Abstract
Background
We assessed the humoral immune response of both ChAdOx1-nCOV (CovishieldTM) and BBV-152 (CovaxinTM) vaccines in Indian health care workers (HCW).
Methods
A Pan-India, Cross-sectional, Coronavirus Vaccine-induced Antibody Titre (COVAT) study was conducted that measured SARS-CoV-2 anti-spike binding antibody quantitatively, 21 days or more after the first and second dose of two vaccines in both severe acute respiratory syndrome (SARS-CoV-2) naïve and recovered HCW. Primary aim was to analyze antibody response (seropositivity rate, Geometric Mean Titre [GMT] and 95% Confidence Interval [CI]) following each dose of both vaccines and its correlation to age, sex, blood group, body mass index (BMI) and comorbidities. Here we report the results of anti-spike antibody response after first and two completed doses.
Results
Among the 515 HCW (305 Male, 210 Female) who took two doses of both vaccines, 95.0% showed seropositivity to anti-spike antibody. However, both seropositivity rate and GMT (95% CI) of anti-spike antibody was significantly higher in Covishield vs. Covaxin recipients (98.1 vs. 80.0%; 129.3 vs. 48.3 AU/mL; both p < 0.001). This difference persisted in 457 SARS-CoV-2 naïve and propensity-matched (age, sex and BMI) analysis of 116 participants. Age > 60-years, males, people with any comorbidities, and history of hypertension (HTN) had a significantly less anti-spike antibody GMT compared to age ≤ 60 years, females, no comorbidities and no HTN respectively, after the completion of two doses of either vaccine. Gender, presence of comorbidities, and vaccine type were independent predictors of antibody seropositivity rate and anti-spike antibody titre levels in multiple logistic and log transformed linear regression analysis. Both vaccine recipients had similar solicited mild to moderate adverse events and none had severe or unsolicited side effects.
Conclusions
Both vaccines elicited good immune response after two doses, although seropositivity rates and GMT of anti-spike antibody titre was significantly higher in Covishield compared to Covaxin recipients.
Keywords: SARS-CoV-2, Vaccines, Immunogenicity, Anti-spike antibody, Comorbidities
1. Introduction
Nation-wide vaccination against the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, the cause of Coronavirus disease 2019 (COVID-19) pandemic is currently ongoing across the globe. Vaccination with two candidate vaccines namely CovishieldTM and CovaxinTM in India has started from January 16, 2021 after the Emergency Use Approval (EUA). CovishieldTM (ChAdOx1-nCOV or AZD1222, acquired from Oxford University and AstraZeneca, manufactured by Serum Institute of India, Pune) is a recombinant replication-deficient chimpanzee adenovirus-vectored vaccine encoding SARS-CoV-2 spike antigen produced in genetically modified human embryonic kidney (HEK) 293 cells. Each dose (0.5 ml) of Covishield contains 5 × 1010 viral spike particles. CovaxinTM (BBV-152, manufactured by Bharat Biotech, Hyderabad in collaboration with Indian Council of Medical Research [ICMR], India) is a ß-propiolactone inactivated whole virion vaccine having all SARS-CoV-2 proteins adjuvanted with imidazoquinoline, a Toll-like receptor 7/8 (TLR 7/8) agonist, to boost the immune response. Each dose (0.5 ml) of Covaxin contains 6 µg dose of whole virion inactivated corona virus protein of strain NIV-2020–770. The exact proportion of spike antigen in this whole-virus vaccine is not exactly known. While, early data from available phase 3 randomized clinical trials (RCTs) suggested that these two vaccines are safe and effective [1], [2], [3], there is still a paucity of information as to how much and how long, these novel vaccines can elicit an immune response, both at humoral and cellular level in the real-world settings. Moreover, the antibody kinetics after the first dose and completion of both doses of Covishield and Covaxin and thereafter, over a period of time is less well known. Here, we report the final results of ongoing Cross-sectional Coronavirus Vaccine-induced Antibody Titre (COVAT) study that assessed the anti-spike antibody humoral response 21-day after the first dose but before the second dose, and also after the completion of two doses of both Covishield and Covaxin [4].
2. Methods
2.1. Study design and participants
This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies [5]. COVAT study is an ongoing, pan-India, cross-sectional study that was approved by the ethical committee of Thakershy Charitable Trust, Ahmedabad, Gujarat, India. Written informed consent were taken from all the participants who participated in this study, voluntarily. All adult health care workers of > 18 years of age who received the first dose of vaccine were eligible to participate in this study including those who had recovered from the COVID-19 in the recent past (> 6 weeks before the first dose). Individuals with current confirmed SARS-CoV-2 infection and those diagnosed within 6-weeks were excluded from the study. All subjects have received the first and second dose of either vaccine, 0.5 ml intramuscularly in the deltoid region. Data collection for this analysis started since the January 16, 2021 (first day of vaccination amongst HCW) until May 15, 2021 (data-lock date). The participants who acquired SARS-CoV-2 infection during the study period before the second sampling were excluded from the final analysis. India had nearly 25 million cases of COVID-19 with an average case reported per day ranging from 0.1 to 0.4 million during this period, with a peak of > 0.4 million cases on a single day on May 7, 2021.
2.2. Measurements
Clinical data was collected from all eligible participants including age, sex, blood groups, body mass index (BMI), past history of confirmed SARS-CoV-2 infection, any comorbidities, presence of diabetes mellitus (type 1 [T1DM] and type 2 [T2DM]), hypertension (HTN) including its duration and treatment received, dyslipidemia, ischemic heart disease (IHD), chronic kidney disease (CKD) and cancer. Data was collected for any adverse events post-vaccination after the first and second dose. Additional data was also collected for subsequent SARS-CoV-2 infection including breakthrough infections. Accordingly, all participants were instructed to record and report the severity of conventional or solicited adverse events (fever; injection site pain, induration, swelling, redness; muscle pain; headache; excess fatigue; rash; pruritus or acute allergic reaction) to site investigators occurring within a week of vaccination after first and second dose. Severity of solicited adverse events was graded as nil, mild (recovered within 24 to 48-hour) to moderate (> 48-hour to < 7-days) and severe (requiring hospitalization), depending upon the intensity and duration of adverse event(s). Similarly, a record of unexpected or unsolicited adverse events of bleeding, thrombo-embolic episode, bells’ palsy, seizure or other neurological manifestations, occurring from day 0 to 6 months after the second dose was also being recorded. Data collection for unsolicited adverse events and breakthrough infections is still ongoing and will be continued for 6-months after the second dose. Breakthrough infections are reported as per the given proforma that include- timing and severity, defined as mild (in-house or hospital treatment in anticipation of possible worsening but not actually requiring oxygen therapy), moderate (requiring oxygen supplementation but not requiring assisted ventilation, and severe (requiring ventilator).
Anti-spike antibody titre is being measured at four time-points: day 21 after the first dose until the day before the second dose; day 21–28 of second dose, day 83–97 (3-months) and day 173–187 (6-months) after the second dose. First blood sample (5 ml) were collected from eligible participants from day 21 after the first dose but before the second dose and second blood sample from day 21–28 after the second dose of each vaccine. An additional 7 days (21–36) for collection of second blood sample was allowed due to widespread lockdown. All samples were collected as either serum or plasma using EDTA vials from each participant and analyzed at Central laboratory of Neuberg, Supratech at Ahmedabad, Gujarat, India. The IgG antibodies to SARS-CoV-2 directed against the spike protein (S-antigen, both S1 and S2 protein) were assayed with LIASON® S1/S2 quantitative antibody detection kit (DiaSorin Saluggia, Italy) using indirect chemiluminescence immunoassay (CLIA). The diagnostic sensitivity of this kit has been reported to be 97.4% (95% Confidence Interval [CI], 86.8–99.5%) > 15 days after SARS-CoV-2 infection with a specificity of 98.5% (95% CI, 97.5–99.2%) as per manufacturer’s protocol. Antibody levels > 15.0 arbitrary unit (AU)/mL were considered as sero-positive, while antibody level ≤ 15 AU/mL were considered as seronegative, as per the manufacturer’s kit. This kit has found to have a concordance to neutralizing antibody (NAb) done by Plaque Reduction Neutralization Test (PRNT) with a positive agreement of 94.4% (95% CI, 88.8–97.2%) and negative agreement of 97.8% (95% CI, 94.4–99.1%) at a cut-off of > 15 AU/mL. The lower and upper limit of this quantitative spike antibody kit is 3.8 and 400 AU/mL respectively, as per the manufacture’s brochure [6].
2.3. Statistical analysis
Descriptive and inferential statistical analysis has been carried out for the present study. Normality of the data was assessed by Shapiro-Wilk test and visually by QQ plot for Covishield and Covaxin subgroups. Data on continuous scale was presented as Median (Interquartile range, IQR) and categorical data were presented as number (%). Mann-Whitney test was utilized to assess two non-parametric groups and Kruskal-Wallis test was used to compare the differences among two or multiple data group for data on continuous scale where it is presented as Median (Interquartile range, IQR). We also presented the log-transformed antibody titre data as geometric means along with its 95% confidence interval. For data presented as geometric means, we used parametric tests (independent sample t-test and ANOVA test). We performed the analyses for the entire dataset and reported the main results. In order to confirm that the convenience sampling methodology did not creep in any bias in the results, we repeated the above analyses on the propensity matched dataset. A propensity score was generated taking into consideration age, sex and BMI of the all cohorts after the first dose and amongst the SARS-CoV-2 naïve cohorts after two complete doses of either vaccine. Participants having similar scores were matched and the two groups were compared accordingly. Chi-square test or Fischer’s exact test was used to find the significance of study parameters on categorical scale between two or more groups. Furthermore, multiple logistic regression analysis was also conducted to find out whether any independent factors were associated with a blunted response to vaccine in anti-spike antibody generation following the first and after two doses of either vaccine. Additionally, in order to provide more reliable information on the independent predictors of antibody levels, explanatory variables were dummy coded and a log transformation of the outcome variable was done to perform multiple regression analysis. The β coefficients of the significant predictors were back transformed by taking an antilog to facilitate information of the data. A two-sided P value of < 0.05 was considered as statistically significant. To compare the antibody kinetics between two vaccines, we also presented the data as dot plot (present individual titers as points) as well as box-whisker plots, both after the first and the second dose. Entire statistical analysis was carried out with Statistical Package for Social Sciences (SPSS Complex Samples) Software Version 22.0 for windows, SPSS Inc., Chicago, IL, USA, with Microsoft Word and Excel being used to generate graphs and tables.
3. Results
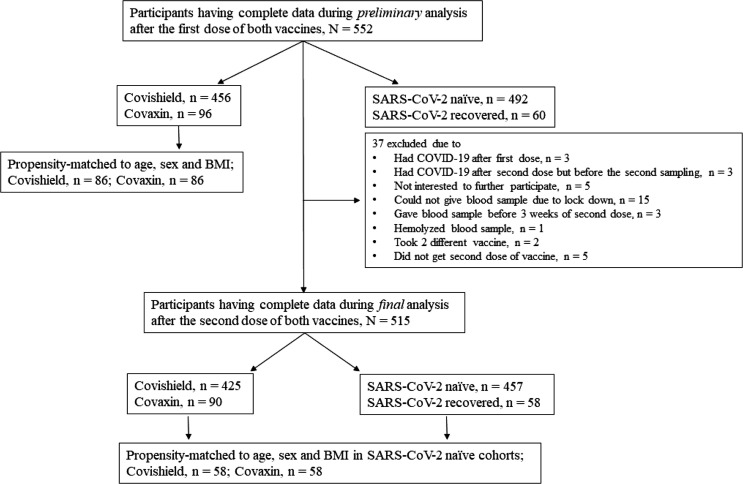
Five hundred fifty-two participants who received first dose of either of the vaccine had complete set of data including anti-spike antibody, although only 515 who received two complete doses were included in the final statistical analysis after the exclusion of 37 cohorts from preliminary analysis. Fig. 1 depicts the flow diagram of participants disposition that entered from first to final analysis. The mean age of the participants was 44.8 ± 13.1 years, with 59.2% males (305/515) and 40.8% females (210/515). Out of 515 participants, 437 (84.9%) were aged ≤ 60 years and 78 (15.1%) were of age > 60 years. While 24.3% (125/515) had one or more comorbidities, 10.1% had T2DM, 18.3% had HTN, 4.7% had dyslipidemia and 2.5% had IHD. We had no participant having T1DM, CKD or cancer in our study cohort. Among all participants who completed two doses of either of the vaccine, 11.3% (58/515) had past history of confirmed SARS-CoV-2 (2-week prior to the first dose of vaccine). Participants who acquired SARS-CoV-2 infection after the first dose of either vaccine but before the collection of second blood sample (after second dose) were excluded. While there was no imbalance in baseline characteristics, a significantly larger proportion of participants had higher BMI, any comorbidities and past history of COVID-19 in Covishield compared to the Covaxin recipients. Table 1 summarizes the baseline characteristics of both 552 cohorts (who completed first dose of either of the vaccine) and 515 cohorts (who completed two doses of either of the vaccine).
Fig. 1.
Flow diagram for participants disposition after first and second dose of each vaccine.
Table 1.
