Abstract
Complexes between the retinoblastoma protein (pRb) and the transcription factor E2F-1 are thought to be important for regulating cell proliferation. We have shown previously that the E7 oncoprotein from human papillomavirus type 16, dependent upon its binding to pRb proteins, induces proliferation, disrupts differentiation, and induces apoptosis when expressed in the differentiating, or fiber, cells of the ocular lenses in transgenic mice. Mice that carry a null mutation in E2F-1 do not exhibit any defects in proliferation and differentiation in the lens. By examining the lens phenotype in mice that express E7 on an E2F-1 null background, we now show genetic evidence that E7’s ability to alter the fate of fiber cells is partially dependent on E2F-1. On the other hand, E2F-1 status does not affect E7-induced proliferation in the undifferentiated lens epithelium. These data provide genetic evidence that E2F-1, while dispensible for normal fiber cell differentiation, is one mediator of E7’s activity in vivo and that the requirement for E2F-1 is context dependent. These data suggest that an important role for pRb-E2F-1 complex during fiber cell differentiation is to negatively regulate cell cycle progression, thereby allowing completion of the differentiation program to occur.
Normal growth, development, and homeostasis of a multicellular organism requires precise balancing of cellular proliferation, differentiation, and apoptosis. Signals that regulate proliferation are thought to ultimately control passage of cells through the cell cycle in which the retinoblastoma (RB) family of pocket proteins and the E2F/DP (hereafter referred to as E2F) family of transcription factors reside as central regulators. A broadly defined model suggests that E2F factors act directly downstream of RB family members and that proliferation occurs when E2F activity promotes S-phase entry while RB family members suppress this proliferation primarily through repression (23, 29). Under normal cell cycle regulation, proliferation is thought to occur when pRb-E2F-DNA repressor complexes are disrupted by cyclin-dependent kinase-mediated phosphorylation (6). Cell cycle regulation can be altered by the binding of oncoproteins from DNA tumor viruses to RB family members, which disrupts these complexes, leading to deregulated E2F activity, uncontrolled proliferation, and perhaps tumor formation (7). E2F-1 has been implicated as an oncogene from studies in cultured cells in which E2F-1 overexpression drove quiescent cells through the G1 into the S phase of the cell cycle, ultimately leading to apoptosis or neoplastic transformation (1). However, more recently, mice that carry an E2F-1 null mutation were documented to develop tumors in certain tissues, suggesting a tumor suppressor function for E2F-1 (15, 58). Thus, in tumorigenesis, E2F-1 can act as either a positive or negative regulator of cell growth, depending on the context. How this model relates to control of proliferation and differentiation during normal development in vivo is largely undefined.
The role of the pRb:E2F-1 interaction in the control of development has recently been addressed by studies in Drosophila. Proteins homologous to both the RB family, i.e., RBF (9), and the E2F family, i.e., dE2F/dDP (12, 40), have been identified. During Drosophila development in vivo, dE2F is required for the normal expression of RNR2 and the normal rate of DNA synthesis (11, 49). RBF associates with dE2F and regulates dE2F activity, as shown by experiments in which retina-specific expression of RBF suppressed ectopically driven proliferation caused by retina-specific expression of dE2F/dDP in normally postmitotic cells (10).
In mouse development, the embryonic lens of the eye has been used as a model system for elucidating the molecular requirements for control of proliferation and differentiation. In this organ composed entirely of epithelial tissue, undifferentiated anterior cells in a region referred to as the central epithelium acquire the capacity to divide as they migrate posteriorly into a proliferation (germinative) zone. Influenced by their position in the lens and signals from other ocular tissues, these cells continue to divide and migrate further towards the posterior into a transitional zone, where they cease cell cycle progression prior to differentiating into fiber cells. As they differentiate, they migrate away from the epithelium and into the fiber cell compartment in the interior of the lens, elongate into lens fibers, and eventually lose membrane-bound organelles, such as the nucleus. This pattern of growth and differentiation in the lens results in a large mass of highly elongated, differentiated fiber cells bordered anteriorly by a single cell layer of undifferentiated cuboidal epithelial cells (33, 46).
Recently, studies in the mouse have begun to address the role of pRb in lens development. The E7 oncoprotein of human papillomavirus type 16 (HPV-16) is known to bind to and inactivate pRb (4, 14, 38) and to lead to pRb’s degradation (27). Lens-specific expression of E7, dependent upon its ability to associate with the RB family of proteins, leads to the continued proliferation of cells residing in the differentiated, or fiber, cell compartment of the lens, the failure of these cells to take on the morphological characteristics of the differentiated fiber cell, and the induction of apoptosis through both p53-dependent and p53-independent pathways (42, 43). Similarly, lens-specific expression of a related oncogene, a truncated simian virus 40 (SV40) large T antigen which can bind pRb but not p53, also leads to proliferation in spatially inappropriate regions of the lens and apoptosis (16). Lastly, RB-null embryos, generated by gene targeting, exhibit a lens phenotype similar to that observed in E7 transgenic embryos at a similar developmental age (36). Taken together, these in vivo studies indicate that pRb is essential for appropriate cell cycle control during mouse lens fiber cell differentiation.
Interestingly, RB and E2F family members are contextually expressed in the rodent lens. In the undifferentiated epithelium all known RB (pRb, p107, and p130) and E2F (E2F-1, -2, -3, -4, and -5) family members are expressed, whereas in the differentiated lens fibers only subsets of these families (including pRb, p107, E2F-1, E2F-3, and E2F-5) are expressed (41, 48). While the presence of these E2Fs and complexes between RB family members and E2Fs in the lens have been documented, their in vivo functions in controlling cell proliferation and/or differentiation have not been elucidated.
In order to determine whether the developmental defects in the lens elicited by E7’s inactivation of pRb are mediated by E2F-1, E7 transgenic mice that are also E2F-1 deficient were generated by crossing the E7 transgenic mice (42) with mice that carry a null mutation in the E2F-1 locus (58) and the effects of E2F-1 status on E7-induced proliferation, disrupted differentiation, and apoptosis were assessed. Results indicate that E2F-1 is dispensable for normal lens development. E7-induced proliferation in the undifferentiated epithelium also appears to be independent of E2F-1. However, in the differentiated fibers, the E7-induced phenotype is partially dependent on E2F-1. Thus, the genetic requirement for E2F-1 during lens development appears to be context dependent, i.e., correlating with the positional or differentiation state of the cell.
MATERIALS AND METHODS
Generation of E7 transgenic mice deficient at the E2F-1 locus.
The transgenic mice expressing HPV-16 E7 specifically in the lens (42) and the mice carrying the E2F-1 null mutation (58) have been described. E7/E2F-1−/− mice were generated by crossing homozygous E7 transgenic mice from line 75a to mice carrying a null mutation in the E2F-1 locus, producing E7/E2F-1+/− F1 mice, which were then intercrossed to generate mice of E7/E2F-1+/+, E7/E2F-1+/−, and E7/E2F-1−/− genotypes. Mice were screened for E7 and E2F-1 status by PCR analysis of DNA prepared from tail biopsy specimens as described (42, 58).
Histological analysis.
Day E13.5 embryos (embryos at day 13.5 of embryogenesis), heads from day E15.5 embryos, and eyes from neonates were fixed in 4% paraformaldehyde overnight at 4°C, transferred to phosphate-buffered saline (PBS), and embedded in paraffin. Embedded samples were sectioned (5 μm thickness), deparaffinized in xylenes, rehydrated through a graded ethanol series, and stained with hematoxylin and eosin. Embryos were staged by designating midday on the day that the vaginal plug was observed as day 0.5 in development. At least 10 sections from at least five different animals for each developmental time point were examined.
In situ detection of proliferation.
