Abstract
d-Allulose, a C-3 epimer of d-fructose, is a rare sugar that has no calories. Although d-allulose has been reported to have several health benefits, such as anti-obesity and anti-diabetic effects, there have been no reports evaluating the effects of d-allulose on insulin resistance using a hyperinsulinemic-euglycemic clamp (HE-clamp). Therefore, we investigated the effects of d-allulose on a high-sucrose diet (HSD)-induced insulin resistance model. Wistar rats were randomly divided into three dietary groups: HSD containing 5% cellulose (HSC), 5% d-allulose (HSA), and a commercial diet. The insulin tolerance test (ITT) and HE-clamp were performed after administration of the diets for 4 and 7 weeks. After 7 weeks, the muscle and adipose tissues of rats were obtained to analyze Akt signaling via western blotting, and plasma adipocytokine levels were measured. ITT revealed that d-allulose ameliorated systemic insulin resistance. Furthermore, the results of the 2-step HE-clamp procedure indicated that d-allulose reversed systemic and muscular insulin resistance. d-Allulose reversed the insulin-induced suppression of Akt phosphorylation in the soleus muscle and epididymal fat tissues and reduced plasma TNF-α levels. This study is the first to show that d-allulose improves systemic and muscle insulin sensitivity in conscious rats.
Keywords: d-allulose, Rare sugar, Insulin resistance, Hyperinsulinemic-euglycemic clamp, Soleus muscle, Epididymal fat
d-allulose, rare sugar, insulin resistance, hyperinsulinemic-euglycemic clamp, soleus muscle, epididymal fat
1. Introduction
Insulin resistance is a condition in which insulin cannot exert its effect in insulin-sensitive target tissues, including skeletal muscles, adipose tissues, and the liver [1]. Insulin resistance is associated with obesity, hyperinsulinemia, hypertension, hyperglycemia, and cardiovascular disease [2]. Regular consumption of beverages sweetened with caloric sugars has been reported to lead to insulin resistance [3, 4]. Rats fed a high-sucrose diet (HSD) have been used as a model for insulin resistance in conjunction with hypertriglyceridemia [5, 6, 7]. The balance between proinflammatory adipokines (i.e., TNF α) and anti-inflammatory adipokine (i.e., adiponectin) may play a critical role in insulin resistance of this model [8, 9].
d-Allulose, a C-3 epimer of d-fructose, is a rare sugar that has no calories. d-allulose is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA) for use as a food ingredient and with other sweeteners [10]. d-allulose is very small quantities in nature, but it exists in food products, and heat processing produces d-allulose in high sugar products [11]. d-Allulose was reported to have multiple beneficial effects, such as anti-obesity, anti-hyperglycemia, and anti-hyperlipidemic activities in rodents [12, 13, 14] and humans [15, 16]. Results from previous studies indicate that d-allulose affects fat and glucose metabolism in the liver. Itoh et al. demonstrated that d-allulose reduced hepatic fat accumulation in leptin-deficient mice, an obesity model [17]. Kanasaki et al. reported that d-allulose enhances HDL-cholesterol uptake into the liver by increasing hepatic receptor expression in rats [18]. d-Allulose suppresses the activity of hepatic enzymes, fatty acid synthase, and glucose-6 phosphate dehydrogenase and decreases lipogenesis in the liver of rats [19]. Glucokinase is crucial for glucose metabolism in the liver [20], and studies have shown that d-allulose promotes glucokinase translocation from the nucleus to the cytoplasm in the liver and hepatic glycogen accumulation [21, 22]. Hossain et al. showed that d-allulose prevents insulin resistance, as determined via homeostasis model assessment of insulin resistance (HOMA-IR) or the oral glucose tolerance test, in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a type 2 diabetes mellitus model [23, 24]. Some methods for evaluating insulin resistance have been suggested but the HE-clamp method is the gold standard for assessing insulin action [25]. Furthermore, there is still a lack of direct evidence regarding the effect of d-allulose on insulin resistance in skeletal muscles. In this study, we first tried to evaluate the effects of d-allulose on insulin resistance by using a 2-step HE-clamp method in an HSD-induced insulin-resistant rat model, and examined the insulin signaling molecules in insulin target tissues, especially the muscles.
2. Materials and methods
2.1. Chemicals
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), glycerol, SDS, phenylmethylsulfonyl fluoride, and magnesium chloride were purchased from Wako Pure Chemical Industries (Tokyo, Japan). NaCl was purchased from Fuji film (Tokyo, Japan). Sodium pentobarbital was purchased from Kyoritsu Seiyaku Corp (Tokyo, Japan). Trypsin inhibitor, Triton X-100, and 2-mercaptoethanol were purchased from MP Biomedicals, LLC (Irvine, CA, USA). Tween 20, Trizma hydrochloride, and bovine serum albumin were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Bromophenol blue was purchased from Katayama Chemicals (Nagoya, Japan).
