Abstract
The bacterial genotoxin colibactin interferes with the eukaryotic cell cycle by causing dsDNA breaks. It has been linked to bacterially induced colorectal cancer in humans. Colibactin is encoded by a 54 kb genomic region in Enterobacteriaceae. The colibactin genes commonly co-occur with the yersiniabactin biosynthetic determinant. Investigating the prevalence and sequence diversity of the colibactin determinant and its linkage to the yersiniabactin operon in prokaryotic genomes, we discovered mainly species-specific lineages of the colibactin determinant and classified three main structural settings of the colibactin–yersiniabactin genomic region in Enterobacteriaceae. The colibactin gene cluster has a similar but not identical evolutionary track to that of the yersiniabactin operon. Both determinants could have been acquired on several occasions and/or exchanged independently between enterobacteria by horizontal gene transfer. Integrative and conjugative elements play(ed) a central role in the evolution and structural diversity of the colibactin–yersiniabactin genomic region. Addition of an activating and regulating module (clbAR) to the biosynthesis and transport module (clbB-S) represents the most recent step in the evolution of the colibactin determinant. In a first attempt to correlate colibactin expression with individual lineages of colibactin determinants and different bacterial genetic backgrounds, we compared colibactin expression of selected enterobacterial isolates in vitro. Colibactin production in the tested Klebsiella species and Citrobacter koseri strains was more homogeneous and generally higher than that in most of the Escherichia coli isolates studied. Our results improve the understanding of the diversity of colibactin determinants and its expression level, and may contribute to risk assessment of colibactin-producing enterobacteria.
Keywords: high pathogenicity island, secondary metabolite, polyketide, cytopathic effect, Escherichia coli, Klebsiella, Citrobacter
Data Summary
DNA sequences are publicly available from the SRA and Assembly databases at the National Center for Biotechnology Information (NCBI). The corresponding accession numbers are listed in Table S2 (available in the online version of this article).
Impact Statement.
Colibactin can act as a bacterial genotoxin and thus promote colorectal cancer development. Little is known about the origin, diversity and prevalence of the colibactin genes (clb) within prokaryotes. The clb genes are closely associated with pathogenicity islands or integrative and conjugative elements (ICEs). We screened roughly 375000 prokaryotic genomes to analyse the diversity and evolution of such mobile genetic elements among bacterial populations. Interestingly, we only detected clb genes in subgroups of the order Enterobacterales, namely the Enterobacter–Escherichia, Yersinia–Serratia and Erwinia–Pantoea clades, mainly in Escherichia coli, Klebsiella species and Citrobacter koseri. The clb determinant, together with the yersiniabactin (ybt) gene cluster, belong to an ICE in most of the clb-positive enterobacteria, especially in Klebsiella. We show that both determinants, though in principle freely transferable within bacteria, have a mainly species-specific phylogeny, and that colibactin expression levels were species-independent. Recombination promoted the structural diversification of the ICE in different species, including its successive degeneration that led to the establishment of the colibactin and yersiniabactin islands in Escherichia coli phylogroup B2 strains. Our results not only illustrate differing evolutionary tracks of the clb and ybt determinants in different species of the Enterobacterales, but also highlight the importance of ICEs for genomic variability and the evolution of archetypal pathogenicity islands.
Introduction
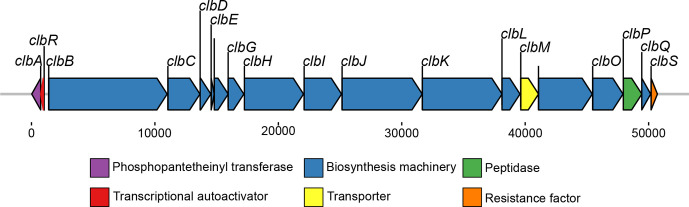
The non-ribosomal peptide/polyketide hybrid colibactin is a secondary metabolite found in a variety of bacterial species of the family Enterobacteriaceae. The colibactin biosynthetic machinery is encoded by a 54 kb polyketide synthase (pks) or clb genomic island [1], which includes 19 genes. The largest part of the island consists of a section of overlapping or closely spaced genes: clbB to clbL and clbN to clbQ, which are aligned on the same strand and code for components of the biosynthesis complex. The colibactin assembly line is supplemented with a dedicated transporter, encoded by clbM, and a resistance-conferring protein encoded by clbS [2, 3]. Two additional genes required for colibactin production are located ca. 400 bp upstream of the first biosynthesis gene clbB in the opposing reading direction: the clbR gene coding for an auto-activating, pks island-specific transcription factor and the phosphopantetheinyl transferase-encoding gene clbA, which is crucial for activation of polyketide biosynthesis complexes (Fig. 1) [4–6]. Between these two divergent transcription units, there is a ‘variable number of tandem repeat’ (VNTR) region, which comprises a varying number of a repeating octanucleotide sequence (5′-ACAGATAC-3′) depending on the isolate [2].
Fig. 1.
Schematic representation of the genomic architecture of the pks island (ca. 54 kb) present in Escherichia coli strain M1/5. The 19 genes within the island are coloured with respect to their function. The island codes for a phosphopantetheinyl transferase (clbA, in purple), a transcriptional autoactivator (clbR, in red), multiple core biosynthetic genes (clbB–clbL, clbN, clbO and clbQ in blue), a transporter (clbM, yellow), a peptidase (clbP, in green) and a resistance factor (clbS, orange).
Recently, the structure of the colibactin molecule has been proposed [3, 7–9]. Yet, the biological role of colibactin is still under discussion. Colibactin can interfere with the progression of the eukaryotic cell cycle, presumably by cross-linking DNA resulting in dsDNA breaks in genomic instability in eukaryotes [1, 10, 11]. The ability to produce colibactin has been described to increase the pathogenic potential of the producing bacteria and to promote colorectal cancer development [12–17], but has also been related to beneficial effects to the host [3, 18–20].
Initially, the pks island has been described in extraintestinal pathogenic Escherichia coli to be chromosomally inserted into the asnW tRNA locus in close proximity to another tRNA(Asn) gene-associated pathogenicity island, the so-called ‘high pathogenicity island’ (HPI). The HPI harbours an additional polyketide determinant coding for the metallophore yersiniabactin biosynthetic machinery [1, 21]. As members of the Enterobacteriaceae are generally not known as archetypal secondary metabolite producers, the origin of the pks island remains to be further investigated. Interestingly, the colibactin determinant has also been detected as part of an ‘integrative and conjugative element’ (ICE) in different enterobacteria. This ICE also integrates near a tRNA(Asn) locus into the bacterial chromosome and commonly carries the yersiniabactin gene cluster [2, 22]. It has been suggested that the close linkage observed between the colibactin and yersiniabactin gene clusters results from the functional interconnection between the colibactin and yersiniabactin biosynthetic pathways via the phosphopantetheinyl transferase ClbA, which can also contribute to the biosynthesis of yersiniabactin [5, 23]. The highly conserved colibactin determinant has so far been detected in a spectrum of strains belonging to the order Enterobacteriales: most commonly among members of the family Enterobacteriaceae, including Escherichia coli strains of phylogroup B2, followed by Klebsiella pneumoniae isolates, but also in Citrobacter koseri and Klebsiella aerogenes [2, 18, 24]. Less conserved variants or homologues of the colibactin gene cluster have been described phenotypically or based on nucleotide sequence data in another member of the Enterobacterales, an Erwinia oleae strain, but also the honey bee symbiont Frischella perrara, and the marine alphaproteobacterium Pseudovibrio [24, 25].
Due to the low sequence similarity of the colibactin genes present in Enterobacterales and the two homologous polyketide determinants in F. perrara and Pseudovibrio, and because of their association with mobile genetic elements (MGEs) or at least mobility-associated genes, one can hypothesize that the clb gene cluster is spread by horizontal gene transfer, perhaps via an ICE-like element [2, 24]. While in most Enterobacteriaceae the colibactin determinant is typically associated with an ICE, the characteristic mobility and transfer features of an ICE are absent in the sequence context of the pks island in Escherichia coli phylogroup B2 strains. Nevertheless, the pks island in Escherichia coli remains mobilizable and transferable through external factors, supporting the hypothesis that former MGEs can undergo a stabilization (homing) process upon their chromosomal integration [26–28].
Studies addressing the prevalence of the colibactin genes have so far mainly focused on Klebsiella species or Escherichia coli backgrounds. The scarcity of data in other prokaryotic species regarding its distribution and the structure of the associated MGE makes it challenging to reliably further characterize the transmission and evolution of this polyketide determinant [18]. Previous data show that the prevalence varied from 5.3 to 25.6 % in Klebsiella and from 9.5 to 58 % in Escherichia, highlighting an enrichment of the pks island in specific ecological niches, whereas studies with a broader screening approach resulted in a prevalence of 14 % in Klebsiella and 9.5 % in Escherichia isolates, respectively [2, 29–34]. Notably, in health-related studies, a higher association of the clb genes was observed amongst strains with an increased virulence potential, with a prevalence of as much as 78.8 % for Klebsiella subgroups and 72.7 % for colorectal cancer-associated Escherichia coli isolates [29, 32, 35–37]. The colibactin genes are frequently found in hyper-virulent and multidrug-resistant K. pneumoniae isolates [38, 39].