5-Bromo-2′-deoxyuridine (BrdU) (100 μg/g of body weight) plus 5-fluoro-2′-deoxyuridine (FrdU) (6.7 μg/g of body weight) was dissolved in PBS and injected into either pregnant mothers or neonates and allowed to incorporate for 1 h. Upon sacrifice, day E13.5 embryos, heads from day E15.5 embryos, and eyes from neonates were fixed in 10% formalin overnight at 4°C, transferred to PBS, and embedded in paraffin. Nuclei which had incorporated BrdU were identified immunohistochemically by using a BrdU Staining kit (Oncogene Research), as described (53). The numbers of BrdU-positive nuclei (brown) and total nuclei in the fiber cell compartment and epithelium were counted separately on at least six different sections per lens from each of three to five animals, and the data were averaged. From these data, a proliferative index (percent BrdU-positive cells) was calculated, and finally the proliferative index for lenses from nontransgenic or E7/E2F-1−/− mice relative to proliferation in lenses from E7/E2F-1+/− or E7/E2F-1+/+ transgenic mice was calculated. The proliferation (or germinative) and transitional zones of the epithelium in nontransgenic (and similarly E7 transgenic) mice were defined according to the definitions of McAvoy for the postnatal day 1 rat lens (32, 33). The proliferation zone extends from the lens equator where the last epithelial cell with the long axis perpendicular to the epithelium-fiber junction is located anteriorly to the point where the percent BrdU-positive cells became markedly reduced. The epithelial portion of the transitional zone was defined as extending from the equator posteriorly to the point where nuclei became positioned off the capsular surface and where the cells curved anteriorly so that their apices touched the epithelium. This position corresponds approximately to the point at which β-crystallin proteins are first detected in the lenses of neonatal mice (20). Standard errors and statistical significance were determined by using an unpaired Student’s t test with Instat computer software (Graphpad Software).
In situ detection of apoptosis.
Upon sacrifice, day E13.5 embryos, heads from day E15.5 embryos, and eyes from neonates were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned (5-μm thickness). Apoptosis was detected in situ by using an ApopTag kit (Oncor) as previously described (43). For each genotype at each developmental stage, the number of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL)-positive cells in the fiber cell compartment was counted on each of at least six different sections per individual lens from each of three to five animals. For time points for studies in embryos, the numbers of TUNEL-positive and total nuclei were counted. From these data, an apoptotic index (percent TUNEL-positive cells) was calculated, and finally the apoptotic index for lenses from nontransgenic or E7/E2F-1−/− mice relative to apoptosis for lenses from E7/E2F-1+/− or E7/E2F-1+/+ mice was calculated. Standard errors and statistical significance were determined as described above.
Isolation of lens DNA and analysis of nucleosomal-length fragments.
Total genomic DNA (2 or 4 μg) from lenses of mice of the various genotypes was isolated and nucleosomal-length fragments were resolved on 2% agarose gels as previously described (42). Computer-based densitometric scanning was performed on negatives from three independent gels, and peak areas for the low-molecular-weight nucleosomal-length bands and the high-molecular-weight band of uncleaved DNA were determined by using NIH image computer software (DCRT; NIH). The ratios of peak area for low-molecular-weight DNA to peak area for high-molecular-weight DNA were calculated. The data were averaged, and standard deviations and statistical significance were determined as described above.
Immunoblot analysis of crystallin and MIP26 proteins.
Water-soluble and water-insoluble fractions of lens proteins were isolated as described by Morgenbesser et al. (35). Lenses from several neonatal mice of each genotype were pooled and homogenized in ice-cold 0.1 M Tris, pH 7.4. The water-soluble fraction was separated from the water-insoluble fraction by centrifugation, ice-cold urea buffer (0.1 M Tris [pH 8.0], 7 M urea, 5 mM EDTA) was added to the water-insoluble fraction, and the samples were incubated on ice for 20 min. Protein lysates (0.1, 0.5, and 1 μg) for each genotype were dispensed onto a prewet immobilon-PSQ membrane (Millipore) in a slot blot apparatus (BioRad). The membrane was blocked in 5% milk–0.1% Tween in PBS for 1 h at room temperature (r.t.) followed by incubation with primary antibody to MIP26 or γ-crystallin diluted in the blocking solution (1:5,000) for 1 h at r.t. Blots were washed three times in 0.1% Tween–PBS for 10 min at r.t. followed by incubation with goat anti-rabbit biotinylated antibody (diluted 1:5,000 in blocking solution) for 30 min at r.t. Washed blots were then incubated with streptavidin-horseradish peroxidase-conjugate (2 μg/ml) in 0.1% Tween–PBS for 30 min. Peroxidase activity was detected by chemiluminescence (Amersham). Individual blots were stripped and reblotted for the other protein. Densitometric analysis of at least three blots per protein was performed within the linear range of the film by using NIH image computer software (DCRT; NIH). Standard errors and statistical significance were determined as described above.
RESULTS
Effect of an E2F-1 null mutation on E7’s disruption of lens fiber cell differentiation.
To determine if the lenticular defects elicited by E7’s inactivation of pRb were mediated by E2F-1, we crossed E7 transgenic mice (42) with mice homozygous for an E2F-1 null mutation (58). Lenses from embryonic and neonatal mice that were E7 transgenic and E2F-1 wild type (hereafter referred to as E7 transgenic), E7 transgenic and E2F-1 heterozygous (E7/E2F-1+/−), or E7 transgenic and E2F-1 null (E7/E2F-1−/−) were examined. For comparison, lenses from transgenic mice that were E2F-1 wild type (nontransgenic), E2F-1 heterozygous (E2F-1+/−), or E2F-1 null (E2F-1−/−) were also examined.
Initially the eyes of weanlings were examined for an overt change in the E7 phenotype of the eye when the transgene was placed on the E2F-1−/− background. The eyes of weanling mice that were E2F-1−/− or E2F-1+/− were indistinguishable from those of a nontransgenic weanling. E7 transgenic mice exhibit microphthalmia and cataracts (42). Interestingly, E7/E2F-1−/− weanlings exhibited less severe microphthalmia and cataracts than those exhibited by E7 or E7/E2F-1+/− weanlings.