Baseline subject characteristics.
|
First dose of either vaccine |
Two completed doses of either vaccine |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall, N = 552 |
Covishield, N = 456 |
Covaxin, N = 96 |
P value | Overall, N = 515 |
Covishield, N = 425 |
Covaxin, N = 90 |
P value |
| Age | ||||||||
| Age (in years), Mean ± SD | 44.85 ± 13.23 | 45.32 ± 12.97 | 42.25 ± 15.24 | 0.087 | 44.82 ± 13.09 | 45.15 ± 12.26 | 43.44 ± 16.04 | 0.286 |
| Age ≤ 60 years, n (%) Age > 60 years, n (%) |
464 (84.06) 88 (15.94) |
384 (84.21) 72 (15.79) |
80 (83.33) 16 (16.67) |
0.831 | 437 (84.9) 78 (15.1) |
361 (84.9) 64 (15.1) |
76 (84.4) 14 (15.6) |
0.905 |
| Sex | ||||||||
| Male, n (%) Female, n (%) |
325 (58.88) 227 (41.12) |
268 (58.77) 188 (41.23) |
57 (59.38) 39 (40.63) |
0.913 | 305 (59.2) 210 (40.8) |
252 (59.3) 173 (40.7) |
53 (58.9) 37 (41.1) |
0.943 |
| Body mass index (BMI) | ||||||||
| BMI (kg/m2), Mean ± SD | 25.61 ± 4.36 | 25.57 ± 4.53 | 25.75 ± 3.31 | 0.751 | 25.42 ± 4.43 | 25.96 ± 4.87 | 24.02 ± 3.66 | 0.001 |
| BMI < 25 kg/m2, n (%) BMI 25–29.9 kg/m2, n (%) BMI ≥ 30 kg/m2, n (%) |
379 (68.66) 106 (19.2) 67 (12.13) |
309 (67.76) 89 (19.52) 58 (12.72) |
70 (72.92) 17 (17.71) 9 (9.38) |
0.556 | 353 (68.5) 96 (18.6) 66 (12.8) |
287 (67.5) 81 (19.1) 57 (13.4) |
66 (73.3) 15 (16.7) 9 (10) |
0.531 |
| Blood group (%) | ||||||||
| A + ve B + ve AB + ve O + ve A-ve B-ve AB-ve O-ve |
108 (19.6) 159 (28.8) 30 (5.4) 225 (40.8) 7 (1.3) 10 (1.8) 2 (0.4) 11 (2) |
89 (19.5) 141 (30.9) 25 (5.5) 172 (37.7) 7 (1.5) 10 (2.2) 2 (0.4) 10 (2.2) |
19 (19.8) 18 (18.8) 5 (5.2) 53 (55.2) 0 0 0 1 (1) |
0.052 | 104 (20.2) 148 (28.7) 29 (5.6) 205 (39.8) 7 (1.4) 9 (1.7) 2 (0.4) 11 (2.1) |
86 (20.2) 132 (31.1) 24 (5.6) 155 (36.5) 7 (1.6) 9 (2.1) 2 (0.5) 10 (2.4) |
18 (20) 16 (17.8) 5 (5.6) 50 (55.6) 0 0 0 1 (1.1) |
0.319 |
| Comorbidities | ||||||||
| Any Co-morbidities, n (%) No Co-morbidities, n (%) |
131 (23.73) 421 (76.27) |
118 (25.88) 338 (74.12) |
13 (13.54) 83 (86.45) |
0.010 | 125 (24.3) 390 (75.7) |
114 (26.8) 311 (73.2) |
11 (12.2) 79 (87.8) |
0.003 |
| Type 2 diabetes mellitus (T2DM) | ||||||||
| T2DM, n (%) No T2DM, n (%) |
57 (10.33) 495 (89.67) |
49 (10.75) 407 (89.25) |
8 (8.33) 88 (91.66) |
0.480 | 52 (10.1) 463 (89.9) |
46 (10.8) 379 (89.2) |
6 (6.7) 84 (93.3) |
0.234 |
| T2DM Duration | ||||||||
| Duration < 5 years, n (%) Duration 5–10 years, n (%) Duration > 10 years, n (%) |
11 (1.99) 31 (5.61) 15 (2.71) |
10 (2.19) 26 (5.7) 14 (3.07) |
1 (1.04) 5 (5.2) 1 (1.04) |
0.605 | 8 (1.6) 30 (5.8) 14 (2.7) |
8 (1.9) 25 (5.9) 13 (3.1) |
0 5 (5.6) 1 (1.1) |
0.409 |
| T2DM Control | ||||||||
| Optimum control, n (%) No optimum control, n (%) |
55 (9.96) 2 (0.36) |
48 (10.53) 1 (0.21) |
7 (7.29) 1 (1.04) |
0.307 | 51 (9.9) 1 (0.1) |
45 (10.6) 1 (0.2) |
6 (6.7) 0 |
0.470 |
| T2DM Management | ||||||||
| Monotherapy, n (%) Combination therapy, n (%) Insulin, n (%) No medication, n (%) |
16 (2.89) 35 (6.34) 1 (0.18) 5 (0.9) |
13 (2.85) 32 (7.01) 1 (0.21) 4 (0.87) |
3 (3.13) 3 (3.13) 0 1 (1.04) |
0.563 | 15 (2.9) 32 (6.2) 0 4 (0.1) |
13 (3.1) 29 (6.8) 0 4 (0.9) |
2 (2.2) 3 (3.3) 0 0 |
0.615 |
| Hypertension (HTN) | ||||||||
| Yes, n (%) No, n (%) |
99 (17.93) 453 (82.07) |
77 (16.89) 379 (83.11) |
22 (22.91) 74 (77.08) |
0.162 | 94 (18.3) 421 (81.7) |
75 (17.6) 350 (82.4) |
19 (21.1) 71 (78.9) |
0.440 |
| HTN Duration | ||||||||
| Duration < 5 years, n (%) Duration 5–10 years, n (%) Duration > 10 years, n (%) |
29 (5.25) 48 (8.69) 22 (3.99) |
26 (5.7) 31 (6.8) 20 (4.39) |
3 (3.13) 17 (17.7) 2 (2.08) |
0.067 | 27 (5.2) 46 (8.9) 21 (4.1) |
24 (5.6) 31 (7.3) 20 (4.7) |
3 (3.3) 15 (16.7) 1 (1.1) |
0.053 |
| HTN Management | ||||||||
| RAAS Blockers, n (%) CCBs, n (%) Combined, n (%) No Medicine, n (%) |
41 (7.43) 22 (3.99) 21 (3.81) 2 (0.36) |
37 (8.11) 20 (4.39) 19 (4.17) 1 (0.21) |
4 (4.16) 2 (2.08) 2 (2.08) 1 (1.04) |
0.228 | 40 (7.8) 21 (4.1) 20 (3.9) 2 (0.4) |
37 (8.7) 19 (4.5) 18 (4.2) 1 (0.2) |
3 (3.3) 2 (2.2) 2 (2.2) 1 (1.1) |
0.158 |
| Lipid abnormalities | ||||||||
| Dyslipidemia, n (%) No Dyslipidemia, n (%) |
26 (4.71) 526 (95.28) |
26 (4.71) 430 (94.29) |
0 96 (1 0 0) |
0.050 | 24 (4.7) 491 (95.3) |
24 (5.6) 401 (94.4) |
0 90 (1 0 0) |
0.062 |
| Ischemic heart disease (IHD) | ||||||||
| IHD, n (%) No IHD, n (%) |
13 (2.35) 539 (97.64) |
13 (2.85) 443 (97.15) |
0 96 (1 0 0) |
0.094 | 13 (2.5) 502 (97.5) |
13 (3.1) 412 (96.9) |
0 90 (1 0 0) |
0.093 |
| History of SARS-CoV-2 infection | ||||||||
| Past H/o COVID-19, n (%) No Past H/o COVID-19, n (%) |
60 (10.87) 492 (89.13) |
57 (12.5) 399 (72.28) |
3 (3.12) 93 (96.88) |
0.006 | 58 (11.3) 457 (88.7) |
55 (12.9) 370 (87.1) |
3 (3.3) 87 (96.7) |
0.009 |
p < 0.05 considered as statistically significant, p computed by chi-square test or independent sample t-test to test the significance between Covaxin and Covishield, BMI- body mass index, T2DM- type 2 diabetes mellitus, HTN- hypertension, RAAS- Renin angiotensin aldosterone system, CCB- calcium channel blocker, IHD- ischemic heart disease, SARS-CoV-2- severe acute respiratory syndrome coronavirus-2, COVID-19- coronavirus disease 2019.
3.1. SARS-COV-2 anti-spike antibody positivity rates following one or two doses of vaccine
Of the 522 participant (325 male, 227 female), 456 and 96 received the first dose of Covishield and Covaxin respectively. Overall, 79.3% (438/552) had seropositivity (defined as anti-spike antibody titre > 15 AU/mL measured at day 21 until day before second dose) for anti-spike antibody. The seropositivity rate was significantly higher in Covishield vs. Covaxin recipient (86.8 vs. 43.8% respectively, p < 0.001) after the first dose. Importantly, past history of SARS-CoV-2 infection resulted in a significantly greater antibody seropositivity rate compared to SARS-CoV-2 naïve individuals in overall cohort (96.7% vs. 77.2%, p < 0.001) after the first dose, irrespective of the type of vaccine received. Among the 515 participants (305 male, 210 female) who completed both doses of Covishield (n = 425) and Covaxin (n = 90) respectively, 95.0% (489/515) had seropositivity (defined as anti-spike antibody titre > 15 AU/mL measured at day 21–36 after the second dose) for anti-spike antibody. Notably, the seropositivity rate was significantly higher in Covishield vs. Covaxin recipients (98.1 vs. 80.0% respectively, p < 0.001) after two complete doses. Seropositivity rate was significantly (p = 0.001) higher in participants aged ≤ 60 years (96.3%) vs. > 60 years (87.2%), in overall cohorts. While no difference in seropositivity rate was observed for gender, BMI and presence of any comorbidities in overall study participants; females compared to males (100.0 vs. 96.8%, p = 0.02) and those having no comorbidities (any) compared to ones with any comorbidities had a significantly higher seropositivity rate (99.0 vs. 95.6%, p = 0.02) amongst Covishield recipients. Amongst the captured co-morbidities, people with T2DM had a significantly lower seropositivity rate compared to those without (84.6 vs. 96.1%, p = 0.002) in overall study participants, and this was consistent in both Covishield (91.3 vs. 98.9%, p < 0.001) and Covaxin recipients (33.3 vs. 83.3%, p = 0.003), respectively after the completion of two doses. No differential antibody seropositivity rate was observed in relation to types of blood group, presence or absence of dyslipidemia, IHD and treatment regime of DM and HTN. Table 2 summarizes the seropositivity rates after the first and second doses with either vaccine.
Table 2.
Seropositivity to anti-spike antibody after the first dose and second dose of either vaccine.
