Abstract
Objective:
The bioactivities of TotalFill BC and the MTA Fillapex sealers were evaluated.
Methods:
Sixty horizontal root sections were enlarged to size 5 Gates Glidden and randomly divided into six groups (n=10 in each group). In Groups 1–3, sections were filled with TFBCS, while sections in groups 4–6 were filled with MTAFS. Specimens from groups 1 and 4 were soaked in simulated body fluid (SBF) for one day, those from groups 2 and 5 for one week, and those from groups 3 and 6 for two months. All specimens were processed for scanning electron microscope (SEM) examination. Apatite precipitation on sealer and sealer–dentine interfaces was quantified using image analysis software (ImageJ). Energy dispersive X-ray (EDX) was used to analyse calcium (Ca) and phosphate (P) contents of surface precipitation on which calculation of the calcium phosphate (Ca/P) ratio was based.
Results:
TFBCS samples, regardless of the duration of SBF soaking, yielded a significantly higher surface area of precipitation compared to MTAFS (P<0.05); such precipitation increased over time, and the differences among the three time-points were also statistically significant. Following one day of SBF soaking, MTAFS samples showed only limited precipitation that started to appear after one week. EDX showed that Ca content and the Ca/P ratio of surface deposits on TFBCS samples increased over time with no difference between one week and two months of SBF soaking. The Ca content and Ca/P ratio of surface deposits on MTAFS were significantly lower than that of TFBCS samples regardless of the SBF soaking time.
Conclusion:
Ageing TotalFill BC sealer in SPF can induce considerable apatite formation. In addition, the TotalFill BC sealer surface showed high Ca2+ ion release as reflected by the formation of apatite with a high Ca/P ratio. These bioactivity features increased over time. In comparison, the MTAFS appears to have lower and delayed bioactivity.
Keywords: Bioactivity, bioceramic sealer, calcium phosphate ratio, MTA Fillapex, TotalFill BC sealer
HIGHLIGHTS.
In simulated body fluid TotalFill BC bioceramic sealer induced surface apatite which could result in higher biological sealing.
These surface apatite of TotalFill BC sealer with high Ca content and elevated Ca/P ratio could directly stimulate the deposition of new bone in the periapical lesions if the sealer slightly extruded beyond the apex.
MTA Fillapex sealer showed lower and delayed apatite formation ability, and this may be attributed to its high resin content and lower calcium silicate content.
INTRODUCTION
Currently available endodontic bioceramic materials are dimensionally stable and highly hydrophilic, and their setting is mainly dependent on the physiological moisture of the root canal and dentinal tubules. Such benefits hold bioceramic sealers as one of the best endodontic sealing materials that has been developed to date (1–3). EndoSequence BC (Brasseler, Savannah, GA, USA) is a premixed calcium silicate-based sealer, which was launched in 2009 and is available outside North America under the name of “TotalFill BC Sealer”(1, 4, 5). TotalFill BC contains both tricalcium and dicalcium silicate, calcium hydroxide, colloidal silica, monobasic calcium phosphate, and zirconium oxide (6). Zirconium oxide acts as a radio-pacifier that can substitute for bismuth oxide, used in mineral trioxide aggregate (7). Mineral trioxide aggregate (MTA) was approved for endodontic use by the United States Food and Drug Administration in 1998, and MTA is considered by some authors as a classic bioceramic material with the addition of some heavy metals (1, 8, 9). MTA was shown to promote favourable tissue reaction (10, 11) and provides a biological seal by forming a hydroxyapatite layer (12). Nevertheless, poor handling of MTA has precluded its use as a stand-alone sealer that does not require additional chemicals to provide sufficient flow (13). Accordingly, some ingredients, such as resin and thickening agents, were added to MTA to produce MTA-based sealers, such as MTA Fillapex from Angelus Solucões Odontológicas, Londrina, PR, Brazil (14).
Bioceramic materials are often classified according to their interactions with the surrounding living tissues as either bioactive or bioinert materials (15). Bioactive materials include calcium silicate, calcium phosphate, and glass. Bioactive bioceramics can undergo interactions with the surrounding tissue and are capable of forming hydroxyapatite or carbonated apatite on their surfaces (16). In addition, when a bioactive sealer is compacted against dentine, a dentine–sealer interface layer forms in the presence of phosphate. Accordingly, studies that evaluate the bioactivity of different types of sealers are essential as bioactivity is considered the keystone for important functional properties, such as the sealing ability, osteoconductivity, and biocompatibility.
