Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated disease, coronavirus disease 2019 (COVID-19), has caused a devastating pandemic worldwide. Here, we explain basic concepts underlying the transition from an epidemic to an endemic state, where a pathogen is stably maintained in a population. We discuss how the number of infections and the severity of disease change in the transition from the epidemic to the endemic phase and consider the implications of this transition in the context of COVID-19.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated disease, coronavirus disease 2019 (COVID-19), has caused a devastating pandemic worldwide. Here, we explain basic concepts underlying the transition from an epidemic to an endemic state, where a pathogen is stably maintained in a population. We discuss how the number of infections and the severity of disease change in the transition from the epidemic to the endemic phase and consider the implications of this transition in the context of COVID-19.
Basic concepts of epidemic and endemic diseases
An epidemic refers to the rapid spread of a pathogen in a population, while the endemic state refers to the stable maintenance of the pathogen, typically at a lower prevalence. When a new virus emerges into a human population, it can ignite an epidemic. The virus can be introduced from a different part of the world (e.g., viruses brought by the conquistadors to the Americas), or it can be a newly emerged zoonosis (e.g., Ebola virus or severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]). If the virus spreads worldwide, then the epidemic is a pandemic. An epidemic requires the basic reproductive number of the virus, R0, which equals the typical number of secondary infections produced by each infected individual when the population is completely susceptible, to be greater than one. The number of infections grows exponentially. The exponential growth phase cannot last for very long, as the virus runs out of susceptible individuals: infected individuals recover and are, at least temporarily, immune to infection. Thus, as the epidemic progresses, the effective reproductive number, Reff, for the infection falls. The epidemic subsides, and the number of infected individuals can fall to very low levels. The epidemic can fade out (i.e., the virus can go extinct) if the population is below a critical size, as is frequently the case for epidemics in island populations.
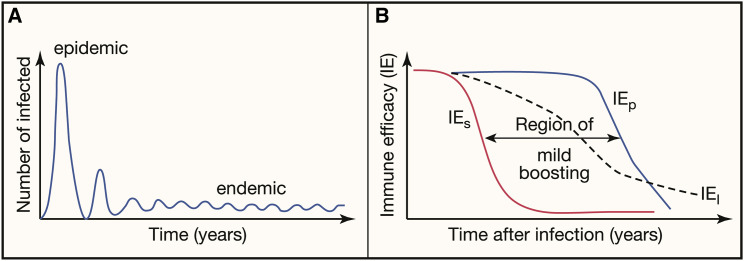
If the virus does not go extinct, it can persist for an extended period of time at a lower prevalence than at the peak of the epidemic. The latter is the endemic phase. The Reff on average equals one during this phase. The endemic phase is characterized by a dynamic equilibrium where susceptible individuals arise by birth, by immigration, or by waning of immunity in previously immune individuals. In addition, seasonal fluctuations in transmission can result in oscillations in the number of infections. Figure 1 A shows how the number of infections changes during the transition from epidemic to endemic phase. Importantly, the efficacy of the immune response to an infection is central to shaping the endemic phase.
Figure 1.
Prevalence of infection and impact of immune efficacy in endemicity
(A) Changes in number of infections during the transition between epidemic and endemic phase in the absence of interventions or virus evolution. The number of infected individuals peaks during the epidemic phase. If the epidemic does not fade out, then the virus can reach an endemic phase with a much lower number of infections.
(B) Different measures of immune efficacy (IE) are expected to decrease over time. IES, IEP, and IEI describe how immunity reduces susceptibility to infection, pathology, and infectiousness of infected individuals, respectively. In the region of mild boosting, individuals can become reinfected (because IES has waned), but these reinfections will be mild (because IEP is still high).
Will SARS-CoV-2 die out after the current epidemic phase, or will it persist in an endemic state? How do we expect the number of infections (prevalence) and the severity of disease to change as we transition to endemicity? How will prevalence and severity be affected by vaccination? Understanding the transition from epidemic to endemicity and how it is affected by vaccination requires taking into account that protection against reinfection and protection against disease wane at different rates.