|
First dose of either vaccine |
Two completed doses of either vaccine |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall, N = 552 |
Covishield, N = 456 |
Covaxin, N = 96 |
Overall, N = 515 |
Covishield, N = 425 |
Covaxin, N = 90 |
||||||
| Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | |
| Age | ||||||||||||
| Age ≤ 60 years, n (%) Age > 60 years, n (%) |
372/464 (80.2) 66/88 (74.4) |
0.272 | 339/384 (88.3) 57/72 (79.2) |
0.036 | 33/80 (41.2) 9/16 (56.2) |
0.270 | 421/437 (96.3) 68/78 (87.2) |
0.001 | 358/361 (99.2) 59/64 (92.2) |
<0.001 | 63/76 (82.9) 9/14 (64.3) |
0.110 |
| Sex | ||||||||||||
| Male, n (%) Female, n (%) |
251/325 (77.2) 187/227 (82.4) |
0.142 | 228/268 (85.1) 168/188 (89.4) |
0.182 | 23/57 (40.4) 19/39 (48.7) |
0.417 | 286/305 (93.8) 203/210 (96.7) |
0.140 | 244/252 (96.8) 173/173 (1 0 0) |
0.018 | 42/53 (79.2) 30/37 (81.1) |
0.830 |
| Body mass index (BMI) | ||||||||||||
| BMI < 25 kg/m2 BMI 25–29.9 kg/m2 BMI ≥ 30 kg/m2 |
301/379 (79.4) 85/106 (80.2) 57/67 (85.1) |
0.731 | 273/309 (88.3) 74/89 (83.1) 49/58 (84.5) |
0.375 | 27/70 (38.6) 11/17 (64.7) 4/9 (44.4) |
0.150 | 338/353 (95.8) 90/96 (93.8) 61/66 (92.4) |
0.441 | 285/287 (99.3) 78/81 (96.3) 54/57 (94.7) |
0.028 | 53/66 (80.3) 12/15 (80) 7/9 (77.8) |
0.984 |
| Blood groups | ||||||||||||
| A + ve, n (%) B + ve, n (%) AB + ve, n (%) O + ve, n (%) A-ve, n (%) B-ve, n (%) AB-ve, n (%) O-ve, n (%) |
84/108 (77.8) 130/159 (81.8) 23/30 (76.7) 175/225 (77.8) 7/7 (1 0 0) 8/10 (80) 2/2 (1 0 0) 9/11 (81.8) |
0.826 | 79/89 (88.8) 121/141 (85.8) 21/25 (84) 149/172 (86.6) 7/7 (1 0 0) 8/10 (80) 2/2 (1 0 0) 9/10 (90) |
0.930 | 5/19 (26.3) 9/18 (50) 2/5 (40) 26/53 (49.1) - - - 0/1 (0) |
0.400 | 99/104 (95.2) 142/148 (81.8) 26/29 (89.7) 193/205 (94.1) 7/7 (1 0 0) 9/9 (1 0 0) 2/2 (1 0 0) 11/11 (1 0 0) |
0.799 | 86/86 (1 0 0) 127/132 (96.2) 23/24 (95.8) 153/155 (98.7) 7/7 (1 0 0) 9/9 (1 0 0) 2/2 (1 0 0) 10/10 (1 0 0) |
0.569 | 13/18 (72.2) 15/16 (93.8) 3/5 (60) 40/50 (80) - - - 0/1 (0) |
0.396 |
| Comorbidities | ||||||||||||
| Any Co-morbidities, n (%) No Co-morbidities, n (%) |
95/131 (72.5) 343/421 (81.5) |
0.027 | 92/118 (78) 304/338 (89.9) |
0.001 | 3/13 (23.1) 39/83 (47) |
0.092 | 116/125 (92.8) 373/390 (95.6) |
0.207 | 109/114 (95.6) 308/311 (99) |
0.021 | 7/11 (63.6) 65/79 (82.3) |
0.148 |
| Type 2 diabetes mellitus (T2DM) | ||||||||||||
| T2DM, n (%) No T2DM, n (%) |
42/57 (73.7) 396/495 (80) |
0.265 | 39/49 (79.6) 357/407 (87.7) |
0.112 | 3/8 (37.5) 39/88 (44.3) |
0.710 | 44/52 (84.6) 445/463 (96.1) |
0.002 | 42/46 (91.3) 375/379 (98.9) |
<0.001 | 2/6 (33.3) 70/84 (83.3) |
0.003 |
| T2DM Duration | ||||||||||||
| T2DM Duration < 5 years, n (%) T2DM Duration 5–10 years, n (%) T2DM Duration > 10 years, n (%) |
9/11 (81.8) 21/31 (67.74) 11/15 (73.3) |
0.751 | 8/10 (80) 20/26 (76.92) 11/14 (78.6) |
0.994 | 1/1 (1 0 0) 1/5 (20) 0/1 (0) |
0.214 | 8/8 (1 0 0) 23/30 (76.7) 13/14 (92.9) |
<0.001 | 8/8 (1 0 0) 21/25 (84) 13/13 (1 0 0) |
<0.001 | - 2/5 (40) 0/1 (0) |
0.008 |
| T2DM Control | ||||||||||||
| T2DM-Optimum control, n (%) T2DM-No optimum control, n (%) |
40/55 (72.7) 0/2 (0) |
0.390 | 38/48 (79.2) 1/1 (1 0 0) |
0.609 | 2/7 (28.6) 1/1 (1 0 0) |
0.168 | 43/51 (84.3) 1/1 (1 0 0) |
0.369 | 41/45 (91.1) 1/1 (1 0 0) |
0.568 | 2/6 (33.3) 0 (0/0) |
0.762 |
| T2DM Management | ||||||||||||
| Monotherapy, n (%) Combination therapy, n (%) Insulin, n (%) No medication, n (%) |
14/16 (87.5) 24/35 (68.6) 1/1 (1 0 0) 3/5 (60) |
0.287 | 12/13 (92.3) 24/32 (75) 1/1 (1 0 0) 3/4 (75) |
0.414 | 2/3 (66.7) 0/3 (0) - 0/1 (0) |
0.155 | 14/15 (93.3) 26/32 (81.2) - 4/5 (80) |
0.151 | 13/13 (1 0 0) 26/29 (89.7) - 3/4 (75) |
0.201 | 1/2 (50) 0/3 (0) - 1/1 (1 0 0) |
0.103 |
| Hypertension (HTN) | ||||||||||||
| HTN, n (%) No HTN, n (%) |
65/99 (65.7) 373/453 (82.3) |
0.001 | 56/77 (72.7) 340/379 (89.7) |
<0.001 | 13/22 (55.4) 41/74 (88.9) |
0.760 | 86/94 (91.5) 403/421 (95.7) |
0.090 | 72/75 (96) 345/350 (98.6) |
0.137 | 14/19 (73.7) 58/71 (81.7) |
0.438 |
| HTN Duration < 5 years, n (%) HTN Duration 5–10 years, n (%) HTN Duration > 10 years, n (%) |
17/29 (58.6) 33/48 (68.8) 15/22 (68.2) |
0.549 | 17/26 (65.4) 25/31 (80.6) 14/20 (70) |
0.414 | 0/3 (0) 8/17 (47.1) 1/2 (50) |
0.482 | 27/27 (1 0 0) 39/46 (84.8) 20/21 (95.2) |
0.008 | 24/24 (1 0 0) 28/31 (90.3) 20/20 (1 0 0) |
0.010 | 3/3 (1 0 0) 11/15 (73.3) 0/1 (0) |
0.152 |
| HTN Management | ||||||||||||
| RAAS Blockers, n (%) CCBs, n (%) Combined, n (%) No Medicine, n (%) |
28/41 (68.3) 14/22 (63.6) 14/21 (66.7) 1/2 (50) |
0.944 | 27/37 (73) 14/20 (70) 14/19 (73.7) 1/1 (1 0 0) |
0.928 | 1/4 (25) 0/2 (0) 0/2 (0) 0/1 (0) |
0.704 | 37/40 (92.5) 20/21 (95.2) 19/20 (95) 2/2 (1 0 0) |
0.958 | 35/37 (94.6) 18/19 (94.7) 18/18 (1 0 0) 1/1 (1 0 0) |
0.352 | 2/3 (66.7) 2/2 (1 0 0) 1/2 (50) 1/1 (1 0 0) |
0.695 |
| Dyslipidemia | ||||||||||||
| Dyslipidemia, n (%) No Dyslipidemia, n (%) |
23/26 (88.5) 415/526 (78.89) |
0.437 | 23/26 (88.5) 373/430 (86.7) |
0.897 | - 42/96 (43.8) |
(*) | 23/24 (95.8) 466/491 (94.9) |
0.954 | 23/24 (95.8) 394/401 (98.2) |
0.692 | - 72/90 (80) |
(*) |
| Ischemic heart disease (IHD) | ||||||||||||
| IHD, n (%) No IHD, n (%) |
11/13 (84.6) 427/539 (79.2) |
0.635 | 11/13 (84.6) 385/443 (86.9) |
0.810 | - 42/96 (43.8) |
13/13 (1 0 0) 476/502 (94.8) |
0.400 | 13/13 (1 0 0) 404/412 (98.1) |
0.612 | - 72/90 (80) |
(*) | |
| History of SARS-CoV-2 infection | ||||||||||||
| Past H/o COVID-19, n (%) No Past H/o COVID-19, n (%) |
58/60 (96.7) 380/492 (77.2) |
<0.001 | 55/57 (96.5) 341/399 (85.5) |
0.023 | 3/3 (1 0 0) 39/93 (41.9) |
0.046 | 58/58 (1 0 0) 431/457 (94.3) |
0.062 | 55/55 (1 0 0) 362/370 (97.8) |
0.271 | 3/3 (1 0 0) 69/87 (79.3) |
0.378 |
| Types of vaccine | ||||||||||||
| Covishield, n (%) Covaxin, n (%) |
396/456 (86.8) 42/96 (43.8) |
<0.001 | 417/425 (98.1) 72/90 (80) |
<0.001 | ||||||||
p < 0.05 considered as statistically significant, p computed by chi-square test; BMI- Body mass index, T2DM- Type 2 diabetes mellitus, HTN- Hypertension, RAAS- Renin angiotensin aldosterone system, CCB- Calcium channel blocker, IHD- Ischemic heart disease, SARS-CoV-2- Severe acute respiratory syndrome coronavirus-2, COVID-19- Coronavirus disease 2019, NA- Not applicable, (*) - No statistics is computed.
Interestingly, in a repeat analysis of 492 SARS-CoV-2 naïve participants after the first dose (after exclusion of subjects with a past history of COVID-19, n = 60), and 457 SARS-CoV-2 naïve participants after the two complete doses (excluding past history of COVID-19, n = 58) results were similar and consistent. Seropositivity rate to anti-spike antibody was significantly higher in SARS-CoV-2 naïve Covishield recipients compared to Covaxin, both after the first (85.5 vs. 41.9% respectively; p < 0.001) and two completed second dose (97.8 vs. 79.3% respectively; p < 0.001) (Supplementary table 1).
3.2. Comparison of SARS-CoV-2 anti-spike antibody seropositivity rate following one or two doses of vaccine in propensity matched analysis
In the propensity-matched analysis of 172 participants (86 participants in each arm) after the adjustment for age, sex and BMI who received the first dose of either vaccine; past history of SARS-CoV-2 infection and type of vaccine received were found to be associated with a different antibody seropositivity rate. Seropositive rates were found to be significantly higher in Covishield compared to the Covaxin recipients (88.4% vs. 43.0%, p < 0.001) in the propensity-matched analysis. Similarly, in the propensity-matched analysis of 116 SARS-CoV-2 naïve participants (58 recipients with each vaccine) after the adjustment for age, sex and BMI who received the two complete doses of either vaccine; seropositivity rates were significantly higher in Covishield compared to the Covaxin (98.3% vs. 82.8%, p = 0.004) recipients. Notably, age ≤ 60 years had higher seropositivity rate vs. > 60-years (98.5 vs. 79.6%, p = 0.001) and the presence of T2DM was associated with a lower seropositivity rate compared to those without (92.0 vs. 50.0%, p = 0.005) in the propensity matched analysis after completion of two doses (Table 3 ).
Table 3.
