Abstract
Summary
Background
Fibroblast growth factor receptor (FGFR) 2 gene alterations are involved in the pathogenesis of cholangiocarcinoma. Pemigatinib is a selective, potent, oral inhibitor of FGFR1, 2, and 3. This study evaluated the safety and antitumour activity of pemigatinib in patients with previously treated, locally advanced or metastatic cholangiocarcinoma with and without FGFR2 fusions or rearrangements.
Methods
In this multicentre, open-label, single-arm, multicohort, phase 2 study (FIGHT-202), patients aged 18 years or older with disease progression following at least one previous treatment and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 recruited from 146 academic or community-based sitesin the USA, Europe, the Middle East, and Asia were assigned to one of three cohorts: patients with FGFR2 fusions or rearrangements, patients with other FGF/FGFR alterations, or patients with no FGF/FGFR alterations. All enrolled patients received a starting dose of 13·5 mg oral pemigatinib once daily (21-day cycle; 2 weeks on, 1 week off) until disease progression, unacceptable toxicity, withdrawal of consent, or physician decision. The primary endpoint was the proportion of patients who achieved an objective response among those with FGFR2 fusions or rearrangements, assessed centrally in all patients who received at least one dose of pemigatinib. This study is registered with ClinicalTrials.gov, NCT02924376, and enrolment is completed.
Findings
Between Jan 17, 2017, and March 22, 2019, 146 patients were enrolled: 107 with FGFR2 fusions or rearrangements, 20 with other FGF/FGFR alterations, 18 with no FGF/FGFR alterations, and one with an undetermined FGF/FGFR alteration. The median follow-up was 17·8 months (IQR 11·6–21·3). 38 (35·5% [95% CI 26·5–45·4]) patients with FGFR2 fusions or rearrangements achieved an objective response (three complete responses and 35 partial responses). Overall, hyperphosphataemia was the most common all-grade adverse event irrespective of cause(88 [60%] of 146 patients). 93 (64%) patients had a grade 3 or worse adverse event (irrespective of cause); the most frequent were hypophosphataemia (18 [12%]), arthralgia (nine [6%]), stomatitis (eight [5%]),hyponatraemia (eight [5%]), abdominal pain (seven [5%]), and fatigue (seven [5%]). 65 (45%) patients had serious adverse events;the most frequent were abdominal pain (seven [5%]), pyrexia (seven [5%]), cholangitis (five [3%]), and pleural effusion (five [3%]). Overall, 71 (49%) patients died during the study, most frequently because of disease progression (61 [42%]); no deaths were deemed to be treatment related.
Interpretation
These data support the therapeutic potential of pemigatinib in previously treated patients with cholangiocarcinoma who have FGFR2 fusions or rearrangements.
Funding
Incyte Corporation.
Introduction
Cholangiocarcinomas are a group of heterogeneous tumours classified as intrahepatic or extrahepatic (perihilar and distal) based on the tumour location in the biliary tract.1 Comprehensive genomic profiling has identified several potentially actionable oncogenic alterations in patients with cholangiocarcinoma,2 including in genes encoding fibroblast growth factor receptor (FGFR). Somatic alterations in FGFR can lead to aberrant FGFR signalling, which can drive tumorigenesis by enhancing cellular proliferation, migration, survival, and invasion, as well as angiogenesis.3FGFR2 fusions and rearrangements are found almost exclusively in intrahepatic cholangiocarcinoma, occurring in 10–16% of patients.4–6 Consequently, in addition to other targeted agents, FGFR inhibitors are garnering interest as potential therapeutics for cholangiocarcinoma.7
Surgery is currently the only curative treatment for cholangiocarcinoma; however, surgery is an option for only around 35% of patients8 and, of those who undergo potentially curative resection, approximately 35% subsequently relapse within 2 years.9 The standard-of-care first-line treatment for locally advanced or metastatic cholangiocarcinoma is gemcitabine plus cisplatin.10 There is no established standard-of-care after failure of first-line chemotherapy, and the efficacy of second-linechemo therapy regimens for advanced biliary cancer remains low.11–13
Pemigatinib is a selective, potent, oral competitive inhibitor of FGFR1, FGFR2, and FGFR3.14 We report the final results from the multicentre, open-label phase 2 FIbroblast Growth factor receptor inhibitor in oncology and Hematology Trial (FIGHT-202), evaluating the safety and antitumour activity of pemigatinib in previously treated patients with locally advanced or metastatic cholangio carcinoma, with or without FGF/FGFR alterations.
Methods
Study design and participants
This open-label, single-arm phase 2 trial was done at 146 academic or community-based sitesin the USA, Europe, the Middle East, and Asia (appendix pp 2–4).
Patients were identified during routine clinical practice. Eligible patients were aged 18 years or older, had a histological or cytological diagnosis of locally advanced or metastatic cholangiocarcinoma with documented disease progression following at least one previous systemic cancer therapy (previous treatment with selective FGFR inhibitors was not permitted), radiologically measurable disease according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, life expectancy of at least 12 weeks, and previously treated and clinically stable brain or CNS metastases without corticosteroids for at least 4 weeks (corticosteroids were otherwise allowed without restriction). Patients were also required to have adequate hepatic and renal function (total bilirubin <1·5 × upper limit of normal [ULN], or ≥2·5 × ULN for Gilbert syndrome or a disease involving the liver; aspartate aminotransferase and alanine aminotransferase ≤2·5 × ULN; and creatinine clearance >30 mL/min calculated with the Cockcroft-Gault formula), serum phosphate less than or equal to the institutional ULN, and serum calcium within the institutional normal range. Eligible patients had no history of HIV infection, did not have active hepatitis B or C virus infection, did not have an abnormal echocardiogram or uncontrolled cardiac disease, had no history or current evidence of ectopic mineralisation or calcification, and had no clinically significant corneal or retinal disorders confirmed by ophthalmological examination.
The protocol (appendix p 26) was approved by each institutional review board or independent ethics committee; the trial was performed in accordance with the Declaration of Helsinki.Patients gave written, informed consent for inclusion in the study.
Procedures
Before assessment for eligibility, patients were pre screened centrally for FGF/FGFR status using massively parallel DNA sequencing (FoundationOne, Foundation Medicine, Cambridge, MA, USA; appendix p 4).15 Patients who already had an FGF/FGFR status report based on local assessment (Clinical Laboratory Improvement Amendments [CLIA]-certified) or an existing FoundationOne report were also included. Retrospective central confirmation of locally documented FGF/FGFR status with FoundationOne was required for cohort assignment. Based on the centrally confirmed results, patients were assigned to one of three cohorts: patients with FGFR2 fusions or rearrangements, patients with other FGF/FGFR alterations, or patients with no FGF/FGFR alterations.
All patients self-administered oral pemigatinib at a starting dose of 13·5 mg once daily (21-day cycle; 2 weeks on, 1 week off), irrespective of cohort assignment, until radiological disease progression, unacceptable toxicity, withdrawal of consent,or physician choice. This dose regimen was supported by pharmacokinetic and pharmacodynamic results from a phase 1/2 study of pemigatinib for advanced malignancies.16Pemigatinib dosing could be interrupted for up to 14 days to allow for resolution of toxicities (appendix p 7). Hyper phosphataemia, an expected on-target pharmacological effect of FGFR inhibition, was managed using dietary modifications, phosphate-lowering therapy, or dose modifications (appendix p 4).
Tumour response was assessed by independent review according to RECIST 1.1. Disease was assessed by CT (or MRI, according to the investigator’s discretion) every 6 weeks for the first 12 weeks, and every 9 weeks thereafter, until disease progression or discontinuation for any reason other than disease progression. Safety was assessed based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03,17 vital signs, 12-lead electrocardiograms, and clinical laboratory tests including mean serum phosphate levels. Mean 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) and parathyroid hormone levels were also determined as they are central in phosphate homoeostasis.18 Safety was assessed at screening, during treatment (day 1, 8, and 15 of cycle 1, and day 1 of subsequent cycles), at the end of treatment, and during follow-up (30 days after discontinuation of treatment).
Outcomes
The primary endpoint was the proportion of patients with FGFR2 fusions or rearrangements who achieved an objective response (best overall response of confirmed complete response or confirmed partial response), assessed by independent central review.
Secondary endpoints were the proportion of patients with an objective response in patients with other FGF/FGFR alterations, in all patients with FGF/FGFR alterations, and in patients with no FGF/FGFR alterations, and duration of response, the proportion of patients with disease control, progression-free survival, overall survival, safety in all cohorts, and population pharmacokinetics (data to be reported separately). Progression-free survival was defined as the time from first dose to progressive disease or death, overall survival was defined as the time from first dose to death from any cause, duration of response was defined as the time from complete response or partial response to progressive disease or death, and disease control was defined as complete response, partial response, or stable disease.