MATERIALS AND METHODS
Sixty recently extracted single-rooted human teeth were collected. The use of the extracted teeth and the protocol of the study were approved by the institutional research ethics board. All teeth were checked for the absence of internal resorption, cracks, and any other defects that could affect the outcome of this study. After removing the crowns, the middle portion of each root was sectioned transversely using a diamond saw to obtain 2.00±0.05 mm-thick root sections. The lumen of each section was enlarged with size 2–5 Gates Glidden to obtain a standardised diameter of 1.3 mm. The specimens were immersed in 5.25% NaOCl, followed by soaking in 17% EDTA for 1 min and then washed with distilled water and dried with absorbent paper points. The root sections were randomly divided into six groups- ten root sections each as follows:
Groups 1, 2, and 3: Root sections were filled with TFBCS (Lot number 4003SP-V 09/2016). Group 4, 5, and 6: Root sections were filled with MTAFS (Lot number 35867, 35760, 35267-V 05/2017).
Sealers were injected into the prepared root canal spaces using minimal pressure. Sealers were left to set for 24 hours at a relative humidity of 100% to allow the initial setting of the materials. Specimens were soaked in 3 ml of Ca and Mg-free physiological-like buffered (pH 7.4) solution (Dulbecco’s Phosphate Buffered Saline-BE17-512, Lonza Walkersville Inc, MD, USA) (DPBS) and hold within sterile tightly sealed plastic tubes. The root sections were stored for three different durations as follows: for 1 day (Groups 1 and 4), for 1 week (Groups 2 and 5), and for 2 months (Groups 3 and 6) All specimens were incubated at 37 oC and the solution were renewed weekly.
Evaluation of bioactivity
After each endpoint, the root sections were removed from the solution and dried. Specimens were mounted on metallic stubs and gold-sputtered, and examined by the scanning electron microscopy (SEM) connected to energy dispersive X-ray analysis (EDX) (JEOL–JFC1100E Tokyo, Japan). Specimens were examined to identify the formation of precipitates on six randomly selected areas on the cement surface and at the sealer-dentine interfaces at (100-1000x) magnification. The percentage of the surface area covered by spherical or globular particles of apatite precipitation was calculated relative to the total surface area using the digital image analysis software ImageJ. EDX was utilized to determine the calcium and phosphate content of surface precipitations (X-ray electron beam penetration of EDX is about 2.98 µm), then the calcium phosphate ratio (Ca/P) was calculated from the atomic ratio data obtained.
Statistical analysis
Quantitative data were presented as Mean±SD. The outcome measures data were analysed by the non-parametric Mann-Whitney test to compare paired independent samples. The repeated-measures ANOVA was used for multiple compared groups and repeated time points. Significance level was set at p-value<0.05.
RESULTS
SEM analysis
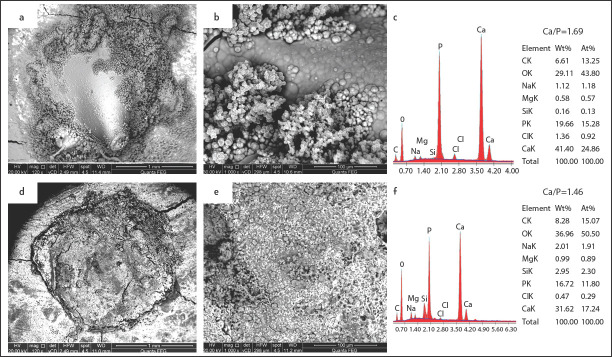
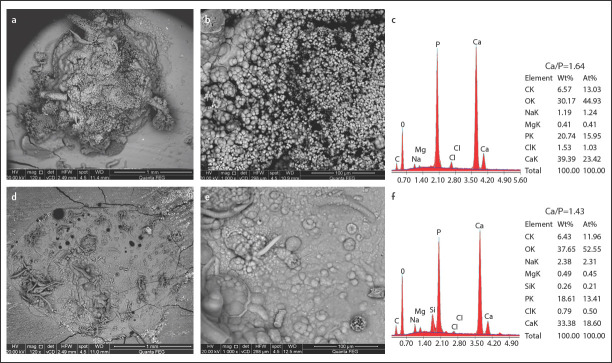
SEM image analysis of surface precipitation and percentage of apatite globules covering the surface showed a statistically significant difference between TFBCS and MTAFS treated samples at each time period (Table 1). After one day of immersion in DPBS, TFBCS samples showed an amorphous outer surface and obvious bioactivity (Fig. 1a, b). Sealer dentine junction stared to be covered by precipitates. After one week, the bioactivity of TotalFill BC was noticeable (Fig. 2a, b). A dense homogeneous structure with porosities was observed covering the surface. Sealer expansion was observed in some samples (Fig. 2a). After 2 months, TotalFill BC surfaces showed significant precipitates on the surface creating a coating of micro to nanospherulites. Rounded-globular Ca-rich crystals varying in size were observed (Fig. 3a, b). There was a statistically significant difference between the three-time points.