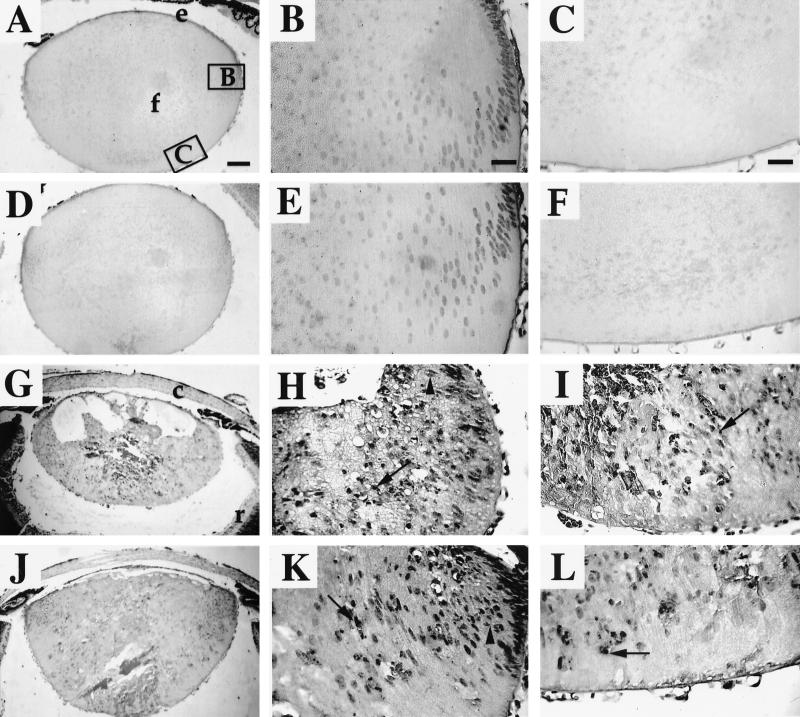
To understand the cellular basis for the differences in the sizes of the eyes, we examined the effect of the mouse’s E2F-1 status on the lens phenotype of the E7 transgenic mice by microscopic analysis. Hematoxylin and eosin-stained eye sections from neonatal mice were examined. The lenses of E2F-1−/− mice were indistinguishable from those of nontransgenic mice (compare Fig. 1A to C with Fig. 1D to F) in that the epithelial cells differentiated into highly elongated fiber cells with appropriate denucleation. The fiber cell compartment was devoid of mitotic cells, which is consistent with the postmitotic state (Fig. 1F). By contrast, the lenses of E7 transgenic mice (Fig. 1G to I) were much smaller and had noticeable large vacuoles in the anterior regions and smaller vacuoles throughout the cortical and posterior regions. In the lenses of the E7 transgenic mice, the cells remained small, rounded, and nucleated rather than differentiating into elongated fiber cells. Mitotic cells were apparent throughout the fiber cell compartment, indicating that proliferation was occurring in an inappropriate region of the lens. Cells with fragmented and pyknotic nuclei were also apparent, suggesting that apoptosis was occurring. Lenses of E7/E2F-1+/− neonates were indistinguishable from those of E7 transgenic littermates (i.e., E7/E2F-1+/+ mice). By contrast, lenses of neonatal E7/E2F-1−/− mice (Fig. 1J to L) exhibited a phenotype intermediate between those of the lenses of nontransgenic mice and E7/E2F-1+/− mice (compare Fig. 1A to C with Fig. 1G to I and Fig. 1J to L). Overall the lenses from the E7/E2F-1−/− mice appeared larger than those from the E7/E2F-1+/− mice. Morphometric measurements indicated that the lenses of E7/E2F-1−/− mice (Fig. 1J) were 20% larger than those of E7 transgenic mice (Fig. 1G), confirming this impression. The large vacuolated regions observed in the lenses of E7/E2F-1+/− mice were reduced in size and number in the lenses of E7/E2F-1−/− mice. Some lens fiber cells of E7/E2F-1−/− mice appeared to be more elongated than those of E7/E2F-1+/− littermates. A decrease in the numbers of mitotic cells in the lenses of the E7/E2F-1−/− mice was observed compared to those of the E7/E2F-1+/− littermates. A decrease in the number of pyknotic and fragmented nuclei in the lenses of E7/E2F-1−/− mice compared to those of E7/E2F-1+/− littermates was also noted. However, mitotic cells and pyknotic nuclei were still noted on the E7/E2F-1−/− background. Similar histological changes were observed at earlier stages of lens development, including days E13.5 and E15.5 (data not shown). These data indicate that E2F-1 is itself dispensable for normal fiber cell differentiation; however, they suggest that E2F-1 is required in part to mediate E7’s effects on fiber cell differentiation.
FIG. 1.
Histology of lenses from neonatal E7 transgenic mice on E2F-1-sufficient or -deficient backgrounds. Paraffin sections (5-μm thickness) of eyes from nontransgenic (A, B, and C), E2F-1−/− (D, E, and F), E7/E2F-1+/− (G, H, and I), and E7/E2F-1−/− (J, K, and L) mice were stained with hematoxylin and eosin. Representative sections are shown. Panels B, E, H, and K show higher magnifications of the right equatorial regions of the lenses shown in A, D, G, and J, respectively (see box B in panel A). Panels C, F, I, and L show higher magnifications of the posterior regions of the lenses shown in panels A, D, G, and J, respectively (see box C in panel A). e, epithelial cells; f, fiber cells; c, cornea; r, retina; arrows point to pyknotic nuclei; arrowhead indicates mitotic figure. Bar, 100 μm for panels A, D, G, and J and 25 μm for other panels. In all panels the anterior of the lens is oriented at the top.
The effect of the E2F-1 null mutation on proliferation in the lens fiber cell compartment in the E7 transgenic mice.
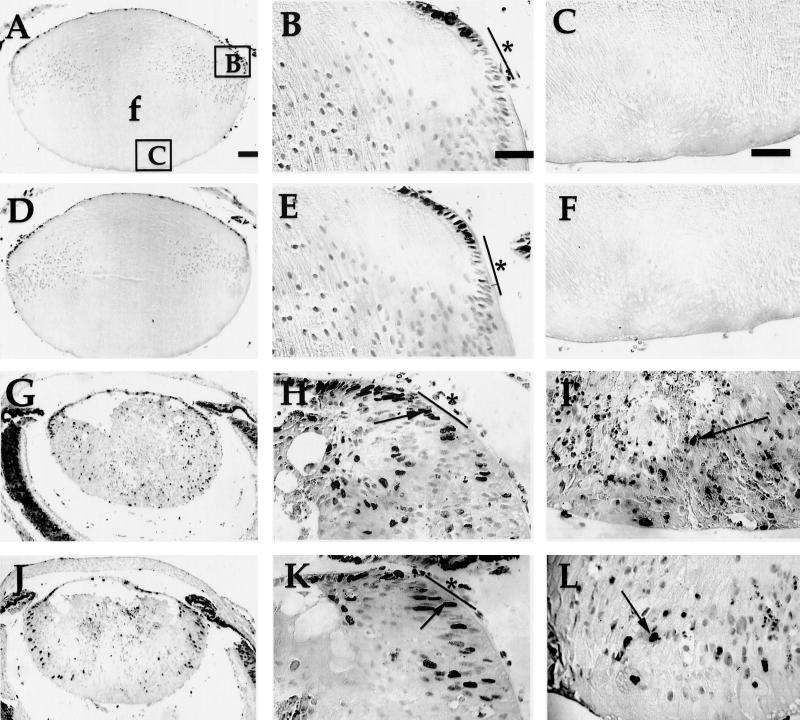
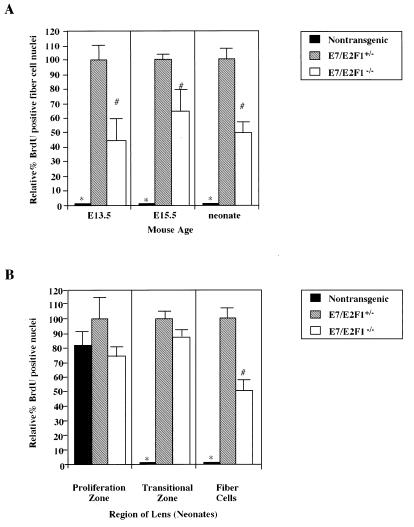
We have shown previously that inactivation of the pRb family by E7 expression in the lens leads to proliferation throughout the fiber cell compartment, the region that normally contains only postmitotic, differentiated cells (42, 43, 53). To determine if the E2F-1 status affected the level of E7-induced proliferation in the fiber cell compartment, we measured the numbers of proliferating cells in this region of lenses from both embryos and neonatal mice using BrdU incorporation assays. In the lenses of E2F-1−/− neonates, the number and pattern of BrdU-labeled nuclei were identical to those displayed in the lenses of nontransgenic neonates (compare Fig. 2D with Fig. 2A). In these lenses, there was no proliferation in the fiber cell compartment (compare Fig. 2E and F with Fig. 2B and C). By contrast, nuclei are BrdU-labeled throughout this compartment in the lenses of E7/E2F-1+/− neonates (Fig. 2G to I). On the E2F-1−/− background, E7-induced proliferation was reduced throughout this compartment (compare Fig. 2G to I with Fig. 2J to L). To quantify this effect of E2F-1 status on E7-induced proliferation, the numbers of BrdU-positive and total nuclei in the fiber cell compartment were counted in multiple sections from mice of the different genotypes and the percentages of nuclei that were BrdU positive (referred to as the proliferative indices) were calculated. The proliferative index for the lenses from the E7/E2F-1+/− mice was 16.3% ± 1.2%, whereas the proliferative index for the lenses from the E7/E2F-1−/− mice was 8.0% ± 0.6%. Thus, the proliferative index for the fiber cells of lenses from the E7/E2F-1−/− mice was 49% of that of lenses from E7/E2F-1+/− mice (see Fig. 5A). At day E13.5 the proliferative index for the lenses from E7/E2F-1−/− embryos was 44% of that found for the lenses of E7/E2F-1+/− littermates (6.3% ± 0.7% and 14.3% ± 2.1%, respectively), and at day E15.5 the proliferative index for the lenses from E7/E2F-1−/− mice was 66% of that found for the lenses of E7/E2F-1+/− littermates (11.3% ± 0.6% and 17.0% ± 1.7%, respectively). The proliferative index for lens sections from E7/E2F-1+/+ mice, determined for a limited number of samples only, appeared to be similar to that for sections from E7/E2F-1+/− mice (data not shown). These data indicate that E7-induced proliferation in the fiber cell compartment is dependent in part on E2F-1 throughout the developmental window examined. Thus, we conclude that E2F-1 is one mediator of E7’s effects on proliferation in a population of cells that normally are differentiated.
