérin (BCG) vaccination and COVID-19: scientific brief, 12 April 2020. [Google Scholar]
- World Health Organization. Manual for case management of cutaneous leishmaniasis in the WHO Eastern Mediterranean Region 2014.
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to any reader directly upon reasonable request.