Measures of immune efficacy
The notion of immune protection is frequently associated with infections (or vaccination) that generate lifelong protection from infection and disease. This view of immune protection does not effectively describe the immunology and epidemiology of coronaviruses (CoVs), where immunity gradually wanes, and individuals get reinfected (Box 1 ). For these viruses, we need a more nuanced understanding of how an individual’s immunity affects different aspects of protection, and how this immunity wanes. Based on concepts developed for vaccine efficacy, the immune efficacy generated by infection or vaccination has three components:
-
•
Immunity can reduce susceptibility to infection. This reduction is termed IES, and it describes how immunity reduces the probability of infection. IES takes values between 0 and 1, with 0 corresponding to no reduction in susceptibility and thus no protection, and 1 corresponding to perfect protection from infection (sometimes called sterilizing immunity), where the individual cannot get infected.
-
•
Immunity can reduce infectiousness. This reduction is termed IEI, and it describes the extent to which those who do get infected (because IES < 1) are less infectious for others. IEI takes values between 0 and 1, with 0 corresponding to no reduction in infectiousness, and 1 corresponding to complete blocking of transmission from the infected individual.
-
•
Immunity can reduce pathology. Pathology can be defined variously, from getting symptoms to death of the infected individual. This is termed IEP, and it describes how immunity prior to infection affects the extent of pathology during the course of reinfection. IEP takes values between 0 and 1, with 0 corresponding to no reduction in pathology, and 1 corresponding to a mild or asymptomatic infection.
Box 1. Characteristics of immunity to coronavirus.
| An important characteristic of coronaviruses in general (and likely SARS-CoV-2, in particular) is that immunity to these viruses gradually wanes, so individuals can get reinfected—potentially every few years. This is in contrast with infections such as measles, where infection and vaccination likely induce life-long immunity that blocks transmission of the virus from immune individuals. Life-long transmission-blocking immunity greatly simplifies epidemiological models for the spread of infections, particularly during the endemic phase. Our current understanding of herd immunity and the effect of vaccination pertains to viruses such as measles. Herd immunity describes the collective immunological status of a population of hosts, as opposed to an individual host, with respect to a given pathogen. Herd immunity of a population can be high if a large fraction of the population has acquired immunity (by vaccination or infection) that prevents these individuals from transmitting the virus. If transmission-blocking immunity gradually wanes, as is the case for coronaviruses, herd immunity will be transient. Describing what happens during the endemic phase for these infections requires integrating our understanding of the within-host dynamics of infection and immunity with epidemiology. | Coronaviruses are single-stranded positive-sense RNA viruses. Human coronaviruses (hCoVs) are transmitted by the respiratory route, and most virus replication occurs in the upper and lower respiratory tract. The age of first infection of hCoVs is low—by age 4, most children have turned seropositive for immunoglobulin G antibodies to hCoVs. These primary infections of children typically cause mild disease that often looks like the common cold. Infection with a given coronavirus strain elicits both antibody and T cell immunity, which peaks after infection and provides transient protection from reinfection from the same strain when antibody levels are high. A number of studies have shown frequent natural reinfections with hCoVs, as measured by detection of viral RNA or observation of serospikes induced by infection. The loss of protection against reinfection has also been confirmed by experimental infection of volunteers. Collectively, these studies indicate that shortly after infection, individuals are refractory to reinfection, but this protection wanes with time, and individuals can get reinfected with circulating hCoVs every few years. |
We also note that while the measures of immune efficacy typically take values between 0 and 1, in rare cases they can have negative values. Negative values of the components of immune efficacy correspond to scenarios where prior immunity has a detrimental effect. This has been suggested for the case of antibody-dependent enhancement of dengue infections, where prior immunity can, in some circumstances, exacerbate the severity of subsequent infections with different strains of dengue (IEP < 0).