Seropositivity to anti-spike antibody in propensity score (age, sex, BMI) matched analysis after the first dose of either vaccine in total cohorts and after the second doses of either vaccine in SARS-CoV-2 naïve cohorts#
|
First dose of either vaccine |
Two completed doses of either vaccine# |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall, N = 172 |
Covishield, N = 86 |
Covaxin, N = 86 |
Overall, N = 116 |
Covishield, N = 58 |
Covaxin, N = 58 |
||||||
| Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | Seropositivity Rate | P | |
| Age | ||||||||||||
| Age ≤ 60 years, n (%) Age > 60 years, n (%) |
98/148 (66.2) 15/24 (62.5) |
0.722 | 67/73 (91.2) 9/13 (69.2) |
0.019 | 31/75 (41.3) 6/11 (54.5) |
0.409 | 66/67 (98.5) 39/49 (79.6) |
0.001 | 43/43 (1 0 0) 14/15 (93.3) |
0.088 | 23/24 (95.8) 25/34 (73.5) |
0.027 |
| Sex | ||||||||||||
| Male, n (%) Female, n (%) |
52/81 (64.2) 61/91 (67) |
0.696 | 36/40 (90) 40/46 (87) |
0.661 | 16/41 (39) 21/45 (46.7) |
0.475 | 47/51 (92.2) 58/65 (89.2) |
0.593 | 23/23 (1 0 0) 34/35 (97.1) |
0.414 | 24/28 (85.7) 24/30 (80) |
0.565 |
| Body mass index (BMI) | ||||||||||||
| BMI < 25 kg/m2 BMI 25–29.9 kg/m2 BMI ≥ 30 kg/m2 |
78/124 (62.9) 22/29 (75.9) 13/19 (68.4) |
0.402 | 55/62 (88.7) 12/14 (85.7) 9/10 (90) |
0.938 | 23/62 (37.1) 10/15 (66.7) 4/9 (44.4) |
0.116 | 84/92 (91.3) 15/17 (88.2) 6/7 (85.7) |
0.836 | 50/51 (98) 6/6 (1 0 0) 1/1 (1 0 0) |
0.933 | 34/41 (82.9) 9/11 (81.8) 5/6 (83.3) |
0.996 |
| Blood group | ||||||||||||
| A + ve, n (%) B + ve, n (%) AB + ve, n (%) O + ve, n (%) A-ve, n (%) B-ve, n (%) AB-ve, n (%) O-ve, n (%) |
22/37 (59.5) 34/47 (72.3) 3/6 (50) 53/80 (66.2) - 1/2 (50) - - |
0.655 | 17/18 (94.4) 28/33 (84.8) 2/2 (1 0 0) 28/31 (90.3) - 1/2 (50) - - |
0.368 | 5/19 (26.3) 6/14 (42.9) 1/4 (25) 25/49 (51) - - - - |
0.264 | 22/24 (91.7) 30/32 (93.8) 8/9 (88.9) 40/46 (87) 1/1 (1 0 0) 2/2 (1 0 0) - 2/2 (1 0 0) |
0.948 | 14/14 (1 0 0) 17/18 (94.4) 7/7 (1 0 0) 15/15 (1 0 0) 1/1 (1 0 0) 2/2 (1 0 0) - 1/1 (1 0 0) |
0.894 | 8/10 (80) 13/14 (92.9) 1/2 (50) 25/31 (80.6) - - - 1/1 (1 0 0) |
0.581 |
| Comorbidities | ||||||||||||
| Any Co-morbidities, n (%) No Co-morbidities, n (%) |
19/33 (57.6) 94/139 (67.6) |
0.274 | 18/22 (81.8) 58/64 (90.6) |
0.266 | 1/11 (9.1) 36/75 (48) |
0.015 | 7/9 (77.8) 98/107 (91.6) |
0.174 | 4/4 (1 0 0) 53/54 (98.1) |
0.784 | 3/5 (60) 45/53 (84.9) |
0.202 |
| Type 2 diabetes mellitus (T2DM) | ||||||||||||
| T2DM, n (%) No T2DM, n (%) |
8/17 (47.1) 105/155 (67.7) |
0.088 | 7/11 (63.6) 69/75 (92) |
0.006 | 1/6 (16.7) 36/80 (45) |
0.176 | 2/4 (50) 103/112 (92) |
0.005 | 2/2 (1 0 0) 55/56 (98.2) |
0.849 | 0/2 (0) 48/56 (85.7) |
0.002 |
| T2DM Duration | ||||||||||||
| T2DM Duration < 5 years, n (%) T2DM Duration 5–10 years, n (%) T2DM Duration > 10 years, n (%) |
3/4 (75) 4/8 (50) 1/5 (20) |
0.114 | 2/3 (66.7) 4/4 (1 0 0) 1/4 (25) |
0.059 | 1/1 (1 0 0) 0/4 (0) 0/1 (0) |
0.156 | 1/1 (1 0 0) 1/3 (33.3) - |
0.143 | 1/1 (1 0 0) 1/1 (1 0 0) - |
0.982 | 0/0 (0) 0/2 (0) - |
0.902 |
| T2DM Control | ||||||||||||
| Optimum control, n (%) No optimum control, n (%) |
7/16 (43.8) 1/1 (1 0 0) |
0.121 | 6/10 (60) 1/1 (1 0 0) |
0.052 | 1/6 (16.7) |
0.176 | 2/4 (50) - |
(*) | 2/2 (1 0 0) - |
(*) | 0/2 - |
(*) |
| T2DM Management | ||||||||||||
| Monotherapy, n (%) Combination therapy, n (%) Insulin, n (%) No medication, n (%) |
4/6 (66.6) 3/10 (30) 1/1 (1 0 0) - |
0.085 | 3/3 (1 0 0) 3/7 (42.9) 1/1 (1 0 0) - |
0.198 | 1/3 (33.3) 0/3 (0) 0/1 (0) - |
0.364 | 1/1 (1 0 0) 1/3 (33.3) - - |
0.933 | 1/1 (1 0 0) 1/1 (1 0 0) - - |
0.982 | - 0/2 - - |
(*) |
| Hypertension (HTN) | ||||||||||||
| HTN, n (%) No HTN, n (%) |
93/137 (67.9) 20/35 (57.1) |
0.232 | 64/72 (88.9) 12/14 (85.7) |
0.735 | 29/65 (44.6) 8/21 (38.1) |
0.600 | 9/12 (75) 96/104 (92.3) |
0.053 | 1/1 (1 0 0) 56/57 (98.2) |
0.894 | 8/11 (72.7) 40/47 (85.1) |
0.328 |
| HTN Duration | ||||||||||||
| HTN Duration < 5 years, n (%) HTN Duration 5–10 years, n (%) HTN Duration > 10 years, n (%) |
4/8 (50) 13/22 (59.1) 3/5 (60) |
0.645 | 4/5 (80) 5/5 (1 0 0) 3/4 (75) |
0.634 | 0/3 (0) 8/17 (47.1) 1/1 (0) |
0.362 | 4/4 (1 0 0) 5/7 (71.4) 0/1 (0) |
0.054 | 1/1 (1 0 0) - - |
(*) | 3/3 (1 0 0) 5/7 (71.4) 0/1 (0) |
0.101 |
| HTN Management | ||||||||||||
| RAAS Blockers, n (%) CCBs, n (%) Combined, n (%) No Medicine, n (%) |
4/8 (50) 3/6 (50) 4/6 (66.7) 1/2 (50) |
0.749 | 4/5 (80) 3/4 (75) 4/4 (1 0 0) 1/1 (1 0 0) |
0.788 | 0/3 0/2 0/2 0/1 |
0.155 | 1/2 (50) 3/3 (1 0 0) - - |
0.124 | 1/1 (1 0 0) - - - |
0.894 | 0/1 (0) 3/3 (1 0 0) - - |
0.380 |
| Dyslipidemia | ||||||||||||
| Dyslipidemia, n (%) No Dyslipidemia, n (%) |
8/10 (80) 107/162 (66.04) |
0.768 | 6/6 (1 0 0) 63/80 (78.75) |
0.102 | 2/4 (50) 44/82 (53.69) |
0.864 | - 105/116 (90.52) |
(*) | - 57/58 (98.3) |
(*) | - 48/58 (82.8) |
(*) |
| Ischemic heart diseases (IHD) | ||||||||||||
| IHD, n (%) No IHD, n (%) |
3/4 (75) 110/168 (65.5) |
0.692 | 3/4 (75) 73/82 (89) |
0.393 | 0/0 37/86 (43) |
0.393 | - 105/116 (90.52) |
(*) | - 57/58 (98.3) |
(*) | - 48/58 (82.8) |
(*) |
| History of SARS-CoV-2 infection | ||||||||||||
| Past H/o COVID-19, n (%) No Past H/o COVID-19, n (%) |
12/12 (1 0 0) 101/160 (63.1) |
0.009 | 9/9 (1 0 0) 67/77 (87) |
0.250 | 3/3 (1 0 0) 34/83 (41) |
0.042 | NA |
|||||
| Types of vaccine | ||||||||||||
| Covishield, n (%) Covaxin, n (%) |
76/86 (88.4) 37/86 (43) |
<0.001 | 57/58 (98.3) 48/58 (82.8) |
0.004 | ||||||||
p < 0.05 considered as statistically significant, p computed by chi-square test; BMI- Body mass index, T2DM- Type 2 diabetes mellitus, HTN- Hypertension, RAAS- Renin angiotensin aldosterone system, CCB- Calcium channel blocker, IHD- Ischemic heart disease, SARS-CoV-2- Severe acute respiratory syndrome coronavirus-2, COVID-19- Coronavirus disease 2019, NA- Not applicable, (*) - No statistics is computed.
3.3. Assessment of SARS-CoV-2 spike antibody titre following one or two doses of vaccine
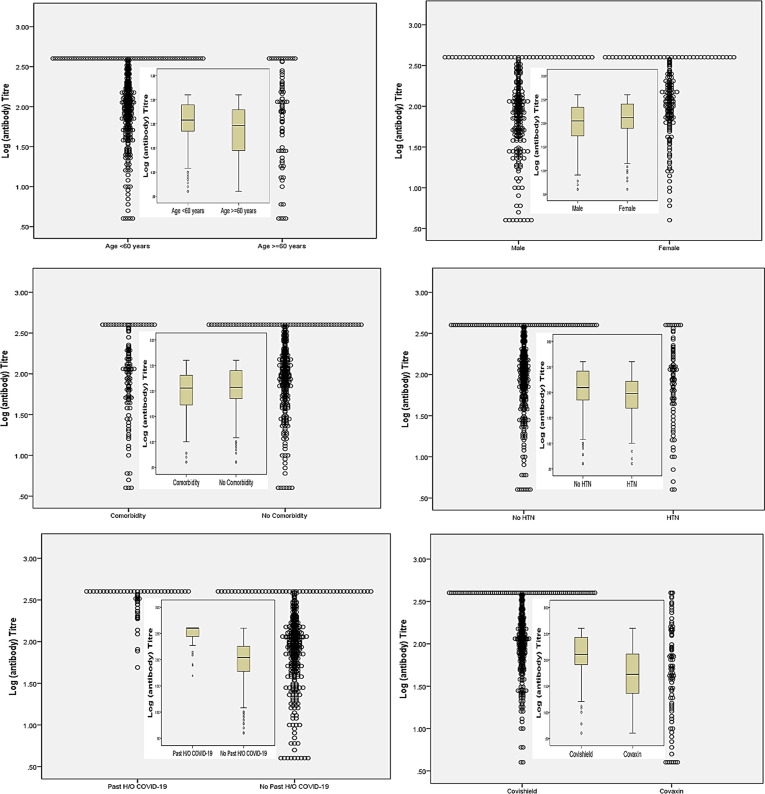
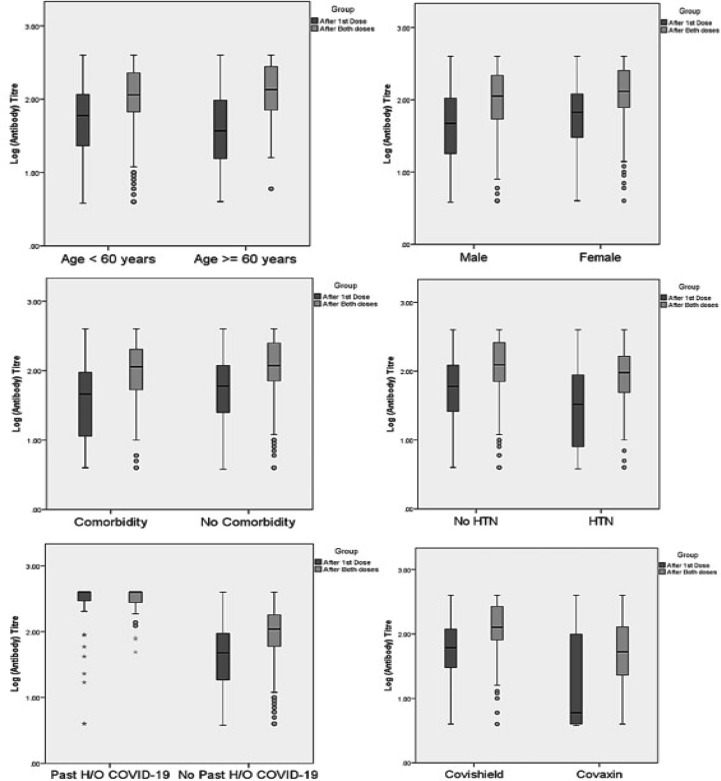
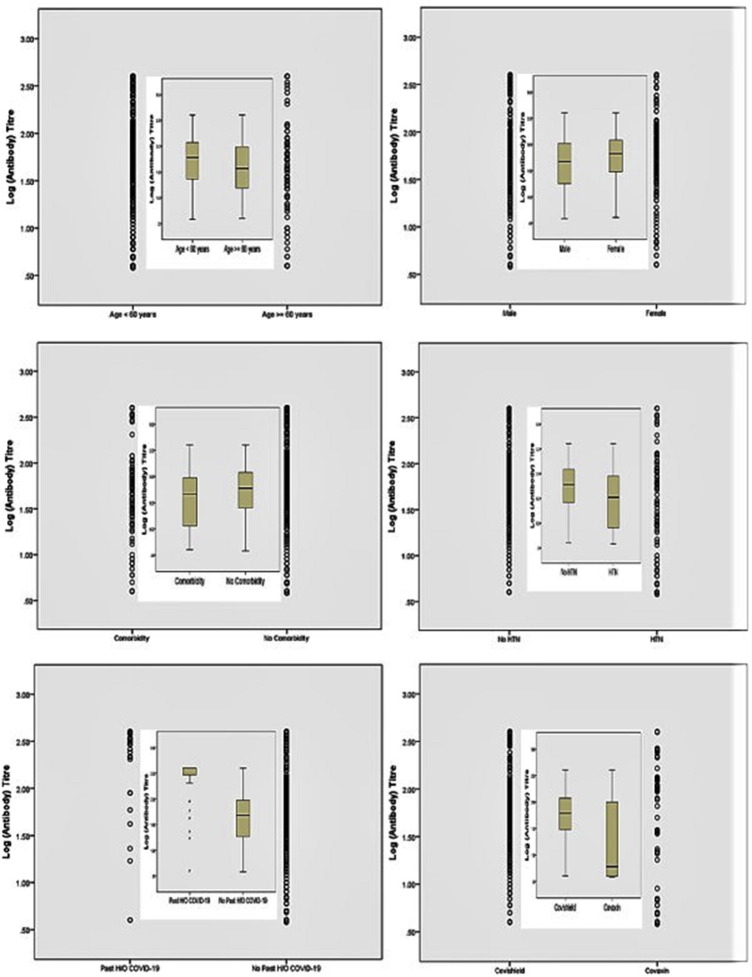
The Geometric mean titre (GMT, 95% Confidence Interval) of anti-spike antibody was significantly higher in Covishield vs. Covaxin recipients, both after the first dose (GMT, 62.4 vs. 16.8 AU/mL, respectively; p < 0.001) and after the two complete doses (GMT, 129.3 vs. 48.3 AU/mL, respectively; p < 0.001). Subjects aged > 60 years, or HTN or of male sex had significantly lower anti-spike antibody GMT compared to those aged ≤ 60 years, without HTN or females, respectively after the completion of two doses of either vaccine (Table 4 ). Indeed, past history of SARS-CoV-2 infection elicited significantly higher antibody titre compared to SARS-CoV-2 naïve subjects, both after the first (GMT, 252.0 vs. 40.8 AU/mL; p < 0.001) and second dose (GMT, 302.7 vs. 95.6 AU/mL; p < 0.001), irrespective of the type of vaccine received. Notably, the GMT (95% CI) of anti-spike antibody was significantly higher in SARS-CoV-2 naïve participant who received Covishield compared to Covaxin both after first (GMT, 51.1 vs. 15.4 AU/mL, respectively; p < 0.001) and the completion of second dose (GMT, 114.0 vs. 45.3 AU/mL, respectively; p < 0.001) (Supplementary table 2). Furthermore, these GMT findings are consistent and concordant with an additionally calculated median (IQR) titre of anti-spike antibody among overall (Supplementary table 3) and the SARS-CoV-2 naïve cohorts (Supplementary table 4). Dot plot and Box-whisker plot (inbox) (Fig. 2 ) depicts the log antibody titre for different study parameters that were significantly different after the two complete doses of vaccine. Box-whisker plot (Fig. 3 ) depicts the long antibody titre for study parameters that were significantly different after first and second dose of vaccine. Supplementary Fig. 1 depicts the Dot plot and Box-whisker plot (inbox) for the log antibody titre for different study parameters that were significantly different after the first dose of vaccine. No significant difference in the median (IQR) anti-spike antibody titre was observed when samples were analyzed between day 21–28 vs. day 29–36 vs. day 36–45 after the first dose of either vaccine (Supplementary table 5).