TABLE 1.
Comparison between percentages of globular precipitation surface area in the two groups at three-time points
| 1 Day | 1 Week | 2 Months | |
|---|---|---|---|
| TotalFill BC | 41.79%Ca±6.14 | 65.02%Ba±5.68 | 90.70%Aa±3.34 |
| MTA Fillapex | 4.04%Cb±1.49 | 12.37%Bb±2.91 | 34.92%Ab±6.03 |
Mean values with different capital superscript letters in the same raw are statistic significantly different. Mean values with different small letters in the same column are statistic significantly different. (P<0.05)
Figure 1.
Morphology and elemental composition of surface precipitates on TotalFill BC sealer (a, b and c) and MTA Fillapex sealer (d, e, and f) after 1 day of immersion in simulated body fluid (SPF)
Figure 2.
Morphology and elemental composition of surface precipitates on TotalFill BC sealer (a, b and c) and MTA Fillapex sealer (d, e, and f) after I week of immersion in SPF
Figure 3.
Morphology and elemental composition of surface precipitates on TotalFill BC sealer (a, b and c) and MTA Fillapex sealer (d, e, and f) after 2 months of immersion in SPF
Samples of MTAFS aged for one day showed an amorphous irregular surface that was almost free from visible deposits as well as the sealer-dentine interface (Fig. 1d, e). Ingredients of titanium oxide traces were scattered throughout the sealer surface. After one week of immersion, samples showed limited precipitate of isolated islands of rounded globules. Many thin bright cloud-like deposits were also observed, minimal precipitations at the sealer-dentine interface and gaps were observed (Fig. 2d, e). After 2 months, surfaces were partially covered with deposits and occasionally with prismatic or needle-like crystals, likewise little precipitates were observed at the dentine–material interface (Fig. 3d, e). There was a statistically significant difference between the three-time points.
EDX analysis
After one day of immersion of TFBCS specimens, EDX revealed a low level of Si, and significantly higher Ca peaks levels of the surface deposits than MTAFS (Fig. 1c). Whereas after two months, Ca levels remained high, and Si reflexes were no longer detectable (Fig. 3c). As shown in Table 2, Ca/P ratios in specimens immersed in PBS were 1.58 1.69, and 1.64 for one day, one week, and two months respectively. There was an increase in Ca/P ratios with increasing immersion time (Table 2). Samples of MTAFS aged in (DPBS) for one day showed scattered aggregates with low Ca levels, high Si peakes, and traces of Ti peaks (Fig. 1f). After one week, EDX analysis revealed increasing in Ca reflexes, while the Si level was decreased with time (Fig. 2f). MTA Fillapex samples aged for two months presented high Ca reflexes with a low Si level. Ca/P ratio were 1.50, 1.46, and 1.43 for samples immersed for one day, one week, and two months respectively. Regardless of any variable, results showed that the atomic ratio of Ca content of the surface precipitations in the TFBCS group was 21±2.7 which was higher than that of MTAFS 15.5±3.5 and that difference was statistically significant (P<0.05) (Table 2).
TABLE 2.