FIG. 2.
In situ detection of proliferation in lenses from neonatal E7 transgenic mice on E2F-1-sufficient or -deficient backgrounds by using a BrdU incorporation assay. BrdU incorporated into newly synthesized DNA for 1 h was detected in paraffin sections (5-μm thickness) of neonatal eyes from nontransgenic (A, B, and C), E2F-1−/− (D, E, and F), E7/E2F-1+/− (G, H, and I), and E7/E2F-1−/− (J, K, and L) mice. Representative sections are shown. Panels B, E, H, and K show higher magnifications of the right equatorial regions of the lenses shown in panels A, D, G, and J, respectively (see box B in panel A). Panels C, F, I, and L show higher magnifications of the posterior regions of the lenses shown in panels A, D, G, and J, respectively (see box C in panel A). No detectable signal was observed in control sections from neonates in which BrdU was not injected (data not shown). Arrows point to dark diaminobenzidene-stained nuclei indicating BrdU-positive nuclei; light nuclei are BrdU-negative nuclei. e, epithelial cells; f, fiber cells; bars and asterisks in panels B, E, H, and K denote approximate boundaries of the transitional zone; the proliferation zone is anterior to the transitional zone. (See Materials and Methods section for a more complete definition of these zones.) Bar, 100 μm for panels A, D, G, and J and 50 μm for other panels. In all panels the anterior of the lens is oriented at the top.
FIG. 5.
E2F-1 status affects E7-induced proliferation during lens development. (A) BrdU incorporation assays were performed on sections from day E13.5 and E15.5 embryos and neonatal nontransgenic, E7/E2F-1+/−, and E7/E2F-1−/− mice. The numbers of BrdU-positive and total nuclei in the fiber cell compartment (means ± standard errors of the means) from at least six central lens sections from each of three to five independent samples per genotype were counted. The data were averaged, proliferative indices (percent BrdU-positive nuclei) and the percent BrdU-positive nuclei in each genotype relative to that in lenses from E7/E2F-1+/− mice were calculated and subjected to statistical analyses (see the Materials and Methods and Results sections). The relative percents BrdU-positive nuclei for genotypes marked (with ∗ and #) were significantly different (P < 0.05) from those for the other groups. (B) The BrdU-labeled sections for which data are presented in panel A were used to assess spatial patterns of proliferating cells in the lens. The relative percent BrdU-positive nuclei in the proliferation and transitional zones of the epithelium was determined separately for each region. The data for each region were averaged and subjected to statistical analysis. The relative percents BrdU-positive nuclei for genotypes marked (with ∗ or #) were significantly different (P < 0.05) from those for other groups.
The effect of the E2F-1 null mutation on apoptosis in the lens fiber cell compartment of the E7 transgenic mice.
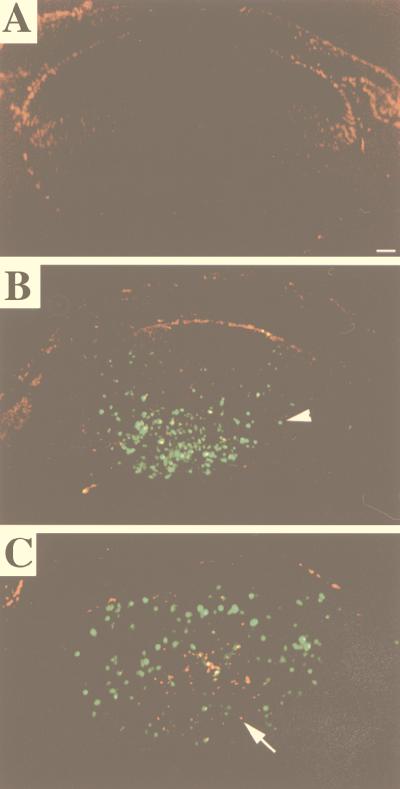
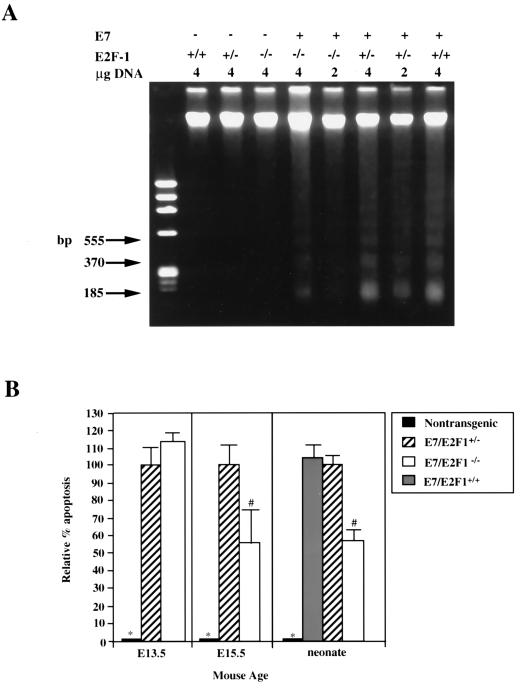
We have shown previously that E7 expression in the lens leads to apoptosis in the fiber cell compartment (42, 43). To determine if the E2F-1 status affected the extent of E7-induced apoptosis, we performed both TUNEL and DNA ladder analyses on lenses of nontransgenic, E7/E2F-1+/−, and E7/E2F-1−/− mice. TUNEL analysis indicated that E7 expression induced apoptosis in the fiber cell compartment whereas apoptosis is not observed in the fiber cell compartment in the lenses of nontransgenic neonates (compare Fig. 3A and B) and that E7-induced apoptosis was reduced in the E2F-1−/− background. This reduction appeared to occur uniformly across the fiber cell compartment (compare Fig. 3B and C). To quantify the effect of E2F-1 status on E7-induced apoptosis, DNA ladder analyses were performed on 2- or 4-μg samples of DNA isolated from the lenses of neonatal mice of various genotypes (Fig. 4). The amount of DNA in the low-molecular-weight range relative to the amount of high-molecular-weight DNA for each sample was calculated, and this ratio was compared to that for the E7/E2F-1+/− mice. E2F-1 status alone (E2F-1+/+, E2F-1+/−, or E2F-1−/−) did not alter the level of apoptosis as no DNA fragmentation was observed in any of these three samples. The levels of apoptosis in lenses of E7/E2F-1+/+ and E7/E2F-1+/− neonates also did not differ (104% and 100%, respectively). However, the level of apoptosis in lenses of E7/E2F-1−/− neonates was 57% of that found in lenses of E7/E2F-1+/− littermates. A similar level of reduction was observed by using TUNEL analyses (data not shown).
FIG. 3.
In situ detection of apoptosis in lenses from neonatal E7 transgenic mice on E2F-1-sufficient or -deficient backgrounds by using TUNEL analysis. Paraffin sections (5-μm thickness) of neonatal eyes from nontransgenic (A), E7/E2F-1+/− (B), and E7/E2F-1−/− (C) mice were subjected to a fluorescein-TUNEL assay and counterstained with propidium iodide. Representative sections are shown. No detectable signal was observed in control sections from which terminal deoxytransferase was omitted (data not shown). The arrowhead indicates TUNEL-positive nuclei, which are green or yellow, whereas the arrow points to TUNEL-negative nuclei, which are red. Bar, 100 μm. In all panels the anterior of the lens is oriented at the top.
FIG. 4.