Table 4.
Geometric Mean (SD) anti-spike antibody titre after the first dose and second doses of either vaccine.
|
First dose of either vaccine |
Two completed doses of either vaccine |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall, N = 552 |
Covishield, N = 456 |
Covaxin, N = 96 |
Overall, N = 515 |
Covishield, N = 425 |
Covaxin, N = 90 |
||||||
| Antibody Titer, Geometric Mean (95% CI), in AU/mL |
P value |
Antibody Titer, Geometric Mean (95% CI), in AU/mL |
P value |
Antibody Titer, Geometric Mean (95% CI), in AU/mL |
P value |
Antibody Titer, Geometric Mean (95% CI), in AU/mL |
P value |
Antibody Titer, Geometric Mean (95% CI), in AU/mL |
P value |
Antibody Titer, Geometric Mean (95% CI), in AU/mL |
P value |
|
| Age | ||||||||||||
| Age ≤ 60 years Age > 60 years |
51.84 (51.47–52.21) 39.63 (38.75–40.51) |
0.10 | 66.17 (65.83–66.51) 45.51 (44.62–46.39) |
0.02 | 16.07 (14.94–17.19) 21.28 (18.56–23.99) |
0.54 | 114.81 (114.56–115.06) 77.62 (76.79–78.44) |
0.002 | 136.99 (136.75–137.22) 93.28 (92.49–94.07) |
0.001 | 51.76 (51.01–52.50) 32.94 (30.37–35.51) |
0.22 |
| Sex | ||||||||||||
| Male Female |
44.15 (43.69–44.61) 58.82 (58.31–59.33) |
0.02 | 55.44 (55.01–55.87) 73.79 (73.32–74.26) |
0.02 |
15.12 (13.77–16.47) 19.71 (18.08–21.34) |
0.44 | 97.72 (97.39–98.05) 125.89 (125.54–126.24) |
0.009 | 117.49 (117.17–117.81) 147.57 (147.24–147.89) |
0.01 | 41.69 (40.71–42.67) 58.61 (57.52–59.69) |
0.22 |
| Body-mass Index (BMI), Kg/m2 | ||||||||||||
| < 25 25–29.9 ≥ 30 |
47.98 (47.57–48.39) 53.62 (52.86–54.38) 53.54 (52.46–54.62) |
0.70 | 63.25 (62.88–63.62) 59.58 (58.82–60.34) 62.09 (60.99–63.18) |
0.92 | 14.17 (12.99–15.35) 30.86 (28.17–33.54) 20.61 (17.22–23.99) |
0.20 | 107.15 (106.86–107.43) 109.65 (109.04–110.25) 117.49 (116.75–118.22) |
0.75 | 128.82 (128.56–129.08) 123.03 (122.44–123.61) 134.89 (134.14–135.64) |
0.87 | 45.71 (44.84–46.58) 53.70 (51.69–55.70) 51.29 (49.38–53.19) |
0.91 |
| Blood group | ||||||||||||
| A + ve B + ve AB + ve O + ve A-ve B-ve AB-ve O-ve |
53.74 (52.85–54.63) 52.48 (51.89–53.07) 46.30 (44.71–47.89) 45.69 (45.16–46.22) 98.88 (97.01–100.75) 48.64 (45.53–51.75) 43.83 (38.53–49.12) 46.61 (44.15–49.07) |
0.87 |
76.54 (75.77–77.31) 59.43 (58.85–60.01) 64.99 (63.46–66.52) 58.17 (57.68–58.66) 98.88 (97.01–100.75) 48.64 (45.53–51.75) 43.83 (38.53–49.12) 59.59 (57.46–61.71) |
0.70 | 10.25 (8.09–12.41) 19.84 (17.55–22.13) 8.50 (6.53–10.47) 20.86 (19.35–22.37 - - - 4.00 |
0.36 | 120.23 (119.71–120.75) 117.22 (116.79–117.65) 91.62 (90.27–92.97) 95.50 (95.1–95.9) 156.67 (155.24–158.09) 131.52 (129.60–133.43) 77.27 (75.41–79.13) 169.39 (168.39–170.54) |
0.23 | 149.97 (149.50–150.43) 125.31 (124.85–125.76) 118.85 (117.68–120.02) 120.50 (120.13–120.87) 156.67 (155.24–158.09) 131.52 (129.60–133.43) 77.27 (75.41–79.13) 186.21 (185.06–187.36) |
0.50 | 45.08 (43.49–46.66) 67.76 (66.52–68.99) 26.12 (21.12–31.12) 46.77 (45.71–47.83) - - - 64.86 (NP) |
0.66 |
| Any Comorbidities | ||||||||||||
| Yes No |
40.71 (39.94–41.48) 52.84 (52.46–53.22) |
0.07 | 49.37 (48.62–50.12) 67.69 (67.34–68.03) |
0.02 | 7.06 (5.59–8.53) 19.30 (18.14–20.46) |
0.04 | 98.15 (97.61–98.69) 112.20 (111.93–112.47) |
0.20 | 114.71 (114.21–115.20) 135.11 (134.85–135.37) |
0.09 | 19.49 (17.27–21.71) 54.70 (53.96–55.44) |
0.01 |
| Type 2 diabetes mellitus (T2DM) | ||||||||||||
| Yes No |
39.52 (38.31–40.73) 51.00 (50.64–51.36) |
0.20 | 50.37 (49.17–51.56) 64.00 (63.67–64.33) |
0.21 | 8.93 (6.75–11.10) 17.84 (16.73–18.95) |
0.26 | 92.47 (91.36–93.58) 110.84 (110.59–111.08) |
0.23 | 121.25 (120.33–122.17) 130.32 (130.08–130.56) |
0.61 | 11.63 (7.64–15.62) 53.63 (52.93–54.33) |
0.004 |
| Duration of DM | ||||||||||||
| < 5 yrs. 5–10 yrs. > 10 yrs. |
52.42 (50.39–54.44) 32.97 (31.27–34.67) 45.28 (42.42–48.14) |
0.43 | 53.58 (51.17–55.98) 47.34 (45.71–48.97) 53.84 (51.09–56.58) |
0.58 | 5.50 (42.64–51.28) 4.00 (3.78–7.22) - |
0.26 | 164.06 (162.33–165.78) 67.29 (165.71–168.87) 131.52 (129.33–133.71) |
0.04 | 162.18 (160.45–163.90) 91.20 (89.85–92.54) 172.19 (170.62–173.75) |
0.13 | - 14.39 (9.59–19.18) 4.00 |
(*) |
| Control of DM | ||||||||||||
| Optimum Not optimal |
39.24 (37.98–40.49) 48.06 (43.87–52.24) |
0.86 |
51.25 (50.03–52.47) 21.99 |
(*) | 6.28 (4.68–7.88) 105.00 |
(*) | 90.99 (89.95–92.13) 212.81 |
(*) | 119.67 (118.73–120.60) 212.81 |
(*) | 11.63 (7.64–15.62) - |
(*) |
| T2DM Management | ||||||||||||
| Monotherapy Combination Insulin No medication |
46.10 (44.39–47.80) 38.33 (36.67–39.99) - 30.09 (26.54–33.64 |
0.56 |
63.53 (61.89–65.16) 45.90 (44.26–47.54) - 49.83 (43.20–56.45) |
0.53 |
11.48 (8.65–14.31) 9.05 (4.93–14.97) - 4.00 (*) |
0.67 |
125.89 (124.32–127.46) 84.14 (82.56–85.72) - 65.31 (61.14–69.48) |
0.31 |
177.34 (176.38–178.29) 114.55 (113.30–115.79) - 52.84 (47.37–58.31) |
0.10 |
14.28 (5.89–22.66) 4.31 (3.02–5.6) - 151.98 |
0.002 |
| Hypertension (HTN) | ||||||||||||
| HTN No HTN |
31.46 (30.61–32.31) 54.89 (54.52–55.25) |
<0.001 | 37.91 (37.16–38.66) 69.00 (68.62–69.38) |
<0.001 |
16.38 (14.09–18.66) 16.98 (15.80–18.15 |
0.93 |
80.72 (80.12–81.32 116.14 (115.88–116.40) |
0.002 |
100.51 (109.96–111.06 136.46 (136.20–136.71) |
<0.001 |
34.17 (32.40–35.94) 52.84 (52.04–53.63) |
<0.001 |
| Duration of HTN | ||||||||||||
| < 5 yrs. 5–10 yrs. > 10 yrs. |
24.56 (23.18–25.93) 35.22 (33.94–36.49) 34.10 (32.15–36.05) |
0.003 |
30.28 (28.95–31.61) 47.42 (46.13–48.71) 35.88 (33.87–37.89) |
0.001 |
4.00 (*) 20.46 (17.75–23.17) 20.49 (6.52–34.46) |
0.47 |
80.35 (79.23–81.47) 72.44 (71.46–73.42) 103.28 (101.92–104.63) |
0.01 |
89.35 (88.53–90.17) 98.08 (97.14–99.02) 120.23 (119.09–121.36) |
0.04 |
34.72 (33.02–36.42) 38.72 (36.55–40.89 5.00 (*) |
0.24 |
| HTN Management | ||||||||||||
| RAASB CCBs Combined No Medicine |
31.36 (30.22–32.49) 27.72 (25.69–29.75) 37.96 (36.04–39.88) 15.62 (6.11–25.12) |
0.02 |
35.33 (34.21–36.45) 33.64 (31.63–35.65) 48.11 (46.33–49.89) 60.99 (*) |
0.003 | 10.41 (5.73–15.08) 4.00 4.00 4.00 |
(*) |
81.28 (80.46–82.09) 90.99 (89.73–92.25) 97.49 (96.24–98.73) 51.17 (45.94–56.39) |
0.23 | 92.17 (91.41–92.93) 98.56 (97.20–99.92) 120.78 (119.81–121.74) 130.92 (*) |
0.08 |
19.94 (15.71–24.17) 42.66 (40.83–44.49) 14.28 (7.43–21.13) 19.93 (*) |
0.40 |
| Dyslipidemia | ||||||||||||
| Yes No |
52.18 (50–85-53.51) 21.99 |
(*) | 52.18 (50.85–53.51) 21.99 |
(*) | – - |
(*) | 97.72 (96.61–98.83) 212.81 |
(*) | 98.63 (97.52–99.74) 212.81 |
(*) | – - |
(*) |
| Ischemic heart disease (IHD) | ||||||||||||
| Yes No |
41.13 (39.21–43.05) 49.89 (49.54–50.24) |
0.63 |
41.13 (39.20–43.05) 63.14 (62.81–63.46) |
0.22 |
16.84 (15.80–17.88) - |
(*) | 112.25 (111.03–113.47) 108.64 (108.39–108.89) |
0.91 |
112.25 (111.03–113.47) 129.89 (129.65–130.13) |
0.56 |
- 48.26 (47.53–48.99) |
(*) |
| History of SARS-CoV-2 infection | ||||||||||||
| Past H/O -Yes Past H/O –No |
251.99 (251.28–252.70) 40.75 (40.42–41.08) |
<0.001 |
250.55 (249.80–251.30) 51.13 (50.82–51.44) |
<0.001 |
239.75 (239.26–240.03) 15.38 (14.37–16.39) |
0.002 |
302.69 (302.28–303.09) 95.59 (95.34–95.84) |
<0.001 |
302.90 (301.58–303.42) 113.95 (113.71–114.19) |
<0.001 |
256.25 (254.72–256.68) 45.28 (44.55–46.00) |
0.009 |
| Types of vaccines | ||||||||||||
| Covishield Covaxin |
62.37 (62.05–62.69) 16.84 (15.80–17.88) |
<0.001 | 129.30 (129.07–129.53) 48.26 (47.46–48.92) |
<0.001 | ||||||||
p < 0.05 considered as statistically significant, p computed by independent sample t-test or ANOVA test, BMI- body mass index, T2DM- type 2 diabetes mellitus, HTN- hypertension, RAAS- Renin angiotensin aldosterone system, CCB- calcium channel blocker, IHD- ischemic heart disease, SARS-CoV-2- severe acute respiratory syndrome coronavirus-2, COVID-19- coronavirus disease 2019, (*) - No statistics is computed.
Fig. 2.
Dot plot and Box-whisker plot (inbox) demonstrating log antibody titer by study parameters after two complete doses of either vaccine.
Fig. 3.
Box-whisker plot demonstrating log antibody titer by study parameters after first and second doses of either vaccine.
