Abstract
The purpose of this study was to explore the stability and toxicity of the herbicides and their degradation byproduct after exposure to different environmental factors. Triazines (atrazine, propazine, simazine) and chloroacetanilides (acetochlor, alachlor, metolachlor) which are commonly used herbicides were evaluated for cytotoxicity in different UV (254 nm and 365 nm) and temperature (4 °C, 23 °C, and 40 °C) conditions as well as degradation rates. Atrazine with the highest LD50 (4.23 μg mL−1) was less toxic than the other tested triazine herbicides Chloroacetanilides tested were more toxic than tested triazines, with LD50 0.08–1.42 μg mL−1 vs 1.44–4.23 μg mL−1, respectively. Alachlor with LD50 0.08 μg mL−1 showed the strongest toxic response as compared with other tested herbicides. Temperatures only did not alter cytotoxicity of the tested herbicides, except for acetochlor and alachlor showing about 45 % more cell death after exposure to 40 °C for 2 h. At all 3 tested temperatures, 2 h of UV treatments did not affect cytotoxic effects of the tested herbicides, except for acetochlor and alachlor. At 4 °C, acetochlor toxicity was attenuated about 63 % after UV 365 nm exposure; but alachlor toxicity was enhanced after either UV 254 or 365 nm exposure for about 40 % and 24 %, respectively. At 23 °C, acetochlor toxicity was enhanced about 35 % after UV 254 nm exposure, but attenuated about 48 % after UV 365 nm exposure. Alachlor toxicity was enhanced about 34 % after UV 254 nm and 23 °C exposure. In combination of UV 254 nm and 40 °C, acetochlor toxicity was lowered by 63 % and alachlor toxicity was no change as compared with 4 °C, no UV group. After co-treatment with UV 365 nm and 40 °C both acetochlor and alachlor toxicity was enhanced 55 % and 80 %, respectively. Through degradation analysis by LC-MS/MS, alachlor showed the most dramatic degradation (only 0.58 %–10.58 % remaining) after heat and UV treatments.
Keywords: Atrazine, Acetochlor, Alachlor, Metolachlor, Environmental toxicology, Photodegradation
Atrazine, Acetochlor, Alachlor, Metolachlor, Environmental toxicology, Photodegradation.
1. Introduction
The United States’ herbicide use has surged 25.6 % in just a 4-year time span from 540 million pounds in 2008 to 678 million pounds in 2012 [1]. While being beneficial to crop growth, the dramatic increase in herbicides use over recent decades poses a serious threat to the environment, due to residue remains left on crops, posing a direct threat to humans through consumption, as well as collection into water systems through runoff [2, 3, 4, 5, 6]. This, in turn, leads to the consumption of harmful chemicals by organisms in and around the ecosystem. Surface runoff from agricultural areas is the main reason for contamination of surface water by herbicides and causes serious environmental impacts. Transformation of herbicides due to nature environmental factors could produce more hazardous transformed byproducts [7]. The toxicity of herbicides, however, after transformation by natural environmental factors are not well studied.
Triazine and chloroacetanilide herbicides are some of the most commonly used in agriculture in the United States of America. They are frequently found in soil and aquatic systems due to their high usages and the persistence through physiochemical degradation [8]. Atrazine has been found to have the highest concentration of 30 μg L−1 in ground and surface water [9]. Kalkhoff et at. (1998) detected chloroacetanilides in ground and surface water with concentration of 0.05 μg L−1 and 0.13 μg L−1, respectively [10]. Chloroacetanilides are known to transform rapidly in soil under aerobic conditions [11]. In water with microorganisms, chloroacetanilides are degraded via various pathways to form a large number of degradation byproducts [12, 13, 14]. Triazines, which are relatively more stable than chloroacetanilides, can undergo dealkylation and dechlorination simultaneously with faster rates under UV and ozonation [15].
The triazine herbicides used in this study specifically are atrazine, propazine, and simazine are regarded as more stable compounds [16]. Atrazine, one of most widely used triazines, has up a half-life of up to 6 months in soil [17], and its degradation is known to be affected by temperature and moisture. The main photodegradation processes for triazines at the early phases are through dechlorination and dealkylation. Different triazines have different degradation rates depending on chemical structure [7,18]. Simazine with one less alkyl group and a more symmetric structure is degraded slower as compared with atrazine. Atrazine at low dose exhibits carcinogenic properties and disrupts endocrine system [19]. Studies have demonstrated that some triazine degradation byproducts, such as deisopropyl-atrazine, deethyl-atrazine and deethyldeisopropylatrazine, change toxicity and are persistence as compared to their parent chemicals [18].
Chloroacetanilide herbicides, another widely used group of herbicides, have been frequently detected in ground and surface waters and are toxic to a wide range of organisms [20]. Even at very low concentration, exposure to chloroacetanilide herbicides via contamination of water or agricultural products would be a great public health and environment risk. Ma et al. showed chloroacetanilides can increase oxidative stress by increasing reactive oxygen species level and trigger apoptosis [21]. Studies also showed that utilization of chloroacetanilides links to caner and Parkinson's disease [22,23]. To minimize the impact of herbicide residue and its byproducts in the environment and risk it poses to human health, unveiling the physiochemical effect on herbicide degradation will provide the comprehensive information for improving current pesticide management strategies.
It is known that these chemicals, while in the environment, undergo degradation via different environmental factors [24, 25, 26, 27, 28]. There is a plethora of degradation factors on the average farm and neighboring aquatic ecosystems through soil leaching, such as moisture levels, light intensity, temperature, soil pH, and UV rays. Additional factors can include microorganisms, which can alter chemical composition through degradation, mineralization, or conjugation [24].
Photodegradation is the differentiation (breakdown, derivatization, decomposition) of a substance by exposure to sunlight, usually in tandem with air. This type of degradation is responsible for oxidation and reduction of environmental materials [26]. The degradation factors focused on in this experiment include UV radiation and temperature, both of which individually can cause degradation of organic molecules [28, 29, 30]. When temperature and UV effects are applied in tandem to degrade pesticides and herbicides, the exposure can result in different byproducts, which can alter toxicity in relation to parent compounds. Moreira's study showed that atrazine after radiation could generate 3 main hydroxyl byproducts: atrazine-2-hydroxy, atrazine-desethyl-2-hidroxy, and atrazine-desisopropyl-2-hydroxy [31].