2.2. Treatment of animals
Five-week-old male Wistar rats were purchased from Chubu Kagakushizai Co., Ltd. (Aichi, Japan). All rats were individually housed under a controlled temperature of 24 ± 1 °C and artificial lighting (12-h:12-h reverse light-dark cycle) with free access to diet and water during the entire experimental period. After 1 week of acclimation, the rats were divided into three groups. Rats were fed an HSD (based on AIN-93M; Oriental Yeast Co., Ltd., Tokyo, Japan) containing 5% (w/w) cellulose (Oriental Yeast) or 5% (w/w) d-allulose (Matsutani Chemical Industry Co., Ltd., Hyogo, Japan) based on a previous report [26], or a commercial diet (MF; Oriental Yeast). To equalize the calories of these HSDs, we replaced d-allulose with cellulose. MF is a standard chow diet, which is made from raw materials from natural products, and we compared MF as a control with a high-sucrose diet [27, 28]. The concentration of d-allulose was determined according to previous reports [8, 29]. The composition (w/w) of each diet is shown in Table 1. All experimental procedures complied with the Guide for the Care and Use of Laboratory Animals of Nagoya University, and ethical approval was granted by the Animal Experiment Committee of Nagoya University (HPFS No. 16).
Table 1.
Composition of diets fed to rats in this study.
| MF | HSC | HSA | |
|---|---|---|---|
| g/kg | |||
| Casein | 133.0 | 133.0 | |
| Soybean oil | 38.0 | 38.0 | |
| Cornstarch | 95.0 | 95.0 | |
| α-Cornstarch | 19.7 | 19.7 | |
| Sucrose | 570.0 | 570.0 | |
| Cellulose powder | 97.5 | 47.5 | |
| d-Allulose | 0 | 50.0 | |
| AIN93M mineral mix | 33.3 | 33.3 | |
| AIN93M vitamin mix | 9.5 | 9.5 | |
| L-Cystine | 1.7 | 1.7 | |
| Choline bitartrate | 2.4 | 2.4 | |
| Tertiary butylhydroquinone | 0.008 | 0.008 | |
| % kcal | |||
| Protein | 26.2 | 12.7 | 12.7 |
| Lipid | 13.3 | 9.7 | 9.7 |
| Carbohydrate | 60.5 | 77.6 | 77.6 |
2.3. Insulin tolerance test
At 4 weeks, ITT was performed in all groups after 12 h of fasting. Insulin (0.5 IU/kg BW) (Novolin R, Novo Nordisk Pharma Ltd., Copenhagen, Denmark) was injected intraperitoneally (i.p.). Blood was collected from the tail vein by snipping the tip of the tail before and 30, 60, 90, and 120 min after insulin loading. Plasma glucose concentration was measured using a BF-5S glucose analyzer (Oji Scientific Instruments Co., Ltd., Hyogo, Japan).
2.4. HE-clamp study
The surgeries for cannulation and the HE-clamp were performed as previously described [30, 31]. After 6 weeks on their respective diets, the rats were anesthetized with sodium pentobarbital (50 mg/kg BW i.p.), and the right jugular vein (for 20% glucose solution and insulin infusion) and left carotid artery (for blood sampling) were catheterized. After approximately 1 week, when the body weights of the rats recovered to pre-surgery levels, HE-clamp was performed. After fasting for 16 h, the rats were awake and allowed to move freely in a cage. Blood glucose levels were maintained at a stable level for approximately 30 min before the initial blood sample was collected to measure the basal blood glucose level. A continuous 2-step clamp study was conducted. In the first step, a constant infusion of insulin was administered at a rate of 3 mU/kg BW/min (low-dose, 0–90 min). In the second step, the insulin infusion rate was increased to 30 mU/kg BW/min (high-dose, 90–180 min). During the clamp, blood glucose concentrations were measured every 10 min, and the glucose infusion was adjusted to maintain the basal level of blood glucose. The average glucose infusion rate (GIR) for the last 30 min of each clamp, when the glucose level reached a plateau at the basal level, was regarded as the index of insulin resistance. After the clamp, the rats were anesthetized, and abdominal fat tissues (epididymal, mesenteric, and perirenal fat) were taken and weighed.