Statistical analysis
Efficacy was assessed in all patients with centrally confirmed FGF/FGFR status who received at least one dose of pemigatinib. The safety population included all patients who received at least one dose of pemigatinib. A futility analysis was planned for when approximately 25 patients with FGFR2 fusions or rearrangements had enrolled and had at least one post-baseline tumour assessment or had permanently discontinued treatment. Enrolment in this cohort could have been stopped if two or fewer of the 25 patients enrolled had achieved a response, for which there was a less than 10% probability of the proportion of patients with an objective response being greater than 15% based on a 60-patient cohort. Although the trial was initially designed to enrol 60 patients with FGFR2 fusions or rearrangements, a protocol amendment was later approved (Oct 3, 2017) that allowed enrolment in this cohort to be increased to approximately 100 patients. This sample size was estimated based on the current treatment landscape of cholangiocarcinoma,19 to ensure an adequate population for safety assessments and robust response data. A sensitivity analysis of the proportion of patients with FGFR2 fusions or rearrangements who achieved an objective response was done based on the first 60 patients enrolled as per the original protocol, and on the additional patients enrolled after the protocol amendment to increase the sample size. With an assumed proportion of patients with an objective response of 33%, a sample size of 100 patients was estimated to provide a greater than 95% probability of having a 95% CI with a lower limit of 15%. 15% was considered the minimum clinically meaningful proportion of patients with an objective response, based on proportions of patients with an objective response reported by previous studies of patients with cholangio carcinoma.11–13 In patients with other FGF/FGFR alter ations or no FGF/FGFR alterations, up to 20 patients were planned to be enrolled to provide a greater than 80% probability of observing at least four responders in each cohort if the underlying proportion of patients with an objective response was 30%. The study was not designed to make statistical comparisons between cohorts.
For the primary endpoint, patients with insufficient baseline or on-study disease assessment data were considered non-responders and were included in the denominators for the calculation of the proportion of Figure 1: Trial profile patients with an objective response. No formal hypothesis testing or inferential analyses were done. The 95% CIs for effect sizes were estimated using the Clopper-Pearson method.20 Exploratory subgroup analyses of the proportion of patients with an objective response and progression-free survival were done for patients with FGFR2 fusions or rearrangements to assess the consistency of the pemigatinib treatment effect on the basis of predefined demographic and disease characteristics. Exploratory (post-hoc) pharmacodynamic analyses were also done to assess the associations between exposure and changes in serum phosphate from baseline, and between changes in serum phosphate from baseline and the proportion of patients with an objective response (appendix pp 4–6).
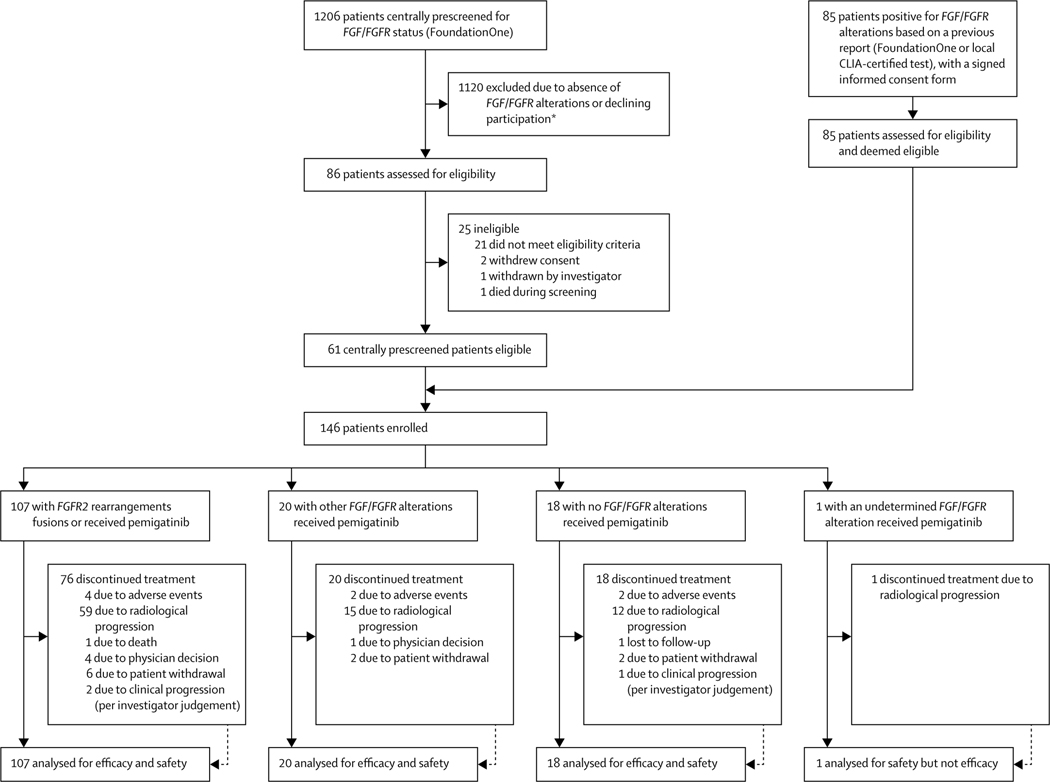
Figure 1: Trial profile.
CLIA=Clinical Laboratory Improvement Amendments. FGFR=fibroblast growth factor receptor. *Numbers of patients excluded for each reason not available.
Statistical analyses were done with SAS (version 9.4). This study is registered with ClinicalTrials.gov, NCT02924376, and enrolment is completed.
Role of the funding source
The study was designed by the funder (Incyte Corporation) with input from the lead investigators (GKA-A and AV). The funder compiled and analysed the study data in collaboration with the corresponding author (GKA-A), and interpreted data in collaboration with all authors. The corresponding author and all coauthors had full access to all the data in the study and provided input on additional analyses before the writing of the report. The corresponding author wrote the first draft of the report and the funder commissioned medical writing services (Envision Pharma Group, Philadelphia, PA, USA) to support subsequent drafts developed under the direction of all authors. All authors reviewed and critically revised each draft and approved the final submitted version. The corresponding author had final responsibility for the decision to submit for publication.
Results
1206 patients were centrally prescreened for FGF/FGFR status using FoundationOne. 1120 patients did not harborFGF/FGFR alterations or declined participation; the remaining 86 were assessed for eligibility (figure 1). An additional 85 patients already had an FGF/FGFR report from FoundationOne or from a local CLIA-certified test, all of whom were assessed for eligibility. Among these 171 patients, 146 were enrolled (25 patients who were centrally prescreened for FGF/FGFR status did not meet other eligibility criteria) between Jan 17, 2017, and the data cutoff date of March 22, 2019: 107 with FGFR2 fusions or rearrangements, 20 with other FGF/FGFR alterations, and 18 with no FGF/FGFR alterations. One patient with FGFR2 rearrangement based on local assessment was not assigned to any cohort because their FGF/FGFR status could not be centrally confirmed by FoundationOne owing to an inadequate tissue sample. Protocol deviations were most commonly due to missed assessments (129 [88%] patients; appendix p 8). Three patients had major protocol deviations: one had received previous FGFR inhibitor therapy for 11 days, ending 30 days before the first pemigatinib dose, and two had resection during the study. Of these two patients, one had a right hepatectomy, which was not resectable before study enrolment but became so during the study, and the other had surgery to remove a lung lesion. All patients with protocol deviations remained in our efficacy and safety populations.
All enrolled patients received at least one dose of pemigatinib and were included in the safety and efficacy populations, except for the one patient with undetermined FGF/FGFR status who was not included in the efficacy analysis(figure1). Overall median follow-up was 17·8 months (IQR 11·6–21·3). Median follow-up was 15·4 months (IQR 9·3–19·0) in patients with FGFR2 fusions or rearrangements, 19·9 months (19·2–21·1) in patients with other FGF/FGFR alterations, and 24·2 months (23·7–24·7) in patients with no FGF/FGFRalterations. Median duration of treatment was 7·2 months (3·9–10·9) in patients with FGFR2 fusions or rearrangements, 1·4 months (1·0–6·1) in patients with other FGF/FGFR alterations, and 1·3 months (0·7–1·9) in patients with no FGF/FGFR alterations.