Ca atomic ratio and Ca/P ratio in surface apatite precipitations in the two groups at three-time points
| 1 Day | 1 Week | 2 Months | |
|---|---|---|---|
| Ca content at % | |||
| TotalFill BC | 18.27Ba±1.7 | 22.71Aa±2.3 | 22.04Aa±1.7 |
| MTA Fillapex | 10.91Bb±1.2 | 18.22Ab±1.5 | 17.49Ab±1.0 |
| Ca/P ratio | |||
| TotalFill BC | 1.58Ba±0.08 | 1.69Aa±0.06 | 1.64Aa±0.08 |
| MTA Fillapex | 1.50Ab±0.11 | 1.46Ab±0.05 | 1.43Ab±0.08 |
Ca: calcium, At%: atomic Wight percentage, Ca/P: calcium phosphate ratio. Mean values with different capital superscript letters in the same raw are statistically significantly different. Mean values with different small letters in the same column are statistically significantly different. (P<0.05)
DISCUSSION
Bioactivity is the ability to form apatite of calcium phosphate deposits after soaking in simulated body fluids; such ability presents the ground for the biocompatibility of inorganic biomaterials. Bioactive materials create an environment that can enhance osteogenesis, mostly through bonding to living bone tissue using the apatite layer formed on its surface once introduced into the living body (17-20). Moreover, deposition of calcium phosphates at the interface and inside the dentinal tubules improve the sealing ability of root canal filling materials (21-23). The present study aimed to evaluate the bioactivity of a bioceramic sealer (TFBCS) in comparison to a sealer that contains MTA (MTAFS).
Following only one day of immersion in DBPS, TotalFill BC filled samples showed an amorphous outer surface and obvious bioactivity. Such an initial layer of deposits seems to serve as nucleation sites for further precipitation, a response that was observed by other researchers using dicalcium silicate cement (24). In contrast, Gandolfi et al. (25) used Hank’s balanced salt solution (HBSS) and found that depositions were not detected except after 7 days, suggesting that the process was slower, dependent on the lower phosphate concentration of this medium. In our study, only after one week of immersion, the bioactivity of TotalFill BC was noticeable, Previous studies have shown a similar reaction (25). After 2 months of immersion in DBPS, the TotalFill BC surfaces became entirely covered by spherical apatite agglomerates. At higher magnification, the agglomerate consisted of tiny ball-like particles. The sealer-dentine interface was entirely covered with deposits. This finding comes in constant with Shokouhinejad et al. (26), who showed that after 2 months, heavy precipitates of spherical particles were observed.
We observed different responses over immersion time with MTAFS. Samples aged in DPBS for 1 day showed an amorphous irregular outer surface agreeing with previous observations (27). Whereas, limited surface precipitate and islands of globular precipitations were observed following one week of immersion. MTAFS showed a high gap within the sealer-dentine interface with little deposits, this observation comes in agreement with the finding of Silva et al. (28). This may be attributed to the polymer ingredients in MTAFS, which make it susceptible to polymerisation shrinkage (4). Samples aged for two months presented surfaces partially covered with deposits and occasionally prismatic or needle-like crystals, Such incomplete coverage with deposits may be attributed to the resinous and bismuth oxide component of MTAFS, which affect MTA bioactivity through inhibiting the hydration reaction (29-31).
It is recognized that the more the Ca content of a biomaterial, the higher the chance of the dentine uptake of Ca. This reaction, in turn, can increase acid resistance and physical strength of dentine and lead to early considerable precipitation of apatite (25, 32). As well, it was observed that precipitates with a high Ca/P ratio mainly include the hydroxyapatite (33, 34). The Ca/P ratio could act as an indicator of the precipitation phase. The formation of carbonated apatite correlates with increased Ca/P ratios (26, 35), therefore, calcium carbonate formation can be presumed when the Ca/P ratio exceeded 1.67 (19).
Analysis of EDX examination showed also variable response between TotalFill BC and MTA Fillapex treated samples. Following one day of immersion, TFBCS deposits revealed high Ca peaks levels, with a low level of silica. There seems to be a synergistic effect between Si as an effective apatite nucleator and Ca as an apatite precipitation accelerator, leading to fast apatite precipitation (36). When HAN et al. (37) examined MTA in Ca- and Mg-free SBF for one day, the Ca/P ratio was only 1.42. After one week, EDX analysis showed precipitate, rich in Ca with a high atomic ratio 22.7. Ca/P ratio showed the highest value. After two months of immersion, TotalFill BC samples developed high Ca and P peaks. Gandolfi et al., (38) examined calcium silicate material (Tech Biosealer root end ) and found that the Ca/P atomic ratio was 2.07 after 28 days. In contrast, Han and Okiji, (39) who compared the Endosequence BC sealer with Biodentine and White ProRoot MTA showed that Endosequence BC sealer - with 90 days of soaking- has the lowest Ca ions and Ca/P ratio was only about 1.3.