Analysis of apoptosis in lenses from neonatal E7 transgenic mice on E2F-1-sufficient or -deficient backgrounds by using DNA ladder and TUNEL analysis. (A) Total genomic DNA was isolated from lenses of neonatal mice, fractionated on a 2% agarose gel, and stained with ethidium bromide. Indicated for each lane are the genotype of the sample and amount of total genomic DNA loaded on the gel. φX174/HaeIII DNA was used as a molecular weight marker (left lane). The migration positions of the three lowest nucleosomal-length bands that correspond with monomers (185 bp), dimers (370 bp), and trimers (555 bp) are indicated. The abundance of small nucleosomal-length DNA fragments and that of high-molecular-weight fragments (means ± standard errors of the means) were quantified from scans of three independent negatives. Ratios were calculated, and the data were averaged and subjected to statistical analysis (see the Materials and Methods section and the Results section). The levels of apoptosis for the E7/E2F-1+/− mice and E7/E2F-1−/− mice were significantly different from those for the three genotypes lacking E7 (P < 0.01; see panel B, right). (B) TUNEL analyses were performed on sections from day E13.5 and day E15.5 embryos (left and middle, respectively) from nontransgenic, E7/E2F-1+/−, and E7/E2F-1−/− mice. Apoptotic indices (percent apoptosis) and relative apoptotic indices for each genotype compared to the apoptosis in lenses from E7/E2F-1+/− mice were calculated and subjected to statistical analyses (see the Materials and Methods and Results sections). The relative percents apoptosis for lenses from neonates (right) were calculated from DNA ladder analyses (see the Materials and Methods and Results sections and panel A). The relative percents apoptosis for the genotypes marked (with ∗ or #) were significantly different (P < 0.05) from that for other groups.
To quantify the effect of E2F-1 status on E7-induced apoptosis at earlier developmental stages, the numbers of TUNEL-positive nuclei and total nuclei in the fiber cell compartment were counted in multiple sections of lenses from several E7/E2F-1+/− and E7/E2F-1−/− mice. The percent of nuclei in the fiber cell compartment that were TUNEL-positive was calculated (referred to as the apoptotic index). The apoptotic index for the lenses from the day E13.5 E7/E2F-1+/− embryos was similar to that for the lenses from E7/E2F-1−/− mice (apoptotic indices of 6.2% ± 0.7% and 7.2% ± 0.6%, respectively; see Fig. 4B). At day E15.5 the apoptotic index for lenses from E7/E2F-1−/− mice was 56% of that found for the lenses of E7/E2F-1+/− littermates (11.6% ± 0.6% and 20.8% ± 1.7%, respectively; see Fig. 4B). The apoptotic index for lenses from E7/E2F-1+/+ embryos was similar to that for lenses from E2F-1+/− embryos (data not shown). Thus, the E2F-1 null mutation partially rescues the E7-induced apoptosis at least by day E15.5. Because loss of E2F-1 partially rescues the lens from E7-induced apoptosis, these results indicate that E2F-1 is one mediator of E7-induced apoptosis in the fiber cell compartment or that E7-induced apoptosis is partially dependent on E2F-1.
The effect of the E2F-1 null mutation on proliferation and apoptosis in the lens epithelium in the E7 transgenic mice.
To determine if the E7 or E2F-1 status affected the level of proliferation in the epithelium, the proliferative indices for this cell layer of lenses from both embryonic and neonatal mice were measured by BrdU incorporation. The numbers of BrdU-positive nuclei and total nuclei in the proliferation (germinative) zone and the epithelial portion of the transitional zone were counted separately in multiple sections from lenses of several mice of each genotype. The proliferative index was determined for each zone for each genotype and compared to that for the E7/E2F-1+/− mice to determine the relative proliferative index (Fig. 5B). The proliferative indices for in the proliferation zone did not differ significantly among the nontransgenic, E7/E2F-1+/−, and E7/E2F-1−/− genotypes (21% ± 2.1%, 26% ± 3.6%, and 19% ± 1.1%; Fig. 5B) (compare Fig. 2B and D with Fig. 2H and K). For the transitional zone the proliferative index for lenses from the E7/E2F-1−/− mice was 87% of that for lenses from E7/E2F-1+/− mice, which was only marginally significantly different (38.7% ± 1.9% and 44.7% ± 1.8%, respectively; P = 0.08). However, the proliferative index for the transitional zone in lenses from the nontransgenic mice was <2%, similar to estimates made for the lenses of neonatal rats (32). Therefore, the proliferative index in this region of the nontransgenic mouse lens is significantly different from that of the E7/E2F-1+/− or E7/E2F-1−/− mice. The E7-induced proliferation observed in the transitional zone is consistent with the fact that E7 transcripts were easily detected in this zone in lenses from E7 transgenic neonates of this line (41). Analyses performed on sections from BrdU-injected day E13.5 and day E15.5 embryos provided findings similar to those for neonatal mice (data not shown). These data indicate that the E2F-1 null mutation did not significantly affect the E7-induced proliferation in the transitional zone of the epithelium, in contrast to its requirement in the fiber cell compartment. Thus, despite the fact that cells in both the fiber cell compartment and the transitional zone of the epithelium are normally postmitotic, proliferation in the transitional zone of the epithelium appeared to be not dependent on E2F-1.
To determine if the E7 or E2F-1 status affected the level of apoptosis in the epithelium, we counted the numbers of TUNEL-positive cells in this region of lenses from neonatal mice (data not shown). The expression of E7 led to minimal increases in the numbers of TUNEL-positive cells in both the proliferation and transitional zones compared to those in the same regions in lenses of nontransgenic neonates. However, the absence of E2F-1 in the E7 transgenic mice did not significantly alter the numbers of TUNEL-positive cells counted in these zones. These data indicate that the small increase in apoptosis in the epithelium caused by E7 is not dependent on E2F-1. Therefore, induced proliferation and apoptosis in these cells must require a set of factors different from those required by these processes in cells of the fiber cell compartment.
Effect of the E2F-1 null mutation on differentiation in the lens of E7 transgenic mice.
The more-normal histological appearance of the lenses of E7/E2F-1−/− mice as compared to the lenses of E7/E2F-1+/− mice (Fig. 1) suggests that loss of E2F-1 might correlate with a partial rescue of E7-disrupted differentiation. The 26-kDa major intrinsic membrane protein, MIP26 (5), and γ-crystallin (34, 46) are two differentiation-specific lens proteins that are normally distributed subcellularly in a distinct proportion in fiber cells. MIP26 is primarily a water-insoluble protein that is localized to the plasma membrane of differentiated fiber cells (3). Normally, a large percentage of γ-crystallins is soluble protein; however, some is found to be water insoluble. Disruption of differentiation and formation of cataracts have been associated with mutations in MIP26 (52) or γ-crystallin (19) genes and with alterations in the amounts or distribution of MIP26 (35) and γ-crystallins (18, 50) in water-soluble and membrane-bound fractions.
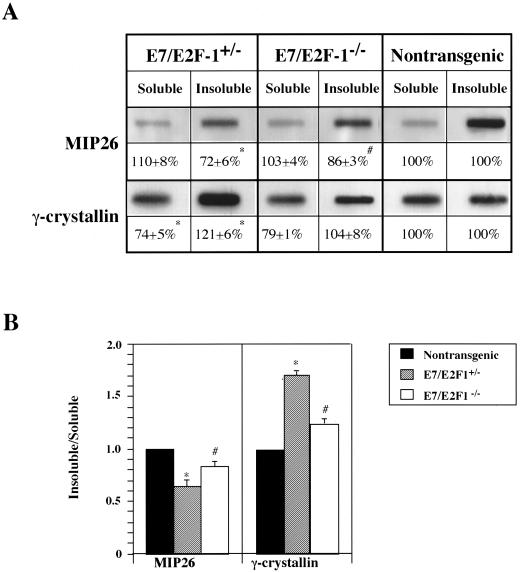
To determine at the biochemical level if E2F-1 loss correlated with improved differentiation of fiber cells in the E7 transgenic mice, the distribution of MIP26 and γ-crystallin proteins to water-soluble and water-insoluble, urea-soluble fractions of the cell was measured in varying amounts of lens lysates (0.1, 0.5, and 1 μg) from E7/E2F-1+/−, E7/E2F-1−/−, and nontransgenic neonates by immunoblot analysis. Relative to the lens lysates from nontransgenic mice (set at 100%), lysates from the E7 transgenic (not shown) or E7/E2F-1+/− mice contained a reduced amount of water-insoluble MIP26 (72% ± 6%) and lysates from the E7/E2F-1−/− mice contained an intermediate amount of water-insoluble MIP26 (86% ± 3%; Fig. 6A). The levels of water-soluble MIP26 did not significantly differ between genotypes (Fig. 6A). Therefore, the ratio of water-insoluble MIP26 to water-soluble MIP26 in the lens lysates from the E7/E2F-1+/− mice (0.65) was lower than that observed in lysates from E7/E2F-1−/− mice (0.84; Fig. 6B), indicating inappropriate localization of MIP26. These data indicate that E7 action, in part mediated by E2F-1, results in a loss of MIP26 from the membrane-bound fraction of the fiber cell.