Across all cohorts, the median age was 59 years (26–78), 135 (92%) of 146 patients had an ECOG performance status of 0 or 1, 126 (86%) had metastatic disease, and 57 (39%) had received two or more previous systemic therapies (table 1). Most patients had received treatment with platinum-based chemotherapy regimens immediately before study entry, most commonly gemcitabine plus cisplatin (68 [47%] patients; appendix p 10). Eight (7%) patients with FGFR2 fusions or rearrangements received locoregional radioembolisation before enrolment. Of the 107 patients with FGFR2 fusions or rearrangements, 105 (98%) had intrahepatic cholangiocarcinoma. This cohort included greater proportions of women, patients aged younger than 65 years, and patients with disease confined to the liver, and included a smaller proportion of patients with an ECOG performance status of 2 than patients in the other cohorts (table 1).
Table 1:
Baseline demographics and disease characteristics
| FGFR2 fusions or rearrangements (n=107) | Other FGF/FGFR alterations (n=20) | No FGF/FGFR alterations (n=18) | All patients (N=146)* | |
|---|---|---|---|---|
| Age, median (range), years | 56 (26 to 77) | 63 (45 to 78) | 65 (31 to 78) | 59 (26 to 78) |
| <65 | 82 (77%) | 10 (50%) | 7 (39%) | 100 (68%) |
| 65 to <75 | 20 (19%) | 7 (35%) | 8 (44%) | 35 (24%) |
| ≥75 | 5 (5%) | 3 (15%) | 3 (17%) | 11 (8%) |
| Sex | ||||
| Male | 42 (39%) | 9 (45%) | 10 (56%) | 62 (42%) |
| Female | 65 (61%) | 11 (55%) | 8 (44%) | 84 (58%) |
| Region | ||||
| North America | 64 (60%) | 6 (30%) | 18 (100%) | 89 (61%) |
| Western Europe | 32 (30%) | 3 (15%) | 0 | 35 (24%) |
| Rest of world† | 11 (10%) | 11 (55%) | 0 | 22 (15%) |
| Race | ||||
| White | 79 (74%) | 9 (45%) | 15 (83%) | 104 (71%) |
| Asian | 11 (10%) | 11 (55%) | 0 | 22 (15%) |
| Black or African American | 7 (7%) | 0 | 1 (6%) | 8 (6%) |
| American Indian or Alaska | 0 | 0 | 1 (6%) | 1 (1%) |
| Native | ||||
| Other or data missing | 10 (9%) | 0 | 1 (6%) | 11 (8%) |
| ECOG performance status | ||||
| 0 | 45 (42%) | 7 (35%) | 7 (39%) | 59 (40%) |
| 1 | 57 (53%) | 10 (50%) | 8 (44%) | 76 (52%) |
| 2 | 5 (5%) | 3 (15%) | 3 (17%) | 11 (8%) |
| Metastatic disease | ||||
| Yes | 88 (82%) | 20 (100%) | 16 (89%) | 125 (86%) |
| No | 16 (15%) | 0 | 2 (11%) | 18 (12%) |
| Missing or not evaluable | 3 (3%) | 0 | 0 | 3 (2%) |
| Number of previous systemic therapies for advanced metastatic disease‡ | ||||
| l | 65 (61%) | 12 (60%) | 12 (67%) | 89 (61%) |
| 2 | 29 (27%) | 7 (35%) | 2 (11%) | 38 (26%) |
| ≥3 | 13 (12%) | 1 (5%) | 4 (22%) | 19 (13%) |
| Previous cancer surgery | 38 (36%) | 6 (30%) | 4 (22%) | 48 (33%) |
| Previous radiotherapy | 28 (26%) | 3 (15%) | 5 (28%) | 36 (25%) |
| Cholangiocarcinoma location§ | ||||
| Intrahepatic | 105 (98%) | 13 (65%) | 11 (61%) | 130 (89%) |
| Extrahepatic | 1 (1%) | 4 (20%) | 7 (39%) | 12 (8%) |
| Other or data missing | l (l%)§ | 3 (15%)¶ | 0 | 4 (3%) |
| History of hepatitis | ||||
| Hepatitis B | 4 (4%) | 1 (5%) | 0 | 5 (3%) |
| Hepatitis C | 1 (1%) | 1 (5%) | 0 | 2 (1%) |
| Sites of disease | ||||
| Liver | 101 (94%) | 17 (85%) | 18 (100%) | 136 (93%) |
| Lymph nodes | 57 (53%) | 11 (55%) | 10 (56%) | 78 (53%) |
| Lung | 58 (54%) | 9 (45%) | 10 (56%) | 77 (53%) |
| Bone | 21 (20%) | 4 (20%) | 2 (11%) | 27 (18%) |
| Ascites | 8 (7%) | 5 (25%) | 2 (11%) | 15 (10%) |
| Pancreas | 7 (7%) | 1 (5%) | 2 (11%) | 10 (7%) |
| Pleural effusion | 4 (4%) | 2 (10%) | 0 | 6 (4%) |
| Skin or subcutaneous tissue | 2 (2%) | 0 | 0 | 2 (1%) |
| Bladder | 0 | 1 (5%) | 0 | 1 (1%) |
| Colon | 1 (1%) | 0 | 0 | 1 (1%) |
| Other | 31 (29%) | 7 (35%) | 12 (67%) | 51 (35%) |
Data are n (%) unless otherwise stated. FGFR=fibroblast growth factor receptor. ECOG=Eastern Cooperative Oncology Group.
The total includes one patient who did not have confirmed FGFftGFR status by central laboratory and was not assigned to any cohort.
Rest of world consists of Israel, South Korea, Taiwan, Thailand, and Japan.
Maximum number of five therapies in patients with FGFR2 fusions or rearrangements and three in the other patient cohorts.
Cholangiocarcinoma location was initially missing for one patient at the data cutoff date; however, this patient was later assessed as having intrahepatic cholangiocarcinoma after the data cutoff date.
The other locations were the gallbladder (n=2) and ampulla of Vater (n=1).
107 (9%) of the 1206 prescreened patients had centrally confirmed FGFR2 fusions or rearrangements. In these 107 patients, 56 different partners were identified, 42 (75%) of which were unique to individual patients. The most common FGFR2 partner was BICC1 (31 [29%] patients; appendix p 11). In five (5%) patients, intron 17 of FGFR2 was rearranged to an intergenic sequence so no partner gene could be identified.
Overall, 115 (79%) of 146 patients had discontinued treatment by the data cutoff date: 76 (71%) of 107 patients with FGFR2 fusions or rearrangements, 20 (100%) of 20 with other FGF/FGFRalterations, 18 (100%) of 18 patients with no FGF/FGFR alterations, and the single patient (100%) with undetermined FGF/FGFR alteration. Patients most commonly discontinued treatment because of progressive disease (59 [55%]) of 107 patients with FGFR2 fusions or rearrangements, 15 [75%] of 20 with other FGF/FGFR alterations, and 12 [67%] of 18 with no FGF/FGFR alterations;figure1).