As previously stated, pesticides have become more popular in agricultural practices worldwide. The adverse effects that these chemicals have on humans and other organisms around the world are dramatic and highly complex. The toxic effects of the herbicides on animals and plants were insufficiently investigated. Moreover, the toxicity of herbicide degradation products is rarely taken into account for its toxicity. Many of the degraded products can exert similar acute and chronic toxicities as the parent compound [32]. The purpose of exposing the herbicides to different environmental factors is to test their stability and to evaluate the toxicity of degraded products together with their parent chemicals. This study was aimed to elucidate the cytotoxicity of the degraded byproducts of 2 classes of herbicides, triazines and chloroacetanilides after exposure of UV and various temperatures. This study is critical to public health as it allows us to build an understanding of the fate and the toxicity of pesticides under the influence of environmental factors. Hopefully it also highlights the need for further experimentation on these and other hazardous chemicals. This study will be beneficial to farmers and other crop growers in order to ensure safer produce.
2. Materials and methods
2.1. Cell culture
Human embryonic kidney cells (HEK-293) were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured as descripted in previous study with Dulbecco Modified Eagle Media (DMEM) supplemented by 10 % fetal bovine serum, 2 mM L-glutamine, and gentamicin (50 μg mL−1) [33]. Incubation occurred at 37 °C, in a 5 % CO2 humidified incubator. The cells were cultured into 96-well plates at 50,000 cells per 200 μL on the day before chemical treatments. Cells were about medium density (∼80 % confluence) when chemicals were added.
2.2. LD50 determination: neutral red uptake assay
Herbicides (triazines: atrazine, simazine, propazine; chloroacetanilides: acetochlor, alachlor, and metolachlor) were obtained from Sigma Aldrich (St. Louis, USA) at a stock of 1 mg mL−1 in methanol. These herbicides were diluted in phosphate buffered saline (PBS) or fresh media to concentrations as indicated in cytotoxicity study for determining LD50 values which represent the chemical concentration needed to inhibit 50 % cell proliferation by neutral red uptake assay. After LD50 was determined for each herbicide, specific LD50 results were used as the concentration for UV and temperature treatments.
LD50 was determined by neutral red assay which is based on the lysosome uptake of neutral red dye [34,35]. Briefly, 200 μL cell suspension (2 × 104 cells per well) were seeded onto 96-well plate on the day before chemical treatments. Chemicals in methanol (1 mg mL−1) were diluted in fresh media to obtain various concentrations for each chemical: acetochlor and alachlor (5–500 ng mL−1), metolachlor and simazine (100–2000 ng mL−1); atrazine and propazine (500–5000 ng mL−1). After 24 h of chemical treatments, 20 μL of 0.33 % Neutral Red Solution (Sigma Aldrich) was added onto wells. After 2 h incubation at 37 ˚C/5 % CO2 incubator, dye solution was carefully removed and cells were rinsed with 200 μL Neutral Red Assay Fixative (0.1 % CaCl2 in 0.5 % formaldehyde) (Sigma Aldrich) twice. The absorbed dye was then solubilized in 200 μL of Neutral Red Assay Solubilization Solution (1 % acetic acid in 50 % ethanol) (Sigma Aldrich) for 10 min at room temperature on a shaker. Absorbance at 540 nm and 690 nm (background) was measured by BioTek Synergy Mx microplate reader.
Each concentration in each experiment was done with at least triplicate. Multiple experiments were done to obtain LD50 values for each herbicide. The viability was determined based on a comparison with untreated cells which were set as 100 % cell viability. The LD50 values were calculated from the dose-response curve.
2.3. UV and temperature treatments
The temperatures chosen for this study are to mimic our real environmental situation. To study temperature effect on herbicide degradation, each herbicide solution at LD50 was solely placed in a cold room (4 °C), laboratory (RT, 23 °C), or an incubator (40 °C) for up to 2 h and then kept in a -20 °C freezer until analysis. Temperature conditions were chosen to be the most relevant representation of real-world situations, in which crops will be grown. The exposure time of UV was chosen based on Mermama's study which showed 2 h of photocatalysis treatment significantly altered IC50 of S-metolachlor [36].
To study UV effects on herbicide degradation, each herbicide solution at LD50 concentration was solely exposed to 2 h of UV radiation from UV lamps (Spectroline, Model ENF-280C). JAZ Spectrometer JAZA 1464 (Ocean Optics Inc) detected the intensity of UV-A (365 nm; 470 μW/cm2) and UV-C (254 nm; 650 μW/cm2) for consistency. UV-A is the main UV ray which can penetrate the atmosphere and reach earth. UV-C is almost all blocked by the atmosphere. When preparing chemicals for cell culture exposure, the herbicides were set in a space with a UV lamp overhead (height of 27 cm) for up to 2 h and then kept in a -20 °C freezer until analysis.
2.4. Cytotoxicity: neutral red uptake assay and MTT assay
In order to determine the HEK-293 cells ability to survive exposure to the treated herbicides, two viability assays were performed: neutral red uptake assay and MTT assay as described in previous studies by Cheng [34,37]. The neutral red uptake assay is to test cell viability through monitoring cellular lysosomal activity. The MTT viability assay is another assay commonly used for quantifying culture viability by measuring metabolic activity and in turn mitochondrial functionality. The cells were treated with herbicides for heat and UV studies at the concentrations based on the LD50.
2.4.1. Neutral red uptake assay for monitoring of cell lysosomal activity
The assay was conducted as descripted in Section 2.2. LD50 determination: Neutral Red Uptake Assay. The relative cell survival rate was calculated by comparing with untreated cells which were set as 100 % cell viability.
2.4.2. MTT assay for the estimation of cell mitochondrial activity
The assay was conducted by following the manufacturer's protocol (Sigma-Aldrich) with slight modification to improve the formazan solubility (ATCC, 2011). Briefly, cells were introduced to 20 μL of MTT reagent (5 mg mL−1 in PBS) after chemical treatments. After 2 h of incubation (37 °C and 5% CO2) with MTT solution, MTT reagent was removed and 100 μL dimethyl sulfoxide (DMSO) was added to the wells to dissolve the formazan. The plates were then shaken for 10 min and read in BioTek Synergy Mx microplate reader spectrophotometrically at a wavelength of 540 nm and 690 nm for background. The relative cell survival rate was calculated by comparing with untreated cells which were set as 100 % cell viability.