2.5. Insulin signaling analysis in skeletal muscle and fat
In the present study, we used the same rats for ITT and HE-clamp study. We waited for the recovery of the rats from the ITT test, did the operation, and followed by the HE-clamp analysis. We conducted the insulin signaling analysis using the different groups of rats which are the similar age as the rats used for the HE-clamp analysis. The rats were intraperitoneally anesthetized with sodium pentobarbital. Insulin (0.5 IU/kg BW) was injected into the inferior vena cava, and the muscles were harvested after 2 min and fats were harvested after 5 min. Samples were snap-frozen in liquid nitrogen and stored at -80 °C for western blotting.
2.6. Western blotting
Proteins in the soleus muscle and epididymal fat were extracted using homogenization buffer (50 mM HEPES, pH 7.4; 150 mM NaCl, 1.5 mM MgCl2, 0.01% trypsin inhibitor, 10% glycerol, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride). After centrifugation (16,000 × g, 30 min, 4 °C), the supernatants were obtained as samples. Protein concentrations in the lysate were determined using the Bradford assay using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were boiled in treatment buffer (62.5 mM Tris, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, and 0.025% bromophenol blue) for 5 min. After SDS-PAGE (10% or 12%), the proteins were transferred onto PVDF membranes (GE Healthcare, UK) using a semi-dry transfer gel method. Membranes were blocked in 5% non-fat milk in TBS-T buffer (20 mM Tris, pH 7.6; 0.8% NaCl, 0.1% Tween 20) for 1 h at room temperature and then incubated overnight at 4 °C with the following primary antibodies: phospho-Akt (Ser473), phospho-Akt (Thr308) (Cell Signaling Technology, Inc., Danvers, MA, USA), and Akt1/2/3 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). These primary antibodies were dissolved in TBS-T containing 5% bovine serum albumin (1:1000). After incubation with the corresponding secondary antibodies, protein bands were detected using an ECL reagent (GE Healthcare UK Limited, Buckinghamshire, UK), and western blot images were quantified using Image J software (NIH, Bethesda, MD, USA).
2.7. Blood levels of adipocytokines
The levels of TNF-α, leptin, and adiponectin were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Abcam, USA).
2.8. Statistical analysis
Data are expressed as mean ± SD. Statistical significance was set at p < 0.05. Statistical significance between three groups was determined using one-way analysis of variance (ANOVA) with Tukey's post hoc test. Statistical significance between the HSC and HSA groups was determined using Student's t-test. IBM SPSS Advanced Statistics 25 (IBM Corp., Armonk, New York, USA) was used for the statistical analyses.
3. Results
3.1. Body weight and fat
Body weight and abdominal fat weight were determined, as shown in Figure 1. There was no difference in body weight between the MF and HSC groups, whereas the body weight of the HSA group was significantly lower than the other groups throughout the experiment (vs MF: week 5, p < 0.05; week 1, 3, 6, p < 0.01; week 2, 4, p < 0.001, vs HSC: week 1, p < 0.01; week 2–6, p < 0.001) (Figure 1A). The average energy intake per day in the HSA group (77 ± 5 kcal) was significantly lower than that of the other two groups (90 ± 6 in the MF group and 85 ± 5 kcal in the HSC group) (p < 0.01). The total amount of abdominal fat (p < 0.01), including epididymal (p < 0.001), mesenteric (p < 0.05), and perirenal fat (p < 0.05), was significantly lower in the HSA group than in the HSC group, whereas there was no difference between the HSA and MF groups (Figure 1B). The relative weights of epididymal fat (p < 0.01) and abdominal fat (p < 0.05) were significantly lower in the HSA group than in the HSC group (Figure 1C).
Figure 1.
Effect of d-allulose on the body weight (A), fat weight (B), and fat weight adjusted to the body weight (C) in rats. Abdominal fat (B and C) represents the total weights of epididymal, mesenteric, and perirenal fats. Results are expressed as mean ± SD; n = 5 (MF), n = 7 (HSC), n = 7 (HSA). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. the MF group; &p < 0.05, &&p < 0.01, &&&p < 0.001 vs. the HSC group.
3.2. Insulin tolerant test and high-dose insulin clamp test
Figure 2A shows the ITT results in terms of the percent change in plasma glucose from the baseline. The HSA group exhibited a larger decrease from the baseline than the HSC group at all time points, and the difference between the groups was significant at 90 min (p < 0.05). In the low-dose insulin clamp test, the GIR of the HSA group was significantly higher than that of the other two groups (p < 0.001). Similarly, in the high-dose insulin clamp test, the GIR of the HSA group was significantly higher than that of the other two groups (vs MF: p < 0.01; vs HSC: p < 0.001) (Figure 2B). These findings indicate that d-allulose improves HSD-induced insulin resistance in the systemic and muscle tissues.
