Key Points
Question
Does ivosidenib treatment improve overall survival outcomes vs placebo among patients with chemotherapy-refractory cholangiocarcinoma with IDH1 mutation?
Findings
In this phase 3 randomized clinical trial including 187 previously treated patients with advanced cholangiocarcinoma with IDH1 mutation, ivosidenib treatment resulted in numerically improved overall survival benefits vs placebo, despite a high rate of crossover. Ivosidenib preserved certain quality of life subscales and was well tolerated.
Meaning
The combined efficacy data and tolerable safety profile, as well as corroborating quality of life data, support the clinical benefit of ivosidenib relative to placebo in cholangiocarcinoma with IDH1 mutation, which has an unmet need for new treatments.
Abstract
Importance
Isocitrate dehydrogenase 1 (IDH1) variations occur in up to approximately 20% of patients with intrahepatic cholangiocarcinoma. In the ClarIDHy trial, progression-free survival as determined by central review was significantly improved with ivosidenib vs placebo.
Objective
To report the final overall survival (OS) results from the ClarIDHy trial, which aimed to demonstrate the efficacy of ivosidenib (AG-120)—a first-in-class, oral, small-molecule inhibitor of mutant IDH1—vs placebo for patients with unresectable or metastatic cholangiocarcinoma with IDH1 mutation.
Design, Setting, and Participants
This multicenter, randomized, double-blind, placebo-controlled, clinical phase 3 trial was conducted from February 20, 2017, to May 31, 2020, at 49 hospitals across 6 countries among patients aged 18 years or older with cholangiocarcinoma with IDH1 mutation whose disease progressed with prior therapy.
Interventions
Patients were randomized 2:1 to receive ivosidenib, 500 mg, once daily or matched placebo. Crossover from placebo to ivosidenib was permitted if patients had disease progression as determined by radiographic findings.
Main Outcomes and Measures
The primary end point was progression-free survival as determined by blinded independent radiology center (reported previously). Overall survival was a key secondary end point. The primary analysis of OS followed the intent-to-treat principle. Other secondary end points included objective response rate, safety and tolerability, and quality of life.
Results
Overall, 187 patients (median age, 62 years [range, 33-83 years]) were randomly assigned to receive ivosidenib (n = 126; 82 women [65%]; median age, 61 years [range, 33-80 years]) or placebo (n = 61; 37 women [61%]; median age, 63 years [range, 40-83 years]); 43 patients crossed over from placebo to ivosidenib. The primary end point of progression-free survival was reported elsewhere. Median OS was 10.3 months (95% CI, 7.8-12.4 months) with ivosidenib vs 7.5 months (95% CI, 4.8-11.1 months) with placebo (hazard ratio, 0.79 [95% CI, 0.56-1.12]; 1-sided P = .09). When adjusted for crossover, median OS with placebo was 5.1 months (95% CI, 3.8-7.6 months; hazard ratio, 0.49 [95% CI, 0.34-0.70]; 1-sided P < .001). The most common grade 3 or higher treatment-emergent adverse event (≥5%) reported in both groups was ascites (11 patients [9%] receiving ivosidenib and 4 patients [7%] receiving placebo). Serious treatment-emergent adverse events considered ivosidenib related were reported in 3 patients (2%). There were no treatment-related deaths. Patients receiving ivosidenib reported no apparent decline in quality of life compared with placebo.
Conclusions and Relevance
This randomized clinical trial found that ivosidenib was well tolerated and resulted in a favorable OS benefit vs placebo, despite a high rate of crossover. These data, coupled with supportive quality of life data and a tolerable safety profile, demonstrate the clinical benefit of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation.
Trial Registration
ClinicalTrials.gov Identifier: NCT02989857
This randomized clinical trial reports the final overall survival results of ivosidenib (AG-120)—a first-in-class, oral, small-molecule inhibitor of mutant IDH1—vs placebo for patients with unresectable or metastatic cholangiocarcinoma with IDH1 mutation.