The obvious prevalence of the colibactin gene cluster in specific enterobacterial species combined with the description of more distantly related homologous determinants has sparked our interest in a better understanding of the spread and evolution of the colibactin determinant and its genetic context in bacteria. Therefore, we aimed to investigate the prevalence and diversity of the colibactin determinant also in isolates outside of the family Enterobacteriaceae. Furthermore, we compared colibactin expression levels among selected Escherichia coli, Klebsiella species and C. koseri isolates carrying different lineages of clb determinants as a first attempt to assess the functional context of the bacterial genetic background, pathogenicity and colibactin expression.
Methods
Bacterial strains and media
For cultivation, bacteria were grown as batch cultures in lysogeny broth (LB) (per litre: 10 g tryptone, 5 g yeast extract, 5 g NaCl) at 37 °C. Strains used in this study are listed in the (Table S1) .
DNA extraction and sequencing
DNA extraction of the enterobacterial strains was performed using the MagAttract HMW DNA Kit (Qiagen) according to the manufacturer’s recommendations. To prepare paired-end libraries we used the Nextera XT DNA Library Preparation kit (Illumina). Libraries were sequenced on the Illumina MiSeq sequencing platform using v2 sequencing chemistry (500 cycles) or on the Illumina NextSeq500 system using v2.5 chemistry (300 cycles). Accession numbers of in-house sequences submitted to the NCBI GenBank database are included in (Table S1) .
Genome selection and phylogenetic analysis
All genome sequences not generated in this study were obtained from publicly available prokaryotic genomes (NCBI GenBank). The quality of in-house sequenced genomes was checked with FastQC v0.11.5 (https://github.com/chgibb/FastQC0.11.5/blob/master/fastqc), and low-quality reads were trimmed using Sickle v1.33 (https://github.com/najoshi/sickle). The processed reads were de novo assembled with SPAdes v3.13.1 [40] and annotated with prokka v1.12 [41]. The genomes were screened for the presence of >45 kb of the complete pks genomic island using standalone BLAST +v2.8.1 [42] and antiSMASH v5.0.0 [43]. The pks island found in the genome of the Escherichia coli strain M1/5 (accession no. CP053296) was used as a reference sequence. The VNTR and the sequence stretch of the pks island that spans between clbJ and clbK were excluded from analysis as these regions are prone to misassembly.
The contigs that align to the colibactin genes were ordered using ABACAS v1.3.1 [44] and multiple sequence alignment was generated using Kalign v3.1.1 [45]. Recombinant regions were detected and removed using Gubbins v2.4.1 [46]. The recombination filtered polymorphisms were then used to generate a maximum-likelihood phylogeny of the colibactin determinant using RAxML v8.2.11 [47] under the GTR-GAMMAX model from 9974 polymorphic sites. The branch support of the maximum-likelihood tree was estimated by bootstrap analysis of 200 replicate trees. The homologous gene cluster found in F. perrara was used as an outgroup. The phylogeny of the corresponding ybt islands was generated with a similar approach. Additionally, a core genome-based phylogeny of all strains that harboured the clb gene cluster was inferred using the approach described by Adeolu and colleagues [48]. The generated trees were visualized using itoL (https://itol.embl.de).
Phylo-grouping of pks-positive strains
The Escherichia coli and Klebsiella strains that harboured the colibactin gene cluster were allocated to their corresponding sequence types using mlst v2.16.1 (https://github.com/tseemann/mlst), which detects sequence types using the PubMLST typing schemes. The Escherichia strains were further classified into their phylogenetic lineages using the standalone tool, EzClermont v0.4.5 (https://github.com/nickp60/EzClermont). The phylogeny of all the clb-positive strains was reconstructed using the whole genome sequence-based approach published by Adeolu and co-workers [48].
Analysis of the diversity of the colibactin and yersiniabactin gene clusters involved virulence gene multi-locus sequence typing (MLST) for both polyketide determinants as previously described [38]. Briefly, the allele sequences of 16 genes of the colibactin gene cluster (clbACDEFGHILMNOPQR) as well as of 11 genes of the yersiniabactin determinant (fyuA, ybtE, ybtT, ybtU, irp1, irp2, ybtA, ybtP, ybtQ, ybtX, ybtS) were extracted from the individual genomes and analysed for allelic variations. Each observed combination of alleles was assigned a unique colibactin sequence type (CbST, listed in Table S5) or yersiniabactin sequence type (YbST, listed in Table S6).
Variable number tandem repeat detection
The VNTR copy number present within the colibactin determinant (upstream of clbR) was detected using the standalone version of tandem repeats finder v4.09 [49]. The VNTR copy number distribution was visualized using R v3.4.3 (https://www.r-project.org/index.html).
Detection of Escherichia coli virulence markers for pathotyping
For pathotyping, the clb-positive Escherichia coli strains were in silico screened for the presence of different E. coli pathotype marker genes using blast +v2.8.1 (Table S1). These genes were used as markers for Escherichia coli pathotypes: enteroaggregative Escherichia coli (EAEC), enterohaemorrhagic Escherichia coli (EHEC), enteropathogenic Escherichia coli (EPEC), enterotoxigenic Escherichia coli (ETEC), diffusely adhering Escherichia coli (DAEC), uropathogenic Escherichia coli (UPEC) and newborn meningitis-causing Escherichia coli (NMEC).
Quantification of colibactin expression through N-myristoyl-d-asparagine
Following an approach described by Bian and colleagues [50], a collection of colibactin-producing strains of the main species harbouring the colibactin determinant was characterized for their ability to produce colibactin under in vitro growth conditions. For this purpose, we quantified N-myristoyl-d-asparagine (N-Myr-d-asparagine) a byproduct during colibactin maturation. The amount of this intermediate extrapolates the resulting colibactin amount produced. After growing the bacteria for 24 h at 37 °C in glass tubes in 5 ml LB supplemented with 200 µl of a water/XAD-16-resin slurry, bacterial cells were harvested by centrifugation. The pelleted bacteria-slurry mixes were sedimented, filtered and dissolved three times in acetone with increasing volume (12 ml, 100 ml and finally 200 ml). Afterward, the solvent was exchanged by rotary evaporation and replaced by 1.6 ml methanol. The sample was further concentrated by centrifugation (10 min, 15000 r.p.m. at 4 °C), followed by drying 1,5 ml of the solution in a vacuum centrifuge and subsequent resuspension in 50 µl methanol. Then, 30 µl of these processed samples was measured by ultra performance liquid chromatography coupled to high-resolution mass spectrometry (UPLC-HRMS) conducted on a Thermo Scientific Ultimate 3000 RS with a Waters Acquity BEH 100×2.1 mm 1.7 µm 130A column (eluent A: 0.1 % formic acid in ddH2O, eluent B: 0.1 % formic acid in acetonitrile), where a flow rate of 0.6 ml min−1 followed by a Bruker Maxis II-4G, 150–2500 m/z and a scan rate of 2 Hz was applied. To enable quantification of N-Myr-d-asparagine, we used 250 mM cinnarizine as an internal standard and normalized peak areas based on the internal standard and the optical density (OD600) of the bacterial culture.
Results
Prevalence of colibactin determinant
Of the 374 754 publicly accessible prokaryotic genomes (as of 30 June 2019) that were screened for the presence of the colibactin gene cluster, 1969 genomes carried this polyketide-encoding operon. An additional 198 clb-positive enterobacterial genomes determined in-house were added to the analysis (Tables 1, S2 and S3). The clb gene cluster was detected in several species of the Enterobacter–Escherichia clade of Enterobacterales, most frequently in Escherichia coli and K. pneumoniae isolates, but also to a lesser extent in K. aerogenes, C. koseri, Erwinia cloacae, Enterobacter hormaechei, K. michiganensis as well as in members of the Yersinia–Serratia clade (Serratia marcescens), and the Erwinia–Pantoea clade (Erwinia oleae). The colibactin determinant was, however, not detectable in 112 546 Salmonella enterica and 41 Salmonella bongori genomes, but in one out of eight genomes of unspecified Salmonella isolates. We did not detect the clb genes in 2634 Shigella species, 861 Yersinia species, 677 Serratia species, 186 Proteus species and 69 Morganella species genomes (Table S4). A less well-conserved homologue of the colibactin determinant was detected in three F. perrara genomes. It should be noted that the number of genomes of Klebsiella species and Escherichia coli analysed in this study are markedly higher than those of the other species and lineages due to the sequencing bias towards Klebsiella species and Escherichia coli strains. Accordingly, a reliable statement on the prevalence of the colibactin determinant in the different species cannot be made.
Table 1.