2.5. Degradation rate measurement by LC-MS/MS
To further understanding of the degradation, the samples were subjected to LC-MS/MS analysis. The use of LC-MS/MS becomes vital in verifying degradation rate. To begin, the herbicide samples, after treatments, were dried in Turbovac using N2 gas for 45 min at 23 °C. After drying, the samples were reconstituted with 0.1 % formic acid in water, with 50 ng mL−1 butachlor as the internal standard (IS) based on EPA 535 method which used butachlor-ESA as an internal standard to detect chloroacetanilides in drinking waters by LC-MS/MS [38]. Finally, the samples were injected into ultra-high-performance liquid chromatography-tandem mass spectrometer (UPLC-MSMS) LCMS 8030 (Shimadzu Inc., Columbia, USA). LCMS 8030 with a Phenomenex KinetexTM C18 column (2.1 × 100 mm, 1.7 um) was used to separate compounds under the following condition: gradient mobile phase system (A: 0.1 % formic acid in water: methanol and B: 0.1 % formic acid in acetonitrile; 0 min 5 % B, 3 min 40 % B, 3.5 min 90 % B, 4 min 5 %); column heater: 30 °C; 10 μL injection volume; flow rate 0.5 mL min−1. The triple quadrupole mass spectrometer with Dual Ionization Source (DUIS) was operated in the positive ionization mode with spray voltage at 4.5 kV; corona pin voltage at 4.5 kV; desolvation line temperature at 250 °C; heat block temperature at 400 °C; nebulizing gas flow rate at 1.5 L min−1 and drying gas flow rate at 15 L min−1. Each compound was monitored by at least two transitions in multiple reaction monitoring (MRM) mode (Table 1). The chromatograms and mass spectra were shown in the Supplementary data. The percentage of remaining herbicide after treatments was calculated by dividing the ratio of treated herbicide with IS to the ratio of untreated herbicide with IS.
Table 1.
Multiple reaction monitoring (MRM) transitions, collision energies, retention time of pesticides and internal standard (IS) included in the method. The quantifier transition is underlined.
| Analyte | MRM Transition (m/z) | Collision energy (eV) | Retention time (min) | Internal Standard |
|---|---|---|---|---|
| Acetochlor | 270.3 > 224.1 | -12 | 5.4 | Butachlor |
| 270.3 > 148.1 | -23 | |||
| Alachlor | 270.3 > 238.2 | -12 | 5.4 | Butachlor |
| 270.3 > 162.3 | -21 | |||
| Metolachlor | 284.3 > 252.1 | -13 | 5.4 | Butachlor |
| 284.3 > 176.1 | -23 | |||
| Atrazine | 216.2 > 174.1 | -20 | 4.1 | Butachlor |
| 216.2 > 104.1 | -30 | |||
| Propazine | 230.2 > 188.1 | -18 | 4.6 | Butachlor |
| 230.2 > 146.1 | -25 | |||
| Simazine | 202.1 > 132.0 | -18 | 3.4 | Butachlor |
| 202.1 > 124.1 | -21 | |||
| Butachlor | 312.4 > 238.1 | -13 | 4.8 | |
| 312.4 > 162.2 | -23 |
2.6. Statistical analysis
All the experiments were performed in triplicates at least. The data was calculated from the mean of at least three separate experiments. The results are reported as means ± SEM. The data were evaluated using the analysis of variance (ANOVA) and the means were analyzed using student's t-test. Statistical significance was determined using student's t-test (p < 0.05, with control at 100 %).
3. Results and discussion
3.1. LD50 determination by neutral red uptake assay
In order to study whether degradation products have altered cytotoxicity as compared to parent compounds, LD50 was chosen as the experimental dosage for UV and temperature treatments. Before testing the environmental factors on herbicide degradation on cytotoxicity, the lethal dose which can kill 50 % of tested cells (LD50) was needed to be determined first. To determine the LD50 for subsequent studies, cells were treated with various concentrations (5–5000 ng mL−1) of herbicides for 24 h before the neutral red uptake assay. The concentrations of herbicides which caused 50 % of cell death (LD50) was calculated and shown in Table 2. The LD50 for triazine herbicides used in this study were from 1.44 μg mL−1 to 4.23 μg mL−1. Simazine showed stronger toxic effect with the lowest LD50 (1.44 μg mL−1) as compared with atrazine which showed weaker toxicity, LD50 4.23 μg mL−1. The LD50 for chloroacetanilide herbicides used in this study were from 0.08 μg mL−1 to 1.42 μg mL−1. Alachlor showed stronger toxic effect with the lowest LD50 (0.08 μg mL−1) as compared with metolachlor which showed weaker toxicity, LD50 1.42 μg mL−1.
Table 2.
LD50 of chloroacetanilide and triazine herbicides determined by neutral red uptake assay in HEK-293 cells.
| LD50 of Chloroacetanilide herbicides (μg mL−1) | LD50 of Triazine herbicides (μg mL−1) | ||
|---|---|---|---|
| Acetochlor | 0.22 | Atrazine | 4.23 |
| Alachlor | 0.08 | Propazine | 2.51 |
| Metolachlor | 1.42 | Simazine | 1.44 |
Moreover, chloroacetanilide herbicides which have lower LD50 values (0.08–1.42 μg mL−1) showed stronger toxic effects as compared with triazine herbicides which have higher LD50 values (1.44–4.23 μg mL−1). It is consistent with NIH data in NIH TOXNET: Chloroacetanilides: Acetochlor 1929–2148 mg kg-1; Alachlor 790–1350 mg kg-1; Metolachlor 2780–2877 mg kg-1; Triazines: Atrazine 1869–3090 mg kg-1; Propazine >5000 mg kg-1; Simazine >5000 mg kg-1 [39], except Atrazine. Among of chloroacetanilide herbicides in this study, the order of herbicide toxicity based on neural red study was alachlor > acetochlor > metolachlor. Among of triazine herbicides in this study, the order of triazines toxicity based on neutral red study was simazine > propazine > atrazine. Atrazine was much less toxic as compared with the other two tested triazines in this study. However, atrazine's results in this study were not consistent with NIH data which atrazine is much more toxic as compared with other tested triazines. Since NIH data is based on in vivo studies, it could be due to different testing systems. The toxicokinetic factors, such as metabolism, must be included in the consideration of chemical toxicity. Jin et al. [40] demonstrated that the metabolites of atrazine by cytochrome P450 can increase oxidative stress and disrupt the endocrine system in mice. Abarikwu and Farombi [41] suggested that atrazine toxicity is cell-type specific. Studies observed that human SH-SY5Y cells shows toxic responses to lower levels of atrazine; but HepG2 liver cells to higher levels of atrazine. Kale et al. [42] suggests that acetochlor and alachlor, but not metolachlor, have hepatotoxicity in rats and dogs at the lowest tested dose 100–200 μmole L-1 for 2–4 h; but all three chloroacetanilides have the same potency in human hepatic cells at 400–800 μmole L-1 for 2 h.