For MTAFS treated samples, EDX analysis of aggregate composition after 1 day of soaking showed low Ca levels, with high silica peaks. EDX revealed traces of titanium peaks on sealer surfaces, possibly due to the deep X-ray penetration under the deposits since the electron beam penetration at 20-keV acceleration was 2.98 μm. A low Ca/P ratio of about 1.49 was also observed. Whereas one week of immersion showed a significant increase in Ca reflexes to 18.2, silica levels significantly decreased with time, and the Ca/P ratio remained at the same level of around 1.45. Precipitations on MTAFS, after 2 months, showed the same level of Ca reflexes, while the Ca/P ratio slightly decreased to 1.43. The study by Viapiana et al. (27) supports our findings, by their demonstration of the effect of MTAFS, which exhibited low Ca ion compared with calcium silicate sealer (experimental sealer based on Portland cement). While Marciano et al., (14) found that the MTAFS did not release calcium hydroxide. MTAFS produced precipitates with a lower Ca/P ratio compared to TotalFill BC. This may be a result of the lower Ca-releasing ability of MTAFS (40). It is possible that if MTAFS contains less resin by volume and more calcium silicate ingredients, it would have shown more bioactive properties.
The obvious variability in outcome among bioactivity studies could be explained by differences in experimental designs and lack of technique standardisation. For example, in some studies, soaking solutions were refreshed daily (41, 42), or every three days (43-45), or even every seven days (19, 39) or soaked for 21 days without refreshing (46). Finally, the detection of apatite crystals in in-vitro models for the bioactivity assessment is still questionable, and the role of environmental factors, such as temperature, pH, carbon dioxide partial pressure, and agitation, has often been overlooked as reported by several authors (47, 48).
CONCLUSION
Compared with MTAFS, the TFBCS induced higher and earlier apatite formation which increased over time. Such features could be reflected in better biological sealing ability. In addition, the surface apatite of TFBCS sealer showed higher Ca content and elevated Ca/P ratio which can stimulate new bone tissue deposition. MTAFS sealer showed lower and delayed apatite formation ability.
Footnotes
Conflict of interest: The authors deny any conflicts of interest related to this study.
Ethics Committee Approval: This study were approved by the Ain shams University Ethics Committee (Date: 28/12/2015, Number: 151).
Peer-review: Externally peer-reviewed.
Financial Disclosure: None declared.
Authorship contributions: Concept – M.A.E., E.E.H., A.A.E.E.; Design – M.A.E., A.A.E.E.; Supervision – M.A.E., E.E.H., A.A.E.E.; Funding - M.A.E.; Materials - M.A.E.; Data collection &/or processing – M.A.E.; Analysis and/or interpretation – M.A.E., A.A.E.E.; Literature search – M.A.E.; Writing – M.A.E., A.A.E.E.; Critical Review – E.E.H., A.A.E.E.
REFERENCES
- 1.Trope M, Bunes A, Debelian G. Root filling materials and techniques:bioceramics a new hope? Endod Topics. 2015;32(1):86–96. [Google Scholar]
- 2.Jain P, Ranjan M. The rise of biocramics in endodontics:A review. Int J Pharm Bio Sci. 2015;6(1):416–22. [Google Scholar]
- 3.Gandolfi MG, Iacono F, Agee K, Siboni F, Tay F, Pashley DH, et al. Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(6):e39–45. doi: 10.1016/j.tripleo.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Al-Haddad A, Abu Kasim NH, Che Ab Aziz ZA. Interfacial adaptation and thickness of bioceramic-based root canal sealers. Dent Mater J. 2015;34(4):516–21. doi: 10.4012/dmj.2015-049. [DOI] [PubMed] [Google Scholar]
- 5.Silva Almeida LH, Moraes RR, Morgental RD, Pappen FG. Are premixed calcium silicate-based endodontic sealers comparable to conventional materials?a systematic review of in vitro studies. J Endod. 2017;43(4):527–35. doi: 10.1016/j.joen.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Hess D, Solomon E, Spears R, He J. Retreatability of a bioceramic root canal sealing material. J Endod. 2011;37(11):1547–9. doi: 10.1016/j.joen.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Cutajar A, Mallia B, Abela S, Camilleri J. Replacement of radiopacifier in mineral trioxide aggregate;characterization and determination of physical properties. Dent Mater. 2011;27(9):879–91. doi: 10.1016/j.dental.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Haapasalo M, Parhar M, Huang X, Wei X, Lin J, Shen Y. Clinical use of bioceramic materials. Endod Topics. 2015;32(1):97–117. [Google Scholar]