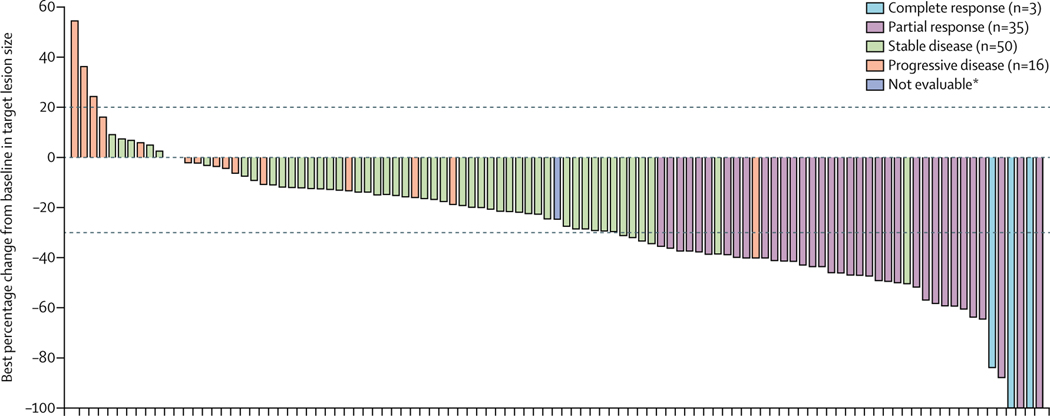
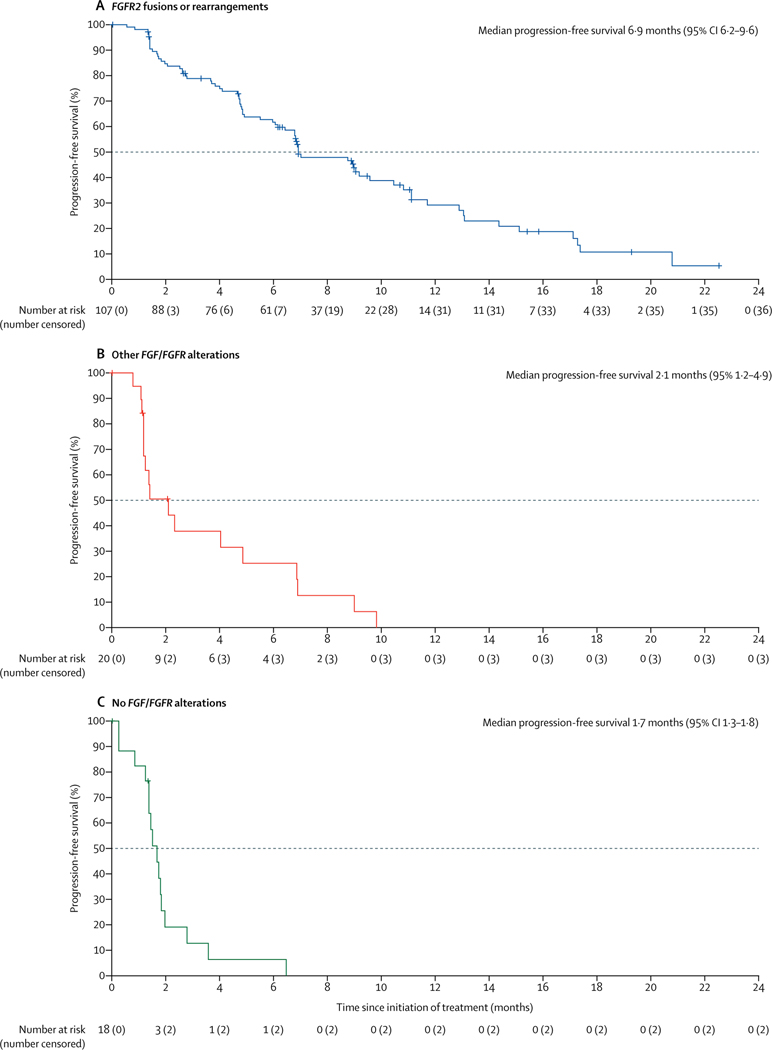
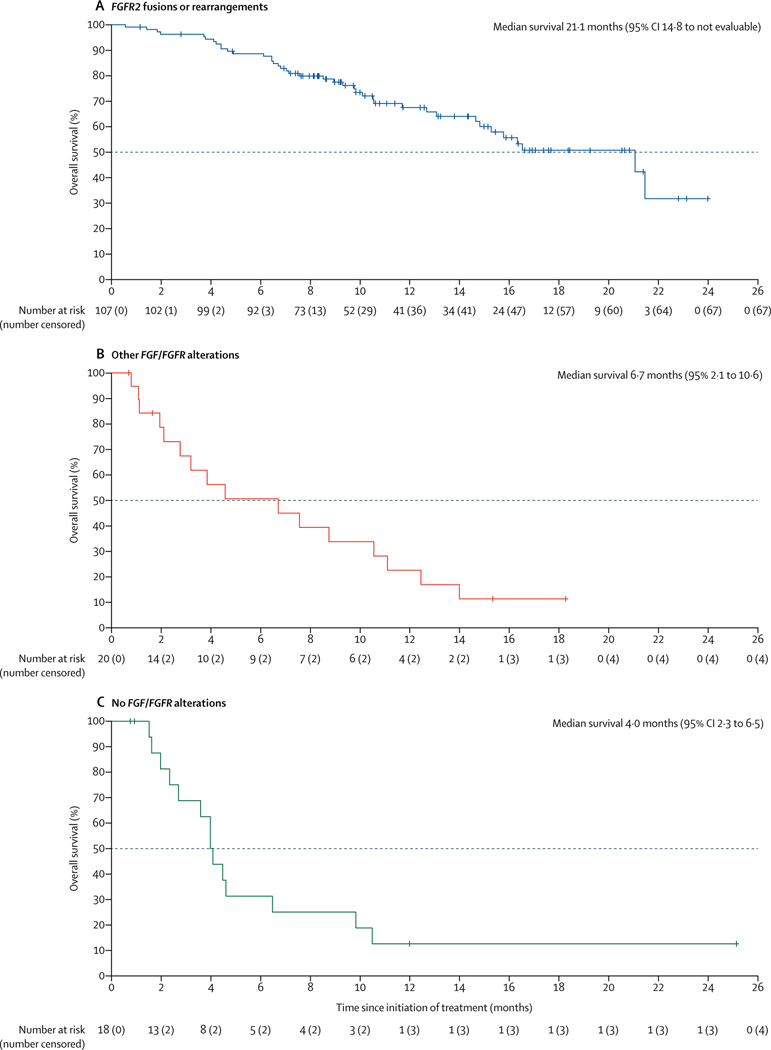
38 (35·5% [95% CI 26·5–45·4]) of 107 patients with FGFR2 fusions or rearrangements achieved a centrally confirmed objective response (table 2). Three (2·8%) patients had confirmed complete responses and 35 (32·7%) had confirmed partial responses. 88 (82% [95% CI 74–89]) of 107 patients achieved disease control. A sensitivity analysis done at the time of the primary analysis showed that the proportion of the first 60 patients with FGFR2 fusions or rearrangements (enrolled as per the original protocol) who achieved an objective response was consistent with the proportion of patients with an objective response among the 47 patients enrolled in this cohort after the protocol amendment to increase the sample size (21 [35·0%, 23·1–48·4] of 60 patients vs 17 [36·2%, 22·7–51·5] of 47 patients). Median time to first response was 2·7 months (IQR 1·4–3·9); median duration of response among responders was 7·5 months (95% CI 5·7–14·5; table 2, appendix p 18). 91 (88%) of 103 patients with FGFR2 fusions or rearrangements who had post-baseline measurements had reductions in centrally assessed best percentage change from baseline in target lesion size (sum of diameters; figure2). Median progression-free survival was 6·9 months (95% CI 6·2–9·6; figure 3, table 2); the overall survival data were not mature at the data cutoff (40 [37%] of 107 patients had died; median overall survival was 21·1 months [95% CI 14·8 to not estimable]; figure 4, table 2). Objective responses in patients with FGFR2 fusions or rearrangements were seen across all demographic and disease subgroups assessed (appendix p 19), and median progression-free survival was generally similar across these subgroups (appendix p 20), including in patients who had received one, two, or three or more previous lines of therapy and patients with FGFR2–BICC1 fusionsversus those with any other FGFR2 fusions or rearrangements.
Table 2:
Primary and secondary efficacy outcomes
| FGFR2 fusions or rearrangements (n=107) | Other FGF/FGFR alterations (n=20) | No FGF/FGFR alterations (n=18) | |
|---|---|---|---|
| Proportion of patients with an objective response | 35·5% (26·5 to 45·4) | 0 | 0 |
| Best overall response* | |||
| Complete response | 3 (2·8%) | 0 | 0 |
| Partial response | 35 (32·7%) | 0 | 0 |
| Stable disease | 50 (46·7%) | 8 (40·0%) | 4 (22·2%) |
| Progressive disease | 16 (14·9%) | 7 (35·0%) | 11 (61·1%) |
| Not evaluable | 3 (2·8%) | 5 (25·0%) | 3 (16·7%) |
| Duration of response | |||
| Patients with events | 21/38 (55%) | 0 | 0 |
| Patients censored | 17/38 (45%) | 0 | 0 |
| Median duration of response, months | 7·5 (5·7 to 14·5) | .. | .. |
| Kaplan-Meier estimated probability of retaining a response | |||
| At 6 months | 68% (49 to 82) | .. | .. |
| At 12 months | 37% (19 to 56) | .. | .. |
| Proportion of patients with disease control | 82% (74 to 89) | 40% (19 to 64) | 22% (6 to 48) |
| Progression-free survival | |||
| Patients with events | 71 (66%) | 17 (85%) | 16 (89%) |
| Patients censored | 36 (34%) | 3 (15%) | 2 (11%) |
| Median, months | 6·9 (6·2 to 9·6) | 2·1 (1·2 to 4·9) | 1·7 (1·3 to 1·8) |
| Kaplan-Meier estimates of progression-free survival | |||
| At 6 months | 62% (52 to 70) | 25% (8 to 47) | 6% (<1 to 25) |
| At 12 months | 29% (19 to 40) | 0 | 0 |
| Overall survival† | |||
| Patients with events | 40 (37%) | 16 (80%) | 14 (78%) |
| Patients censored | 67 (63%) | 4 (20%) | 4 (22%) |
| Median overall survival, months | 21·1 (14·8 to not estimable) | 6·7 (2·1 to 10·6) | 4·0 (2·3 to 6·5) |
| Kaplan-Meier estimates of overall survival | |||
| At 6 months | 89% (81 to 93) | 51% (26 to 71) | 31% (11 to 54) |
| At 12 months | 68% (56 to 76) | 23% (7 to 43) | 13% (2 to 33) |
Data are % (95% CI), n (%), or months (95% CI). FGFR=fibroblast growth factor receptor.