Bacterial species testing positive for the presence of the colibactin determinant
|
No. of strains screened |
clb-positive strains |
Predominant sequence types |
|
|---|---|---|---|
|
19 200 |
1462 |
ST12, ST127, ST141, ST2015, ST372, ST404, ST550, ST625, ST73, ST80, ST95, ST998 |
|
|
8038 |
572 |
ST11, ST23, ST234, ST258, ST3, ST48 |
|
|
Klebsiella aerogenes |
222 |
101 |
ST4, ST93 |
|
35 |
27 |
na |
|
|
711 |
2 |
na |
|
|
610 |
2 |
na |
|
|
515 |
1 |
na |
|
|
94 |
1 |
na |
|
|
Salmonella sp. |
8 |
1* |
na |
|
1 |
1 |
na |
|
|
3 |
3 |
na |
*Unverified source organism (excluded from Refseq).
Diversity of the colibactin determinant
To determine whether the prevalence of the colibactin gene cluster is restricted to specific phylogenetic lineages of Escherichia coli and Klebsiella species, the sequence types of the corresponding Escherichia coli and Klebsiella species isolates were further analysed. As shown in (Fig. S1), the clb gene cluster was enriched in a small subset of Escherichia coli STs (12 out of 11 537 STs, as of 30 October 2020), K. aerogenes STs (two out of 214 STs, as of 30 October 2020), and K. pneumoniae STs (six out of 5237 STs, as of 30 October 2020), respectively. In these 12 Escherichia coli STs, between 58 and 94 % of the allocated isolates carry the clb determinant. A high percentage (ca. 96 %) of the K. aerogenes ST4 and ST93 included in our study harboured the colibactin genes. In the tested K. pneumoniae strains, all ST3 isolates were clb-positive, and more than 75 % of the analysed ST23 and ST234 isolates carried the colibactin gene cluster, whereas this was only the case for a significantly lower percentage of the K. pneumoniae isolates allocated to ST11, ST258 and ST48. Table S2 contains a complete list of STs to which colibactin-positive Escherichia coli and Klebsiella isolates have been assigned.
The nucleotide sequences of the clb gene cluster extracted from the 2169 strains (Table S2) were used to generate a recombination-free phylogeny of the colibactin determinant as shown in Fig. 2a (see Figs S2 and S3 for branch support values and strain labels/assembly IDs, and Fig. S4 indicates predicted recombination events in the clb gene cluster). Serratia marcescens strain MSU97 isolated from a plant source, Erwinia oleae strain DAPP-PG531 isolated from olive tree knot, Klebsiella michiganensis strain NCTC10261 of an unknown source, and the Escherichia coli phylogroup E strain 14696–7 isolated from the pericardial sac of a white-tailed deer (Odocoileus virginianus) harbour the most genetically distant variants of the colibactin determinant (Fig. 2a). Within the Enterobacteriaceae, a large group of clb gene clusters can be defined, which is dominated by two highly conserved clades present in Escherichia coli phylogenetic lineage B2 and different Klebsiella species isolates, respectively. The colibactin determinants detected in Erwinia cloacae and Erwinia hormaechei belong to the Klebsiella clades of clb loci, whereas the clb gene clusters found in C. koseri and in an unspecified Salmonella isolate represent an independent clade, i.e. clb6 (Fig. 3). In a few other Escherichia coli and Klebsiella species genomes, the clb determinant can be distinguished from the two major conserved clades of colibactin determinants observed in Klebsiella or Escherichia coli. An even more divergent group comprises the clb gene clusters of mainly Escherichia coli phylogroup A, B1 and a few B2 isolates, but also of some K. pneumoniae strains (Fig. 3, belonging to clb clades clb1 and clb2).
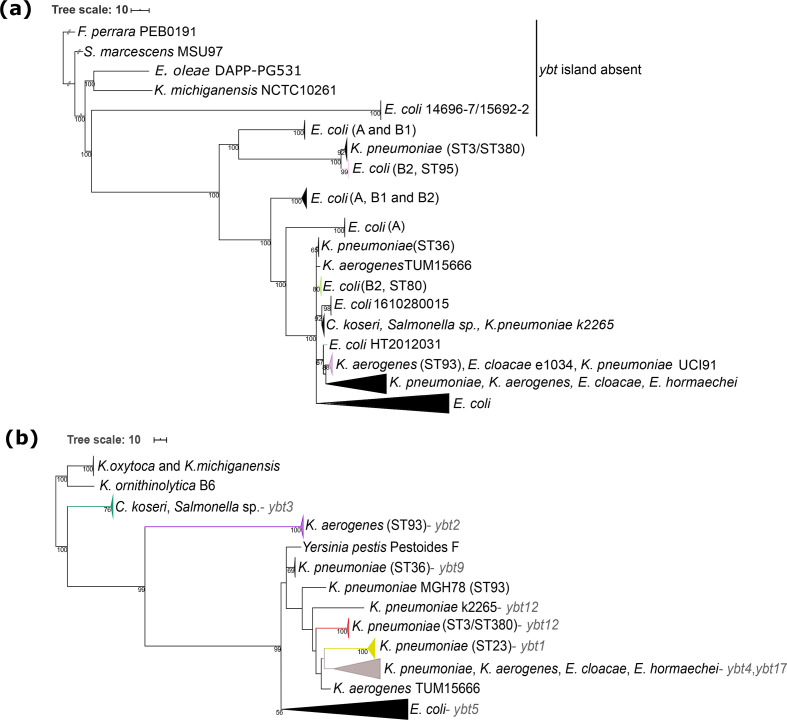
Fig. 2.
Maximum-likelihood-based phylogenetic analysis of the colibactin and yersiniabactin determinants. (a) Phylogenetic tree of the colibactin gene cluster (collapsed), and (b) phylogenetic tree of the corresponding ybt determinants (collapsed) using the genetically distant K. michiganensis strains as an outgroup [38]. Additionally, the yersiniabactin sequence type (YbST) as defined by Lam and colleagues [38] associated with individual bacterial clades is indicated. The branch colours in both trees depict the prominent bacterial sequence type of the clade.
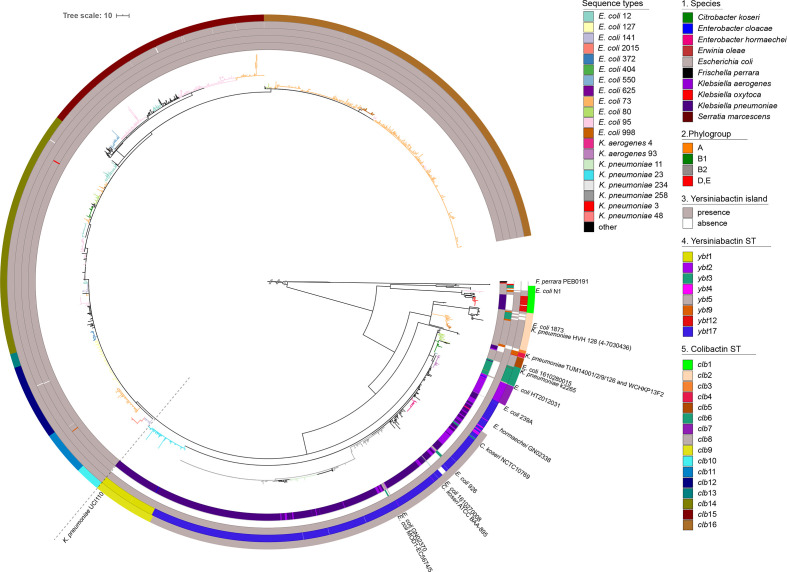
Fig. 3.
Maximum-likelihood-based phylogeny of the colibactin gene cluster detected in 2169 enterobacterial genomes. Every leaf represents a single sequence variant of the clb gene cluster, which can be allocated to different lineages and clades. From innermost to outermost, the first circle indicates the species harbouring the clb determinant; the second circle shows the Escherichia coli phylogroup, the third circle shows the presence/absence of the ybt operon; the fourth circle shows the yersiniabactin sequence types (YbST) of the ybt determinant (from Fig. 1b) that correspond to the pks island lineage present in the individual genome. The fifth circle shows the different colibactin sequence types (CbST) of the clb gene cluster. The branch colours in the centre of the tree depict the prominent bacterial sequence types (Fig. 1). The large conserved Escherichia coli phylogroup B2 clade is separated from the large Klebsiella clade with a faint broken line.
Although the clb gene clusters found in the large clade of Escherichia coli B2 strains are highly conserved, individual ST-specific lineages, such as clb10 (from Escherichia coli ST141 and ST2015 strains) and clb12 (from Escherichia coli ST121 strains) can still be described within this clade. Beyond that, we also observed multiple lineages per sequence type, such as clb2, clb11, clb15 and clb16 found in Escherichia coli strains of ST73 (Fig. 3). Three lineages of the clb locus were predominantly detectable in Klebsiella pneumoniae strains. They belong to the most distant Klebsiella ST3/ST380 clade (clb1), the remaining large and diverse ST258/ST11 clade (clb8), and finally the hypervirulent ST23 clade (clb9) (Fig. 3). A phylogeny of the colibactin gene cluster inferred from concatenated amino acid sequences of the 17 clb genes (Fig. S10) was very similar to the aforementioned recombination-free nucleotide-based phylogeny (Fig. 3).