- 9.Lee JK, Kwak SW, Ha JH, Lee W, Kim HC. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg Chem Appl. 2017;2017:2582849. doi: 10.1155/2017/2582849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernabé PF, Holland R, Morandi R, de Souza V, Nery MJ, Otoboni Filho JA, et al. Comparative study of MTA and other materials in retrofilling of pulpless dogs'teeth. Braz Dent J. 2005;16(2):149–55. doi: 10.1590/s0103-64402005000200012. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q, Haglund R, Safavi KE, Spangberg LS. Adhesion of human osteoblasts on root-end filling materials. J Endod. 2000;26(7):404–6. doi: 10.1097/00004770-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Parirokh M, Torabinejad M. Mineral trioxide aggregate:a comprehensive literature review--Part III:Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Vitti RP, Prati C, Silva EJ, Sinhoreti MA, Zanchi CH, de Souza e Silva MG, et al. Physical properties of MTA Fillapex sealer. J Endod. 2013;39(7):915–8. doi: 10.1016/j.joen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Marciano MA, Duarte MA, Camilleri J. Calcium silicate-based sealers:Assessment of physicochemical properties, porosity and hydration. Dent Mater. 2016;32(2):e30–40. doi: 10.1016/j.dental.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Best SM, Porter AE, Thian ES, Huangc J. Bioceramics:Past, present and for the future. J Euro Ceram Soci. 2008;28(7):1319–27. [Google Scholar]
- 16.Wu C, Chang J. A review of bioactive silicate ceramics. Biomed Mater. 2013;8(3):032001. doi: 10.1088/1748-6041/8/3/032001. [DOI] [PubMed] [Google Scholar]
- 17.Gandolfi MG, Siboni F, Polimeni A, Bossù M, Riccitiello F, Rengo S, et al. In vitro screening of the apatite-forming ability, biointeractivity and physical properties of a tricalcium silicate material for endodontics and restorative dentistry. Dent J. 2013;1(4):41–60. [Google Scholar]
- 18.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–15. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Gandolfi MG, Siboni F, Primus CM, Prati C. Ion release, porosity, solubility, and bioactivity of MTA Plus tricalcium silicate. J Endod. 2014;40(10):1632–7. doi: 10.1016/j.joen.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Niu LN, Jiao K, Wang TD, Zhang W, Camilleri J, Bergeron BE, et al. A review of the bioactivity of hydraulic calcium silicate cements. J Dent. 2014;42(5):517–33. doi: 10.1016/j.jdent.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandolfi MG, Parrilli AP, Fini M, Prati C, Dummer PM. 3D micro-CT analysis of the interface voids associated with Thermafil root fillings used with AH Plus or a flowable MTA sealer. Int Endod J. 2013;46(3):253–63. doi: 10.1111/j.1365-2591.2012.02124.x. [DOI] [PubMed] [Google Scholar]
- 22.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15:1–201. [PubMed] [Google Scholar]
- 23.Kim JR, Nosrat A, Fouad AF. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J Dent. 2015;43(2):241–7. doi: 10.1016/j.jdent.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen CC, Ho CC, David Chen CH, Wang WC, Ding SJ. In vitro bioactivity and biocompatibility of dicalcium silicate cements for endodontic use. J Endod. 2009;35(11):1554–7. doi: 10.1016/j.joen.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Gandolfi MG, Taddei P, Tinti A, Dorigo EDS, Prati C. Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Mat Sci Eng. 2011;31(7):1412–22. [Google Scholar]