3.4. Comparison of SARS-CoV-2 anti-spike antibody titre following one or two doses of vaccine in propensity matched analysis
In the propensity (age, sex and BMI) matched analysis of 172 participants (86 participants in each arm) who received first dose of either vaccine; gender, past history of SARS-CoV-2 infection and type of vaccine received were found to be associated with a significantly different anti-spike antibody GMT. Anti-spike antibody was found to be significantly higher in Covishield compared to the Covaxin recipients (GMT, 62.0 vs. 16.4 AU/mL, respectively; p < 0.001) even in the propensity-matched analysis after the first dose. Furthermore, in the propensity-matched analysis of 116 SARS-CoV-2 naïve participants (58 recipients with each vaccine) after the adjustment for age, sex and BMI and after the two complete doses of either vaccine; GMT (95% CI) to anti-spike antibody were significantly higher in Covishield compared to the Covaxin (GMT, 112.6 vs. 46.8 AU/mL, respectively; p < 0.001) recipients. Notably, age > 60-years, males, people with any comorbidities, and history of HTN had a significantly less anti-spike antibody GMT compared to age ≤ 60 years (76.8 vs. 111.5 AU/mL, respectively; p = 0.02), females (83.2 vs. 89.1 AU/mL, respectively; p < 0.001), no comorbidities (68.4 vs. 91.2 AU/mL, respectively; p = 0.04) and no HTN (79.7 vs. 116.2 AU/mL, respectively; p < 0.001) respectively, after the completion of two doses of either vaccine (Table 5 ).
Table 5.
Propensity score (age, sex, BMI) matched analysis of Geometric Mean (95% CI) anti-spike antibody titre after the first dose of either vaccine in total cohorts and after the second doses of either vaccine in SARS-CoV-2 naïve cohorts#
|
First dose of either vaccine |
Two completed doses of either vaccine# |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall, N = 172 |
Covishield, N = 86 |
Covaxin, N = 86 |
Overall, N = 116 |
Covishield, N = 58 |
Covaxin, N = 58 |
||||||
| Antibody Titer, Geometric Mean (95 %CI) in AU/mL |
P |
Antibody Titer, Geometric Mean (95 %CI) in AU/mL |
P |
Antibody Titer, Geometric Mean (95 %CI) in AU/mL |
P | Antibody Titer, Geometric Mean (95 %CI) in AU/mL |
P |
Antibody Titer, Geometric Mean (95 %CI) in AU/mL |
P |
Antibody Titer, Geometric Mean (95 %CI) in AU/mL |
P | |
| Age (Years) | ||||||||||||
| ≤ 60 > 60 years |
51.48 (49.17–52.59) 38.84 (36.72–39.11) |
0.102 | 67.95 (63.44–69.38) 48.62 (47.29–48.87) |
0.021 | 18.08 (17.92–19.90) 21.73 (21.04–22.92) |
0.612 | 111.48 (111.17–112.56) 76.84 (76.79–77.01) |
0.022 |
132.65 (131.88–134.36) 88.35 (87.19–88.74) |
0.002 |
49.76 (48.92–49.90) 31.73 (31.04–32.29) |
0.223 |
| Sex | ||||||||||||
| Male Female |
43.25 (43.05–45.74) 59.72 (58.81–60.91) |
0.018 | 41.25 (40.69–42.85) 54.54 (54.15–56.64) |
0.034 | 16.19 (15.29–17.12) 19.56 (17.69–21.04) |
0.496 | 83.17 (83.05–83.41) 89.13 (88.91–89.57) |
<0.001 |
114.54 (114.45–114.64) 141.35 (140.44–142.75) |
0.012 |
41.48 (41.29–41.67) 54.88 (54.69–55.04) |
0.446 |
| Body-mass Index (BMI) | ||||||||||||
| < 25 kg/m2 25–29.9 kg/m2 ≥ 30 kg/m2 |
47.09 (46.67–47.54) 51.92 (50.86–52.07) 53.86 (51.83–54.18) |
0.792 |
64.04 (63.48–65.32) 61.9 (60.47–62.39) 62.38 (61.35–63.28) |
0.601 |
13.3 (12.04–14.82) 26.42 (23.89–24.82) 20.62 (19.21–21.39) |
0.359 |
87.09 (86.66–87.14) 91.2 (90.96–92.07) 79.18 (78.83–80.18) |
0.728 |
124.82 (123.48–125.18) 121.03 (120.64–121.76) 138.89 (137.7–139.14) |
0.933 | 43.1 (42.84–43.27) 52.81 (51.89–53.47) 50.83 (49.05–51.79) |
0.323 |
| Blood group | ||||||||||||
| A + ve B + ve AB + ve O + ve A-ve B-ve AB-ve O-ve |
54.72 (52.07–55.75) 51.23 (50.49–52.44) 46.06 (44.54–48.24) 45.17 (44.65–46.04) 96.34 (94.51–97.28) 47.29 (46.84–48.31) 43.52 (41.69–43.99) 46.78 (44.29–47.93) |
0.902 | 73.12 (71.76–73.73) 61.43 (60.95–62.32) 64.48 (64.25–65.98) 55.11 (54.43–56.79) 99.67 (94.21–100.19) 43.52 (42.62–45.03) 43.96 (42.12–44.57) 57.21 (55.2–58.97) |
0.712 |
11.16 (10.49–13.43) 18.76 (16.52–18.99) 7.12 (6.12–9.12) 20.77 (19.71–22.43) - - - - |
0.393 |
119.63 (118.07–120.75) 117.22 (115.91–117.88) 91.03 (90.27–92.54) 95.08 (94.33–96.02) 156.67 131.52 (129.84–132.03) - 169.39 (168.23–170.24) |
0.710 | 147.12 (146.37–148.73) 124.43 (123.95–125.16) 117.48 (116.25–117.92) 118.11 (117.83–119.57) 156.67 131.52 (129.84–132.03) - 172.21 |
0.467 | 45.08 (43.49–46.66) 67.76 (66.52–68.99) 26.12 (21.12–31.12) 46.77 (45.71–47.83) - - - 64.86 |
0.282 |
| Any Comorbidities | ||||||||||||
| Yes No |
40.63 (38.38–40.89) 53.67 (52.39–53.97) |
0.595 | 49.71 (47.58–50.54) 67.81 (65.75–69.92) |
0.022 | 9.48 (8.37–10.93) 19.23 (17.91–21.2) |
0.065 | 68.4 (68.05–69.63) 91.20 (90.12–92.68) |
0.042 |
107.71 (106.68–108.31) 131.11 (129.87–132.92) |
0.022 |
15.48 (14.03–15.93) 53.23 (52.91–53.96) |
0.010 |
| Type 2 diabetes mellitus (T2DM) | ||||||||||||
| T2DM No T2DM |
40.63 (38.38–40.89) 53.67 (52.39–53.97) |
0.187 | 48.55 (47.32–49.28) 67.32 (64.16–69.95) |
0.202 | 9.47 (9.15–11.85) 17.09 (16.7–18.75) |
0.262 | 79.20 (78.62–80.44) 103.84 (102.61–103.90) |
0.129 |
88.55 (87.32–89.52) 132.32 (131.46–132.95) |
0.141 |
9.47 (9.15–11.85) 57.09 (56.27–58.66) |
0.079 |
| Duration of DM | ||||||||||||
| < 5 years 5–10 years > 10 years |
51.62 (50.68–53.81) 29.73 (27.71–31.83) 44.75 (42.58–45.68) |
0.393 |
54.62 (53.21–55.63) 47.20 (46.19–48.3) 53.12 (51.7–54.89) |
0.612 |
46 5.5 (4.46–12.8) 4 |
(*) |
145.62 59.73 (57.71–61.93) - |
(*) |
145.62 71.20 - |
(*) |
- 12.2 (7.46–22.8) - |
(*) |
| Control of DM | ||||||||||||
| Optimum Not optimum |
36.83 (34.43–39.84) 24.65 |
(*) |
57.42 (53.73–60.93) 24.65 |
(*) | 6.84 (5.81–7.94) - |
(*) |
86.54 (84.62–89.78) - |
(*) |
106.42 (103.21–107.36) - |
(*) | 9.42 (7.81–10.69) - |
(*) |
| T2DM Management | ||||||||||||
| Monotherapy Combination Insulin No medication |
46.91 (44.35–48.09) 38.46 (37.43–40.27) - - |
0.197 | 62.91 (59.48–64.31) 44.55 (43.59–45.24) - - |
0.245 | 11.53 (10.58–13.17) 9.09 (7.92–9.96) - - |
0.262 | 112.91 80.46 (78.84–84.13) - - |
(*) |
112.91 114.55 - - |
(*) |
- 4.09 (2.92–4.96) - - |
(*) |
| Hypertension (HTN) | ||||||||||||
| HTN No HTN |
32.92 (30.01–33.6) 56.37 (54.14–57.08) |
<0.001 | 38.26 (37.6–39.74) 70.32 (68.97–71.88) |
<0.001 | 15.07 (14.14–18.28) 15.98 (14.03–17.83) |
0.871 | 79.72 (79.01–80.65) 116.19 (114.28–117.04) |
<0.001 |
100.51 (99.6–102.93) 135.65 (133.7–136.58) |
<0.001 |
34.07 (32.86–38.28) 52.98 (50.12–53.54) |
0.456 |
| Duration of HTN | ||||||||||||
| < 5 years 5–10 years > 10 years |
24.35 (22.31–25.41) 34.14 (32.96–36.42) 35.5 (32.83–36.57) |
0.004 | 28.61 (26.51–29.87) - 46.56 (44.12–46.25) 37.83 (36.48–38.54) |
0.003 | 4 19.72 (18.55–21.89) 19.5 (17.86–20.38) |
0.395 | 79.85 (77.31–80.76) 72.44 (71.46–73.42) 5.5 |
0.034 |
87.61 - - |
(*) | 39.72 (37.02–41.42) 35.72 (34.55–37.89) 5.5 |
0.158 |
| HTN Management | ||||||||||||
| RAAS Blockers CCBs Combined No Medicine |
32.28 (30.46–33.18) 26.87 (24.47–28.51) 36.84 (35.24–38.08) 14.67 (14.01–16.15) - |
0.001 | 34.17 (32.08–36.54) - 32.6 (31.58–33.24) 48.25 (46.8–49.27) 60.99 |
0.002 | 9.98 (7.02–11.05) 4 4 4 - |
(*) |
74.28 (70.46–78.09) 36.12 (34.73–39.25) - - - |
0.215 | 82.17 - - - - |
(*) |
39.84 36.12 (34.73–39.25) - - - |
(*) |
| Dyslipidemia | ||||||||||||
| Yes No |
52.92 (51.48–53.69) 59.11 (58.17–60.2) |
0.071 |
52.92 (51.48–53.69) 114.81 (113.12–115.41) |
0.001 |
- 47.13 (45.94–48.18) |
(*) |
- 95.11 (92.17–98.2) |
(*) |
- 114.81 (113.12–115.41) |
(*) |
- 47.13 (45.94–48.18) |
(*) |
| Ischemic heart disease (IHD) | ||||||||||||
| Yes No |
43.56 (42.19–44.65) 51.67 (50.07–52.75) |
0.581 |
43.56 (42.19–44.65) 63.83 (61.38–64.79) |
0.329 |
- 16.56 (14.27–19.85) |
(*) |
- 101.34 (100.25–105.17) |
(*) |
- 118.48 (116.79–119.29) |
(*) |
- 48.56 (46.02–49.58) |
(*) |
| History of SARS-CoV-2 infection: | ||||||||||||
| Yes No |
251.19 (251.04–251.30) 39.74 (38.71–40.02) |
<0.001 |
251.55 (250.25–302.68) 41.83 (40.58–42.87) |
<0.001 | 256 (255.84–257.91) 15.14 (14.96–15.78) |
0.012 | NA | |||||
| Types of vaccine | ||||||||||||
| Covishield Covaxin |
62.01 (61.19–63.92) 16.38 (15.91–17.46) |
<0.001 |
112.64 (111.50–113.92) 46.79 (45.91–47.83) |
<0.001 |
||||||||
p < 0.05 considered as statistically significant, p computed by independent sample t-test or ANOVA test, BMI- body mass index, T2DM- type 2 diabetes mellitus, HTN- hypertension, RAAS- Renin angiotensin aldosterone system, CCB- calcium channel blocker, IHD- ischemic heart disease, SARS-CoV-2- Severe acute respiratory syndrome coronavirus-2, COVID-19- coronavirus disease 2019, (*) - No statistics is computed.
3.5. Independent variables associated with seropositivity to anti-spike spike antibody and titre level following two doses of vaccine
Multiple logistic regression analysis (to identify the independent predictors for non-seropositivity rate based on development of SARS-CoV-2 Anti-spike antibody) suggests that amongst all the variables analyzed, three variables such as- sex, presence of comorbidities, and vaccine type were independent predictors of antibody seropositivity rate. Associated co-morbidities were significantly related to an increase in the non-seropositivity rate by > 4-fold (Odds Ratio [OR] 4.68; 95% CI, 1.382–15.839; p = 0.013), while male gender also showed a significant increase in the non-seropositivity rate by > 3-folds (OR 3.37; 95% CI, 1.221–9.321; p = 0.019). Notably, recipients of Covishield had a significantly lower non-responder rate by 41% (OR 0.59; 95% CI, 0.021–0.659; p < 0.001), compared to those who received Covaxin (Table 6 ).