Introduction
Cholangiocarcinomas are rare, aggressive tumors, with an increasing incidence (mainly of the intrahepatic subgroup) and poor prognosis.1,2 The median survival among patients with advanced disease is approximately less than 12 months,3,4,5 with 5-year survival rates of 10% or less.4 Most patients with unresectable or metastatic disease undergo palliative systemic therapy.2 Chemotherapy remains the primary treatment option for cholangiocarcinoma management, with a combination of gemcitabine and cisplatin as the current first-line standard of care,3 and FOLFOX (folinic acid, fluorouracil, and oxaliplatin) recommended as the second-line standard of care.2 However, survival outcomes with first- or second-line chemotherapy are modest.3,6,7 Some agents are approved for specific molecularly defined subsets of cholangiocarcinoma (eg, cholangiocarcinoma with fibroblast growth factor receptor [FGFR] fusions, with neurotrophic tyrosine receptor kinase fusions, or microsatellite instability-high cancer); however, isocitrate dehydrogenase 1 (IDH1; OMIM 147700) mutations rarely occur in these subgroups.8,9,10,11 There is only 1 currently approved targeted treatment for patients with unresectable and metastatic cholangiocarcinoma.12,13 These factors highlight the need for new treatment paradigms in this disease.2,4
Mutations in the metabolic enzyme IDH1 are detected in approximately 13% of intrahepatic cholangiocarcinomas and 1% of extrahepatic cholangiocarcinomas.14 Mutations of IDH1 play a central role in cholangiocarcinoma pathogenesis but are not associated with prognosis.14,15 Ivosidenib (AG-120) is an oral, potent, targeted inhibitor of the IDH1 variant, approved for the treatment of acute myeloid leukemia in subsets of patients with a susceptible IDH1 variant.16,17 In a phase 1, dose-escalation and expansion study (NCT02073994), ivosidenib treatment resulted in a median progression-free survival (PFS) of 3.8 months and median overall survival (OS) of 13.8 months, along with a well-tolerated safety profile, among 72 patients with heavily treated advanced cholangiocarcinoma with IDH1 mutation.18 These data supported further evaluation of ivosidenib in the randomized, double-blind, placebo-controlled phase 3 clinical ClarIDHy trial, which enrolled patients with previously treated cholangiocarcinoma with IDH1 mutation. As of the January 31, 2019, data cutoff, the primary objective of the ClarIDHy trial was met, with a statistically significant improvement in PFS with ivosidenib compared with placebo (hazard ratio [HR], 0.37 [95% CI, 0.25-0.54]; 1-sided P < .001 by independent radiology center; HR, 0.47 [95% CI, 0.33-0.68]; 1-sided P < .001 by investigator).19 The disease control rate observed with ivosidenib was due mostly to the stable disease rate (an objective response rate of 2% [3 partial responses] and a stable disease rate of 51% with ivosidenib vs an objective response rate of 0% and a stable disease rate of 28% with placebo). Final analyses of OS data from the ClarIDHy trial (final data cutoff: May 31, 2020), along with updated safety, additional baseline comutation data, and quality of life (QOL) data, are reported herein.
Methods
Study Design and Participants
The phase 3 ClarIDHy trial design has been reported previously.19 This study was conducted from February 20, 2017, to May 31, 2020, at 49 hospitals across 6 countries (France, Italy, South Korea, Spain, the United Kingdom, and the United States),19 among patients aged 18 years or older with histologically confirmed cholangiocarcinoma with IDH1 mutation. Patients must have had documented disease progression after at least 1 but no more than 2 prior treatment regimens for advanced disease (nonresectable or metastatic), including gemcitabine or a fluorouracil-based chemotherapy regimen, and received no prior IDH-variant inhibitor therapy. Additional key eligibility criteria included an Eastern Cooperative Oncology Group performance status score of 0 or 1; an expected survival of at least 3 months; and adequate bone marrow, hepatic, and kidney function. Patient IDH1-variant status was confirmed centrally and prospectively by next-generation sequencing on formalin-fixed paraffin-embedded tumor tissue specimens using the Oncomine Focus Assay (Thermo Fisher Scientific) in a Clinical Laboratory Improvement Amendments–certified laboratory. Patients were evaluated for eligibility and enrolled by the participating investigators at the trial centers. This trial was conducted according to the International Conference on Harmonization of Good Clinical Practice guidelines and principles of the Declaration of Helsinki.20 Approval from the institutional review board and independent ethics committee was obtained at each study site. All patients provided written informed consent before participating in the trial. Information on racial and ethnic categories reported by patients to the study team or from medical records was captured as part of the clinical database for this study according to applicable local regulation. An independent data and safety monitoring board regularly reviewed the safety data to ensure the safety of treatment and proper trial conduct. This trial is registered with ClinicalTrials.gov (NCT02989857). The complete study protocol is available in Supplement 1.