Assessed and response confirmed by independent reviewer (95% CIs not available for individual response values).
Overall survival data were not mature at data cutoff.
Figure 2: Best percentage change from baseline in target lesion size for individual patients with FGFR2 fusions or rearrangements.
Coloured bars indicate confirmed responses assessed by RECIST 1·1. FGFR=fibroblast growth factor receptor. RECIST 1.1=Response Evaluation Criteria in Solid Tumors version 1.1. *Patient had a decrease in target lesion size but was not evaluable for response using RECIST.
Figure 3: Kaplan-Meier estimates of progression-free survival.
FGFR=fibroblast growth factor receptor.
Figure 4: Kaplan-Meier estimates of overall survival.
The median overall survival in patients with FGFR2 fusions or rearrangements was not mature at the data cutoff, at which time 40 overall survival events (deaths) had occurred. FGFR=fibroblast growth factor receptor.
No patients with other FGF/FGFR alterations or no FGF/FGFR alterations achieved a response; eight (40·0%) patients with other FGF/FGFR alterations, 58 (45·7%) patients with any FGF/FGFR alterations (including FGFR2 fusions or rearrangements), and four (22·2%) patients with no FGF/FGFR alterations had stable disease (table 2). Centrally assessed best percentage change from baseline in target lesion size for all individual evaluable patients among those with other FGF/FGFR alterations and those with no FGF/FGFR2 alterations are shown in the appendix (p 21). Median progression-free survival and overall survival in patients with other FGF/FGFR alterations and in patients with no FGF/FGFR alterations are shown in table 2, figure 3, and figure 4.
In 35 patients with information available for therapies received immediately after discontinuing pemigatinib (appendix p 9), most (24 [69%]) proceeded to chemotherapy, primarily FOLFIRI (leucovorin, fluorouracil, and irinotecan; 11 [31%]). Four patients received targeted therapy immediately after pemigatinib, including three with TAS-120, a pan-FGFR tyrosine kinase inhibitor (two with FGFR2 fusions or rearrangements and one with other FGF/FGFR alterations) and one (with FGFR2 fusions or rearrangements) with sulfatinib, a dual tyrosine kinase inhibitor of FGFR1 and the VEGF receptor. Four patients (three with FGFR2 fusions or rearrangements and one with other FGF/FGFR alterations) received immuno therapy with nivolumab and two patients (one with FGFR2 fusions or rearrangements and one with no FGF/FGFR alterations) received immunotherapy with pembrolizumab. One patient (with FGFR2 fusions or rearrangements) received locoregional radio emboli sation immediately after discontinuation of pemigatinib.
Across all three cohorts (n=146), the most common adverse event was hyperphosphataemia (occurring in 88 [60%] of 146 patients irrespective of cause; table 3, appendix p 13). Other common all-cause adverse events (≥40% incidence) of any grade included alopecia, diarrhoea, fatigue, and dysgeusia (appendix p 13).
Table 3:
Treatment-related adverse events*
| Grade 1–2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Hyperphosphataemia† | B1 (55%) | 0 | 0 |
| Alopecia | 67 (46%) | 0 | 0 |
| Dysgeusia | 55 (38%) | 0 | 0 |
| Diarrhoea | 49 (34%) | 4 (3%) | 0 |
| Fatigue | 45 (31%) | 2 (1%) | 0 |
| Stomatitis | 39 (27%) | 8 (5%) | 0 |
| Dry mouth | 42 (29%) | 0 | 0 |
| Nausea | 34 (23%) | 2 (1%) | 0 |
| Decreased appetite | 34 (23%) | 1 (1%) | 0 |
| Dry eye | 30 (21%) | 1 (1%) | 0 |
| Dry skin | 22 (15%) | 1 (1%) | 0 |
| Arthralgia | 16 (11%) | 6 (4%) | 0 |
| Palmar-plantar erythrodysaesthesia | 16 (11%) | 6 (4%) | 0 |
| Constipation | 20 (14%) | 0 | 0 |
| Hypophosphataemia* | 8 (5%) | 10 (7%) | 0 |
| Pain in extremity | 15 (10%) | 0 | 0 |
| Vomiting | 13 (9%) | 1 (1%) | 0 |
| Weight decreased | 13 (9%) | 1 (1%) | 0 |
| Myalgia | 10 (7%) | 1 (1%) | 0 |
| Nail discolouration | 10 (7%) | 1 (1%) | 0 |
| Abdominal pain | 8 (5%) | 1 (1%) | 0 |
| Anaemia | 8 (5%) | 1 (1%) | 0 |
| Onychoclasis | 8 (5%) | 1 (1%) | 0 |
| Paronychia | 8 (5%) | 1 (1%) | 0 |
| Hyponatraemia | 4 (3%) | 3 (2%) | 1 (1%) |
| Urinary tract infection | 7 (5%) | 1 (1%) | 0 |
| Hypercalcaemia | 5 (3%) | 1 (1%) | 0 |
| Skin exfoliation | 5 (3%) | 1 (1%) | 0 |
| Blood alkaline phosphatase increased | 2 (1%) | 2 (1%) | 0 |
| Acute kidney injury | 3 (2%) | 1 (1%) | 0 |
| Erythema | 3 (2%) | 1 (1%) | 0 |
| Nail disorder | 3 (2%) | 1 (1%) | 0 |
| Aspartate aminotransferase increased | 1 (1%) | 2 (1%) | 0 |
| Alanine aminotransferase increased | 2 (1%) | 1 (1%) | 0 |
| Dysphagia | 2 (1%) | 1 (1%) | 0 |
| Keratitis | 2 (1%) | 1 (1%) | 0 |
| Rash pruritic | 1 (1%) | 1 (1%) | 0 |
| Hyperbilirubinaemia | 0 | 1 (1%) | 0 |
| Hypokalaemia | 0 | 1 (1%) | 0 |
| Proteinuria | 0 | 1 (1%) | 0 |
| Skin toxicity | 0 | 1 (1%) | 0 |
| Thrombosis | 0 | 1 (1%) | 0 |
Data include one patient who did not have FGFftGFR status centrally confirmed and was not assigned to any cohort. FGFR=fibroblast growth factor receptor. MedDRA=Medical Dictionary for Regulatory Activities. Shown are treatment-related adverse events occurring in a10% of patients in the total study population of 146 patients (no grade 5 adverse events were reported in this study). Rows are ordered relative to the descending frequency of any grade treatment-related adverse events.
The following MedDRA preferred terms related to hypophosphataemia were combined: blood phosphorus decreased; and hypophosphataemia.
The following MedDRA preferred terms related to hyperphosphataemia were combined: blood phosphorus increased; and hyperphosphataemia.
Hyperphosphataemia occurred early after treatment initiation (median time to onset 15 days [95% CI 8–47]) and was managed with a low-phosphate diet (number of patients not available), concomitant phosphate binders (27 [18%] patients), diuretics (one [1%] patient), dose reduction (one [1%] patient), and dose interruption (two [1%] patients). Notably, in post-hoc analyses, the maximum increases in serum phosphate concentration from baseline occurring after pemigatinib treatment correlated with exposure (appendix p 22), suggesting that phosphate concentration can be used as a surrogate for exposure. A bell-shaped association was observed between change in phosphate levels and the proportion of patients with FGFR2 fusions or rear rangements who had an objective response (appendix p 23). The models suggest that 13·5 mg is an optimal starting dose for treatment of patients with cholangiocarcinoma.