Table 6.
Multiple logistic regression to identify the independent predictors for non-seropositivity after first and second dose (based on development of SARS-CoV-2 Anti-spike antibody).
|
First dose: Variables in the Equation | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | P value | Exp(B) | 95% C.I. for EXP(B) |
||
| Lower | Upper | |||||||
| Age | 0.019 | 0.327 | 0.003 | 1 | 0.954 | 1.019 | 0.537 | 1.935 |
| Sex (ref = female) | 0.281 | 0.250 | 1.263 | 1 | 0.261 | 1.324 | 0.811 | 2.161 |
| BMI | -0.022 | 0.176 | 0.016 | 1 | 0.900 | 0.978 | 0.692 | 1.382 |
| Associated comorbidities | 1.004 | 0.434 | 5.366 | 1 | 0.021 | 2.730 | 1.167 | 6.386 |
| Type 2 diabetes | 0.163 | 0.470 | 0.120 | 1 | 0.729 | 1.177 | 0.468 | 2.958 |
| Hypertension | -0.323 | 0.383 | 0.714 | 1 | 0.398 | 0.724 | 0.342 | 1.532 |
| Ischemic heart disease | -0.236 | 0.950 | 0.062 | 1 | 0.803 | 0.789 | 0.123 | 5.084 |
| Dyslipidaemia | 0.000 | 0.000 | 2.333 | 1 | 0.127 | 1.000 | 1.000 | 1.000 |
| Past H/O Covid-19 | −1.947 | 0.744 | 6.848 | 1 | 0.009 | 0.143 | 0.033 | 0.613 |
| Covishield vs. Covaxin | −2.206 | 0.274 | 64.631 | 1 | <0.001 | 0.110 | 0.064 | 0.189 |
| Constant | 6.929 | 1.884 | 13.528 | 1 | 0.0001 | 1021.857 | ||
| Second dose: Variables in the Final Equation | ||||||||
| Age | -0.425 | 0.472 | 0.810 | 1 | 0.368 | 0.654 | 0.259 | 1.650 |
| Sex (ref = female) | 1.216 | 0.518 | 5.503 | 1 | 0.019 | 3.374 | 1.221 | 9.321 |
| BMI | -0.205 | 0.280 | 0.536 | 1 | 0.464 | 0.815 | 0.471 | 1.410 |
| Associated comorbidities | 1.543 | 0.622 | 6.152 | 1 | 0.013 | 4.679 | 1.382 | 15.838 |
| Type 2 diabetes | -0.552 | 0.620 | 0.793 | 1 | 0.373 | 0.576 | 0.171 | 1.941 |
| Hypertension | 0.718 | 0.595 | 1.458 | 1 | 0.227 | 2.051 | 0.639 | 6.579 |
| Ischemic heart disease | -0.286 | 0.950 | 0.062 | 1 | 0.893 | 0.789 | 0.423 | 6.084 |
| Dyslipidemia | 0.000 | 0.000 | 3.401 | 1 | 0.065 | 1.000 | 1.000 | 1.000 |
| Past H/O Covid-19 | 0.732 | 0.609 | 1.444 | 1 | 0.230 | 2.078 | 0.630 | 6.854 |
| Vaccine type (ref = Covaxin) | −2.825 | 0.522 | 29.296 | 1 | <0.001 | 0.590 | 0.021 | 0.659 |
a. Variable(s) entered on regression step: Age, Sex, BMI (body mass index), Associated Comorbidities, Type 2 diabetes, Hypertension, Ischemic heart disease, Dyslipidaemia, Past H/O Covid-19, Covishield/Covaxin.
In a multiple linear regression model with log transformed antibody levels as outcome variable, we found presence of co-morbidity, sex, vaccine type and past history of COVID-19 infection as independent predictors of antibody titre. The R value of the model represents the simple correlation and is 0.493, which indicates a moderate degree of correlation. The overall R2 value of 0.247 indicating 24.7% of the total variation in the dependent variable (antibody titre) can be explained by our model. The multiple regression analysis (to identify the independent predictors for SARS-CoV-2 Anti-spike antibody titre) suggests that amongst all the variables analyzed, four variables such as- presence of comorbidity, sex, past history of SARS-CoV-2 infection and vaccine type were independent predictors of antibody levels. While presence of comorbidity resulted in decrease in the antibody titre by 13% (β = 0.87; 95% CI, 0.79–0.97; p = 0.010), female gender was found to have 9% greater antibody titre compared to males (β = 1.09; 95% CI,1.02–1.17; p = 0.018). SARS-CoV-2 naïve participants were associated with significantly lower antibody titre by 37% (β = 0.63; 95% CI, 0.57–0.71; p < 0.001). Notably, recipients of Covaxin had a significantly lower antibody titre by 32% ((β = 0.68; 95% CI, 0.62–0.74; p < 0.001), as compared to those who received Covishield (Table 7 ).
Table 7.
Multiple regression to identify the independent predictors for non-responders (log-transformed anti-spike antibody levels were used as outcome variable) after first and second dose of either vaccine.
|
After first dose | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients |
Standardized Coefficients |
t | Sig. | 95.0% Confidence Interval for B | |||
| B | Std. Error | Beta | Lower Bound | Upper Bound | ||||
| FINALSTEP | (Constant) | 2.749 | 0.267 | 10.29 | <0.001 | 2.224 | 3.273 | |
| Age | 0.156 | 0.088 | 0.108 | 1.766 | 0.078 | -0.018 | 0.329 | |
| Sex (ref = male) | 0.110 | 0.046 | 0.088 | 2.377 | 0.018 | 0.019 | 0.200 | |
| BMI | 0.011 | 0.024 | 0.018 | 0.471 | 0.638 | -0.035 | 0.058 | |
| Blood group | -0.001 | 0.015 | -0.004 | -0.096 | 0.924 | -0.031 | 0.028 | |
| Comorbidities | -0.101 | 0.035 | -0.153 | −2.882 | 0.004 | -0.171 | -0.032 | |
| Diabetes mellitus (DM) | 0.006 | 0.097 | 0.003 | 0.065 | 0.948 | -0.184 | 0.196 | |
| DM Duration | 0.113 | 0.087 | 0.068 | 1.309 | 0.191 | -0.057 | 0.284 | |
| Hypertension (HTN) | -0.013 | 0.083 | -0.008 | -0.155 | 0.877 | -0.176 | 0.150 | |
| HTN Duration | -0.127 | 0.069 | -0.145 | −1.858 | 0.064 | -0.262 | 0.007 | |
| Ischemic heart disease (IHD) | -0.122 | 0.174 | -0.030 | -0.697 | 0.486 | -0.464 | 0.221 | |
| Dyslipidaemia | 1.156 | 0.000 | -0.041 | -0.901 | 0.368 | 0.000 | 0.000 | |
| Vaccine Type (ref = Covishield) | -0.539 | 0.062 | -0.333 | −8.684 | 0.001 | -0.661 | -0.417 | |
| Pre-vaccination | -0.752 | 0.073 | -0.381 | −10.33 | 0.001 | -0.895 | -0.609 | |
| After second dose | ||||||||
| FINALSTEP | (Constant) | 3.231 | 0.185 | 17.45 | <0.001 | 2.868 | 3.595 | |
| Age | 0.038 | 0.067 | 0.037 | 0.571 | 0.568 | -0.094 | 0.171 | |
| Sex (ref = male) | 0.085 | 0.036 | 0.093 | 2.370 | 0.018 | 0.015 | 0.155 | |
| BMI | -0.001 | 0.025 | -0.001 | -0.031 | 0.975 | -0.049 | 0.048 | |
| Blood group | 0.000 | 0.012 | 0.001 | 0.026 | 0.980 | -0.023 | 0.023 | |
| Comorbidities | -0.133 | 0.052 | -0.106 | −2.573 | 0.010 | -0.235 | -0.031 | |
| IHD | 0.072 | 0.134 | 0.025 | 0.535 | 0.593 | -0.192 | 0.336 | |
| Dyslipidaemia | 1.156E-005 | 0.000 | -0.041 | -0.901 | 0.368 | 0.000 | 0.000 | |
| Vaccine Type (ref = Covishield) | -0.387 | 0.047 | -0.327 | −8.244 | <0.001 | -0.479 | -0.295 | |
| Pre-vaccination | -0.450 | 0.056 | -0.316 | −8.103 | <0.001 | -0.559 | -0.341 | |
a. Dependent Variable: Log (Antibody Titre), a-coefficient, β coefficients of the significant predictors were reported in the result section after back transformation by taking an antilog to facilitate information of the data with 95% Confidence Interval values describing the unstandardized coefficient.
3.6. Post-vaccination adverse events
Covishield recipients had higher proportions of any mild to moderate side-effects as compared to Covaxin (46.7% vs. 31.2%, p = 0.006) after the first dose. Higher rates of mild to moderate side effects were observed with Covishield compared to the Covaxin recipients (50.0 % vs. 30.2%, p = 0.008) even in the propensity-matched particiapnts after the first dose. Although Covishield recipients also reported a higher incidence of mild to moderate side-effects in 18.1% (77/425) compared to 11.1% (10/90) in Covaxin recipients after the second dose of vaccine, it did not reach statistical significance (p = 0.11). Notably, in the propensity-matched cohort, any side-effects (mild to moderate) post vaccination was also similar (p = 0.13) in the Covishield arm 8/58 (13.79%) vs. Covaxin arm 6/58(10.34%). No serious solicited and any unsolicited side effects were noted after the first and second dose until the data lock (Supplementary table 6). Since this study is still ongoing, we will keep on reporting the details of any unsolicited side effects with either vaccine until 6 months after the second dose.
3.7. Post-vaccination SARS-CoV-2 infection
From Jan 16, 2021 (Day 1 of vaccination) until May 15, 2021 (Day 21–36 after second dose, data-locking date), a total of 30 (30/492, 6.1%) HCW have reported to have COVID-19 among SARS-CoV-2 naïve cohorts (n = 492) after the first dose of vaccination. Of the 30 participants 4 had suspected (symptomatic and positive signs on High Resolution Computed Tomography [HRCT] of chest but negative to either Reverse Transcription Polymerase Chain Reaction [RT-PCR] or Rapid Antigen Test [RAT]) and 26 had confirmed (RT-PCR or RAT positive) COVID-19. Three had SARS-CoV-2 infection before the second dose, while 27 had acquired SARS-CoV-2 infection after the second dose. Breakthrough infections (defined as SARS-CoV-2 infection > 2 weeks after the second dose) were reported in 4.9% (24/492) of cases following both vaccines. Breakthrough infections were noted in 5.5% (22/399) cohorts in Covishield and 2.2% (2/93) of Covaxin recipients, although there were no significant statistical differences (p = 0.21) in breakthrough infections between two vaccine recipients until the data lock. The majority of vaccine recipients had mild (28/30) to moderate (2/30) COVID-19 infections and all recovered. None of the cohort who had COVID-19 following either vaccine had severe COVID requiring mechanical ventilation (Supplementary Table 7). Since this study is still ongoing, we will report every detail of breakthrough infection with either vaccine until 6 months after the second complete doses.
4. Discussion
Summarily, this cross-sectional COVAT study reported an overall 95.0% (489/515) seropositivity rate after two complete doses of both vaccines in entire study participants that include both SARS-CoV-2 naïve and recovered individuals (Covishield 98.1% and Covaxin 80.0%, respectively). While seropositivity rates after two complete doses was 97.8% and 79.3% with Covishield and Covaxin, respectively in SARS-CoV-2 naïve individuals; 100% of study participants with a past history of SARS-CoV-2 were seropositive after two doses of either vaccine. Notably, while both vaccines showed an increase in seropositivity rate, GMT (95% CI) and median (IQR) anti-spike antibody titre after the second dose, Covaxin gained a significant increase in both seropositivity and antibody titre only after the two doses. Contrarily, Covishield showed a good seropositivity rate and a > 3-fold rise in anti-spike antibody GMT even after a single dose. One dose of either vaccine yielded a very high seropositivity and anti-spike antibody titre in SARS-CoV-2 recovered individuals (Table 8 ). There was no significant difference in seropositivity rate with regard to age, sex, BMI, blood group and any comorbidities including its duration and treatment, except that the participants with T2DM had a significantly less seropositive rate compared to those without. Importantly, GMT (95% CI) and median (IQR) titre of ant-spike antibody was significantly lower in age > 60 years, males and people with history of HTN compared to age ≤ 60 years, females and no history of HTN, respectively. Notably, no difference in seropositivity rate was observed amongst SARS-CoV-2 naïve vs. SARS-CoV-2 recovered participants after the two completed doses of either vaccine. Any adverse side effects post-vaccination was similar in Covishield and Covaxin recipients after second dose, although it was higher with former after the first dose compared to later but were mild to moderate in nature. Surprisingly, the seropositivity rate, GMT and median anti-spike antibody titre was significantly higher in Covishield recipients, compared to the Covaxin. Whether this differential finding between two vaccines is related to a lesser number of Covaxin participants compared to the Covishield, or due to the difference in characteristics of participants, or due to the difference between the type of vaccine- vector-based vs. inactivated whole virion, or related to differential immunogenic response due to the difference in spike antigen dose in each vaccine - is not exactly known - and need further studies. Nevertheless, even in age- sex- and BMI-matched propensity analysis, seropositivity rate, GMT and median anti-spike antibody titre continued to remain significantly higher with Covishield compared to Covaxin in SARS-CoV-2 naïve recipients. Notably, sex, presence of comorbidities, and the type of vaccine used were independent predictors of antibody kinetics in multiple regression analysis.