3.2. Herbicides after heat and UV exposures exhibit different effects on cell viability
In order to test the cytotoxicity of degraded herbicides after UV and temperature treatments, HEK-293 cells were treated with those herbicides at LD50 dosage with or without UV and various temperature treatments for 2 h. After 24-h sample exposure, cells were then subjected for neutral red uptake assay for lysosomal activity and MTT assay for mitochondrial activity. The relative cell viability for cells treated with chemicals at LD50 dosage was set up as 100 %. If the relative cell viability is more than 100 %, it indicates cells have higher survival rate after exposure with chemicals. If the relative cell viability is less than 100 %, it indicates cells have lower survival rate after exposure with chemicals.
3.2.1. Temperature or UV effect on cytotoxicity of triazines and chloroacetanilides
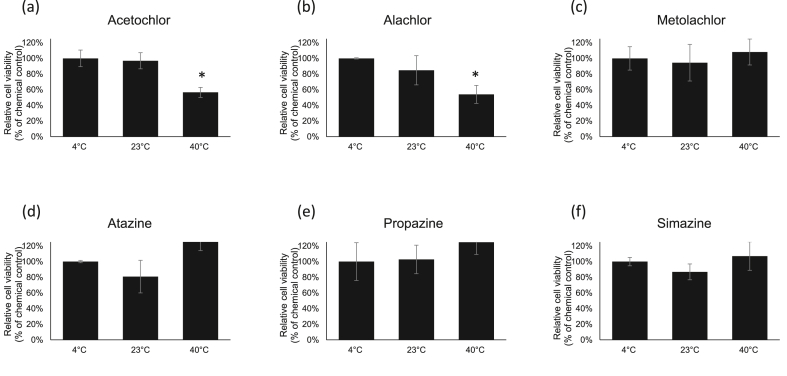
Herbicides after various temperature treatments were used to treat cells to determine cell viability by neutral red assay (Figure 1). In these experiments, the 4 °C group was set up as a control. The results showed that acetochlor (Figure 1a) and alachlor (Figure 1b) exhibited higher toxicity after exposing to 40 °C for 2 h as compared with 4 °C and RT treatment groups with about 45 % more cell death. Temperature has no further effect on metolachlor triggered cytotoxicity (Figure 1c). For triazines, all tested triazine compounds showed no further effect after temperature treatments on cytotoxicity (Figure 1d-f).
Figure 1.
Cytotoxicity assessments by the neutral red uptake assay in HEK-293 cells. The cells were exposed to herbicides with different temperature treatments. Values are the mean ± SEM of experiments. The relative cell viability for cells treated with chemicals at LD50 dosage was set up as 100 %.
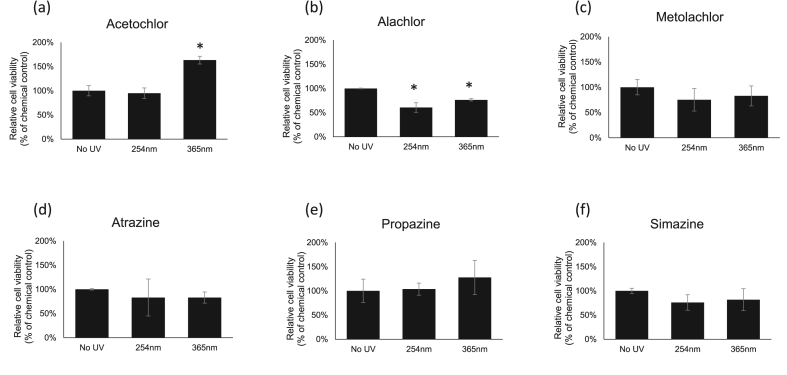
For UV effect on herbicide toxicity, cells were first exposed to UV 254 nm or UV 365 nm for 2 h at 4 °C and then subjected for neutral red cell viability analysis. In these experiments, the no UV group was set up as a control. The results showed that the cell viability increased significantly for about 63 % after acetochlor exposed to UV 365 nm as compared with no UV treatment and UV 254 nm treated groups (Figure 2a). Alachlor after either UV 254 nm or UV 365 nm treatment showed increased toxicity (40 % and 24 %, respectively) as compared with no UV treatment group (Figure 2b). For metolachlor, the cytotoxicity has no further change between no UV, UV 254 nm, and UV 365 nm groups (Figure 2c). On the other hand, 2-h UV treatments at 4 °C showed no further changes on cytotoxicity of all tested triazine compounds (Figure 2d-f). These results are consistent with other studies which found alachlor has shorter half-life than metolachlor has in outdoor aquatic mesocosms, 19.8 days vs 33.8 days at 25 ng mL−1, respectively [43].
Figure 2.
Cytotoxicity assessments by the neutral red uptake assay in HEK-293 cells. The cells were exposed to herbicides with 2 h of UV-C (254 nm) and UV-A (365 nm) treatments at 4 °C. Values are the mean ± SEM of experiments. The relative cell viability for cells treated with chemicals at LD50 dosage was set up as 100 %.