Diversity of the yersiniabactin determinant in colibactin-positive bacteria
The majority (>98 %) of the clb-positive enterobacterial strains also harboured the yersiniabactin genes (ybt) (Fig. 3, third circle). The Escherichia coli strains of phylogroup A, B1 and E as well as the tested K. michiganensis and Erwinia oleae strains carrying the most genetically distant lineages of the colibactin gene cluster, together with the F. perrara strains used as an outgroup, are ybt-negative (Fig. 2b). There were no strains that carried multiple copies of the colibactin gene cluster; however, two well-separated copies of the ybt determinant were found in C. koseri strains ATCC BAA-895 and 0123A_53_520. It should be noted that the latter strain is derived from a metagenome. The phylogenetic analysis indicated that all ybt operons from Escherichia coli clustered together (Fig. 2b). Alike the clb gene cluster, also the ybt locus of the Escherichia coli phylogroup B2 strains was highly conserved. In contrast, the sequence comparison of the ybt determinants of Klebsiella species resulted in different lineages, which correlate with lineages ybt1, 12 and 17 (ICEKp10), ybt9 (ICEKp3), and ybt4 (originating from a plasmid) previously described by Lam and colleagues [38].
Congruent phylogeny of the colibactin and yersiniabactin determinants
The strong coexistence of the colibactin and yersiniabactin determinants on the one hand and the description of different evolutionary lines of clb and ybt determinants in different enterobacterial species on the other hand led us to analyse whether both gene clusters can predominantly be transferred individually or together. Our results indicate that the clades of the evolutionary lineages of the clb and ybt loci are chiefly species-/genus-specific. The phylogeny of clb and ybt determinants is largely congruent, with the ybt gene cluster being, even more, species-/genus-specific than that of the clb gene cluster (Fig. S5). However, in some strains we observed evidence of interspecies transfer of these genes: the clb and corresponding ybt determinants of the C. koseri isolates NCTC10769, ATCC BAA-895 and Escherichia coli strains 239A, 926, GN02370 and MOD1-EC5674/5 were allocated to the large Klebsiella-dominated lineage clb8 (Fig. 3). Additionally, the clb gene cluster of K. pneumoniae strain k2265 was found within lineage clb6, which predominantly represents C. koseri isolates. However, the ybt determinant of K. pneumoniae strain k2265 belonged to the ybt12 lineage represented by K. oxytoca isolates. Similarly, the ybt determinant of the aforementioned strains E. coli GN02370 and C. koseri ATCC BAA-895 belonged to lineage ybt4 (plasmid originating ybt loci) instead of ybt17. Regardless of the aforementioned exceptions, clades of the clb gene cluster usually correlated with the corresponding clade of ybt genes (Fig. 3, third and fourth circle).
Genetic structure of the MGEs harbouring the colibactin determinant
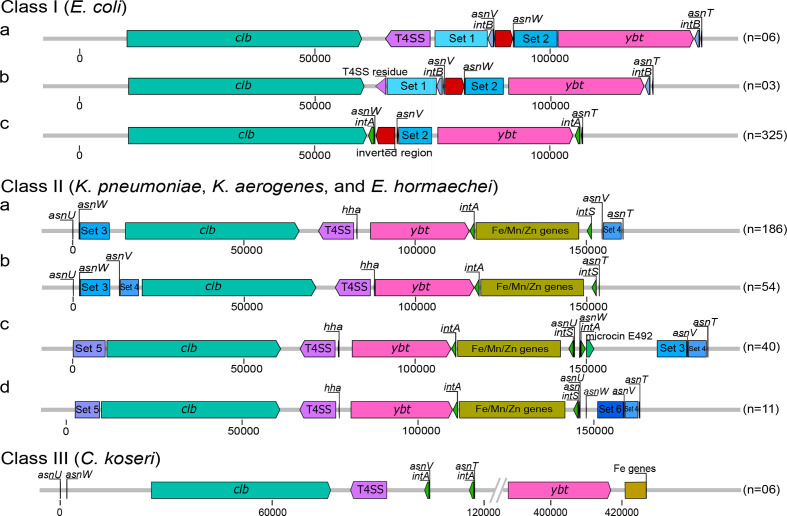
To further investigate whether the colibactin and yersiniabactin determinants are jointly distributed by horizontal gene transfer and to obtain clues to the underlying mechanism, we compared the chromosomal context of the two polyketide biosynthesis gene clusters and the genetic structure of associated MGEs. We observed species-specific structural differences of the chromosomal regions harbouring the clb, and ybt gene clusters (Figs 4 and S6). In Escherichia coli phylogroup B2 strains, the clb and ybt determinants are present as part of two individual pathogenicity islands (PAIs) with their cognate integrase and different tRNA genes (class I of clb-harbouring MGE). Both PAIs are located neighbouring each other in the chromosome. Within class Ia, the two PAIs are separated by a type 4 secretion system (T4SS)-encoding operon (virB) and a region that includes two conserved gene sets (Set 1 and Set 2), different tRNA(Asn) loci and an integrase gene (see Table S3 for genes present in the different conserved gene sets). This region is shown to have been diminished in class Ib structural variants, where only gene virB1 of the T4SS determinant is left alongside the conserved region. In the predominant Escherichia coli structural variant, class Ic, the complete T4SS operon (including virB1) has been lost together with gene set 1, the integrase gene was exchanged and a DNA stretch comprising the genes yeeO, tRNA(Asn), cbl and gltC was inverted (shown in red, Fig. 4). The region separating the pks island and the HPI was reduced from a 40 kb (in class Ia) to a 15 kb stretch in class Ic. In contrast, within all Klebsiella strains, the clb and ybt gene clusters are part of an ICE, and are separated by a T4SS-encoding operon (virB) and the hha gene coding for the haemolysin expression-modulating protein. Downstream of the ybt gene cluster an integrase gene is located followed by a set of genes involved in Fe/Mn/Zn metabolism (structural class II of clb- and ybt-harbouring chromosomal regions) (Fig. 4). Interestingly, one type of such ICEs is located next to genes necessary for microcin E492 biosynthesis (class IIc, Fig. 4). Enterobacter hormaechei strains harbour a structurally similar ICE to that of Klebsiella strains. In most C. koseri strains, however, the two polyketide determinants are separated by a large 250 kb chromosomal region. The T4SS-related genes are closely positioned to the clb genes while the gene set involved in Fe/Mn/Zn metabolism is located downstream of the ybt determinant. Only a minor fraction of enterobacterial isolates analysed displayed some variation regarding gene content and synteny of these three main classes of colibactin and yersiniabactin-encoding chromosomal regions. The structure of clb and ybt regions that do not conform to these major classes are as shown in Fig. S7. Instead of class I, several Escherichia coli strains carried class II-like chromosomal regions where the T4SS and the Fe/Mn/Zn metabolism genes were present. In Escherichia coli strain HVH128 none of the three main classes of colibactin- and yersiniabactin-encoding regions could be identified. Although both polyketide determinants are co-localized with one integrase gene each, they are widely separated on this strain’s chromosome. K. pneumoniae strains TUM14001, TUM14002, TUM14009, TUM14126 and WCHKP13F2 harboured two T4SS-encoding gene clusters in close proximity of the clb and ybt gene clusters and lacked the Fe/Mn/Zn genes, whereas K. pneumoniae strain UCI110 was also missing the Fe/Mn/Zn metabolism-related genes. In contrast to the other C. koseri isolates, we detected a class II- instead of a class III-type clb-ybt region in C. koseri isolate BAA-895.
Fig. 4.
Structural variation of the colibactin and yersiniabactin-encoding chromosomal region in Escherichia coli, Enterobacter hormaechei, K. pneumoniae, K. aerogenes and C. koseri. The different genetic structures and chromosomal insertion sites of the colibactin and/or yersiniabactin determinants found within the three main structural classes are shown. The clb gene cluster (teal green), T4SS module (purple), ybt gene cluster (pink), integrase genes (green), the conserved sets of genes (Table S5) that are present up/downstream of the two polyketide determinants, classed into sets (blue boxes), and the Fe/Mn/Zn module (yellow) are shown. The number of genomes included in the tested set of genomes that harbour the different structural variants is indicated in parentheses. The colibactin–yersiniabactin chromosomal regions that do not conform to these major structures are as shown in Fig. S7.