Table 8.
Comparison of seropositivity rate, geometric mean titre (95% CI) and median (IQR) anti-spike antibody after the first (21-days or more but before second dose) and second dose (day 21–36) in total, SARS-CoV-2 naïve, and recovered participants.
| Characteristics |
Total cohorts |
SARS-CoV-2 naïve participant # |
Past history of SARS-CoV-2 infection |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Covishield, n/N | Covaxin, n/N | Odds ratio, [95% CI], P value | Covishield, n/N |
Covaxin, n/N |
Odds ratio, [95% CI], P value | Covishield, n/N |
Covaxin, n/N |
Odds ratio, [95% CI], P value | |
| First dose | N = 552 | N = 492 | N = 60 | ||||||
| Seropositivity rate (%) | 396/456, (86.8) |
42/96, (43.8) |
8.48 [5.22–13.79] * |
341/399, (85.5) |
39/93, (41.9) |
8.14 [4.95–13.38] * |
55/57 (96.5) |
3/3 (1 0 0) |
3.17 [0.13–79.60] ** |
| Geometric Mean (95% CI) anti-spike antibody titre in AU/mL | 62.37 (62.05–62.69) |
16.84 (15.80–17.88) |
* |
51.13 (50.82–51.44) |
15.38 (14.37–16.39) |
* |
250.55 (249.80–251.30) |
239.75 (239.26–240.03) |
P = 0.85 |
| Median (IQR)anti-spike antibody titre in AU/mL | 61.5 (30.0–119.5) |
6.0 (4.0–99.7) |
* | 55.0 (28.0–94.0) |
5.0 (4.0–85.5) |
* | 400.0 (298.0–400.0) |
260.0 (214.0–260.0) |
P = 0.42 |
| Second dose | N = 515 | N = 457 | N = 58 | ||||||
| Seropositivity rate (%) | 417/425, (98.1) |
72/90, (80.0) |
13.03 [5.46–31.09] * |
362/370, (97.8) |
69/87, (79.3) |
11.80 [4.94–28.22] * |
55/55 (1 0 0) |
3/3 (1 0 0) |
$ |
| Geometric Mean (95% CI) anti-spike antibody titre in AU/mL | 129.30 (129.07–129.53) |
48.26 (47.46–48.92) |
* | 113.95 (113.71–114.19) |
45.28 (44.55–46.00) |
* | 302.90 (301.58–303.42) |
256.25 (254.72–256.68) |
P = 0.97 |
| Median (IQR) anti-spike antibody titre in AU/mL | 127.0 (80.5–268.5) |
53.0 (22.3–131.0) |
* | 115.0 (75.8–199.3) |
51.0 (20.0–125.0) |
* | 400.0 (278.0–400.0) |
308.0 (233.0–308.0) |
P = 0.64 |
# May include asymptomatic and undiagnosed COVID-19.
$ No statistics computed as seropositivity rate is constant.
* P < 0.001.
** P = 0.48.
Our findings are similar to published evidence in randomized controlled trials (RCTs) and real-world studies, although only few studies correlated it with other variables such as age, sex, BMI or presence or absence of comorbidities. In phase 2 RCT, 96.6% (95% CI, 92.8–98.8) had seropositivity (defined as post-vaccination IgG anti-spike antibody titre 4-fold higher than the baseline) to IgG anti-spike antibody titre at day 56 (28-days after the second dose), measured by ELISA in 177 participants who received 6 mcg dose of Algel-IDMG Covaxin. Moreover, seroconversion rate to NAb measured by PRNT50 rose to 98.3% 28-days after the second dose, compared to 47.5% 28-days after the first dose. However, no difference in NAb titre was observed in relation to gender and age [7], [8]. In phase 1/2 RCT, two doses of ChAdOx1 nCoV-19 (Covishield) vaccine elicited a significant increase in anti-spike IgG antibody at day 56 (Median 639 EU, IQR 360–792) compared to its median titre (210.7 EU, 149.4–206.8) at 28-days, as measured by ELISA in 10 prime-boost participants [9]. Similarly, in phase 2/3 RCT there was a significant increase in anti-spike IgG antibody in 1593 cohorts who took 2 standard doses of ChAdOx1 nCoV-19 measured by multiplex immunoassay [1], [2]. In a cross-sectional community survey from England (REal-time Assessment of Community Transmission-2 program, REACT-2) that studied IgG anti-spike antibody kinetics after two doses of Pfizer-BioNTech vaccine, involving 971 participants, found 91.1% seropositivity across all age groups. There was also a consistent decreasing trend in IgG positivity with the increasing age in REACT-2 study [10]. A high IgG seropositivity of 90.1% to a single dose of Pfizer-BioNTech vaccine was observed in people with past confirmed or suspected COVID across all age groups. Notably, none of these studies reported the antibody response in relation to comorbidities. Recent studies have reported a significantly diminished anti-spike antibody titre in hemodialysis patients compared to healthy control even after the two completed doses of both Pfizer-BioNTech vaccine and Moderna vaccines [11], [12], [13]. One study found highly attenuated humoral response to anti-spike antibody after two completed doses of Pfizer-BioNTech vaccine in people receiving disease-modifying drugs such as Ocrelizumab and Fingolimod for the treatment of multiple sclerosis [14]. In a recent small study (n = 136) that assessed immunogenicity with Covishield and Covaxin in Indian people with rheumatoid arthritis reported a significantly lower median (IQR) anti-spike antibody titre with later compared to the former, measured by ELISA [15].
The larger question is whether the humoral antibody response to a vaccine correlates with the efficacy (reduction in attack rates, severity and mortality due to COVID-19). Although the correlation of antibody titre to the vaccine efficacy is less well understood, NAb targeting different epitopes of spike glycoprotein have been found to protect from COVID-19 with ChAdOx1 nCoV-19 (Covishield) and Moderna mRNA-1273 vaccine. Moreover, NAb titre following vaccination highly correlates with the NAb titre in convalescent post-SARS-CoV-2 infection. Furthermore, Anti-spike antibody titre is reported to highly correlate with in-vitro virus neutralization test measured by PNT [16]. Indeed, a strong correlation (r range 0·87–0·94) between NAb responses measured by PNT against the spike glycoprotein and those detected by ELISAs have been reported in patients with RT-PCR confirmed COVID-19 [17]. Contrarily, a longitudinal study found no relationship between post-vaccination serum binding-antibody in SARS-CoV-2 naïve individuals [18]. Collectively, it is not exactly known as to what level of binding and neutralizing antibody protects human from COVID-19 [19].
To the best of our knowledge, this Pan-India cross-sectional COVAT study would be the first of its kind that has involved HCW from 13 States and 22 cities and reporting anti-spike antibody kinetics after two completed doses of two different vaccines. However, we also acknowledge several limitations. First, in the present study, we have used a convenience sampling amounting to selection bias. A community-based study in a larger population with multi-stage sampling would be an ideal sampling method. Second, we did not have dilution and neat values for those sample that hit both the upper and lower limit of kit that assessed anti-spike antibody. This could have likely underestimated the GMT in Covishield recipients, since many participants had undiluted plateaued value when compared to Covaxin recipients. Third, we used a binary logistic regression (to identify the predictors of non-response to vaccines) which primarily assumes linearity between the explanatory variable and the outcome variable, hence this model may miss out any predictor variable which may have non-linear relationship with the outcome variable. Fourth, we have measured only anti-spike binding antibody and could not assess NAb and cell-mediated immune response such as Th-1 and Th-2 dependent antibody or cytokines (primarily due to the lack of standardized commercial labs in India). Although a recent study has demonstrated a high correlation between spike protein-based ELISA and different antibody classes including NAb in COVID-19 patients [20]. Fifth, we could not measure the baseline anti-spike antibody titre prior to the vaccination, because of logistic issue due to lockdown. Due to the missing pre-vaccination titre, both seroconversion rates and mean-fold titre increases could not be calculated. Finally, two values of short-term anti-spike antibody as evaluated in this report may not necessarily predict the efficacy of vaccine, nor the absence of seropositivity confer failure of vaccine in the absence of NAb and T-cell response assessment.
In conclusion, this cross-sectional study suggests that both vaccines induce seropositivity to anti-spike antigen in 95% of SARS-COV-2 naïve and recovered individuals, 3-weeks after completion of two doses. Notably, Covishield recipients had a significantly higher rate of seropositivity and GMT of anti-spike antibody compared to Covaxin both after first and second doses. Whether any real difference in inducing immunogenicity and corresponding efficacy exists between two vaccines can only be meaningfully demonstrated through a head-to-head RCT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank all the participants who volunteered for this study. We express our sincere gratitude and acknowledgment to our Indian regional coordinators for the smooth conduct of this study that include (in alphabetical order) – Drs. Akash Kumar Singh (Vadodara), Amit Gupta (Noida), Anuj Maheshwari (Lucknow), Arvind Kumar Ojha (Kolkata), Bhavtharini (Erode), B. Harish Kumar (Mysore), J K Sharma (New Delhi), Jayant Panda (Cuttack), Kavyachand Yalamudi (Guntur), Kiran Shah (Vadodara), M Gowri Sankar (Coimbatore), Manohar KN (Bangalore), Meena Chhabra (New Delhi), Pratap Jethwani (Rajkot), M Shunmugavelu (Trichy), Rajiv Kovil (Mumbai), Sunil Gupta (Nagpur), Subhash Kumar (Patna), Somnath (Hyderabad), Urman Dhruv (Ahmedabad). Our heartfelt thanks to Ms. Roma Dave (Dietician) and Dr. Priya Phatak (Ahmedabad) for keeping entire data up-to-date and confidential at every step. Special thanks to Dr. Bhavini Shah, Dr. Sandip Shah, and Dr. Krutarth Shah from Neuberg Supratech Laboratory, Ahmedabad, for generously supporting our cause.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.09.055.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharat Biotech Announces Phase 3 Results of COVAXIN®: India’s First COVID-19 Vaccine Demonstrates Interim Clinical Efficacy of 81%. covaxin-phase3-efficacy-results.pdf (bharatbiotech.com). Accessed on May 31, 2021.
- 4.Singh AK, Phatak SR, Singh NK, et al. Antibody Response after First-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) amongst Health Care Workers in India: Preliminary Results of Cross-sectional Coronavirus Vaccine-induced Antibody Titre (COVAT) study. medRxiv preprint doi: 10.1101/2021.04.07.21255078. [DOI] [PMC free article] [PubMed]
- 5.Elm E.V., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LIAISON SARS-CoV-2 S1/S2 IgG. The fully automated serology test for the detection of SARS-CoV-2 IgG antibodies. liaisonr_sars-cov-2_s1s2_igg_brochure.pdf.pdf (diasorin.com). Accessed on May 31, 2021.
- 7.Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis 2021. Published Online January 21, 2021 10.1016/ S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed]
- 8.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis 2021. Published Online March 8, 2021 10.1016/ S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed]
- 9.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward H, Cooke G, Whitaker M, et al. REACT-2 Round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv preprint doi: 10.1101/2021.02.26.21252512; this version posted March 1, 2021.
- 11.Simon B, Rubey H, Treipl A, et al. Hemodialysis Patients Show a Highly Diminished Antibody Response after COVID-19 mRNA Vaccination Compared to Healthy Controls. 10.1101/2021.03.26.21254259 doi: medRxiv preprint. [DOI] [PMC free article] [PubMed]
- 12.Grupper A, Sharon N, Finn T, et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. CJASN April 2021, CJN.03500321; DOI: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed]
- 13.Chan L, Fuca N, Zeldis E, Campbell K, Shaikh A. Antibody Response to mRNA-1273 SARS-CoV-2 Vaccine in Hemodialysis Patients with and without Prior COVID-19. CJASN May 2021, CJN.04080321; DOI: 10.2215/CJN.04080321. [DOI] [PMC free article] [PubMed]
- 14.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021, Vol. 14: 1–8 DOI: 10.1177/ 17562864211012835. [DOI] [PMC free article] [PubMed]
- 15.Shenoy P, Ahmed S, Cherian S, et al. Immunogenicity of the ChAdOx1 nCoV-19 and the BBV152 Vaccines in Patients with Autoimmune Rheumatic Diseases. MedRxiv preprint. 10.1101/2021.06.06.21258417.
- 16.Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goel RR, Apostolidis SA, Painter MM, et al. Longitudinal Analysis Reveals Distinct Antibody and Memory B Cell Responses in SARS-CoV2 Naïve and Recovered Individuals Following mRNA Vaccination. medRxiv preprint doi: 10.1101/2021.03.03.21252872. [DOI] [PMC free article] [PubMed]
- 19.Khoury DS, Cromer D, Reynaldi A, et al. What level of neutralising antibody protects from COVID-19? medRxiv preprint doi: 10.1101/2021.03.09.21252641.
- 20.Marchi S, Viviani S, Remarque EJ, et al. Characterization of antibody response in asymptomatic and symptomatic SARS-CoV-2 infection. PLoS ONE 2021;16(7): e0253977. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.