For infections such as measles, which likely generate lifelong sterilizing immunity, IES = 1 for the lifetime of the individual, and consequently, IEI and IEP can largely be ignored, and a simple binary view of immunity is sufficient for modeling the epidemiology of measles. In contrast, immunity to CoVs wanes over time, and we need to consider how the waning of immunity relates to changes in IES, IEI, and IEP.
The magnitude of these three components of immune efficacy depends on the levels of antibody and T cell immunity in an individual and typically wanes as the levels of antibody and T cells wane. High levels of immunity are required to prevent individuals from getting infected (sterilizing immunity). If there is insufficient immunity to prevent infection, partial immunity can nonetheless allow the individual to mount a more rapid response, which typically results in more rapid control of the infection, and consequently lowers transmission and pathology. Thus, as the level of immunity decays, we expect IES to wane faster than IEI and IEP as shown in Figure 1B.
The course of infection, the functioning of the immune system, and the extent of pathology typically depend strongly on age. Consequently, the demographics of the population and the age at which individuals get their first infection play an important role in determining the burden of disease in the population.
Waning of immunity and protection
The decrease in levels of antibody and T cells after infection or immunization results in the waning of the different components of immune protection (the IEs). For infections that don’t generate lifelong immunity, we do not have quantitative measurements of the magnitude of the different components of immune efficacy after infection and vaccination and how they change over time. Below we discuss what we know about the qualitative features of the different measures of immune efficacy, first for the endemic human CoVs (hCoVs) and then for SARS-CoV-2.
Qualitatively, immunity to hCoVs in adults gives rise to transient protection from infection (indicating high IES after infection that wanes over a few years). Epidemiological calculations suggest that hCoV reinfections result in substantial virus transmission from infected individuals, suggesting intermediate levels of IEI. Less is known about IEP. All the infections observed are mild—we do not know whether the disease is inherently mild in individuals of all ages, or whether prior immunity elicited by mild primary infections during childhood reduces the severity of infections in adults. In other words, we do not know whether hCoVs would cause severe primary infections in adults, as all adults have had prior exposure to the virus in childhood. However, it is likely that primary infections with currently circulating hCoVs would cause severe disease in older individuals. Historical records indicate that OC43, one of the currently circulating hCoVs, probably caused the so-called Russian Flu epidemic in 1889-1890. The disease was severe in adults during the epidemic but subsequently became mild. What is not known is the extent to which the reduction in disease severity, particularly in adults, was due to prior immunity acquired from infections in childhood, or due to virus evolution.
For SARS-CoV-2, recent studies indicate that both natural, and to a greater extent vaccine-induced, immunity result in high levels of all components of immune efficacy—individuals are well protected shortly after infection or vaccination. Direct measurements of SARS-CoV-2-specific antibody and T cell levels show that these levels decrease over time. Epidemiological studies document reinfections but suggest that these are milder than primary infections. These observations suggest that infection-blocking immunity (IES) wanes faster than disease-reducing immunity (IEP).
Disease prevalence and severity during the transition from the epidemic to the endemic phase
The transition from epidemic to endemic phase is associated with many changes as the levels of effective immunity build within the population. These include a change in the number of infected individuals (the prevalence of infection), the age-distribution of primary infections and reinfections, and the severity of disease in infected individuals. Here, we emphasize that the susceptibility of individuals to infection (IES) and the severity of their pathology (IEP) can be separated and depend on the level of the individuals’ immunity with changes over time. This is important because the potential of severe disease upon infection shapes public perception of the infection itself and drives medical and policy decisions.
In this section, we will discuss both the severity of disease in individuals who get infected and the disease burden, which is the total number of sick people in the population. We use the infection fatality rate (IFR, the fraction of infections that result in death) as a surrogate measure for the severity of infections. For simplicity of explanation, here we will use both terms interchangeably. We first consider disease prevalence during the transition from the epidemic to the endemic phase, and then how disease severity changes during this transition, focusing on how the outcome depends on the level of an individuals’ immunity.