Organization of the colibactin gene cluster
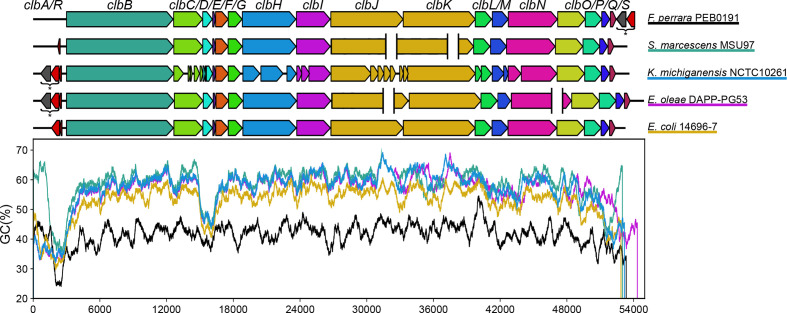
The clb gene cluster is composed of 19 genes, which are required for the regulation, biosynthesis and transport of colibactin. The origin of this gene cluster is unclear. We therefore compared the structure of the gene clusters representing the homologous clb locus found in F. perrara and the phylogenetically most distant and potentially older clb determinants relative to the Echerichia coli B2 type of the clb determinant (Fig. 2a), which are present in Serratia marcescens, Erwinia oleae, K. michiganensis and Escherichia coli phylogroup E strain 14696-7 (Fig. 5). The various clb determinants correspond in terms of the structure of the genes coding for the biosynthesis machinery, transport and resistance to colibactin (clbB-clbS) and resemble the structure of the well-described clb locus in K. pneumoniae/K. aerogenes/Escherichia coli B2 strains. The individual clb determinants differ more clearly in the presence and localization of the genes involved in the regulation and activation of colibactin biosynthesis (clbR and clbA). These two genes are absent in the homologous gene cluster found in F. perrara and in the clb determinant in Serratia marcescens. However, in F. perrara, a phosphopantetheinyl transferase coding for a homologue of ClbA (43 % amino acid similarity) and a radical S'-adenosylmethionine (SAM) enzyme-encoding gene are found directly downstream of clbS. In Serratia marcescens, a helix–turn–helix (HTH)-type regulatory protein homologous to clbR is encoded by a gene located upstream of clbB (78 % amino acid similarity). In Erwinia oleae and K. michiganensis, a SAM enzyme-encoding gene is present directly downstream of clbA. Although the colibactin gene clusters in Erwinia oleae and K. michiganensis have a high nucleotide sequence similarity of ca. 99.77 %, the predicted coding regions of clbC/D and clbH/I/J/K/L/M/N are noticeably different due to multiple frameshift deletions in K. michiganensis. The structure of the clb locus found in phylogroup E Escherichia coli strain 14696-7 already corresponds to the structure of Escherichia coli strains of phylogroup B2 (Figs 1 and 5), yet this gene cluster shows the least sequence similarity of all tested clb gene clusters in non-B2 Escherichia coli isolates (Fig. 2a) to the determinant occurring in Escherichia coli strains of phylogroup B2.
Fig. 5.
The structural organization and GC profiles of the clb determinants in the five most genetically distant bacterial strains (according to Fig. 2a). The genes that make up the homologous pks gene cluster found in F. perrara and the most distant clb determinants present in Serratia marcescens, Erwinia oleae, K. michiganensis and Escherichia coli (phylogroup E) strain 14696-7 are depicted. The GC profile of the gene cluster in the different strains is shown alongside with the colours underlining the different species. SAM genes and clbA homologues (*) are shown downstream of the pks gene cluster in F. perrara and upstream of the clb determinant in K. michiganensis and Erwinia oleae. The gaps in assembly are shown with white spaces.
Looking at the G+C plot of the colibactin gene clusters, it is clear that all investigated enterobacterial clb gene clusters show a very similar G+C plot, which has a significantly higher average G+C content and differs significantly from that of the clb homologous gene cluster in F. perrara (Fig. 5). The G+C content profile of these gene clusters indicates that there are two regions of low G+C content in the enterobacterial clb determinants: the region including clbA and clbR (at position ca. 1500–3000 bp of the colibactin gene cluster) and the region spanning clbD and clbE (at position ca. 15000–16500 bp of the clb gene cluster). The G+C content drop in the region including clbA and clbR (at position ca. 1500–3000 bp of the colibactin gene cluster) is associated with a predicted recombination site, which is located upstream of or interrupting clbB (Fig. S4).
The comparison of structural features of the clb gene cluster also included the VNTR region located upstream of clbR in the clbR–clbB intergenic region. The size of the VNTR region has been described to range from 2 to 20 copy numbers (Putze et al., 2009 [2]). The VNTR copy number distribution in ca. 1300 clb-positive genomes demonstrated that there is a preference for VNTR regions ranging from 7 to 10 copy numbers. Copy numbers from 18 to 34 were present in only a few strains (Fig. S8). Species and/or ST-specific copy number variation was not observed.
Comparative genomic analysis of multiple colibactin-encoding determinants based on (draft) genome sequences led to the observation that the homologous genes clbJ and clbK are prone to fusion/deletion (Lam et al., 2018 [38]). We also observed that in several assemblies of the clb gene cluster 625 bp from the 3′ end of clbJ and 3540 bp from the 5′ end of clbK including the 11 bp intergenic region are missing, for a total of 4174 bp (Fig. S9). The assembly of our internally generated genome sequences produced by short read (Illumina) sequencing showed this clbJ/K fusion/deletion. However, because assemblies of sequence data of the same strains generated by a long-read sequencing technology (PacBio), where the long reads covered both genes, had both clbJ and clbK completely present, we assume that the clbJ/K fusions described are artificial and result from erroneous assemblies of short-read sequencing data.
Quantification of colibactin synthesis in selected strains
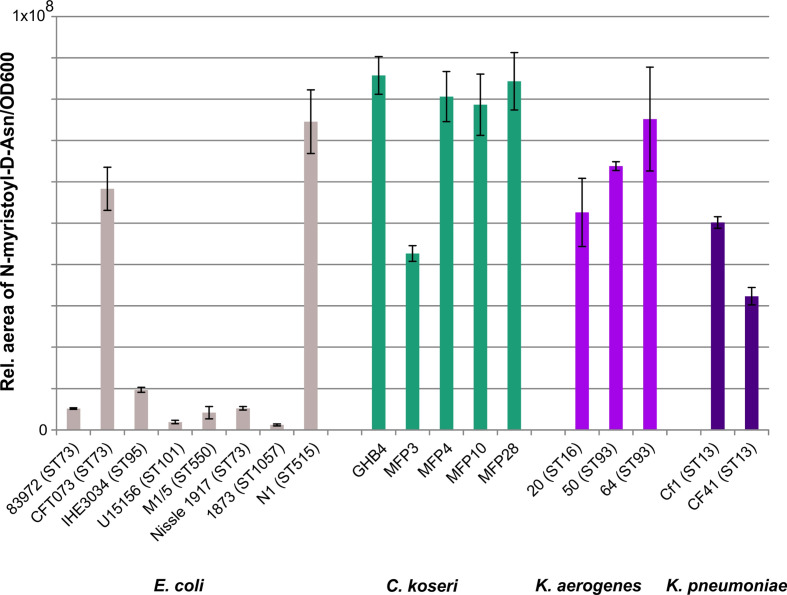
To investigate a possible correlation between the genetic structure of the clb determinant or the genetic background of the corresponding host strain with colibactin expression, we quantified N-Myr-d-asparagine levels produced in vitro by selected clb-positive Escherichia coli, Klebsiella species and C. koseri strains covering the diversity of clb determinants in these species (Fig. 6). Based on the detected relative amount of N-Myr-d-asparagine produced, the investigated isolates can be roughly divided into two groups: one group included most of the measured Escherichia coli strains that produced only very low relative amounts of N-Myr-d-asparagine. In contrast, the tested C. koseri, K. aerogenes and K. pneumoniae isolates and the Escherichia coli isolates CFT073 and N1 showed a 3- to 70-fold higher N-Myr-d-asparagine production. Also within the C. koseri, K. aerogenes and K. pneumoniae isolates, we found differences in the relative N-Myr-d-asparagine levels. However, these differences were not as strong as among the eight Escherichia coli isolates studied. The observed relative N-Myr-d-asparagine levels do not indicate phylogroup, ST or species-specific differences in colibactin production. For example, the Escherichia coli strain 1873, although the clb gene cluster present in this strain is phylogenetically more closely related to that of Escherichia coli strain N1, shows a significantly weaker N-Myr-d-asparagine production than Escherichia coli N1. Similarly, it should be noted that C. koseri MFP3 produces less N-Myr-d-asparagine than other closely related C. koseri.
Fig. 6.
Comparison of colibactin production of different strains accessed by quantification of the precolibactin cleavage product N-Asn-d-myristol. This assay enabled us to compare the ability of different strains and different species to produce colibactin under controlled conditions in vitro. Measurements were conducted based on three biological replicates, and means with standard deviations are as shown.