93 (64%) patients had grade 3 or worse adverse events.The most frequent grade 3 or worse adverse events (irrespective of cause)were hypophosphataemia (18 [12%]), arthralgia (nine [6%]), stomatitis (eight [5%]), hyponatraemia (eight [5%]), abdominal pain (seven [5%]), and fatigue (seven [5%]).
Hypophosphataemia occurred in 33 (23%) of 146 patients and was the most common grade 3 or worse adverse event (ten [7%] treatment related [table 3]; 18 [12%] all-cause [appendix p 13]). Mean changes in phosphate concentration from baseline decreased after day 15 of cycle 1 and were sustained thereafter; 1,25(OH)2D3 and parathyroid hormone concentrations followed similar patterns, albeit with some reversal in parathyroid hormone concentration at longer treat ment durations (appendix p 24). Correspondingly, the proportion of patients with lower-thannormal 1,25(OH)2D3 levels (<18 to 86 pg/mL) increased from 17 (15%) of 117 at baseline to 63 (79%) of 80 on day 1 of cycle 5. The proportion of patients with lower-thannormal parathyroid hormone levels (<10 to 65 pg/mL) increased from 15 (11%) of 137 at baseline to 20 (22%) of 89 on day 1 of cycle 5. These proportions were generally sustained up to the data cutoff date.
Among other clinically notable adverse events, nail toxicities (all related Medical Dictionary for Regulatory Activities [MedDRA] preferred terms combined; appendix p 17) were experienced by 62 (42%) of 146 patients (three [2%] grade ≥3) and occurred with a median time to onset of 6・0 months (95% CI 4・8–8・8). Five (3%) of 146 patients had dose reductions and six (4%) had dose interruptions because of nail toxicities.
Adverse events related to serous retinal detachment due to subretinal fluid accumulation (all related MedDRA preferred terms combined; appendix p 17) occurred in six (4%) of 146 patients; all events were grade 1 or 2, except for one grade 3 event that was classified as of rhegmatogenous origin and unrelated to treatment. One (1%) of 146 patients had a dose interruption due to this adverse event.
Overall, 13 (9%) of 146 patients discontinued treatment owing to adverse events, most frequently (at least two patients) intestinal obstruction (n=2) and acute kidney injury (n=2). Adverse events leading to dose reductions occurred in 20 (14%) patients, most frequently (at least two patients) stomatitis (n=5), palmar-plantarerythrodysaesthesia syndrome (n=5), arthralgia (n=5), asthenia (n=2), and onychomadesis (n=2). 62 (42%) patients had dose interruptions due to adverse events, most frequently (at least four patients) stomatitis (n=11), palmar-plantar erythrodysaesthesia syndrome (n=8), arthralgia (n=7), fatigue (n=6), and abdominal pain (n=4).
65 (45%) patients had serious adverse events.The most frequent serious adverse events (more than three patients) were abdominal pain (n=7), pyrexia (n=7), cholangitis (n=5), and pleural effusion (n=5). Overall, 71 (49%) patients died during the study. The most frequent primary cause of death was disease progression (61 [42%] patients); other primary causes were kidney failure (n=1), respiratory failure (n=1), and brain infarction (n=1); seven deaths were of unknown causes. Among the 71 patients who died, six also had fatal treatment-emergent adverse events (failure to thrive [n=2], bile duct obstruction [n=1], sepsis [n=1], pleural effusion [n=1], and cholangitis [n=1]). No deaths were deemed by the investigators to be treatment related.
Discussion
In this multicentre, open-label, phase 2 study in patients with cholangiocarcinoma who had progressed after at least one previous systemic therapy, 35·5% of patients with FGFR2 fusions or rearrangements treated with pemigatinib achieved an objective response, and responses were durable. No patients with other FGF/FGFR alterations or no FGF/FGFR alterations achieved a response, and overall survival and progression-free survival remained poor in these cohorts. The encouraging antitumour activity in patients with FGFR2 fusions or rearrangements was observed across most demographic and disease subgroups assessed.
The antitumour activity of pemigatinib in patients with FGFR2 fusions or rearrangements compares favourably with that reported for other second-line chemotherapy and targeted therapies, as shown by a previous meta-analysis of efficacy data from studies of second-line therapies13 (proportion of patients with an objective response 9·5% [95% CI 7·2–12·5], progression-free survival 2·6 months [1·6–8·0], overall survival 6·5 months [4·1–31·0]), by a previous retrospective study of second-line chemotherapy in patients with advanced biliary tract cancer (overall survival 11 months [8·8–13·1]),12 and by a phase 3 clinical trial that recruited patients with advanced biliary cancer receiving second-line active symptom control plus mFOLFOX (modified leucovorin, fluorouracil, and oxaliplatin) after progressing on first-line gemcitabine plus cisplatin (median overall survival 6·2 months [95% CI unavailable]).11 However, we advise caution in comparing data across studies because the overall survival data in this study are not mature, and there are differences in study designs and patient populations enrolled. Comparisons are also limited because patients enrolled in this study predominantly had intrahepatic cholangio carcinoma and were molecularly selected for FGF/FGFR alterations, whereas other studies included all biliary tract cancers and did not select patients based on their molecular profiles.11–13 In this regard, a recent post-hoc analysis of data from the ABC-01, ABC-02, and ABC-03 trials21 in patients receiving first-line gemcitabine plus cisplatin combinations suggested that patients with intrahepatic cholangiocarcinoma or liver-only disease might have longer overall survival than do those with other biliary tract cancers. Moreover, another retrospective analysis reported longer overall survival in patients with tumours with FGFR alterations than in those without FGFR alterations.22 Despite these limitations, the observed proportion of patients with an objective response and the progression-free survival in this study suggest that pemigatinib has encouraging clinical activity in patients with FGFR2 fusions or rearrangements. Although this drug class represents a substantial advance in treatment options, response duration and progression-free survival were short in some patients, despite the presence of driver FGFR2 fusions or rearrangements. Emerging research suggests that the short response duration and progression-free survival in these patientsmight result from clonal evolution leading to acquired resistance mutations during treatment with FGFR inhibitors.23,24
Pemigatinib and other targeted therapies could potentially complement chemotherapies that are administered either systemically or directly to the liver via hepatic artery infusion,25,26 as recently studied in patients with intra hepatic cholangiocarcinoma.27–29
The prevalence of FGFR2 alterations of 9% is based on patients centrally prescreened for enrolment, and those who already had an FGF/FGFR status report were not included in the denominator. Nevertheless, this prevalence is broadly consistent with that reported in the published scientific literature (10–16%),4–6 and suggests that a substantial proportion of patients with cholangiocarcinoma might benefit from FGFR2-targeted treatment with pemigatinib. Consistent with previous observations,22 the most common FGFR2 partner gene in this study was BICC1, which did not seem to have a different effect on the proportion of patients with an objective response or progression-free survival compared with any other FGFR2 rearrangement partner. The observation that most FGFR2 fusion or rearrangement partners identified were unique to individual patients is also consistent with previous findings.2,4–6,30 In view of the molecular diversity of cholangiocarcinoma, these results highlight the importance of incorporating DNA-based or RNA-basednext-generation sequencing assays into standard practice, to detect both known and novel FGFR2 fusions or rearrangements, and thus to identify all patients who might benefit from FGFR-targeted therapies.