During the epidemic phase, the large number of susceptible individuals results in a high prevalence of infections at the peak. As susceptible individuals are depleted, the epidemic subsides, and over time, there is a transition to an endemic phase. The change from epidemic to endemic phase is thus characterized by a dramatic reduction in the prevalence of infection. This decrease is not monotonic, and there are typically several waves of infection. The timing and magnitude of these waves are affected by the imposition and release of non-pharmaceutical interventions (such as travel restrictions and the use of masks), as well as virus evolution resulting in increased transmissibility and intrinsic seasonality in transmission. This was seen in the multiple waves of the SARS-CoV-2 pandemic, which occurred at different times in different parts of the world (e.g., UK, Brazil, India, and the US), and virus evolution leading to increased transmissibility, particularly of the delta variant of SARS-CoV-2. Multiple waves of infection were also seen during the 1918 H1N1 influenza pandemic. How long it will take for SARS-CoV-2 to reach an endemic phase depends on factors such as the virus’s R0, the extent of vaccination, and how fast immunity wanes. These factors will also affect the prevalence of SARS-CoV-2 in the endemic phase, with lower R0 and slower waning of immunity leading to a lower prevalence of infections.
During the epidemic phase, virtually all infections are primary infections. The distribution of infections, assuming equal exposure to infection across age-groups, follows the age distribution of the population. The relative contribution of each age class to the disease burden equals the product of the age distribution of individuals in the population and the age-dependent disease severity. For virtually all infections, how sick you get depends on how old you are and on your level of immunity. In general, there are two patterns for the age-dependence of the severity of primary infections (see Figure 2 A). In the first pattern, the disease is mild in the young, and the IFR increases monotonically with age. This pattern is observed for SARS-CoV-1, the first coronavirus associated with SARS that emerged in 2003, and SARS-CoV-2. In the second pattern, the disease is mildest at intermediate age (typically young adolescent individuals) and is more severe in the very young and in older individuals. This pattern is observed for measles, smallpox, and also in the coronavirus that causes the Middle East Respiratory Syndrome (MERS).
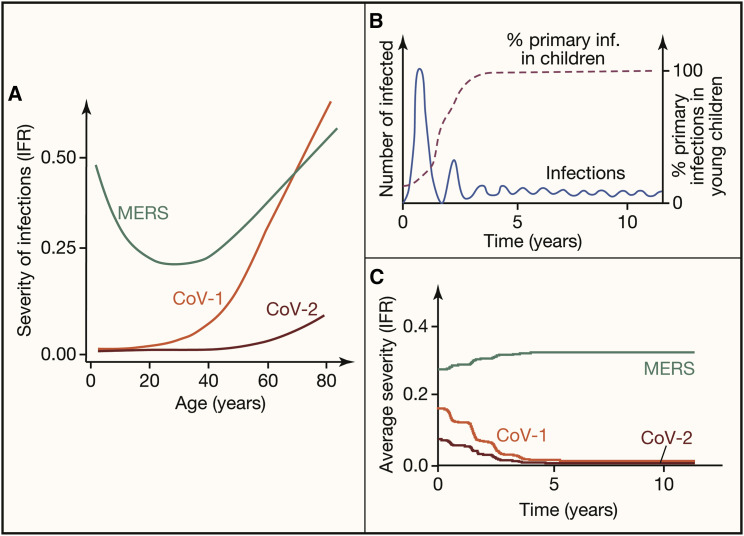
Figure 2.
Different patterns for the severity of primary infections (IFR) of emerging coronaviruses might be expected to affect the prevalence of infections and disease severity if these viruses were to become endemic
For all three infections (SARS-CoV-1, SARS-CoV-2, and MERS), we assume that primary infections provide protection from severe disease following reinfection.