Figure 2.
Percent change in plasma glucose levels during the insulin tolerance test (A), and glucose infusion rates during the clamps (B). A low dose of insulin (3 mU/kg BW/min) was continuously infused for the first 90 min then a high dose of insulin (30 mU/kg BW/min) was infused for the next 90 min. Results are expressed as mean ± SD; n = 5 (MF), n = 7 (HSC), n = 7 (HSA). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. the MF group; &p < 0.05, &&&p < 0.001 vs. the HSC group.
3.3. Phosphorylation of Akt in muscle and fat
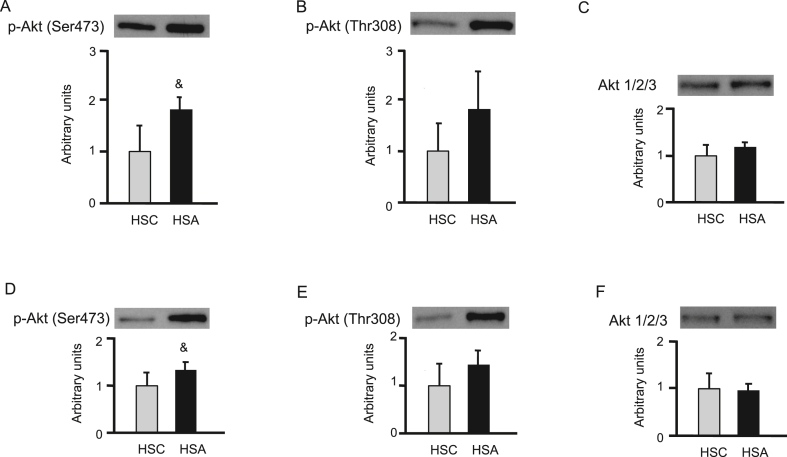
In the western blots, the intensities of phosphorylation at the Ser473 and Thr308 residues of Akt were compared between the HSC and HSA groups in the soleus muscle (Figure 3A-C) and epididymal fat (Figure 3D-F). The phosphorylation of Ser473 of Akt in the HSA group was significantly higher than that in the HSC group (p < 0.05) (Figure 3A, D).
Figure 3.
Effect of d-allulose on the insulin-stimulated phosphorylation of Akt at the Ser473 residue (A) and the Thr308 residue (B), and protein expression of Akt 1/2/3 (C) in the soleus muscles. Effect of d-allulose on the insulin-stimulated phosphorylation of Akt at the Ser473 residue (D) and Thr308 residue (E), and protein expression of Akt 1/2/3 (F) in epididymal fats. Results are expressed as mean ± SD (arbitrary units); n = 5 (HSC), n = 4 (HSA). &p < 0.05 vs. the HSC group. The uncropped versions of western blots used for the analysis are presented in Supplementary Figure 1.
3.4. TNF-α, leptin, and adiponectin
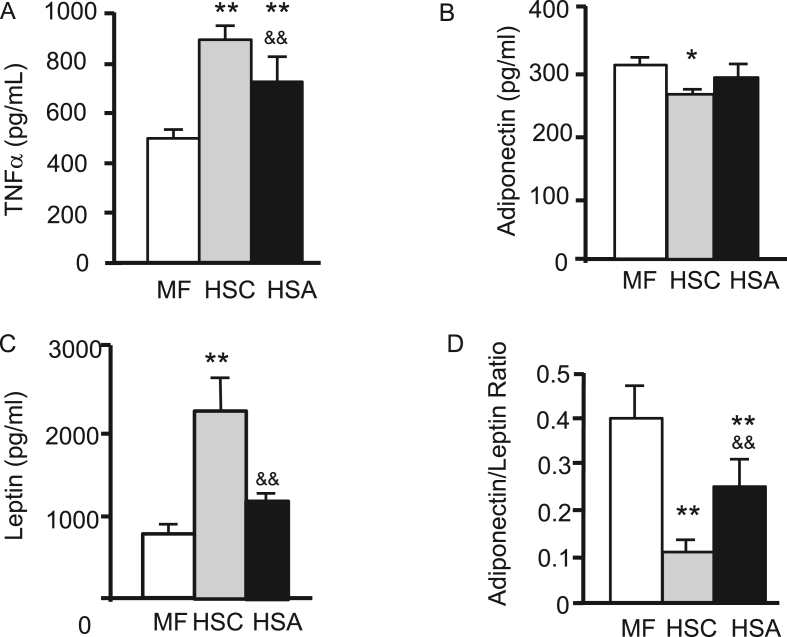
TNF-α levels in the HSA group were significantly lower than those in the HSC group (p < 0.01), whereas those in the MF group were significantly lower than those in the other two groups (p < 0.01) (Figure 4A). Adiponectin levels in the HSA group were not significantly different from those in the HSC group, whereas those in the HSC group were significantly lower than those in the MF group (p < 0.05) (Figure 4B). Leptin levels in the HSA group were significantly lower than those in the HSC group (p < 0.01) (Figure 4C). In addition, adiponectin-leptin ratio in the HSA group was significantly higher than that in the HSC group (p < 0.01) (Figure 4D).