Discussion
Prevalence and mobility of the colibactin gene cluster in Enterobacterales
The ability of MGEs to be exchanged between and within species plays a major role in the extent and speed of microbial evolution. Because MGEs are known to emerge and evolve separately from the host, it is important to explain the development and diversity of MGEs independently. In our study, we investigated on the one hand the nucleotide sequence variability of the colibactin gene cluster with the associated yersiniabactin determinants, and on the other hand the structural diversity of the MGEs hosting the colibactin and yersiniabactin determinants responsible for their horizontal distribution. Both polyketide determinants together could be detected in certain clades of the Enterobacterales, mainly in members of the Enterobacter–Escherichia clade, namely in Escherichia coli, K. pneumoniae, K. aerogenes, and C. koseri, but also in Enterobacter cloacae, Enterobacter hormachei, and possibly in uncharacterized Salmonella species isolates. The colibactin but not the yersiniabactin determinants could also be detected in K. michiganensis, members of other Enterobacterales clades (Erwinia oleae, and Serratia marcescens), and a few phylogroup A and B1 Escherichia coli isolates (Fig. 2a). Interestingly, the colibactin genes have not yet been described in not only archaeal genomes but also Salmonella enterica and Salmonella bongori genomes, although we have screened more than 112000 Salmonella species genomes. Overall, it is remarkable that the clb gene cluster was only found in extraintestinal clinical or in faecal isolates of healthy hosts, but not in enterobacterial diarrhoeal pathogens such as Yersinia species, Salmonella species, Shigella species as well as the various intestinal pathogenic Escherichia coli pathotypes. This observation is consistent with previously published data [2, 51]. In this context, it would be interesting to study whether and in which way colibactin expression supports extraintestinal pathogenicity or intestinal persistence and colonization, but is detrimental to the pathogenesis of diarrhoeal pathogens. Possibly, the genetic background also plays an important role in the horizontal distribution and establishment of MGEs carrying both polyketide determinants. The fact that the colibactin determinant has so far been preferentially distributed in only some, often highly virulent STs in Escherichia coli and K. pneumoniae or K. aerogenes and not more broadly within the respective species (Fig. 2) [38, 39], could also indicate that the transmission, uptake or chromosomal integration of these MGEs is restricted. It is interesting to note that the clb gene cluster as a whole is highly conserved and usually characteristic of the respective species or genus (Figs 3 and S5). Nevertheless, ST-specific variants have been found within a species, e.g. in K. pneumoniae ST3 and ST23. Several groups of sequence variants of the clb gene cluster can also be found within one ST, such as in the Escherichia coli ST73 and ST95 (Figs 3 and S5). These results show that, on the one hand, intraspecies transfer of the colibactin determinant can happen, but on the other hand, certain adaptations of the clb genes to a specific genetic background can also occur at the nucleotide level. In addition, examples of interspecies transfer of the clb and ybt genes can be seen between Klebsiella species and Enterobacter hormachei strains. Similarly and in contrast to the majority of Escherichia coli isolates, we found clb and ybt gene clusters in some Escherichia coli isolates, which are assigned to the large clade of Klebsiella/Enterobacter/Citrobacter-specific clb and ybt variants (Figs 2a and 3). Apart from the interspecies transfer of the entire clb- and ybt-containing MGE, we also identified an example that shows that the two polyketide gene clusters can also be exchanged independently, as in the case of the clb gene cluster of K. pneumoniae strain k2265, which belongs to colibactin clade clb6 (predominantly C. koseri lineage of clb loci), whereas the ybt determinant in this strain is assigned to the yersiniabactin clade ybt12 instead of ybt3, which is usually associated with C. koseri strains carrying clade clb6 (Fig. 3).
Structural diversity of the colibactin–yersiniabactin region
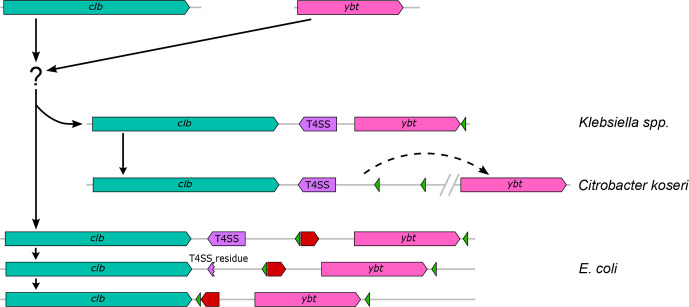
The structural analysis of the genomic region comprising the clb and ybt determinants in their chromosomal sequence context is an important aspect to understand the evolution of these polyketide determinants and their origin. In principle, three structural constellations (classes I to III) can be described, in which the clb and ybt gene clusters are present (Fig. 4). Class I depicts the clb and ybt gene clusters found in the majority of Escherichia coli isolates, each associated with a tRNA(Asn) and an integrase gene. In class Ia, the clb genes are chromosomally inserted at the tRNA locus asnV. Our analyses suggest that in class I, the T4SS gene cluster (‘mobilization module’) and conserved neighbouring genes (set 1) have been lost in a stepwise process, from class Ia to Ic. A further structural modification in this region is represented by the inversion of the yeeO-tRNA(Asn)-cbl-gltC gene set (Fig. 4, red arrow), as a result of which in class Ic, the tRNA gene asnW is located closest to the clb genes. Taking into account that the Escherichia coli strains with class Ia and Ib structures are found in the potentially earlier phylogenetic clades, we hypothesize that the MGE harbouring the clb genes was introduced into the Escherichia coli chromosome separately from that carrying the ybt determinant. Both MGEs were then progressively modified as described above. We do not yet have an explanation for why the resulting class Ic colibactin–yersiniabactin region, which has been described as two PAIs comprising the colibactin and yersiniabactin determinants, respectively [1, 2], is only found in phylogroup B2 strains and only there has it become so successful.
Class II includes different variants of an ICE, in which the two polyketide determinants are present in association with a T4SS-encoding ‘mobilization module’ and a ‘module’ consisting of genes that contribute to Fe/Mn/Zn metabolism (Fig. 4, Table S5). This type of ICE was found in Klebsiella species and Enterobacter hormachei and in a few cases in Escherichia coli and C. koseri (Fig. S6). Unlike in class I and III, the ICE in class II does not only have different tRNA(Asn) loci serving as chromosomal insertion sites, but also lacks tRNA(Asn) and integrase genes in between the clb and ybt genes. In a population-wide analysis of Klebsiella species strains, this ICE was designated ICEKp10 and described as being associated with different combinations of ybt and clb gene lineages [38]. In contrast to class I and II colibactin–yersiniabactin regions, the clb and ybt gene clusters are located far apart on the chromosome in most of the C. koseri genomes studied (class III) (Fig. 4).
The existence of an ICE that unites the clb and ybt gene clusters is the easiest way to explain the co-localization and the joint transfer of the two determinants and thus the high correlation of clb and ybt phylogenetic clades (Fig. 3, fourth circle) in Klebsiella species strains. Despite slight differences in the sequence context and different chromosomal insertion sites (Fig. 4), the ICEs of the four class II variants have an overall identical genetic structure (Fig. 4). The uptake of this ICE thus leads to the acquisition of both the clb and the ybt gene clusters. The presence of the Fe/Mn/Zn metabolic genes neighbouring these ICE variants with an additional integrase gene indicates recombination processes that can alter the genetic structure of the ICE. The clear separation between the clb/T4SS module and the ybt-Fe/Mn/Zn metabolism module in the C. koseri genomes points towards rearrangement/relocation of the ybt region, after ICE integration into the chromosome. The fact that C. koseri strain ATCC BAA-895 possesses in addition to the complete ICE (class II) a second ybt gene cluster (99.98 % nucleotide similarity to the ybt genes present in the ICE) that is located far from the ICE (Fig. S7), supports the hypothesis that the individual polyketide gene clusters can also be integrated into the genome independently of each other. This state could result, for example, from the initial chromosomal integration of different ICE variants, as described by Lam and colleagues in Klebsiella species [38]. As a result of deletion events, through which individual modules are subsequently deleted from one of the two ICEs, the second copy of the ybt gene cluster remains in the genome as a fragment of the degenerated ICE. The presence of two non-identical T4SS modules in the K. pneumoniae strains TUM14001, TUM14002, TUM14009, TUM14126 and WCHKP13F2 associated with the clb or ybt module could be the result of such degeneration of different ICEs (Fig. 3, clb4 and Fig. S7). In this way, our observations on the phylogeny (Fig. 2) and structure (Fig. 4) of the two polyketide determinants and their sequence context can be reconciled, which show that despite the predominant species/genus specificity, there are also ST-specific lineages of the clb genes, which do not necessarily have to match that of the associated ybt genes.
In this context, one could imagine that the arrangement of clb and ybt determinants in Escherichia coli strains (class I) also results from independent integration events of different MGEs, which subsequently degenerated as a result of a stabilization process of these MGEs [26, 28]. This premise is supported by the absence of ybt genes in the clb-positive Escherichia coli strains, which carry the phylogenetically most distant clb determinants compared to the clb genes of phylogroup B2 isolates (Fig. 2a), along with the presence of integrase and tRNA(Asn) genes in close proximity to both polyketide determinants (Fig. 4, class I).