(A) The age-dependent severity of primary infections with emerging coronaviruses as measured by their IFR. Both SARS-CoV-1 and SARS-CoV-2 are mild in children, and disease severity increases with age. In contrast, the IFR for MERS is lowest at intermediate ages.
(B) The transition from initial large epidemic to lower prevalence in the endemic phase (blue line) is associated with a change in the age distribution of primary infections. During the initial epidemic phase, infections occur in all age groups, while during the endemic phase, primary infections occur predominantly in children (dashed line).
(C) Changes in the IFR as we go from the epidemic to the endemic phase. We illustrate how we might expect the severity of SARS-CoV-2, SARS-CoV-1, and MERS might change if they were to become endemic in the human population. The average severity of disease during the endemic stage depends principally on the severity of primary infections of children.
To quantify disease severity during the endemic phase, we need to consider the severity of both primary infections and reinfections. During the endemic phase, most primary infections happen in children, as shown in Figure 2B. Consequently, if primary infections are mild in children and severe only in the old, then primary infections will not contribute substantially to disease severity and disease burden. We expect this to be the case for SARS-CoV-1 and SARS-CoV-2 (see Figure 2C). On the other hand, if disease severity is higher in very young individuals, as is the case for respiratory syncytial virus and MERS, a shift of primary infections to this age group will result in an increase in the number of severe primary cases. We expect this would occur were MERS to become endemic (see Figure 2C).
We also need to consider the severity of disease following reinfection. To do so, we need to know the distribution in the level of immunity in the population, and how immunity affects disease (i.e., IEP). If IEP is high and long-lasting, i.e., if primary infections give rise to long-term protection from pathology, then primary infections are the only ones that can lead to severe disease, and reinfections will be mild. If this is the case for SARS-CoV-2, we would expect the disease to be mild in the population at large because individuals get mild primary infections in childhood, which generate sufficient immunity to prevent severe disease when these individuals get reinfected as adults.
This is not the only way for SARS-CoV-2 to be generally mild in the endemic phase. If both IEP and IES wane at similar rates over time, reinfections can be severe. The severity of reinfections depends on the relative rates of waning of IES and IEP and on the extent of virus transmission in the population. For example, if protection from pathology lasts longer than the time between reinfections, then reinfections will mostly be mild and will also boost immunity (see Figure 1B). If this is the case for SARS-CoV-2, we would expect disease to be mild in the endemic phase. In this optimistic scenario, during the endemic phase, transmission would be large enough that breakthrough cases would be frequent but mild. However, this generally mild outcome may not be obtained if the extent of transmission is sufficiently low during the endemic phase, so that while there would be fewer infections, these would be severe if protection against pathology wanes before reinfection typically occurs. We hope this latter situation will not occur for SARS-CoV-2.
Implications for vaccination
Vaccination remains the preferable way to deal with most infectious diseases. Indeed, for SARS-CoV-2, vaccination during the epidemic phase is essential to reduce disease burden, particularly in older individuals and those with underlying risk factors. Vaccination has both direct and indirect effects. The direct effect is the reduction in the susceptibility, infectiousness, and pathology in the vaccinated individual. The indirect or herd immunity effect is the reduction in the probability that a susceptible (unvaccinated) individual will become infected per unit time. When vaccination leads to long-lasting transmission-blocking immunity (as is the case for measles, chickenpox, and rubella), then childhood vaccination can help reduce the spread of the disease in the population enough to locally eliminate the virus from the population. The reduction in transmission can increase the age of primary infection for unvaccinated individuals, which can have an adverse effect by increasing the average age of first infection and potentially the severity of disease. Because vaccination against SARS-CoV-2, like natural infections, is not likely to produce long-lasting transmission-blocking immunity, vaccination can only generate transient herd immunity. This makes SARS-CoV-2 eradication unlikely, and endemicity the likely long-term outcome.