As reported for other FGFR inhibitors,31,32hyperphosphataemia was the most frequent adverse event associated with pemigatinib, which was anticipated based on the importance of FGFR1 in phosphate homoeostasis via feedback mechanisms also involving FGF23, 1,25(OH)2D3, and parathyroid hormone.18 Hyper phosphataemiaevents were of low severity (grade 1 or 2), few patients required dose reductions or inter ruptions owing to this adverse event, and none of the events was deemed treatment related or led to clinically relevant sequelae. Hypophosphataemia was the most common grade 3 or worse adverse event. The observed hypophosphataemia might have resulted from the continued use of a low-phosphate diet or phosphate binders for hyperphosphataemia during the off-treatment week or from negative-feedback effects on phosphate homoeostasis. As has been observed with other FGFR inhibitors,31,32 nail toxicities and ocular disorders, including serous retinal detachment, were reported. Most of these events were of low severity (grade 1 or 2) and none resulted in clinical sequelae. Accumulation of subretinal fluid leading to serous retinal detachment might reflect dysregulation of the outer retinal barrier or functions of the retinal pigment epithelium resulting from FGFR inhibition and consequent downstream inhibition of the mitogenactivated protein kinase pathway.33
Limitations of this trial include a lack of an active comparator group. The design of the study also precludes comparative assessment of the contribution of FGFRalterations to the survival results, which might be associated with more favourable outcomes in patients with cholangiocarcinoma.21,22 Additionally, although most enrolled patients had intrahepatic disease, the inclusion of patients with extrahepatic disease could have complicated the analyses, given that intrahepatic and extra hepatic tumours have markedly different characteristics. Nevertheless, an advantage of the FGFR2 fusion partner-agnostic design is that it enables focus on effects arising from FGFR2 alterations rather than from anatomical differences. Despite these limitations, the study is strengthened by independent central review of clinical responses and by enrolment of a large number of patients with cholangiocarcinoma with FGF/FGFR alterations, which was facilitated by the international design. Moreover, the large patient accrual achieved by this international reach provides impetus for future clinical trials in cholangiocarcinoma as well as in other rare cancers. On the basis of these encouraging results, an international, phase 3, randomised, active-controlled trial is currently recruiting patients to compare pemigatinib with gemcitabine plus cisplatin chemotherapy as first-line therapy for unresectable or metastatic cholangiocarcinoma with FGFR2 rearrangements (FIGHT-302; NCT03656536). To under stand these data in the context of the efficacy of systemic chemotherapy in patients with FGFR2 fusions or rearrangements, a retrospective post-hoc analysis is currently underway to evaluate progression-free survival in patients enrolled in this study (FIGHT-202) who had received second-line systemic therapy before study enrolment.34
Taken together, these encouraging data demonstrate the potential benefit of pemigatinib in previously treated patients with cholangiocarcinoma and FGFR2 fusions or rearrangements, for whom current second-line systemic therapies offer inadequate efficacy.
Supplementary Material
Research in context.
Evidence before this study
The incidence of intrahepatic cholangiocarcinoma has steadily increased worldwideover the past several decades, as have annual age-adjusted mortality rates. The standard-of-care first-line treatment for locally advanced or metastatic cholangiocarcinoma is gemcitabine plus cisplatin. However, treatment options are limited in the second-line setting. Several potentially actionable oncogenic alterations have been identified in patients with cholangiocarcinoma, including alterations in the fibroblast growth factor receptor (FGFR) gene. We searched PubMed and the American Society of Clinical Oncology and European Society for Medical Oncology abstract databases for manuscripts and abstracts published from database inception to Nov 14, 2019, without language restrictions. The search terms used were (“cholangiocarcinoma” OR “biliary tract cancer”) AND (“fibroblast growth factor receptor” OR “FGFR”). We identified 67 publicationssupporting a role for FGFR inhibitors in the treatment of intrahepatic cholangiocarcinoma, and for clinical trials in molecularly selected patient populations. Preclinical studies show that pemigatinib is a selective, potent, oral competitive inhibitor of FGFR1, FGFR2, and FGFR3. There are currently no published clinical studies of pemigatinib in this patient population.
Added value of this study
The FIbroblast Growth factor receptor inhibitor in oncology and Hematology Trial (FIGHT-202) is an international, open-label phase 2 trial evaluating the safety and antitumour activity of pemigatinib in 146 previously treated patients with locally advanced or metastatic cholangiocarcinoma. Among 107 patients with FGFR2 fusions or rearrangements, 38 (36%) achieved an objective response. This encouraging antitumour activity was observed across demographic and disease subgroups, including in heavily pretreated patients. The most common all-cause adverse event was hyperphosphataemia. Notable aspects of this study include incorporation of FGFR2 fusion partner-agnostic next-generation sequencing (ability to detect both known and novel FGFR2 fusions), and an independent central review of clinical responses. Moreover, the international design facilitated the enrolment of a large number of patients with cholangiocarcinoma with FGF/FGFR alterations (mostly FGFR2 rearrangements), despite this being a relatively rare cancer and a rare genomic alteration. This achievement provides impetus for future clinical trials in cholangiocarcinoma and in other rare cancers, for which achieving sufficient patient enrolment numbers can be challenging. Taken together, the data from this study add to a growing body of evidence supporting a role for FGFR inhibitors and other targeted agents for the treatment of cholangiocarcinoma by demonstrating that pemigatinib possesses antitumour activity and is associated with a manageable safety profile in patients with FGFR2 fusions or rearrangements.
Implications of all the available evidence
Based on the encouraging findings from this study, an international, phase 3, randomised, active-controlled trial is currently recruiting patients to compare pemigatinib with gemcitabine plus cisplatin chemotherapy as first-line therapy for unresectable or metastatic cholangiocarcinoma with FGFR2 rearrangements (FIGHT-302; ClinicalTrials.gov, NCT03656536). Pemigatinib could add to the range of treatments available to patients with cholangiocarcinoma and FGFR2 fusions or rearrangements, for whom current systemic therapies are not sufficiently effective.
Acknowledgments
This study was funded by Incyte Corporation (Wilmington, DE, USA). We thank the study participants and the investigators and research teams who contributed to the study. We also thank Ian M Silverman and Timothy C Burn (Incyte Research Institute, Wilmington, DE, USA) for contributing data from FGF/FGFR genomic analyses and Swamy Yeleswaram (Incyte Research Institute) for contributing to population pharmacokinetic analyses. Medical writing assistance was provided by Simon J Slater (Envision Pharma Group, Philadelphia, PA, USA), funded by Incyte Corporation.
Declaration of interests
GKA-A reports research grants and personal fees for consultingfrom Incyte Corporation, both related and unrelated to the present work; research grants and personal fees for consulting from Agios, AstraZeneca, Bayer, Beigene, Bristol-Myers Squibb, Celgene, Exelixis, Polaris, and QED, unrelated to the present work; research grants from Array, ActaBiologica, Genentech, CASI, Mabvax, Halozyme, Novartis, OncoQuest, Puma Biotechnology, and Roche, unrelated to the present work; and personal fees for consulting from Autem, Berry Gemomics, Bioline, CytomX, Debiopharm, Eisai, Eli Lilly, Flatiron, Genoscience, Ipsen, Jansen, LAM, Loxo, Merck, MINA, Pfizer, RedHill, Silenseed, Sillajen, Sobi, Targovax,Therabionics, Twoxar, and Yiviva, unrelated to the present work. VSreports institutional research grants from Incyte Corporation, both related and unrelated to the present work; institutional research grants from Agios, Bristol-Myers Squibb, Celgene, Clovis, Debiopharm, Fibrogen, Medimmune, Merck, NCI, and Rafael, unrelated to the present work; institutional research grants and honoraria from Halozyme, and Ipsen, unrelated to the present work; and personal fees for consulting from QED, Klus, and NewLink Genetics, unrelated to the present work. AH reportsresearch grants from Incyte Corporation, both related and unrelated to the present work; personal fees for consulting from Amgen, Spectrum Pharmaceuticals, Eli Lilly, Debiopharm, Servier, Bayer, and Eisai, unrelated to the present work; research grants from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Sanofi, and Roche, unrelated to the present work. GV reports personal fees for consultingfrom Bayer, Genentech, Exelixis, Incyte Corporation, Alexion, Amgen, Novartis, Celgene, and Astellas, unrelated to the present work; and institutional research grants from Astellas, Eli Lilly, Merck, Incyte Corporation, EMD Serono, Celgene, ER Squibb and Sons, and Boston Scientific, unrelated to the present work. DM reports research grants and personal fees for consulting from Incyte Corporation, both related and unrelated to the present study; research grants and personal fees for consulting from Shire, Evotec, and iOnctura, unrelated to the present work; research grants from Celgene, unrelated to the present work; and personal fees for consulting from Eli Lilly and Baxter, unrelated to the present work. RA-R reports research grants from Incyte Corporation, related to the present work; equity in Seattle Genetics, unrelated to the present work; equity stake in Actinium Pharmaceutical, unrelated to the present work; and personal fees for consulting from Sirtex, unrelated to the present work. ASP reports research grants from Incyte Corporation, both related and unrelated to the present work; personal fees from AAA, Ipsen, Eisai, and Amgen, unrelated to the present work; grants and personal fees from Taiho and Bristol-Myers Squibb, unrelated to the present work; research grants from Bayer and Exelixis, unrelated to the present work; and stock ownership in Aptose, Seattle Genetics, Immunomedics, Actinium, and Alexion, unrelated to the present work. MJB reports research grants from Incyte Corporation, both related and unrelated to the present work; research grants from Senhwa, Adaptimmune, Agios, Halozyme, Celgene, EMD Serono, Toray, Dicerna, Taiho, Sun BioPharma, Isis Pharmaceuticals, RedHill, Boston Biomedical, Basilea, miRNA Therapeutics, Medimmune, Bioline, Sillajen, ARIAD, Puma Biotechnology, Novartis, and QED, unrelated to the present work; institutional fundingfrom Pieris, unrelated to the present work; shares, stock ownership, and stock options in OncBioMune Pharmaceuticals, Intercept, and AVEO, unrelated to the present work; and personal fees for consultingfrom Exelixis, G1 Therapeutics, Immunovative Therapies, Western Oncolytics, Lynx Group, AstraZeneca, Inspyr Therapeutics, ADC Therapeutics, and Merck, unrelated to the present work.