FIG. 6.
Distribution of MIP26 and γ-crystallin protein to soluble and insoluble fractions in lenses of neonatal E7 transgenic mice on E2F-1-sufficient or -deficient backgrounds determined by using immunoblot analysis. (A) Water-soluble and water-insoluble, urea-soluble lysates (0.1, 0.5, and 1 μg) of lenses from E7/E2F-1+/−, E7/E2F-1−/−, and nontransgenic mice were immunoblotted sequentially with polyclonal murine MIP26 and γ-crystallin antisera. Representative immunoblots of each are shown. Negative controls, i.e., water-soluble and water-insoluble murine brain lysates, revealed no protein binding with either antisera. For each genotype, expression levels for the proteins (means ± standard errors of the means) relative to the nontransgenic level (100%), determined by densitometric analysis, are indicated. The average percents for levels of expression for the genotypes marked (with ∗ or #) were significantly different (P < 0.01) from those for other groups. (B) The ratio of water-insoluble protein relative to water-soluble protein for each genotype for which data are presented in panel A was calculated and plotted. The ratios for genotypes marked (with ∗ or #) were significantly different (P < 0.05) from those for the other groups.
The content of water-insoluble γ-crystallin was 21% greater in lens lysates from the E7/E2F-1+/− mice than that in lysates from nontransgenic mice (121% ± 6%), whereas the level in lysates from E7/E2F-1−/− mice was very similar to that in lysates from nontransgenic mice (104% ± 8%; Fig. 6A). Relative to the lenses of nontransgenic mice the lenses of E7/E2F-1+/− mice contained the least amount of water-soluble γ-crystallin (74% ± 5%), while the lenses of E7/E2F-1−/− mice contained a level (79% ± 1%) marginally higher than that in the lenses of E7/E2F-1+/− mice. Interestingly, the quotient of the ratios of water-insoluble to water-soluble γ-crystallin for E7/E2F-1+/− mice and nontransgenic mice (1.70) was much higher than that for E7/E2F-1−/− mice and nontransgenic mice (1.32; Fig. 6B). These data indicate that a shift in subcellular distribution of γ-crystallin has occurred as a consequence of E7 action and that the shift is mediated in part by E2F-1. Because the E2F-1 null mutation partially rescues the aberrant shift in subcellular distribution of both MIP26 and γ-crystallin that is associated with E7-disrupted fiber cell differentiation, we conclude that E2F-1 is one mediator of E7’s disruption of lens fiber cell differentiation at the biochemical level.
DISCUSSION
Numerous studies document the important role that E2F-1 plays in control of cell proliferation, apoptosis, and transformation in vitro (8, 25, 28, 47, 51, 56). Recent work in vivo in mice carrying a null mutation in E2F-1 have demonstrated that E2F-1 can act positively or negatively to control cell growth in a tissue-specific manner (15, 58). In this study we have asked what function E2F-1 plays as a mediator of the activities of the HPV-16 E7 oncoprotein in vivo in the developing mouse lens. Our study is the first to indicate that in vivo E2F-1, while dispensable for normal lens development, is a mediator of E7 action. Two other recent studies also address the role of E2F-1 in supporting aberrant proliferation, apoptosis, and tumorigenesis in vivo. First, aberrant proliferation and apoptosis in the developing nervous system of RB−/− mouse embryos (30) is mediated in part by E2F-1 (55). Second, tumorigenesis associated with aberrant proliferation and apoptosis in the choroid plexus of mice expressing a truncated SV40 tag (that binds pRb but not p53 [54]) is mediated in part by E2F-1 (44). Collectively, these studies indicate a genetic requirement for E2F-1 in mediating cell proliferation and apoptosis and interfering with normal cell differentiation when RB protein(s) is inactivated. Based upon the knowledge that pRb is a modulator of E2F-1 activity, these genetic analyses strongly suggest that this regulation is important for control of development and tumorigenesis.
E2F-1 is dispensable for normal fiber cell differentiation but is required to mediate E7’s disruption of fiber cell differentiation.
Previously, Yamasaki et al. (58) reported that E2F-1−/− mice developed normally. In this study we have shown that the lenses of the E2F-1−/− mice are indistinguishable from the lenses in their E2F-1 wild-type counterparts, as defined by morphology, proliferation, and apoptosis analyses. The loss of E2F-1 from the lens’ epithelium and fiber cell compartment without consequence to the tissue suggests that either E2F-1 plays no required role in the lens or any function for E2F-1 can be provided by another E2F family member due to either redundancy or compensation when E2F-1 is mutated in the embryo. In the epithelium, all members of the RB and E2F families are expressed (48). Since pRb binds E2F-1, E2F-2, or E2F-3 in vitro and these proteins are also able to induce S phase (8), either E2F-2 or E2F-3 may functionally substitute for the lost function of E2F-1 in the epithelium. The newly differentiating lens fibers express only a subset of RB and E2F family members, including pRb, p107, E2F-1, E2F-3, and E2F-5 (41, 48), and p107 and pRb-containing E2F complexes which may compensate for the lost E2F-1 in these cells have been identified (48). However, an alternative interpretation of these data is that E2F-1 complexed to pRb normally acts as a negative regulator of gene expression during differentiation and therefore, loss of E2F-1 by mutation would have no effect.
It is well known from previous studies in vitro (2, 13, 14, 37) and in vivo (22, 42, 43) that E7, dependent on its ability to bind to and inactivate pRb and/or pRb-like proteins, disrupts normal cell cycle control, interferes with cellular differentiation, and induces apoptosis. Due to pRb’s role in regulating E2F-1 activity, it has been hypothesized that E7’s effects are mediated at least in part through deregulation of E2F-1 (4, 39). In this study we provide the first genetic evidence that in vivo E2F-1 is a mediator of E7 activity. On the cellular level, each aspect of lens fiber cell differentiation that is known to be disrupted by E7 (Fig. 1, 2, and 6) and the consequent apoptosis (Fig. 3 and 4) that ensues is reduced when the E7 transgene is placed on an E2F-1−/− background. Therefore, E2F-1 plays a major role in E7’s disruption of fiber cell differentiation.
In E2F-1−/− mice the lens appears normal, while in E7/E2F-1−/− mice loss of E2F-1 reduces the severity of the E7 phenotype. Because loss of E2F-1 reduces the effects of E7, in fiber cells E2F-1 does have the potential to perform a unique role that cannot be completely compensated for by other family members. These findings are consistent with the simple model in which during normal fiber cell differentiation a subset of genes, such as those promoting cell cycle progression, and/or heretofore unidentified targets are negatively regulated by pRb-E2F-1 complexes. When the putative pRb/E2F-1 complexes are disrupted by E7, free E2F-1 in part is responsible for mediating E7’s effects by activating or derepressing cellular targets promoting cell growth and apoptosis. However, our data to date demonstrate only a genetic requirement for E2F-1. Therefore, other models in which loss of E2F-1 disrupts the regulation of expression of RB and/or other E2F genes may also explain our observations.