3.2.2. Temperature and UV combination effect on cytotoxicity of triazines and chloroacetanilides
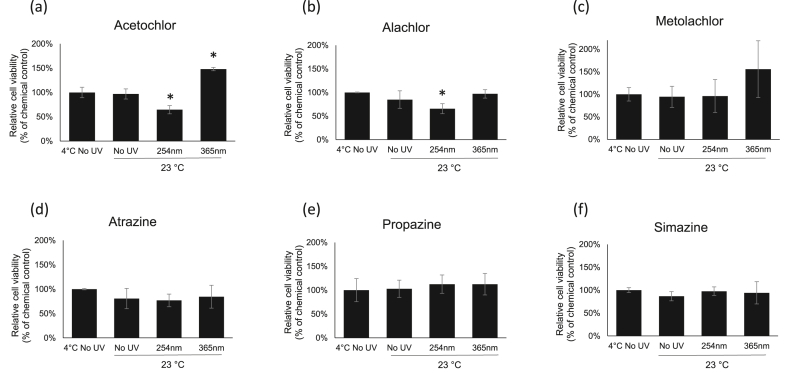
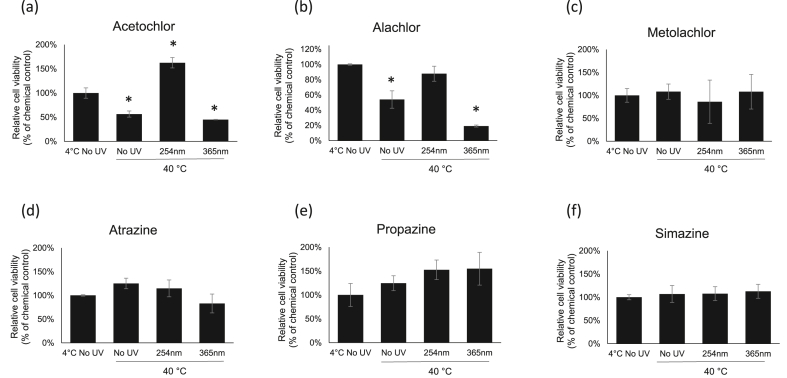
Two of chloroacetanilide herbicides, acetochlor and alachlor, exposed to UV 254 nm and UV 365 nm for 2 h at 23 °C (Figure 3a,b) and 40 °C (Figure 4a,b) have triggered slightly different cellular responses as compared 4 °C groups did (Figure 2a,b). However, there are no differences in cell viability responses for metolachlor and 3 tested triazine herbicides between 4 °C groups (Figure 2c-f), 23 °C groups (Figure 3c-f) and 40 °C (Figure 4c-f).
Figure 3.
Cytotoxicity assessments by the neutral red uptake assay in HEK-293 cells. The cells were exposed to herbicides with 2 h of UV-C (254 nm) and UV-A (365 nm) treatments at 23 °C. Values are the mean ± SEM of experiments. The relative cell viability for cells treated with chemicals at LD50 dosage was set up as 100 %.
Figure 4.
Cytotoxicity assessments by the neutral red uptake assay in HEK-293 cells. The cells were exposed to herbicides with 2 h of UV-C (254 nm) and UV-A (365 nm) treatments at 40 °C. Values are the mean ± SEM of experiments. The relative cell viability for cells treated with chemicals at LD50 dosage was set up as 100 %.
With 2 h of 23 ºC/UV 254 nm exposure, cells in acetochlor (Figure 3a, 35 % lower) and alachlor (Figure 3b; 34 % lower) groups exhibited significant lower viability as compared with cells treated with no UV at 23 °C. With 2 h of 23 ºC/UV 365 nm exposure, cell viability in the acetochlor group was increased about 48 % as compared with 23 ºC/No UV (Figure 3a). This indicated that with the combination of UV 254 nm and 23 °C acetochlor has further degraded into higher-toxic byproducts, but into less-toxic byproducts with 23 ºC/UV 365 nm co-exposure. Cells exposed to the 23 ºC/UV365 nm co-treated alachlor showed no further change in cytotoxicity as compared with 23 ºC/No UV group (Figure 3b). This indicated that at 23 ºC/UV 254 nm alachlor was degraded into higher-toxic byproducts, but not with UV 365 nm.
With 2 h of 40 ºC/UV 254 nm exposure, cells in acetochlor group exhibited a significant change in cell viability (∼63 % increase) as compared with cells in alachlor and metolachlor groups which showed no further changes in cell viability (Figure 4a vs Figure 4b,c). And the cell viability in acetochlor group treated with 40 ºC/UV 254 nm (162.9 %) was significantly higher than 4 ºC/No UV (100 %) and 40 ºC/No UV groups (56.6 %) (Figure 4a). Interestingly, with 2 h of 40 ºC/UV 365 nm exposure, cell viability in the acetochlor group (45.1 %) exhibited the similar level of cytotoxicity as compared with 40 ºC/No UV (56.6 %) (Figure 4a). This indicated that with combination of UV 254 nm and heat (40 °C) acetochlor has further degraded into less-toxic byproducts, but not with UV 365 nm co-exposure. Cells exposed to the 40 ºC/UV254 nm co-treated alachlor (87.9 %) showed less cytotoxicity as compared with 40 ºC/No UV group (54.1 %) (Figure 4b). Cells exposed to the 40 ºC/UV 365 nm co-treated alachlor (19.2 %) showed higher cytotoxicity as compared with 40 ºC/No UV group (54.1 %) (Figure 4b). This indicated that UV 254 nm further degraded alachlor into less-toxic byproducts, but UV 365 nm further degraded alachlor into more-toxic byproducts.
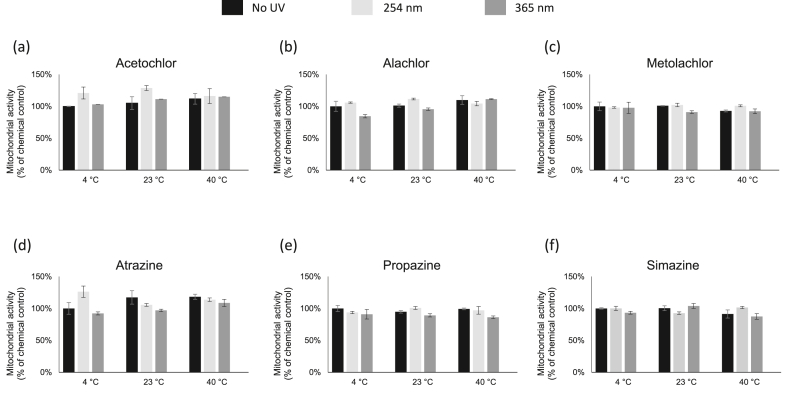
Changes in mitochondrial activity of HEK-293 cells in response to herbicides and its byproducts after the heat and UV exposures were also monitored. However, there were no significant changes in mitochondrial activity in cells after exposed to the tested herbicides and it byproducts for 2 h (Figure 5).
Figure 5.