Randomization and Masking
Randomization and masking details have been described previously.19 In brief, patients were randomly assigned 2:1 to receive ivosidenib or matched placebo, with a block size of 6, and stratified by number of previous systemic treatment regimens for advanced disease (1 vs 2).
Procedures
Procedures followed in the ClarIDHy trial were described previously.19 Ivosidenib, 500 mg, or matched placebo were given orally once daily in continuous 28-day cycles. Treatment continued until disease progression as determined by the investigator, development of other unacceptable toxic effects, confirmed pregnancy, death, withdrawal of consent, loss to follow-up, or trial unblinding or ending. Crossover from the placebo group to the ivosidenib group was allowed for patients with disease progression as confirmed by radiography, per investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.21 Once the primary end point of PFS was met, any patients still receiving placebo were permitted to cross over to the ivosidenib group if they continued to meet eligibility criteria. Radiographic assessment (computed tomography or magnetic resonance imaging) for evaluation of disease response was conducted from day 1 of cycle 1 every 6 weeks (±5 days) through week 48, and every 8 weeks (±5 days) thereafter. Adverse events are reported for patients before crossover, unless otherwise specified.
Outcomes
The primary end point was PFS as determined by blinded independent radiology center per RECIST, version 1.1, and was reported elsewhere.19 Overall survival, defined as the time from date of randomization to the date of death due to any cause, was a key secondary end point. Patients alive at the analysis cutoff date were censored at the date of last contact. Other secondary end points included objective response rate, PFS per investigator assessment, safety and tolerability, and QOL assessed using change from baseline on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and the cholangiocarcinoma and gallbladder cancer module (EORTC QLQ-BIL21) scores and the Patient Global Impression of Change (PGI-C) and Patient Global Impression of Severity (PGI-S) anchor questions; health economic outcomes were assessed using the 5-level EuroQoL 5-dimension (EQ-5D-5L).22,23,24,25 Three QOL domains of interest were prespecified in the statistical analysis plan: physical functioning, pain, and appetite loss. Safety was evaluated by the incidence, severity, and type of treatment-emergent adverse events (TEAEs) (per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03),26 as described previously.19
Statistical Analysis
Overall survival was the first key secondary end point specified in the fixed-sequence testing strategy. It was tested only after statistical significance for PFS, as assessed by the independent radiology center, was achieved, to control the overall type I error in the trial at the 1-sided significance level of .025. Overall survival was planned to be analyzed twice: at the time of the primary PFS analysis and after 150 OS events had been reached (final OS analysis) (eMethods in Supplement 2). Overall survival was compared between the 2 groups by using the 1-sided log-rank test. The HR was estimated from the Cox proportional hazards regression model. The primary analysis of OS followed the intent-to-treat principle, which does not account for the effect of crossover. Consequently, the prespecified rank-preserving structural failure time (RPSFT) model was used to adjust for crossover.27,28,29 The RPSFT method is based on a common treatment assumption: the treatment effect of ivosidenib is the same for all individuals, regardless of when treatment is received.27,28,29
Descriptive statistics were used to summarize safety and comutation data. Details on QOL analysis are provided in the eMethods in Supplement 2.