We have shown that in the lens, E2F-1 is partially responsible for mediating E7’s effects on proliferation, differentiation, and apoptosis. Whereas a reduction in E7-induced proliferation was clearly measurable even at day E13.5, the reduction in apoptosis was not measurable until a later time point. However, at later time points, the level of rescue provided by the E2F-1 null mutation for E7-induced proliferation was approximately equal to that for apoptosis. These observations might tend to favor a model in which the primary effect of E2F-1 in the E7-expressing lens cell is to support proliferation in inappropriate regions of the lens and the effects of E2F-1 on E7-induced apoptosis are secondary. Similarly, the effects of the E2F-1 null mutation on the E7-induced inhibition of fiber cell elongation (Fig. 1) and the subcellular localization of differentiation-specific marker proteins γ-crystallin and MIP26 (Fig. 6) could be secondary because it may not be possible for lens cells to undergo normal differentiation if they cannot withdraw from the cell cycle. However, at the present level of analysis, we cannot discount the possibility that E2F-1 might have direct independent effects on multiple subsets of genes within the E7-expressing cell, i.e., those regulating proliferation, those regulating apoptosis, as has been recently suggested (24, 45), and those regulating differentiation.
The fact that E2F-1 status can modulate all of these aspects of the lens phenotype in E7 transgenic mice argues that E2F-1 is positioned more proximal to E7 than p53 is because loss of p53 leads to a reduction in apoptosis but not a reduction in E7-induced proliferation or inhibition of morphological differentiation (43). While earlier studies argue that E2F-1 lies in a p53-dependent apoptotic pathway (56), more recent studies suggest that E2F-1 can lie in both p53-dependent and p53-independent apoptotic pathways (24, 45, 56). In the lens, there are temporal and spatial distinctions between E7-induced p53-dependent apoptosis and p53-independent apoptosis. E7-induced apoptosis in the early stages of differentiation (at day E13.5) is p53-dependent while later, by day E17.5, E7-induced apoptosis occurs through both p53-dependent and p53-independent pathways. Spatially, p53-independent apoptosis is seen primarily in the nuclear (central) region of the lens while p53-dependent apoptosis is found in the cortical (peripheral) region (43). Phenotypically, the E2F-1 null mutation rescues apoptosis from day E15.5 through the neonate stage (Fig. 5) and reduces apoptosis throughout the lens, with no bias towards rescue in the cortical or nuclear region (Fig. 3). These data might suggest that E2F-1 lies in both p53-dependent and p53-independent pathways leading to apoptosis in the lens and/or that the pathways diverge downstream of E2F-1. Further studies will be required to ascertain with more certainty if this is the case.
Factors in addition to E2F-1 are required to mediate E7’s effects on fiber cell differentiation.
In this study, we have shown that E2F-1 null mutation can partially (50%) rescue E7-induced proliferation, apoptosis, and inhibition of differentiation in the lens. This inability of the E2F-1 null mutation to completely rescue the E7-induced proliferation and apoptosis defects is similar to its inability to completely rescue the same defects in transgenic mice expressing a truncated version of the SV40 T antigen (44), especially where the proliferative defect is concerned. Because rescue is only partial, there must be other factors whose activities are disrupted by these viral oncoproteins. Members of the RB and E2F families in addition to RB and E2F-1 are the most likely candidates. The possibility that RB family members other than pRb are targeted by E7 in the lens is further suggested by the comparison of the ability of E2F-1 null mutation to rescue the E7 lens phenotype at day E13.5 and the ability of this mutation to rescue the effects of the RB null mutation in the lens at this same time in development (55). In the latter case, the proliferation and apoptosis indices on the RB−/−/E2F-1−/− background were 27% and 5%, respectively, of those observed on the solely RB−/− background. This large difference between the efficacies of the E2F-1 null mutation in rescuing the E7 and RB−/− phenotypes, especially with regards to apoptosis, strongly suggests that E7 has effects on factors in the lens in addition to pRb and that these factors may play roles in the lens that heretofore have been unappreciated. Interestingly, the results of Tsai et al. (55) also indicate that RB itself must have targets, in addition to E2F-1, that are involved in mediating aberrant proliferation in the lens. This suggests that pRb itself might normally regulate the activities of other factors during lens differentiation.
Thus, other RB and E2F family members may be affected by E7. Lens fiber cells are known to express p107 (48), which could be a target for E7. It is also possible that the downregulation of p130 that normally occurs during lens fiber cell differentiation (48) has not occurred in lenses expressing E7 and, if so, E7 may also be interfering with p130 function. E7’s disruption of pRb function can be predicted to disrupt the function of E2F-3, which is known to be expressed in lens fiber cells (48), suggesting the simple explanation that E2F-3, which has known functional overlap with E2F-1, may contribute to the residual proliferation in the fiber cell compartment in the lenses of the E7/E2F-1−/− mice. Alternatively, more complicated scenarios in which levels of other E2Fs are altered, allowing these family members to partially compensate for loss of E2F-1, may arise.
It is possible as well that E7 disrupts complexes formed between RB family members and proteins other than E2F family members, such as MDM2, which can bind to and activate E2F-1 (31) and also bind and block pRb function (57), or differentiation-specific factors. For example, pRb binds to MyoD and myogenin during myogenesis (21). Lastly, E7 could affect proliferation and apoptosis by binding to other cell cycle regulators, such as the cyclin-dependent kinase inhibitors p21 (17, 26) and p27 (59).
E2F-1 mediates E7-induced effects on lens development in a context-dependent manner.
Previously, we showed that E7 induced proliferation and apoptosis in cells residing in the differentiated fiber cell compartment of the lens (42). E7 also has been shown to induce proliferation when expressed in the basal layer of the epidermis; however, increased apoptosis in that layer was not observed (22). In the present study we found that in the transitional epithelial cells, E7 induced proliferation and only marginally induced apoptosis and that neither effect was E2F-1 dependent. Thus, the genetic factors required for proliferation and apoptosis differ between the normally postmitotic cells in the epithelium and those in the fiber cell compartment. While transitional epithelial cells are postmitotic, they are positionally different from normal fiber cells or E7-expressing cells in the fiber cell compartment and they have different biochemical characteristics. These data support the concept that the E2F-1 requirement is context dependent.
Recently, Yamasaki et al. (58) reported that loss of E2F-1 reduces tumorigenesis and extends the life span of RB+/− mice in a tissue- and mouse strain-dependent manner. In our study, we noted no convincing effect of E2F-1 gene dosage on phenotype, supporting the idea that the gene dosage effects of E2F-1 are strain specific. Importantly, however, our data suggest that the context dependency of E2F-1 in regulating proliferation and apoptosis at least in the mouse lens may operate at a level even more specific than strain or tissue dependency. Even within the same tissue, composed of one cell type, the requirement for E2F-1 can differ depending on the developmental or positional context of the cell.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01-EY09091 and F32-EY06709 and American Cancer Society grant VM-164.
We thank Angie Buehl and Andrea Frassetto for technical assistance, Terry van Dyke and Tyler Jacks for sharing data prior to publication, and Joe Horwitz (UCLA) and Debbie Carper (NEI) for providing the MIP26 antibodies and γ-crystallin antibodies, respectively.