Mitochondrial cytotoxicity assessment by MTT assay in HEK-293 cells. The cells were exposed to herbicides with 2 h of UV-C (254 nm) and UV-A (365 nm) at different temperature treatments. Values are the mean ± SEM of experiments. The relative cell viability for cells treated with chemicals at LD50 dosage was set up as 100 %.
3.3. Heat and UV exposures trigger different degradation rate
The different cell viability responses to herbicides with heat and UV treatments could be due to the different degradation responses to heat and UV. To quantify the degradation profile, LC-MS/MS was applied to detect the degradation rate of herbicides in response to heat and UV (Table 3).
Table 3.
Degradation rates (% of remaining) of chloroacetanilide and triazine herbicides after 2 h of UV and temperature treatments by LC-MS/MS analysis.
| Acetochlor | % Remaining amount |
|
|---|---|---|
| 254nm UV exposure | 365nm UV exposure | |
| 4 °C | 48.38 ± 34.06 | 84.07 ± 23.29 |
| 23 °C | 11.8 ± 0.01 | 125.1 ± 3.28 |
| 40 °C |
20.31 ± 5.95 |
17.01 ± 10.7 |
| Alachlor |
% Remaining amount | |
| 254nm UV exposure |
365nm UV exposure |
|
| 4 °C | 10.58 ± 4.85 | 4.83 ± 0.15 |
| 23 °C | 1.51 ± 0.10 | 0.58 ± 0.01 |
| 40 °C |
2.33 ± 0.01 |
3.13 ± 0.56 |
| Metolachlor |
% Remaining amount | |
| 254nm UV exposure |
365nm UV exposure |
|
| 4 °C | 16.83 ± 6.40 | 35.39 ± 17.66 |
| 23 °C | 51.70 ± 28.71 | 67.98 ± 6.27 |
| 40 °C |
25.01 ± 15.91 |
46.66 ± 26.12 |
| Atrazine |
% Remaining amount | |
| 254nm UV exposure |
365nm UV exposure |
|
| 4 °C | 34.86 ± 6.06 | 25.75 ± 11.07 |
| 23 °C | 41.81 ± 13.24 | 138.76 ± 23.50 |
| 40 °C |
64.23 ± 0.01 |
34.89 ± 13.50 |
| Propazine |
% Remaining amount | |
| 254nm UV exposure |
365nm UV exposure |
|
| 4 °C | 40.38 ± 6.85 | 5.93 ± 3.07 |
| 23 °C | 15.50 ± 5.07 | 47.17 ± 22.87 |
| 40 °C |
19.15 ± 12.42 |
57.43 ± 12.30 |
| Simazine |
% Remaining amount | |
| 254nm UV exposure |
365nm UV exposure |
|
| 4 °C | 11.67 ± 1.61 | 38.74 ± 14.98 |
| 23 °C | 45.11 ± 13.02 | 30.80 ± 3.52 |
| 40 °C | 64.66 ± 11.20 | 86.15 ± 16.82 |
The degree of degradation measured by LC-MS/MS (Table 3) has shown no specific patents or correlation with specific temperature and UV exposures. All of the tested herbicides have exhibited some degree of degradation. Alachlor was the highest degree of degradation as compared with all other tested herbicides. This signified the instability of alachlor. Since this study was designed to reveal the environmental effects on herbicides toxicity and the mixture of parent compound and degradation products in our environment is what we normally encounter, the formation of specific degradation products was not focused in this study.
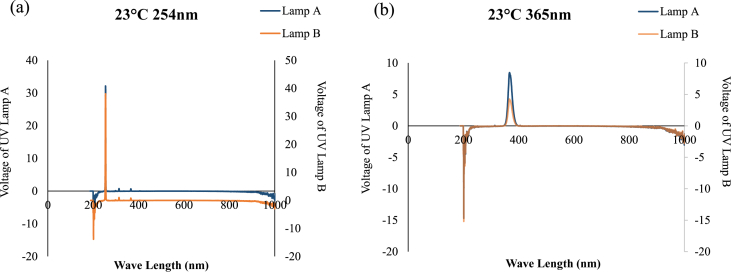
The three tested triazines also showed more resistance to heat and UV treatments (Figures 1d-f and 2d-f). Even in the combination of heat and UV (Figures 3d-f and 4d-f), triazines showed no changes in its cytotoxicity as compared to 4 °C/No UV. Triazines are well known about its persistence in biological and chemical degradation. For example, atrazine's half-life is about 150 days in aerobic condition and more than 2 years in anaerobic condition [44]. The combination treatment of heat and UV has triggered the degradation of acetochlor and alachlor. Especially, more toxic byproducts after exposing to UV 254 nm for 2 h at 23 °C were formed (Figure 3a,b). Interestingly, more toxic byproducts after exposing to UV 365 nm for 2 h at 40 °C were formed (Figure 3,b). In degradation degree study, the results showed alachlor was the most unstable one among the tested herbicides (Table 3). In Kawabata's study [45], nine pharmaceuticals in solution format also show different degradation profiles after UV irradiation at 254 nm, 302 nm or 365 nm (UV–C, UV-B or UV-A, respectively) which are dependent on both chemical structure and the wavelength of UV exposure. Kawabata et al. [45] suggested that UV-A is less effective as compared with UV-C on degrading pharmaceuticals. Another concerning factor for degradation is that UV-C (254 nm) has a higher energy content determined through calculation (Figure 6). For UV-C the energy content was 7.82∗10–24J. For UV-A the energy content for UV-A was 5.49∗10–24J. This is an important point to make as it allows for an understanding of the difference between UV-A (365 nm) and UV-C (254 nm) being 2.33∗10–24J. Due to increased energy content, the degradation of samples exposed to UV-C experienced greater degradation. Moreover, the toxicity study conducted by Kawabata's group also indicated that UV irradiation can reduce the toxicity of some compounds due to the decrease of the amount and also can increase the toxicity of others due to the generations of toxic byproducts [45].
Figure 6.
Representative spectra of UV lamps for UV-C (254 nm) and UV-A (365 nm).