Figure 4.
Effect of d-allulose on the plasma TNF-α (A), plasma adiponectin (B), plasma leptin (C), and adiponectin/leptin ratio (D). Results are expressed as mean ± SD; n = 5 (MF), n = 7 (HSC), n = 7 (HSA). ∗p < 0.05, ∗∗p < 0.01 vs. the MF group; &&p < 0.01 vs. the HSC group.
4. Discussion
In this study, we used the HE-clamp method and found that d-allulose administration reversed the insulin resistance caused by HSD. This method can be used to directly evaluate systemic and muscle insulin sensitivity. Notably, the results of the clamp test indicated that the addition of d-allulose to an HSD improved insulin sensitivity above the level of MF. The GIR obtained using the physiological low-dose insulin infusion clamp is useful for estimating whole-body insulin sensitivity. High-dose insulin infusion is regarded as an index of insulin resistance in skeletal muscle because infusion with a high level of insulin sufficiently suppresses hepatic gluconeogenesis [32]. The skeletal muscles and the liver are the primary tissues that regulate blood glucose levels. Our results from the high-dose clamp indicate that d-allulose can improve muscle-specific insulin resistance induced by HSD.
d-Allulose has been reported to reduce body weight and abdominal fat mass in high-fat diet-fed [33, 34] and HSD-fed [8] rats. Consistently, in our study, the body and abdominal fat weights of the rats in the HSA group were lower than those of the HSC group. There are two reasons for this reduction in body weight. Iwasaki et al. reported that oral administration of d-allulose suppresses food intake by inducing the release of glucagon-like peptide-1 (GLP-1) via vagal afferent signaling [35]. The potent and selective GLP-1-releasing effect of d-allulose in rats has also been demonstrated [36]. Furthermore, Ochiai et al. showed that d-allulose increases resting energy expenditure in HSD-fed rats and contributes to reducing body weight [8]. Notably, recent reports using drinking water containing d-allulose showed improvement in insulin sensitivity in rats fed with a high-fat diet without affecting their body weights or fat masses, indicating that the mechanisms involved are independent of body weight or fat reduction [37, 38].
Several mechanisms other than the reduction of body weight or abdominal fat have been reported to explain the potential benefits of d-allulose in obesity and type 2 diabetes [39]. In liver cells, d-allulose promotes glucokinase translocation from the nucleus to the cytoplasm, resulting in glucose utilization for glycogen synthesis and suppression of d-glucose output [21, 22]. In the intestine, d-allulose suppresses the uptake of d-glucose by intestinal epithelial cells [40]. The preservation of insulin secretion by pancreatic β-cells through administration of d-allulose was demonstrated in OLETF rats [23]. We believe and previous reports support that d-allulose has effects more than energy intake and energy expenditure, however we need other separate study to address this point.
Interestingly, there has been almost no research on the function of d-allulose in muscles. Our data on soleus muscles indicate that d-allulose stimulated the insulin signaling pathway, which was consistent with the results of the HE-clamp method. Whiteman et al. noted that Akt is found in the signaling pathways that mediate the metabolic effects of insulin in several physiologically relevant target tissues [41]. Akt is an essential regulator of insulin signaling and is needed to promote glucose transport. Phosphorylation at both Ser473 and Thr308 is required to activate Akt [42, 43]. In this study, the phosphorylation of the Ser473 and Thr308 residues of Akt from the soleus muscle was higher in the HSA group than in the HSC group. However, phosphorylation of Ser473 and Thr308 residues in the gastrocnemius muscle was not different between the two groups (data not shown), which may have been caused by differences in the properties of the muscles and the conditions of insulin stimulation. Song et al. showed that insulin-stimulated phosphorylation of the Ser473 residue of Akt was higher in the soleus muscle (primarily composed of oxidative fibers: type I muscle) than in either the epitrochlearis muscle (primarily composed of glycolytic fibers: type II muscle) or the extensor digitorum longus muscle (type II muscle). According to the results of a time-course study of Akt phosphorylation, the soleus muscle exhibited the fastest and strongest insulin response among these three muscles [44]. However, Sequea et. al. reported that in the isolated muscles of old rats incubated with physiological levels of insulin, phosphorylation of the Ser473 and Thr308 residues in Akt was higher in the epitrochlearis muscle than in the soleus muscle [45]. In this study, insulin was administered via injection into the inferior vena cava, and the method of insulin administration or sampling time might affect the results of muscle-specific Akt phosphorylation.