Evolution of the colibactin determinant
Homologues of the clb gene cluster were detected in marine alphaproteobacteria such as various Pseudovibrio species (isolates AD26, FO-BEG1, POLY-S9) and Pseudovibrio denitrificans (isolates DSM 17465 and JCM12308) [52]. Despite the general conformity of the genetic structure of these gene clusters, their nucleotide sequence identity to the colibactin gene cluster is quite low (<26 %). Therefore, it was hypothesized that these Pseudovibrio isolates have the potential to produce molecules related to colibactin [52]. Another homologue of the colibactin determinant with a higher (62%) amino acid sequence identity is found in F. perrara [24]. While the genes required for biosynthesis and transport of the polyketide are present, the genes corresponding to clbA and clbR are missing in this gene cluster (Fig. 5). The case is similar with the colibactin gene cluster in S. marcescens. While in F. perrara, a gene coding for a clbA homologue and a gene coding for a SAM enzyme are located immediately downstream of the clbS homologue, a clbR homologue is located upstream of clbB in Serratia marcescens. It is therefore conceivable that the clbA homologue in F. perrara and the clbR homologue in Serratia marcescens are involved in the activation or regulation of colibactin biosynthesis in these bacteria (Fig. 5). It has been described that SAM is used in NRPS modules for colibactin biosynthesis [53]. Looking at the genetic structure of the clbB-S homologous genes cluster in F. perrara and the clb gene cluster in S. marcescens, one can assume that genes involved in the regulation and activation of the biosynthetic pathway including the SAM enzyme gene as well as clbA and clbR homologues have been fused upstream to the already existing part of the island (clbB-clbS) to improve regulation of polyketide biosynthesis. Without having further knowledge about the origin of the colibactin biosynthetic genes themselves, the acquisition of the regulatory/activating genes is obviously among the last evolutionary steps that led to the structural organization of what we currently describe as the colibactin gene cluster. This hypothesis is supported by the abrupt decrease in G+C content and the presence of the predicted recombination site (Fig. S4) directly upstream of clbB in many clb determinants. Furthermore, a module consisting of a gene for a SAM enzyme and a clbA homologue is not only located directly downstream from the clbS homologue in F. perrara, but also upstream from clbR in K. michiganensis and Erwinia oleae, which represent evolutionarily older variants of colibactin-positive Enterobacterales (Fig. 5).
Expression of colibactin in different hosts
Furthermore, we investigated the question of how differently colibactin is expressed within different genera of the Enterobacter–Escherichia clade or even within different lineages of the same species. In an initial approach, we compared colibactin expression in some selected model isolates. Interestingly, we observed an often lower production level of N-Myr-d-asparagine in Escherichia coli isolates compared to K. aerogenes, K. pneumoniae and C. koseri (Fig. 6), which may be expected because Escherichia coli is described as a non-optimal producer of complex secondary metabolites [54]. However, it is of interest that the amount of N-Myr-d-asparagine produced in Escherichia coli strains CFT073 and N1 is comparable to that of the tested Klebsiella species and C. koseri strains (Fig. 6). A species- or lineage-specific ability to produce N-Myr-d-asparagine could not be determined so far. We are fully aware that the data provided in Fig. 6 allow only very limited conclusions and that the comparison of larger sets of isolates will be required to generate more robust results. Future studies will have to investigate which bacterial factors are important for colibactin production and how the strain-specific differences in the expression of this polyketide come about. The systematic comparison of phenotypic colibactin production with information on the genomic context, regulatory and metabolic properties of host strains, and their classification in a phylogenetic context should help us to identify bacterial factors that affect colibactin synthesis.
Conclusion
The colibactin and yersiniabactin gene clusters are highly conserved polyketide determinants present in some clades of Enterobacteriales. They usually coexist together in the genome and are also linked to each other at the biosynthetic level. With the exception of Escherichia coli, the two gene clusters are part of an ICE, which allows the horizontal transfer of both secondary metabolite determinants usually within one species/genus. Bacteria of the genus Klebsiella played an important role in the evolution and distribution of both gene clusters. A large number of different ICEs has been described in Klebsiella species, which besides several other groups of genes include the yersiniabactin determinant [38]. Recombination and rearrangements events between different ICE types may have contributed to the evolution of the ICE variants so far identified in Klebsiella species and other enterobacteria as well as to the further degeneration of such MGEs leading to the colibactin and yersiniabactin-encoding PAIs present in phylogroup B2 Escherichia coli strains (Fig. 7).
Fig. 7.
Schematic representation of the predicted evolution of the colibactin–yersiniabactin genomic region in Enterobacteriaceae. The different elements of this region, i.e. the clb determinant (teal green), T4SS module (purple), ybt gene cluster (pink), integrase genes (green) and an invertible subset of genes (red arrow) are shown. Based on available genome sequence data, we suggest a development from single MGEs containing the clb determinant and the ybt gene cluster, respectively, towards the structural arrangement of both polyketide determinants, which is mainly found in enterobacterial populations. Black arrows (solid or dashed) indicate possible directions of development and DNA rearrangements. After the merge of the clb and ybt gene clusters into one MGE, represented by ICEKp10, there is evidence that three different structural variants have evolved from it: in Klebsiella species strains, the ICEKp10 has remained intact, whereas in C. koseri strains, a DNA rearrangement and re-localization of the ybt determinant to a different chromosomal position has taken place. In Escherichia coli, a gradual loss of the T4SS module and the inversion of a gene set between the two polyketide determinants led to immobilization or stabilization of the ICE, thus resulting the two PAIs known as pks island and HPI, respectively.
The phylogeny of the clb determinants does not determine the level of phenotypic colibactin production. The underlying bacterial factors responsible for the colibactin production efficiency of individual strains need to be identified in future work.
Our investigations provide deeper insights into the evolution of the colibactin gene cluster in Enterobacterales. Based on our findings, we can extend the current explanation for the coexistence and genetic co-localization of both gene clusters. The combination of a PPTase-encoding gene (clbA) with the clbB-S biosynthetic gene cluster during the evolution of the clb determinant not only enabled the efficient activation of the colibactin biosynthesis machinery, but also linked the colibactin and yersiniabactin determinants, which are functionally connected by the activity of PPTase ClbA. This enables the bacteria to synthesize both functionally different secondary metabolites, which leads to a stabilization of the coexistence and co-localization of the two gene clusters in the genome. Our data underpin the importance of MGEs, especially of ICEs, for genomic diversity and variability in enterobacteria as well as for the evolution of more complex bacterial phenotypes, such as the combined expression of the secondary metabolites colibactin and yersiniabactin.
Supplementary Data
Funding information
The work of the Münster team was supported by the German Research Foundation [grant DO789/11-1 and grant 281125614/GRK2220 (EvoPAD project A3)].
Acknowledgements
The data reported in this study appear in part in the PhD theses of A. Wallenstein and H. Wami. We thank M. K. Mammel (United States Food and Drug Administration) for providing Escherichia coli strain N1. We thank K. Tegelkamp and O. Mantel (Münster) for excellent technical support. We gratefully acknowledge CPU time on the high performance cluster PALMA@WWU Münster.
Author contributions
H.W., A.W. and U.D. conceptualized the project. H.W., A.W. and D.S. ran the analyses. R.M., M.S. and E.O. contributed reagents and new tools. H.W., A.W. and U.D. analysed the data. H.W., A.W. and U.D. wrote the manuscript. H.W., A.W., M.S., R.B., E.O., R.M. and U.D. edited and revised the manuscript. All authors read, commented on and approved the final manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: DAEC, diffusely adhering Escherichia coli; EAEC, enteroaggregative Escherichia coli; EHEC, enterohemorrhagic Escherichia coli; EPEC, enteropathogenic Escherichia coli; HPI, high pathogenicity island; HTH, helix-turn-helix; ICE, integrative and conjugative element; MGE, mobile genetic element; NMEC, newborn meningitis-causing Escherichia coli; N-Myr-d-asparagine, N-myristoyl-d-asparagine; PAI, pathogenicity island; SAM, S'-adenosylmethionine; ST, sequence type; T4SS, type 4 secretion system; UPEC, uropathogenic Escherichia coli; VNTR, variable number of tandem repeat.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Seven supplementary tables and ten supplementary figures and are available with the online version of this article.