4. Conclusion
In summary, the triazine herbicides used in this study were more stable than their chloroacetanilide counterparts used in this study under these testing conditions. Acetochlor and alachlor were more sensitive to temperature and UV effects as compared to the other four tested herbicides. At 40 °C, the toxicity of acetochlor and alachlor was further enhanced. The cytotoxicity of acetochlor and alachlor was also altered after the combination treatments of UV and temperature. Since 365nm UV (UV-A) is the main component (about 95 %) of solar UV radiation to earth, it is important to focus on 365 nm UV and heat effects on herbicide toxicity. The study shows that the toxicity of acetochlor and alachlor after 365 nm UV and 23 °C treatments was attenuated, but was enhanced after 365 nm UV and 40 °C treatments. This indicates that degradation byproducts of acetochlor and alachlor after heat and UV-A co-exposure could also have detrimental impact for human health. The future study will focus on identifying the specific degradation products of each test herbicide and their effects on cytotoxicity.
Declarations
Author contribution statement
Johnatan Gideon, Jonathan Mulligan, Christina Hui, Shu-Yuan Cheng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Dr. J. Cohen from Francis Lewis High School for her comments and guidance throughout the work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Atwood D., Paisley-Jones C. Pesticides Industry Sales and Usage 2008 – 2012 Market Estimates. U.S. Environ. Protect. Agency. 2017;3:9–19. [Google Scholar]
- 2.Jonsson C.M., Moura M.A.M., Ferracini V.L., Paraiba L.C., Assalin M.R., Quiroz S.C.N. Bioconcentrations of herbicides used in sugarcane crop in tilapia (Oreochromis niloticus) and the risk for human consumption. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim K.H., Kabir E., Jahan S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017;575:525–535. doi: 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Cornejo J., Nehring R., Osteen C., Wechsler S., Martin A., Vialou A. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960-2008. Econ. Inform. Bull. 2014;124:1–74. [Google Scholar]
- 5.Papadakis E.N., Vryzas Z., Kotopoulou A., Kintzikoglou K., Makris K.C., Papadopoulou-Mourkidou E. A pesticide monitoring survey in rivers and lakes of northen Greece and its human and ecotoxicological risk assessment. Ecotoxicol. Environ. Saf. 2015;116:1–9. doi: 10.1016/j.ecoenv.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Mercado S.A.S., Caleno J.D.Q. Determination of malathion’s toxic effect on Lens culinaris Medik cel cycle. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiss A., Virag D. Photostability and photodegradation pathways of distinctive pesticides. J. Environ. Qual. 2009;38:157–163. doi: 10.2134/jeq2007.0504. [DOI] [PubMed] [Google Scholar]
- 8.Benbrook C.M. Trends in glyphosate herbicide use in the United States and globally: supporting data. Environ. Sci. Eur. 2016;28:3. doi: 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S., Kumar V., Chauhan A. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018;16:211–237. [Google Scholar]
- 10.Kalkhoff S.J., Kolpin D.W., Thurman E.M., Ferrer I., Barcelo D. Degradation of chloroacetanilide herbicides: the prevalence of sulfonic and oxanilic acid metabolites in Iowa groundwaters and surface waters. Environ. Sci. Technol. 1998;32:1738–1740. [Google Scholar]
- 11.Gilliom R.J., Barbash J.E., Crawford C.G., Hamilton P.A., Martin J.D., Nakagaki N., Nowell L.H., Scott J.C., Stackelberg P.E., Thelin G.P., Wolock D.M. US Geological Survey; Washington, DC: 2006. The Quality of Our Nation’s Waters - Pesticides in the Nation’s Streams and Ground Water, 1992–2001, Circular 1291. [Google Scholar]
- 12.Stamper D.M., Tuovinen O.H. Biodegradation of the acetanilide herbicides alachlor, metolachlor, and propachlor. Crit. Rev. Microbiol. 1998;24:1–22. doi: 10.1080/10408419891294163. [DOI] [PubMed] [Google Scholar]
- 13.Lee E.A., Strahan A.P. Methods of analysis by the U.S. geological survey organic geochemistry research group – determination of acetamide herbicides and their degradation products in water using online solid-phase extraction and liquid chromatography/mass spectrometry. US Geol. Surv. Open-File Rep. 2003;2003:173. [Google Scholar]
- 14.Hladik M.L., Hsiao J.J., Roberts A.L. Are neutral chloroacetamide herbicide degradates of potential environmental concern? Anal. Occur. Upper Chesapeake Bay Environ. Sci. Technol. 2005;39:6561–6574. doi: 10.1021/es050268w. [DOI] [PubMed] [Google Scholar]
- 15.Aguera A., Fernandez-Alba A.R. GC-MS and LC-MS evaluation of pesticide degradation products generated through advanced oxidation processes: an overview. Analusis. 1998;26(6):123–130. [Google Scholar]
- 16.Mercurio P., Mueller J.F., Eaglesham G., O’Brien J., Flores F., Negri A.P. Degradation of herbicides in the tropical marine environment: influence of light and sediment. PloS One. 2016;11 doi: 10.1371/journal.pone.0165890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krutz L.J., Shaner D.L., Accinelli C., Zablotowicz R.M., Henry W.B. Atrazine dissipation in s-triazine-adapted and nonadapted soil from Colorado and Mississippi: implications of enhanced degradation on atrazine fate and transport parameters. J. Environ. Qual. 2008;37:848–857. doi: 10.2134/jeq2007.0448. [DOI] [PubMed] [Google Scholar]
- 18.Chan Y.C., Chan H.S., Wong P.K. Integrated photocatalytic-biological treatment of triazine-containing pollutants. Chemosphere. 2019;222:371–380. doi: 10.1016/j.chemosphere.2019.01.127. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Xu M., Yao G., Lai B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymononsulfate (PMS) process: kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018;348:1012–1024. [Google Scholar]
- 20.Chen Z.B., Chen Y., Vymazal J., Kule L., Kozeluh M. Dynamics of chloroacetanilide herbicides in various types of mesocosm wetlands. Sci. Total Environ. 2017;577:386–394. doi: 10.1016/j.scitotenv.2016.10.216. [DOI] [PubMed] [Google Scholar]