We also found that d-allulose increased Akt phosphorylation in the epididymal fat. Although the contribution of glucose uptake into adipose tissue to the GIR during the glucose clamp was minimal, the improvement in insulin sensitivity in the tissue suggests that it contributes to changes in the insulin sensitivity of the liver and skeletal muscle. Obesity-associated changes in the secretion of adipokines such as adiponectin, resistin, and TNF-α can modulate insulin signaling [46] and may play a role in the effects of d-allulose [47, 48, 49]. Changes in the plasma levels of inflammatory adipokines, such as TNF-α, in the HSA group could have affected the improvement in insulin signaling in the muscles.
We showed that d-allulose ameliorated insulin resistance caused by HSD and concluded that d-allulose improves insulin sensitivity in an HSD-induced insulin-resistant rat model by increasing glucose uptake in the skeletal muscle. The anti-insulin resistance effect of d-allulose can be beneficial for the prevention of obesity-related metabolic problems and diabetes.
As a limitation of this study, we fed the rats each diet for 7 weeks, and there were differences in body weight, calorie intake, and abdominal fat among the groups. These factors are known to indirectly affect insulin sensitivity in the skeletal muscle. We need to conduct further studies to confirm these points, such as the evaluation of a single administration of d-allulose on insulin sensitivity using the HE-clamp method. In addition, the use of cellulose powder may affect the present result because dietary fiber may improve glucose metabolism in HSC [50]. However, despite such possible effects, glucose metabolism was even better in HSA than HSC. Thus, this study may underestimate the effect of d-allulose.
5. Conclusion
d-Allulose reversed systemic and muscular insulin resistance induced by a high-sucrose diet in rats. The anti-insulin resistance effect of d-allulose can be beneficial for the prevention of obesity-related metabolic problems and diabetes.
Declarations
Author contribution statement
Yukie Natsume: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Takako Yamada, Tetsuo Iida: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Nobuaki Ozaki, Yoshiharu Oshida: Conceived and designed the experiments.
Yang Gou, Teruhiko Koike: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (17K01842), and Matsutani Chemical Industry Co., Ltd..
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare the following conflict of interests: Takako Yamada; Tetsuo Iida; [are employees of Matsutani Chemical Industry Co., Ltd. (Itami, Japan), who partly funded this research].
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Figure 1
References
- 1.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Lana A., Rodríguez-Artalejo F., Lopez-Garcia E. Consumption of sugar-sweetened beverages is positively related to insulin resistance and higher plasma leptin concentrations in men and nonoverweight women. J. Nutr. 2014;144:1099–1105. doi: 10.3945/jn.114.195230. [DOI] [PubMed] [Google Scholar]
- 4.Ma J., Jacques P.F., Meigs J.B. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J. Nutr. 2016;146:2544–2550. doi: 10.3945/jn.116.234047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chicco A., D'Alessandro M.E., Karabatas L. Muscle lipid metabolism and insulin secretion are altered in insulin-resistant rats fed a high sucrose diet. J. Nutr. 2003;133:127–133. doi: 10.1093/jn/133.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Seböková E., Klimes I., Moss R. Decreased glucose transporter protein (GLUT4) in skeletal muscle of hypertriglyceridaemic insulin-resistant rat. Physiol. Res. 1995;44:87–92. [PubMed] [Google Scholar]
- 7.Taha A.Y., Gao F., Ramadan E. Upregulated expression of brain enzymatic markers of arachidonic and docosahexaenoic acid metabolism in a rat model of the metabolic syndrome. BMC Neurosci. 2012;13:131. doi: 10.1186/1471-2202-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochiai M., Nakanishi Y., Yamada T., Iida T., Matsuo T. Inhibition by dietary d-psicose of body fat accumulation in adult rats fed a high-sucrose diet. Biosci. Biotechnol. Biochem. 2013;77(5):1123–1126. doi: 10.1271/bbb.130019. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira L.S., Santos D.A., Barbosa-da-Silva S., Mandarim-de-Lacerda C.A., Aguila M.B. The inflammatory profile and liver damage of a sucrose-rich diet in mice. J. Nutr. Biochem. 2014;25(2):193–200. doi: 10.1016/j.jnutbio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W., Zhang T., Jiang B., Mu W. Enzymatic approaches to rare sugar production. Biotechnol. Adv. 2017;35(2):267–274. doi: 10.1016/j.biotechadv.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Oshima H., Kimura I., Izumori K. Psicose contents in various food products and its origin. Food Sci. Technol. Res. 2006;12(2):137–143. [Google Scholar]