References
- 1.Nougayrède J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 2.Putze J, Hennequin C, Nougayrède J-P, Zhang W, Homburg S, et al. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae . Infect Immun. 2009;77:4696–4703. doi: 10.1128/IAI.00522-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue M, Kim CS, Healy AR, Wernke KM, Wang Z, et al. Structure elucidation of Colibactin and its DNA cross-links. Science. 2019;365:6457. doi: 10.1126/science.aax2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homburg S, Oswald E, Hacker J, Dobrindt U. Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli . FEMS Microbiol Lett. 2007;275:255–262. doi: 10.1111/j.1574-6968.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin P, Marcq I, Magistro G, Penary M, Garcie C, et al. Interplay between siderophores and colibactin genotoxin biosynthetic pathways in Escherichia coli . PLoS Pathog. 2013;9:e1003437. doi: 10.1371/journal.ppat.1003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallenstein A, Rehm N, Brinkmann M, Selle M, Bossuet-Greif N, et al. ClbR is the key transcriptional activator of colibactin gene expression in Escherichia coli . mSphere. 2020;5:e00591–00520. doi: 10.1128/mSphere.00591-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healy AR, Wernke KM, Kim CS, Lees NR, Crawford JM, et al. Synthesis and reactivity of Precolibactin 886. Nat Chem. 2019;11:890–898. doi: 10.1038/s41557-019-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z-R, Li J, Cai W, Lai JYH, McKinnie SMK, et al. Macrocyclic colibactin induces DNA double-strand breaks via copper-mediated oxidative cleavage. Nat Chem. 2019;11:880–889. doi: 10.1038/s41557-019-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z-R, Li J, Gu J-P, Lai JYH, Duggan BM, et al. Divergent biosynthesis yields a cytotoxic aminomalonate-containing precolibactin. Nat Chem Biol. 2016;12:773–775. doi: 10.1038/nchembio.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bossuet-Greif N, Vignard J, Taieb F, Mirey G, Dubois D, et al. The colibactin genotoxin generates DNA interstrand cross-links in infected cells. mBio. 2018;9:e02317–e02393. doi: 10.1128/mBio.02393-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, et al. Escherichia coli induces dna damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014;63:1932–1942. doi: 10.1136/gutjnl-2013-305257. [DOI] [PubMed] [Google Scholar]
- 14.Dalmasso G, Cougnoux A, Delmas J, Darfeuille-Michaud A, Bonnet R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes. 2014;5:675–680. doi: 10.4161/19490976.2014.969989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dziubańska-Kusibab PJ, Berger H, Battistini F, Bouwman BAM, Iftekhar A, et al. Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat Med. 2020;26:1063–1069. doi: 10.1038/s41591-020-0908-2. [DOI] [PubMed] [Google Scholar]
- 16.Marcq I, Martin P, Payros D, Cuevas-Ramos G, Boury M, et al. The genotoxin colibactin exacerbates lymphopenia and decreases survival rate in mice infected with septicemic Escherichia coli . J Infect Dis. 2014;210:285–294. doi: 10.1093/infdis/jiu071. [DOI] [PubMed] [Google Scholar]
- 17.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. Coli . Nature. 2020;580:269–273. doi: 10.1038/s41586-020-2080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faïs T, Delmas J, Barnich N, Bonnet R, Dalmasso G. Colibactin: more than a new bacterial toxin. Toxins (Basel) 2018;10 doi: 10.3390/toxins10040151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassenaar TM. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit Rev Microbiol, Review. 2018;44:619–632. doi: 10.1080/1040841X.2018.1481013. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019;363:eaar7785. doi: 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brzuszkiewicz E, Brüggemann H, Liesegang H, Emmerth M, Olschläger T, et al. How to become a uropathogen: Comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc Natl Acad Sci U S A. 2006;103:12879–12884. doi: 10.1073/pnas.0603038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellanger X, Payot S, Leblond-Bourget N, Guedon G. Conjugative and mobilizable genomic islands in bacteria: evolution and diversity. FEMS Microbiol Rev. 2014;38:720–760. doi: 10.1111/1574-6976.12058. [DOI] [PubMed] [Google Scholar]
- 23.Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/IAI.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel P, Vizcaino MI, Crawford JM. Gut symbionts from distinct hosts exhibit genotoxic activity via divergent colibactin biosynthesis pathways. Appl Environ Microbiol. 2015;81:1502–1512. doi: 10.1128/AEM.03283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moretti C, Hosni T, Vandemeulebroecke K, Brady C, De Vos P, et al. Erwinia oleae sp. nov., isolated from olive knots caused by Pseudomonas savastanoi pv. savastanoi . Int J Syst Evol Microbiol. 2011;61:2745–2752. doi: 10.1099/ijs.0.026336-0. [DOI] [PubMed] [Google Scholar]
- 26.Hacker J, Kaper JB. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol. 2000;54:641–679. doi: 10.1146/annurev.micro.54.1.641. [DOI] [PubMed] [Google Scholar]
- 27.Messerer M, Fischer W, Schubert S. Investigation of horizontal gene transfer of pathogenicity islands in Escherichia coli using next-generation sequencing. PLoS One. 2017;12:e0179880. doi: 10.1371/journal.pone.0179880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider G, Dobrindt U, Middendorf B, Hochhut B, Szijártó V, et al. Mobilisation and remobilisation of a large archetypal pathogenicity island of uropathogenic Escherichia coli in vitro support the role of conjugation for horizontal transfer of genomic islands. BMC Microbiol. 2011;11:210. doi: 10.1186/1471-2180-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y-T, Lai Y-C, Tan M-C, Hsieh L-Y, Wang J-T, et al. Prevalence and characteristics of pks genotoxin gene cluster-positive clinical Klebsiella pneumoniae isolates in Taiwan. Sci Rep. 2017;7:43120. doi: 10.1038/srep43120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois D, Delmas J, Cady A, Robin F, Sivignon A, et al. Cyclomodulins in Urosepsis strains of Escherichia coli . J Clin Microbiol. 2010;48:2122–2129. doi: 10.1128/JCM.02365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson JR, Johnston B, Kuskowski MA, Nougayrede JP, Oswald E. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J Clin Microbiol. 2008;46:3906–3911. doi: 10.1128/JCM.00949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai Y-C, Lin A-C, Chiang M-K, Dai Y-H, Hsu C-C, et al. Genotoxic Klebsiella pneumoniae in Taiwan. PLoS One. 2014;9:e96292. doi: 10.1371/journal.pone.0096292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Micenkova L, Benova A, Frankovicova L, Bosak J, Vrba M. Human Escherichia coli isolates from hemocultures: Septicemia linked to urogenital tract infections is caused by isolates harboring more virulence genes than bacteraemia linked to other conditions. Int J Med Microbiol. 2017;307:182–189. doi: 10.1016/j.ijmm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Nowrouzian FL, Oswald E. Escherichia coli strains with the capacity for long-term persistence in the bowel microbiota carry the potentially genotoxic pks island. Microb Pathog. 2012;53:180–182. doi: 10.1016/j.micpath.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Krieger JN, Dobrindt U, Riley DE, Oswald E. Acute Escherichia coli prostatitis in previously health young men: bacterial virulence factors, antimicrobial resistance, and clinical outcomes. Urology. 2011;77:1420–1425. doi: 10.1016/j.urology.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy AJ, Martin P, Cloup E, Stabler RA, Oswald E. The genotoxin colibactin is a determinant of virulence in Escherichia coli K1 experimental neonatal systemic infection. Infect Immun. 2015;83:3704–3711. doi: 10.1128/IAI.00716-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshikawa Y, Tsunematsu Y, Matsuzaki N, Hirayama Y, Higashiguchi F, et al. Characterization of colibactin-producing Escherichia coli isolated from Japanese patients with colorectal cancer. Jpn J Infect Dis. 2020;73:437–442. doi: 10.7883/yoken.JJID.2020.066. [DOI] [PubMed] [Google Scholar]
- 38.Lam MMC, Wick RR, Wyres KL, Gorrie CL, Judd LM, et al. Genetic diversity, mobilisation and spread of the yersiniabactin-encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microb Genom. 2018;4:e000196. doi: 10.1099/mgen.0.000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen P, Berglund B, Chen Y, Zhou Y, Xiao T, et al. Hypervirulence markers among non-ST11 strains of carbapenem- and multidrug-resistant Klebsiella pneumoniae isolated from patients with bloodstream infections. Front Microbiol. 2020;11:1199. doi: 10.3389/fmicb.2020.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. Spades: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 42.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, et al. Blast+: Architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blin K, Andreu P, de Los Santos ELC, Del Carratore F, Lee SY, et al. The antiSMASH database version 2: A comprehensive resource on secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2019;47:D630–D625. doi: 10.1093/nar/gky1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assefa S, Keane TM, Otto TD, Newbold C, Berriman M. ABACAS: algorithm-based automatic contiguation of assembled sequences. Bioinformatics. 2009;25:1968–1969. doi: 10.1093/bioinformatics/btp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lassmann T, Sonnhammer EL. Kalign - an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics. 2005;6:298. doi: 10.1186/1471-2105-6-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adeolu M, Alnajar S, Naushad S, Gupta RS. Genome-based phylogeny and taxonomy of the “Enterobacteriales”: Proposal for Enterobacterales ord. Nov. Divided into the families Enterobacteriaceae, Erwiniaceae fam. Nov., Pectobacteriaceae fam. Nov., Yersiniaceae fam. Nov., Hafniaceae fam. Nov., Morganellaceae fam. Nov., and Budviciaceae fam. Nov. Int J Syst Evol Microbiol. 2016;66:5575–5599. doi: 10.1099/ijsem.0.001485. [DOI] [PubMed] [Google Scholar]
- 49.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bian X, Fu J, Plaza A, Herrmann J, Pistorius D, et al. In vivo evidence for a prodrug activation mechanism during colibactin maturation. Chembiochem. 2013;14:1194–1197. doi: 10.1002/cbic.201300208. [DOI] [PubMed] [Google Scholar]
- 51.Morgan RN, Saleh SE, Farrag HA, Aboulwafa MM. Prevalence and pathologic effects of colibactin and cytotoxic necrotizing factor-1 (Cnf 1) in Escherichia coli: experimental and bioinformatics analyses. Gut Pathog. 2019;11:22. doi: 10.1186/s13099-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naughton LM, Romano S, O’Gara F, Dobson ADW. Identification of secondary metabolite gene clusters in the Pseudovibrio genus reveals encouraging biosynthetic potential toward the production of novel bioactive compounds. Front Microbiol. 2017;8:1494. doi: 10.3389/fmicb.2017.01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zha L, Jiang Y, Henke MT, Wilson MR, Wang JX, et al. Colibactin assembly line enzymes use s-adenosylmethionine to build a cyclopropane ring. Nat Chem Biol. 2017;13:1063–1065. doi: 10.1038/nchembio.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Wang Y, Pfeifer BA. Bacterial hosts for natural product production. Mol Pharm. 2008;5:212–225. doi: 10.1021/mp7001329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.