- 21.Ma X.Y., Zhang Y., Guan M.Y., Zhang W.D., Tian H.F., Jiang C.X., Tan X.X., Kang W.J. Genotoxicity of chloroacetamide herbicides and their metabolites in vitro and in vivo. Int. J. Mol. Med. 2021;47:103. doi: 10.3892/ijmm.2021.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coscolla C., Lopez A., Yahyaoui A., Colin P., Robin C., Poinsignon Q., Yusa V. Human exposure and risk assessment to airborne pesticides in a rural French community. Sci. Total Environ. 2017;584–585:856–868. doi: 10.1016/j.scitotenv.2017.01.132. [DOI] [PubMed] [Google Scholar]
- 23.Wan N., Lin G. Parkinson's disease and pesticides exposure: new findings from a comprehensive study in Nebraska, USA. J. Rural Health. 2016;32:303–313. doi: 10.1111/jrh.12154. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S., Mukerji K.G., Lal R. Molecular aspects of pesticide degradation by microorganisms. Crit. Rev. Microbiol. 1996;22:1–26. doi: 10.3109/10408419609106454. [DOI] [PubMed] [Google Scholar]
- 25.Fenner K., Canonica S., Wackett L.P., Elsner M. Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science. 2013;341:752–758. doi: 10.1126/science.1236281. [DOI] [PubMed] [Google Scholar]
- 26.Reddy P.V.L., Kim K. A review of photochemical approaches for the treatment of a wide range of pesticides. J. Hazard Mater. 2015;285:325–335. doi: 10.1016/j.jhazmat.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraj R., Megha P., Sreedev P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscipl. Toxicol. 2016;9:90–100. doi: 10.1515/intox-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matzrafi M. Climate change exacerbates pest damage through reduced pesticide efficacy. Pest Manag. Sci. 2019;75:9–13. doi: 10.1002/ps.5121. [DOI] [PubMed] [Google Scholar]
- 29.Arnosti C., Jorgensen B.B., Sagemann J., Thamdrup B. Temperature dependence of microbial degradation of organic matter in marine sediments: polysaccharide hydrolysis, oxygen consumption, and sulfate reduction. Mar. Ecol. Prog. Ser. 1998;165:59–70. [Google Scholar]
- 30.Almeida A.R., Andrade T.S., Burkina V., Fedorova G., Loureiro S., Soares A.M.V.M., Domingues I. Is UV radiation changing the toxicity of compounds to zebrafish embryos? Ecotoxicol. Environ. Saf. 2015;122:145–152. doi: 10.1016/j.ecoenv.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Moreira A.J., Pinheiro B.S., Araújo A.F., Freschi G.P.G. Evaluation of atrazine degradation applied to different energy systems. Environ. Sci. Pollut. Contr. Ser. 2016;23:18502–18511. doi: 10.1007/s11356-016-6831-x. [DOI] [PubMed] [Google Scholar]
- 32.Kolpin D.W., Thruman E.M., Linhart S.M. The environmental occurrence of herbicides: the importance of degradates in ground water. Arch. Environ. Contam. Toxicol. 1998;35:385–390. doi: 10.1007/s002449900392. [DOI] [PubMed] [Google Scholar]
- 33.Zhen J., Antonio T., Cheng S.Y., Ali S., Jones K.T., Reith M.E.A. Dopamine transporter oligomerization: impact of combining protomers with differential cocaine analog binding affinities. J. Neurochem. 2015;133:167–173. doi: 10.1111/jnc.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang A., Lim H., Cheng S.Y., Xie L. ANTENNA, a multi-rank, multi-layered recommender system for inferring reliable drug-gene-disease associations: repurposing diazoxide as a targeted anti-cancer therapy. IEEE ACM Trans. Comput. Biol. Bioinf. 2018;15:1960–1967. doi: 10.1109/TCBB.2018.2812189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Repetto G., del Peso A., Zurita J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 36.Mermana J., Sutthivaiyakit P., Blaise C., Gagne F., Charnsethikul S., Kidkhunthod P., Sutthivaiyakit S. Photocatalysis of S-metolachlor in aqueous suspension of magnetic cerium-doped mTiO2 core-shell under simulated solar light. Environ. Sci. Pollut. Contr. Ser. 2017;24:4077–4092. doi: 10.1007/s11356-016-8151-6. [DOI] [PubMed] [Google Scholar]
- 37.Cheng S.Y., Oh S., Velasco M., Ta C., Montalvo J., Calderone A. RTP801 regulate maneb- and mancozeb-induced cytotoxicity via NF-Κb. J. Biochem. Mol. Toxicol. 2014;28:302–311. doi: 10.1002/jbt.21566. [DOI] [PubMed] [Google Scholar]
- 38.Shoemaker J.A., Bassett M. U.S. Environmental Protection Agency; Washington, DC: 2005. Method 535: Measurement of Chloroacetanilide and Chloroactamide Herbicide Degradates in Drinking Water by Solid Phase Extraction and Liquid Chromatography/tandem Mass Spectrometry (LC/MS/MS) [Google Scholar]
- 39.Toxnet Toxicology data network. https://toxnet.nlm.nih.gov/ Retrieved on December 8, 2018 from:
- 40.Jin Y.X., Wang L.G., Chen G.L., Lin X.J., Miao W.Y., Fu Z.W. Exposure of mice to atrazine and its metabolite diaminochlorotriazine elicits oxidative stress and endocrine disruption. Environ. Toxicol. Pharmacol. 2014;37:782–790. doi: 10.1016/j.etap.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Abarikwu S.O., Farombi E.O. Atrazine induced apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway. Pestic. Biochem. Physiol. 2014;118:90–98. doi: 10.1016/j.pestbp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Kale V.M., Miranda S.R., Wilbanks M.S., Meyer S.A. Comparative cytotoxicity of alachlor, acetochlor and metolachlor herbicides in isolated rat and cryopreserved human hepatocytes. J. Biochem. Mol. Toxicol. 2013;22:41–50. doi: 10.1002/jbt.20213. [DOI] [PubMed] [Google Scholar]
- 43.Graham W.H., Graham D.W., deNoyelles F., Smith V.H., Larive C.K., Thruman E.M. Metolachlor and alachlor breakdown product formation patterns in aquatic field mesocosms. Environ. Sci. Technol. 1999;33:4471–4476. [Google Scholar]
- 44.Fan A.M., Alexeeff G.V. CA EPA (Office of Environmental Health Hazard Assessment, California Environmental Protection Agency) oehha; 1999. Public Health Goal for Atrazine in Drinking Water, OEHHA.ca.gov/media/downloads/water/chemicals/phg/atrazf.pdf [Google Scholar]
- 45.Kawabata K., Sugihara K., Sanoh S., Kitamura S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J. Toxicol. Sci. 2013;38:215–223. doi: 10.2131/jts.38.215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.