- 26.Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, et al. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endod J. 2012;45(12):1127–34. doi: 10.1111/j.1365-2591.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 27.Viapiana R, Guerreiro-Tanomaru JM, Hungaro-Duarte MA, Tanomaru-Filho M, Camilleri J. Chemical characterization and bioactivity of epoxy resin and Portland cement-based sealers with niobium and zirconium oxide radiopacifiers. Dent Mater. 2014;30(9):1005–20. doi: 10.1016/j.dental.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Amoroso-Silva PA, Guimarães BM, Marciano MA, Duarte MA, Cavenago BC, Ordinola-Zapata R, et al. Microscopic analysis of the quality of obturation and physical properties of MTA Fillapex. Microsc Res Tech. 2014;77(12):1031–6. doi: 10.1002/jemt.22432. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40(6):462–70. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri J. Evaluation of the effect of intrinsic material properties and ambient conditions on the dimensional stability of white mineral trioxide aggregate and Portland cement. J Endod. 2011;37(2):239–45. doi: 10.1016/j.joen.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013;29(5):580–93. doi: 10.1016/j.dental.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Hotta M, Li Y, Sekine I. Mineralization in bovine dentin adjacent to glass-ionomer restorations. J Dent. 2001;29(3):211–5. doi: 10.1016/s0300-5712(01)00005-7. [DOI] [PubMed] [Google Scholar]
- 33.Lu X, Leng Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials. 2005;26(10):1097–108. doi: 10.1016/j.biomaterials.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Yuan H, Li Y, de Bruijn JD, de Groot K, Zhang X. Tissue responses of calcium phosphate cement:a study in dogs. Biomaterials. 2000;21(12):1283–90. doi: 10.1016/s0142-9612(00)00016-8. [DOI] [PubMed] [Google Scholar]
- 35.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J Endod. 2007;33(11):1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Ding SJ, Shie MY, Wang CY. Novel fast-setting calcium silicate bone cements with high bioactivity and enhanced osteogenesis in vitro. J Mat Chem. 2009;19(8):1183–90. [Google Scholar]
- 37.Han L, Okiji T, Okawa S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dent Mater J. 2010;29(5):512–7. doi: 10.4012/dmj.2009-133. [DOI] [PubMed] [Google Scholar]
- 38.Gandolfi MG, Taddei P, Modena E, Siboni F, Prati C. Biointeractivity-related versus chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. J Biomed Mater Res B Appl Biomater. 2013;101(7):1107–23. doi: 10.1002/jbm.b.32920. [DOI] [PubMed] [Google Scholar]
- 39.Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J. 2013;46(9):808–14. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 40.Weng J, Liu Q, Wolke JG, Zhang X, de Groot K. Formation and characteristics of the apatite layer on plasma-sprayed hydroxyapatite coatings in simulated body fluid. Biomaterials. 1997;18(15):1027–35. doi: 10.1016/s0142-9612(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 41.Moinzadeh AT, Aznar Portoles C, Schembri Wismayer P, Camilleri J. Bioactivity potential of EndoSequence BC RRM putty. J Endod. 2016;42(4):615–21. doi: 10.1016/j.joen.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Huan Z, Chang J. Study on physicochemical properties and in vitro bioactivity of tricalcium silicate-calcium carbonate composite bone cement. J Mater Sci Mater Med. 2008;19(8):2913–8. doi: 10.1007/s10856-008-3423-4. [DOI] [PubMed] [Google Scholar]
- 43.Han L, Kodama S, Okiji T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int Endod J. 2015;48(2):124–30. doi: 10.1111/iej.12290. [DOI] [PubMed] [Google Scholar]
- 44.Tian J, Zhang Y, Lai Z, Li M, Huang Y, Jiang H, et al. Ion release, microstructural, and biological properties of iRoot BP plus and ProRoot MTA exposed to an acidic environment. J Endod. 2017;43(1):163–8. doi: 10.1016/j.joen.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Patlolla A, Arinzeh TL. Evaluating apatite formation and osteogenic activity of electrospun composites for bone tissue engineering. Biotechnol Bioeng. 2014;111(5):1000–17. doi: 10.1002/bit.25146. [DOI] [PubMed] [Google Scholar]
- 46.Correa D, Almirall A, García-Carrodeguas R, dos Santos LA, De Aza AH, Parra J, et al. β-Dicalcium silicate-based cement:synthesis, characterization and in vitro bioactivity and biocompatibility studies. J Biomed Mater Res A. 2014;102(10):3693–703. doi: 10.1002/jbm.a.35041. [DOI] [PubMed] [Google Scholar]
- 47.Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials. 2009;30(12):2175–9. doi: 10.1016/j.biomaterials.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Pan H, Zhao X, Darvell BW, Lu WW. Apatite-formation ability--predictor of “bioactivity”? Acta Biomater. 2010;6(11):4181–8. doi: 10.1016/j.actbio.2010.05.013. [DOI] [PubMed] [Google Scholar]