AGM reports research grants from Bristol-Myers Squibb, unrelated to the present work. D-YO reports personal fees for consulting from Genentech– Roche, Novartis, Merck Serono, Bayer, Taiho, ASLAN, Halozyme, and Zymeworks, unrelated to the present work; research grants and personal fees for consulting from AstraZeneca, unrelated to the present work; and researchgrants from Novartis, Array, Eli Lilly, and Green Cross, unrelated to the present work. ED reports institutional researchgrants from Incyte Corporation, both related and unrelated to the present work; institutional research grants and honoraria from Boston Medical and Pfizer, unrelated to the present work; honorariafrom ARMO, unrelated to the present work; and institutional research grants from Merck, AstraZeneca, GlaxoSmithKline, Medimmune, and OncoMed, unrelated to the present work. DVC reports researchgrants from Incyte Corporation, related to the present study; and personal fees for consultingfrom Merck, Bristol-Myers Squibb, Five Prime, Astellas, Taiho, Eli Lilly, Genentech–Roche, Gritstone, Foundation Medicine, Tempus, and Guardant Health, unrelated to the present work. EVC reports personal fees for consultingfrom Incyte Corporation, both related and unrelated to the present work; personal fees for consulting from Astellas, and AstraZeneca, unrelated to the present work; research grants and personal fees for consulting from Bayer, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier, unrelated to the present work; and research grants from Amgen, Boehringer Ingelheim, and Ipsen, unrelated to the present work. TJ, CFL, HZ, and LF are employees of, and hold stock in, Incyte Corporation. AV reports personal fees for consulting from Incyte Corporation, both related and unrelated to the present work; and personal fees for consulting from Amgen, AstraZeneca, Roche, Eli Lilly, Bayer, Medac, Delcath, Shire, Beigene, Bristol-Myers Squibb, Celgene, Eisai, Hengrui, Ipsen, Merck, Pieris, QED, Sanofi, and Servier, unrelated to the present work. DG declares no competing interests.
Footnotes
Data sharing
Access to participant-level data for this study is not available at this time.
Contributor Information
Prof Ghassan K Abou-Alfa, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Medical College, Cornell University, New York, NY, USA.
Vaibhav Sahai, Rogel Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Antoine Hollebecque, Gustave Roussy, Villejuif, France.
Gina Vaccaro, Providence Cancer Center Oncology and Hematology Care Clinic, Portland, OR, USA.
Davide Melisi, Digestive Molecular Clinical Oncology Unit, University of Verona, Verona, Italy.
Raed Al-Rajabi, University of Kansas Cancer Center, Kansas City, KS, USA.
Andrew S Paulson, Baylor Charles A Sammons Cancer Center, Baylor University Medical Center, Dallas, TX, USA.
Mitesh J Borad, Mayo Clinic Cancer Center, Phoenix, AZ, USA.
David Gallinson, Morristown Memorial Hospital, Carol Cancer Center, Morristown, NJ, USA.
Adrian G Murphy, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Prof Do-Youn Oh, Cancer Research Institute, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, South Korea.
Efrat Dotan, Fox Chase Cancer Center, Philadelphia, PA, USA.
Daniel V Catenacci, University of Chicago Medicine, Chicago, IL,USA.
Prof Eric Van Cutsem, University Hospitals Gasthuisberg, Leuven, Belgium; Clinical Digestive Oncology, KU Leuven, Leuven, Belgium.
Tao Ji, Incyte Corporation, Wilmington, DE, USA.
Christine F Lihou, Incyte Corporation, Wilmington, DE, USA.
Huiling Zhen, Incyte Corporation, Wilmington, DE, USA.
Luis Féliz, Incyte Corporation, Wilmington, DE, USA.
Prof Arndt Vogel, Hannover Medical School, Hannover, Germany.
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013; 145: 1215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res 2018; 24: 4154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer 2017; 17: 318–32. [DOI] [PubMed] [Google Scholar]
- 4.Farshidfar F, Zheng S, Gingras MC, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep 2017; 18: 2780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014; 19: 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 2014; 45: 1630–38. [DOI] [PubMed] [Google Scholar]
- 7.Pellino A, Loupakis F, Cadamuro M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol 2018; 3: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012; 61: 1657–69. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J Hepatobiliary PancreatSurg 2001; 8: 154–57. [DOI] [PubMed] [Google Scholar]
- 10.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273–81. [DOI] [PubMed] [Google Scholar]
- 11.Lamarca A, Palmer DH, Singh Wasan H, et al. ABC-06 |A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. Proc Am Soc Clin Oncol 2019;37 (suppl 15): 4003 (abstr). [Google Scholar]
- 12.Lowery MA, Goff LW, Keenan BP, et al. Second-line chemotherapy in advanced biliary cancers: a retrospective, multicenter analysis of outcomes. Cancer 2019; 125: 4426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying J, Chen J. Combination versus mono-therapy as salvage treatment for advanced biliary tract cancer: a comprehensive meta-analysis of published data. Crit Rev Oncol Hematol 2019; 139: 134–42. [DOI] [PubMed] [Google Scholar]
- 14.Liu PCC, Wu LX, Koblish H, et al. Preclinical characterization of the selective FGFR inhibitor INCB054828. Cancer Res 2015; 75 (suppl 15): 771 (abstr). [Google Scholar]
- 15.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013; 31: 1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saleh M, Gutierrez M, Subbiah V, et al. Preliminary results from a phase 1/2 study of INCB054828, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced malignancies. Mol Cancer Ther 2018; 17 (suppl 1): A098 (abstr). [Google Scholar]
- 17.National Cancer Institute Division of Cancer Treatment & Diagnostics. Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. June 14, 2010. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed Oct 14, 2019).
- 18.Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 2016; 15: 51–69. [DOI] [PubMed] [Google Scholar]
- 19.Hollebecque A, Borad MJ, Sahai J, et al. Interim results of FIGHT-202, a phase II, open-label, multicenter study of INCB054828 in patients (pts) with previously treated advanced/ metastatic or surgically unresectable cholangiocarcinoma (CCA) with/without fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations. Ann Oncol 2018; 29 (suppl 8): 756P (abstr). [Google Scholar]
- 20.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–13. [Google Scholar]
- 21.Lamarca A, Ross P, Wasan HS, et al. Advanced intrahepatic cholangiocarcinoma: post-hoc analysis of the ABC-01, -02 and -03 clinical trials. J Natl Cancer Inst 2020; 112: 200–10. [DOI] [PubMed] [Google Scholar]
- 22.Jain A, Borad MJ, Kelley RK, et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis Oncol 2018; 2: 1–12. [DOI] [PubMed] [Google Scholar]
- 23.Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov 2017; 7: 252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krook MA, Bonneville R, Chen HZ, et al. Tumor heterogeneity and acquired drug resistance in FGFR2-fusion-positive cholangiocarcinoma through rapid research autopsy. Cold Spring Harb Mol Case Stud 2019; 5: a004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol 1984; 2: 595–600. [DOI] [PubMed] [Google Scholar]
- 26.Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist 2003; 8: 553–66. [DOI] [PubMed] [Google Scholar]
- 27.Cercek A, Boerner T, Tan BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2019; 6: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol 2009; 20: 1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemeny NE, Schwartz L, Gönen M, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology 2011; 80: 153–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014; 59: 1427–34. [DOI] [PubMed] [Google Scholar]
- 31.Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2018; 36: 276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer 2019; 120: 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nti A, Serrano L, Sandhu H, et al. Frequent subclinical macular changes in combined BRAF/MEK inhibition with high-dose hydroxychloroquine as treatment for advanced metastatic BRAF mutant melanoma: preliminary results from a phase I/II clinical treatment trial. Retina 2019; 39: 502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bibeau K, Féliz L, Barrett S, Na L, Lihou CF, Asatiani E. Progression-free survival in patients with cholangiocarcinoma with FGFR2 fusions or rearrangements: an exploration of response to systemic therapy. Proc Am Soc Clin Oncol 2020; 38 (suppl 4): 588 (abstr). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.