We need to address a number of questions in order to understand how vaccination can optimally facilitate the transition of SARS-CoV-2 to endemicity. Clearly, vaccination is the best way to reduce the disease burden of SARS-CoV-2. Because disease is mild in young children and severe in the old, vaccination of older individuals should be prioritized. While we have measured how the levels of immunity wane after natural primary infections as well as vaccination, there is more uncertainty on how these correlate with the different measures of IEs and how the IEs change for new virus variants. For benign endemicity, it is more important that prior immunity provides protection against pathology (high IEP) rather than protection against infection (high IES). It will be important to know how the breadth of protection against new virus variants differs after vaccination versus natural infection. We also need to determine whether multiple infections or vaccinations are needed to generate long-lasting protection against pathology, and whether this depends on the age of the individual.
Benign endemicity requires mild infections of children. We need to monitor whether, as the virus evolves, it continues to cause relatively mild infections in children. Ongoing vigilance and studies will be needed.
Recap
Emerging infectious pathogens can ignite large epidemics or pandemics as infections spread exponentially through a naive population. Epidemics can fade or transition to an endemic phase. A disease is endemic when there is a relatively stable number of infections in the population. The buildup of immunity in the population leads to a decline in the number of infections and a transition to an endemic phase. If immunity provides lifelong protection from infection, then widespread vaccination can generate sufficient herd immunity to allow for elimination. If, however, immunity wanes over time, then endemicity is the more likely outcome. When an infection becomes endemic, there are different ways in which immunity provides protection without eliminating the virus from the population, such as by a reduction in susceptibility to infection (IES) or by a reduction in pathology (IEP). In the case of viruses against which infection does not generate life-long immunity, including SARS-CoV-2, it will be important to understand how these different aspects of protection wane with time and how they are boosted by natural infection and vaccination.
Recommended reading
- Antia A., Ahmed H., Handel A., Carlson N.E., Amanna I.J., Antia R., Slifka M. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16:e2006601. doi: 10.1371/journal.pbio.2006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar K.M., Reinholt K., Kissler S.M., Lipsitch M., Cobey S., Grad Y.H., Larremore D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371:916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff A., Haggett P. Island epidemics. Sci. Am. 1984;250:138–147. doi: 10.1038/scientificamerican0584-138. [DOI] [PubMed] [Google Scholar]
- Cromer D., Juno J.A., Khoury D., Reynaldi A., Wheatley A.K., Kent S.J., Davenport M.P. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Esther D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti M., Shaman J. Direct observation of repeated infections with endemic coronaviruses. J. Infect. Dis. 2021;223:409–415. doi: 10.1093/infdis/jiaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J.R., Moss P.A.H. Systematic analysis of infectious disease outcomes by age shows lowest severity in school-age children. Sci. Data. 2020;7:329. doi: 10.1038/s41597-020-00668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran M., Longini I., Struchiner C. Springer New York; 2009. Design and Analysis of Vaccine Studies Statistics for Biology and Health. [Google Scholar]
- Keeling M.J., Rohani P. Princeton University Press; 2008. Modeling infectious diseases in humans and animals. [Google Scholar]
- Lavine J.S., Bjornstad O.N., Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R.M. Vaccination programmes and herd immunity. Nature. 1982;300:481–483. doi: 10.1038/300481a0. [DOI] [PubMed] [Google Scholar]
- Saad-Roy C.M., Wagner C.E., Baker R.E., Morris S.E., Farrar J., Graham A.L., Levin S.A., Mina M.J., Metcalf C.J.E., Grenfell B.T. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020;370:811–818. doi: 10.1126/science.abd7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Galanti M. Will SARS-CoV-2 become endemic? Science. 2020;370:527–529. doi: 10.1126/science.abe5960. [DOI] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Moës E., Thoelen I., Wollants E., Lemey P., Vandamme A.-M., Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J.W. Individuals cannot rely on COVID-19 herd immunity: Durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS Pathog. 2021;17:e1009509. doi: 10.1371/journal.ppat.1009509. [DOI] [PMC free article] [PubMed] [Google Scholar]