- 12.Baek S.H., Park S.J., Lee H.G. D-psicose, a sweet monosaccharide, ameliorate hyperglycemia, and dys-lipidemia in C57BL/6J db/db mice. J. Food Sci. 2010;75:H49–53. doi: 10.1111/j.1750-3841.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen J., Huang W., Zhang T. Anti-obesity potential of rare sugar d-psicose by regulating lipid metabolism in rats. Food Funct. 2019;10:2417–2425. doi: 10.1039/c8fo01089g. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo T., Izumori K. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 2006;70:2081–2085. doi: 10.1271/bbb.60036. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi N., Iida T., Yamada T. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 2010;74:510–519. doi: 10.1271/bbb.90707. [DOI] [PubMed] [Google Scholar]
- 16.Noronha J.C., Braunstein C.R., Glenn A.J. The effect of small doses of fructose and allulose on postprandial glucose metabolism in type 2 diabetes: a double-blind, randomized, controlled, acute feeding, equivalence trial. Diabetes Obes. Metabol. 2018;20:2361–2370. doi: 10.1111/dom.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh K., Mizuno S., Hama S. Beneficial effects of supplementation of the rare sugar "d-allulose" against hepatic steatosis and severe obesity in Lep(ob)/Lep(ob) mice. J. Food Sci. 2015;80:H1619–1626. doi: 10.1111/1750-3841.12908. [DOI] [PubMed] [Google Scholar]
- 18.Kanasaki A., Iida T., Murao K. d-Allulose enhances uptake of HDL-cholesterol into rat's primary hepatocyte via SR-B1. Cytotechnology. 2020;72:295–301. doi: 10.1007/s10616-020-00378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuo T., Baba Y., Hashiguchi M. Dietary d-psicose, a C-3 epimer of d-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia Pac. J. Clin. Nutr. 2001;10:233–237. doi: 10.1046/j.1440-6047.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- 20.Matschinsky F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 21.Hossain A., Kitagaki S., Nakano D. Rare sugar d-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 2011;405:7–12. doi: 10.1016/j.bbrc.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda Y., Mori S., Umemura N. Suppression of blood glucose levels by d-psicose in glucose tolerance test in diabetic rats. Jpn. Pharmacol. Ther. 2010;38:261–269. [Google Scholar]
- 23.Hossain A., Yamaguchi F., Hirose K. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model Otsuka Long-Evans Tokushima Fatty rats. Drug Des. Dev. Ther. 2015;9:525–535. doi: 10.2147/DDDT.S71289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain A., Yamaguchi F., Matsunaga T. Rare sugar D-psicose protects pancreas β-islets and thus improves insulin resistance in OLETF rats. Biochem. Biophys. Res. Commun. 2012;425:717–723. doi: 10.1016/j.bbrc.2012.07.135. [DOI] [PubMed] [Google Scholar]
- 25.Borai A., Livingstone C., Ferns G.A. The biochemical assessment of insulin resistance. Ann. Clin. Biochem. 2007;44:324–342. doi: 10.1258/000456307780945778. [DOI] [PubMed] [Google Scholar]
- 26.Yamada T., Iida T., Hayashi N. Effects of D-psicose on body fat accumulation and high fructose corn syrup diets in rats. Nippon Shokuhin Kagaku Kogaku Kaishi. 2010;57:263–267. [Google Scholar]
- 27.Li P., Koike T., Jiang H.Y., Wang Z.H., Kawata Y., Oshida Y. Acute treatment with candesartan cilexetil, an angiotensin II type 1 receptor blocker, improves insulin sensitivity in high-fructose-diet-fed rats. Horm. Metab. Res. 2012;44(4):286–290. doi: 10.1055/s-0032-1304321. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z., Koike T., Li P., Jiang H., Natsume Y., Mu L., Chen T., Oshida Y. Effects of angiotensin II AT1 receptor inhibition and exercise training on insulin action in rats on high-fat diet. Life Sci. 2012;90(9-10):322–327. doi: 10.1016/j.lfs.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Ochiai M., Onishi K., Yamada T. D-psicose increases energy expenditure and decreases body fat accumulation in rats fed a high-sucrose diet. Int. J. Food Sci. Nutr. 2014;65:245–250. doi: 10.3109/09637486.2013.845653. [DOI] [PubMed] [Google Scholar]