REFERENCES
- 1.Adams P, Kaelin W J. The cellular effects of E2F overexpression. In: Farnham P, editor. Transcriptional control of cell growth: the E2F gene family. Vol. 208. New York, N.Y: Springer-Verlag; 1996. pp. 79–94. [DOI] [PubMed] [Google Scholar]
- 2.Banks L, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. [PubMed] [Google Scholar]
- 3.Broekhuyse R M, Kuhlman E D, Stols A L. Lens membranes. VII. MIP is an immunologically specific component of lens fiber membranes and is identical with the 26K band protein. Exp Eye Res. 1979;29:303–313. doi: 10.1016/0014-4835(79)90009-5. [DOI] [PubMed] [Google Scholar]
- 4.Chellappan S, Kraus V B, Kroger B, Munger K, Howley P M, Phelps W C, Nevins J R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chepelinsky A E. The MIP transmembrane channel family. In: Peracchia C, editor. Handbook of membrane channels: molecular and cellular physiology. New York, N.Y: Academic Press; 1994. pp. 413–432. [Google Scholar]
- 6.Cobrinik D. Regulatory interactions among E2Fs and cell cycle control proteins. In: Farnham P, editor. Transcriptional control of cell growth: the E2F gene family. Vol. 208. New York, N.Y: Springer-Verlag; 1996. pp. 31–62. [DOI] [PubMed] [Google Scholar]
- 7.Cress W, Nevins J. Use of the E2F transcription factor by DNA tumor virus regulatory proteins. Curr Top Microbiol Immunol. 1996;208:63–78. doi: 10.1007/978-3-642-79910-5_3. [DOI] [PubMed] [Google Scholar]
- 8.DeGregori J, Leone G, Miron A, Jakoi L, Nevins J R. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du W, Vidal M, Xie J-E, Dyson N. RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila. Genes Dev. 1996;10:1206–1218. doi: 10.1101/gad.10.10.1206. [DOI] [PubMed] [Google Scholar]
- 10.Du W, Xie J, Dyson N. Ectopic expression of dE2F and dDP induces cell proliferation and death in the Drosophila eye. EMBO J. 1996;15:3684–3692. [PMC free article] [PubMed] [Google Scholar]
- 11.Duronio R, O’Farrell P, Xie J-E, Brook A, Dyson N. The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev. 1995;9:1445–1455. doi: 10.1101/gad.9.12.1445. [DOI] [PubMed] [Google Scholar]
- 12.Dynlacht B, Brook A, Dembski M, Yenush L, Dyson N. DNA-binding and trans-activation properties of Drosophila E2F and DP proteins. Proc Natl Acad Sci USA. 1994;91:6359–6363. doi: 10.1073/pnas.91.14.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyson N, Howley P, Munger K, Harlow H E. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 15.Field S, Tsai F, Kuo F, Zubiaga A, Kaelin W G, Jr, Livingston D, Orkin S, Greenberg M. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 16.Fromm L, Shawlot W, Gunning K, Butel J S, Overbeek P A. The retinoblastoma protein-binding region of simian virus 40 large T antigen alters cell cycle regulation in lenses of transgenic mice. Mol Cell Biol. 1994;14:6743–6754. doi: 10.1128/mcb.14.10.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funk J O, Waga S, Harry J B, Espling E, Stillman B, Galloway D A. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11:2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garner W H, Garner M H, Spector A. Gamma-crystallin, a major cytoplasmic polypeptide disulfide linked to membrane proteins in human cataract. Biochem Biophys Res Commun. 1981;98:439–447. doi: 10.1016/0006-291x(81)90859-7. [DOI] [PubMed] [Google Scholar]
- 19.Graw J. Cataract mutations as a tool for developmental genetics. Ophthalmic Res. 1996;28(Suppl. 1):8–18. doi: 10.1159/000267936. [DOI] [PubMed] [Google Scholar]
- 20.Griep A, Westphal H. Differentiation versus proliferation of transgenic mouse lens cells expressing polyoma large T antigen: evidence for regulation by endogenous growth factor. New Biol. 1990;2:727–738. [PubMed] [Google Scholar]
- 21.Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 22.Herber R, Pitot L A, H, Lambert P F. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur J Biochem. 1997;246:581–601. doi: 10.1111/j.1432-1033.1997.t01-2-00581.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh J-K, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- 25.Johnson D G, Schwartz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 26.Jones D L, Alani R M, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11:2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones D L, Thompson D A, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 28.Kowalik T F, DeGregori J, Schwarz J K, Nevins J R. E2F1 overexpression in quiescent fibroblasts leads to an induction of cellular DNA synthesis and apoptosis. J Virol. 1995;69:2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin S-C, Skapek S, Lee E Y-H P. Genes in the RB pathway and their knockout in mice. Semin Cancer Biol. 1996;7:279–289. doi: 10.1006/scbi.1996.0036. [DOI] [PubMed] [Google Scholar]
- 30.MacLeod K F, Hu Y, Jacks T. Loss of RB activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 1996;15:6178–6188. [PMC free article] [PubMed] [Google Scholar]
- 31.Martin K, Trouche D, Hagemeier C, Sorensen T S, Thangue N B L, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995;375:691–697. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- 32.McAvoy J. Cell division, cell elongation and distribution of α, β, and γ-crystallins in the rat lens. J Embryol Exp Morphol. 1978;44:149–165. [PubMed] [Google Scholar]
- 33.McAvoy J. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morphol. 1978;45:271–281. [PubMed] [Google Scholar]
- 34.McAvoy J W. Induction of the eye lens. Differentiation. 1980;17:137–149. doi: 10.1111/j.1432-0436.1980.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 35.Morgenbesser S D, Schreiber A N, Bidder M, Mahon K A, Overbeek P A, Horner J, DePinho R A. Contrasting roles for c-Myc and L-Myc in the regulation of cellular growth and differentiation in vivo. EMBO J. 1995;14:743–756. doi: 10.1002/j.1460-2075.1995.tb07053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenbesser S D, Williams B O, Jacks T, DePinho R A. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- 37.Munger K, Phelps W C, Bubb V, Howley P M, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munger K, Werness B A, Dyson N, Phelps W C, Harlow E, Howley P M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevins J R. E2F; a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 40.Ohtani K, Nevins J. Functional properties of a Drosophila homolog of the E2F1 gene. Mol Cell Biol. 1994;14:1603–1612. doi: 10.1128/mcb.14.3.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan H. Ph.D. thesis. University of Wisconsin, Madison; 1995. [Google Scholar]
- 42.Pan H, Griep A E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994;8:1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- 43.Pan H, Griep A E. Temporally distinct patterns of p53-dependent and p53-independent apoptosis during mouse lens development. Genes Dev. 1995;9:2157–2169. doi: 10.1101/gad.9.17.2157. [DOI] [PubMed] [Google Scholar]
- 44.Pan H, Yni C, Dyson N J, Harlow E, Yamasaki L, Van Dyke T. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol Cell. 1998;2:283–292. doi: 10.1016/s1097-2765(00)80273-7. [DOI] [PubMed] [Google Scholar]
- 45.Phillips A C, Bates S, Ryan K M, Helin K, Vousden K H. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev. 1997;11:1853–1863. doi: 10.1101/gad.11.14.1853. [DOI] [PubMed] [Google Scholar]
- 46.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 47.Qin X Q, Livingston D M, Kaelin W G J, Adams P D. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci USA. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rampalli A M, Gao C Y, Chauthaiwale V M, Zelenka P S. pRb and p107 regulate E2F activity during lens fiber cell differentiation. Oncogene. 1998;16:399–408. doi: 10.1038/sj.onc.1201546. [DOI] [PubMed] [Google Scholar]
- 49.Royzman I, Whittaker A J, Orr-Weaver T L. Mutations in Drosophila DP and E2F distinguish G1-S progression from an associated transcriptional program. Genes Dev. 1997;11:1999–2011. doi: 10.1101/gad.11.15.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell P, Smith S G, Carper D A, Kinoshita J H. Age and cataract-related changes in the heavy molecular weight proteins and gamma crystallin composition of the mouse lens. Exp Eye Res. 1979;29:245–255. doi: 10.1016/0014-4835(79)90005-8. [DOI] [PubMed] [Google Scholar]
- 51.Shan B, Lee W H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiels A, Bassnett S. Mutations in the founder of the MIP family underlie cataract development in the mouse. Nat Genet. 1996;12:212–215. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- 53.Stolen C M, Jackson M W, Griep A E. Overexpression of FGF-2 modulates fiber cell differentiation and survival in the mouse lens. Development. 1997;124:4009–4017. doi: 10.1242/dev.124.20.4009. [DOI] [PubMed] [Google Scholar]
- 54.Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T, van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994;78:703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 55.Tsai K Y, Hu Y, Macleod K F, Crowley D, Yamasaki L, Jacks T. Mutation of E2F-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol Cell. 1998;2:293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Levine A J. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci USA. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao Z-X, Chen J, Levine A J, Modjtahedi N, Xing J, Sellers W R, Livingston D M. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- 58.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson N. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 59.Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz J W, Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13:2323–2330. [PubMed] [Google Scholar]