- 30.Koshinaka K., Oshida Y., Han Y.Q. The effect of nitric oxide synthase inhibitor on improved insulin action by pioglitazone in high-fructose-fed rats. Metabolism. 2004;53:22–27. doi: 10.1016/j.metabol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Qin B., Nagasaki M., Ren M. Effects of keishi-ka-jutsubu-to (traditional herbal medicine: gui-zhi-jia-shu-fu-tang) on in vivo insulin action in streptozotocin-induced diabetic rats. Life Sci. 2003;73:2687–2701. doi: 10.1016/s0024-3205(03)00640-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z., Gardner L.B., Barrett E.J. Insulin and glucose suppress hepatic glycogenolysis by distinct enzymatic mechanisms. Metabolism. 1993;42:1546–1551. doi: 10.1016/0026-0495(93)90149-i. [DOI] [PubMed] [Google Scholar]
- 33.Chung Y.M., Hyun Lee J., Youl Kim D. Dietary D-psicose reduced visceral fat mass in high-fat diet-induced obese rats. J. Food Sci. 2012;77:H53–58. doi: 10.1111/j.1750-3841.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- 34.Han Y., Han H.J., Kim A.H. D-allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 2016;60:1695–1706. doi: 10.1002/mnfr.201500771. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki Y., Sendo M., Dezaki K. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018;9:113. doi: 10.1038/s41467-017-02488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayakawa M., Hira T., Nakamura M. Secretion of GLP-1 but not GIP is potently stimulated by luminal d-Allulose (d-Psicose) in rats. Biochem. Biophys. Res. Commun. 2018;496:898–903. doi: 10.1016/j.bbrc.2018.01.128. [DOI] [PubMed] [Google Scholar]
- 37.Pongkan W., Jinawong K., Pratchayasakul W. D-allulose provides cardioprotective effect by attenuating cardiac mitochondrial dysfunction in obesity-induced insulin-resistant rats. Eur. J. Nutr. 2020 Oct 3 doi: 10.1007/s00394-020-02394-y. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Pratchayasakul W., Jinawong K., Pongkan W. Not only metformin, but also D-allulose, alleviates metabolic disturbance and cognitive decline in prediabetc rats. Nutr. Neurosci. 2020 Nov 5:1–13. doi: 10.1080/1028415X.2020.1840050. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Hossain A., Yamaguchi F., Matsuo T. Rare sugar D-allulose: potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015;155:49–59. doi: 10.1016/j.pharmthera.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Hishiike T., Ogawa M., Hayakawa S. Transepithelial transports of rare sugar D-psicose in human intestine. J. Agric. Food Chem. 2013;61:7381–7386. doi: 10.1021/jf401449m. [DOI] [PubMed] [Google Scholar]
- 41.Whiteman E.L., Cho H., Birnbaum M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metabol. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 42.Alessi D.R., James S.R., Downes C.P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr. Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 43.Vincent E.E., Elder D.J., Thomas E.C. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br. J. Cancer. 2011;104:1755–1761. doi: 10.1038/bjc.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song X.M., Ryder J.W., Kawano Y. Muscle fiber type specificity in insulin signal transduction. Am. J. Physiol. 1999;277:R1690–1696. doi: 10.1152/ajpregu.1999.277.6.R1690. [DOI] [PubMed] [Google Scholar]
- 45.Sequea D.A., Sharma N., Arias E.B. Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats. J. Gerontol. A Biol Sci Med Sci. 2012;67:1279–1285. doi: 10.1093/gerona/gls085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qatanani M., Lazar M.A. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 47.Choi B.R., Kwon E.Y., Kim H.J. Role of synbiotics containing d-allulose in the alteration of body fat and hepatic lipids in diet-induced obese mice. Nutrients. 2018;10:1797. doi: 10.3390/nu10111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acquarone E., Monacelli F., Borghi R. Resistin: a reappraisal. Mech. Ageing Dev. 2019;178:46–63. doi: 10.1016/j.mad.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Steppan C.M., Bailey S.T., Bhat S. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 50.Lattimer J.M., Haub M.D. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2(12):1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Data Availability Statement
Data will be made available on request.