Results
Patients
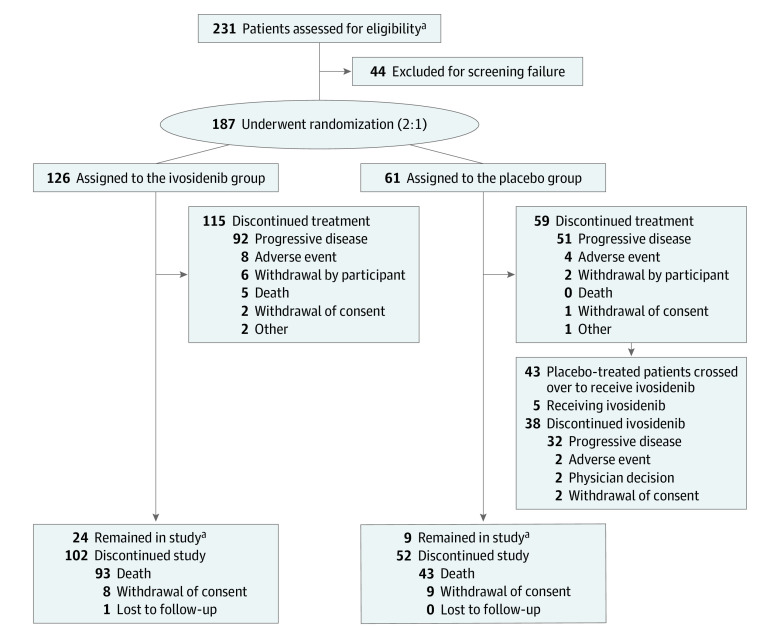
Overall, 231 patients were assessed for eligibility between February 20, 2017, and March 1, 2019. As of May 31, 2020 (data cutoff date for the final OS analysis), 187 patients (median age, 62 years [range, 33-83 years]; American Indian or Alaska Native patients, 1 [0.5%], Asian patients, 23 [12%], Black or African American patients, 2 [1%], Native Hawaiian or other Pacific Islander patients, 1 [0.5%], White patients, 106 [57%], other race, 1 [0.5%], race not reported, 1 [0.5%], missing race, 52 [28%], Hispanic or Latino patients, 9 [5%], not Hispanic or Latino patients, 124 [66%], ethnicity not reported, 2 [1%], missing ethnicity, 52 [28%]) had been randomly assigned to receive ivosidenib (n = 126; 82 women [65%]; median age, 61 years [range, 33-80 years]) or placebo (n = 61; 37 women [61%]; median age, 63 years [range, 40-83 years]). The patient flow diagram is shown in Figure 1. Baseline demographic and disease characteristics were similar in the ivosidenib group and the placebo group (Table). Among all 187 patients, 173 (93%) had metastatic disease, and 88 (47%) had received 2 prior lines of therapy; R132C was the most prevalent IDH1 variant (131 patients [70%]) (Table). As of the data cutoff date, 43 patients (70%) originally randomly assigned to receive placebo had crossed over to receive open-label ivosidenib.
Figure 1. CONSORT Diagram.
aAs of the data cutoff date (May 31, 2020).
Table. Demographic and Baseline Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Ivosidenib (n = 126) | Placebo (n = 61) | |
| Sex | ||
| Female | 82 (65) | 37 (61) |
| Male | 44 (35) | 24 (39) |
| Age, median (range), y | 61 (33-80) | 63 (40-83) |
| Race | ||
| American Indian or Alaska Native | 1 (1) | 0 |
| Asian | 15 (12) | 8 (13) |
| Black or African American | 1 (1) | 1 (2) |
| Native Hawaiian or other Pacific Islander | 1 (1) | 0 |
| White | 71 (56) | 35 (57) |
| Other | 1 (1) | 0 |
| Not reported | 1 (1) | 0 |
| Missing | 35 (28) | 17 (28) |
| Ethnicity | ||
| Hispanic or Latino | 7 (6) | 2 (3) |
| Not Hispanic or Latino | 84 (67) | 40 (66) |
| Not reported | 0 | 2 (3) |
| Missing | 35 (28) | 17 (28) |
| Randomization strata, prior line of therapy | ||
| 1 | 66 (52) | 33 (54) |
| 2 | 60 (48) | 28 (46) |
| IDH1 mutation | ||
| R132C | 86 (68) | 45 (74) |
| R132L | 21 (17) | 7 (11) |
| R132G | 17 (13) | 6 (10) |
| R132S | 2 (2) | 1 (2) |
| R132H | 0 | 2 (3) |
| ECOG PS score at baseline | ||
| 0 | 50 (40) | 19 (31) |
| 1 | 75 (60) | 41 (67) |
| 2 | 0 | 1 (2) |
| 3 | 1 (1) | 0 |
| Cholangiocarcinoma type at diagnosis | ||
| Intrahepatic | 113 (90) | 58 (95) |
| Extrahepatic or perihilar | 5 (4) | 1 (2) |
| Unknown | 8 (6) | 2 (3) |
| Extent of disease at screening | ||
| Local or regional | 9 (7) | 5 (8) |
| Metastatic | 117 (93) | 56 (92) |
| Presence at screening | ||
| Ascites | 34 (27) | 13 (21) |
| Biliary stent | 13 (10) | 7 (11) |
| CA19-9 levels at baseline, median (range), U/mLa | 41.5 (0-61 200)b | 39 (0.1-11 529)b |
Abbreviations: CA19-9, carbohydrate antigen 19-9; ECOG PS, Eastern Cooperative Oncology Group performance status; IDH1, isocitrate dehydrogenase 1.
Patients included in the safety analysis set, before crossover.
Placebo, n = 59; ivosidenib, n = 123.
Efficacy
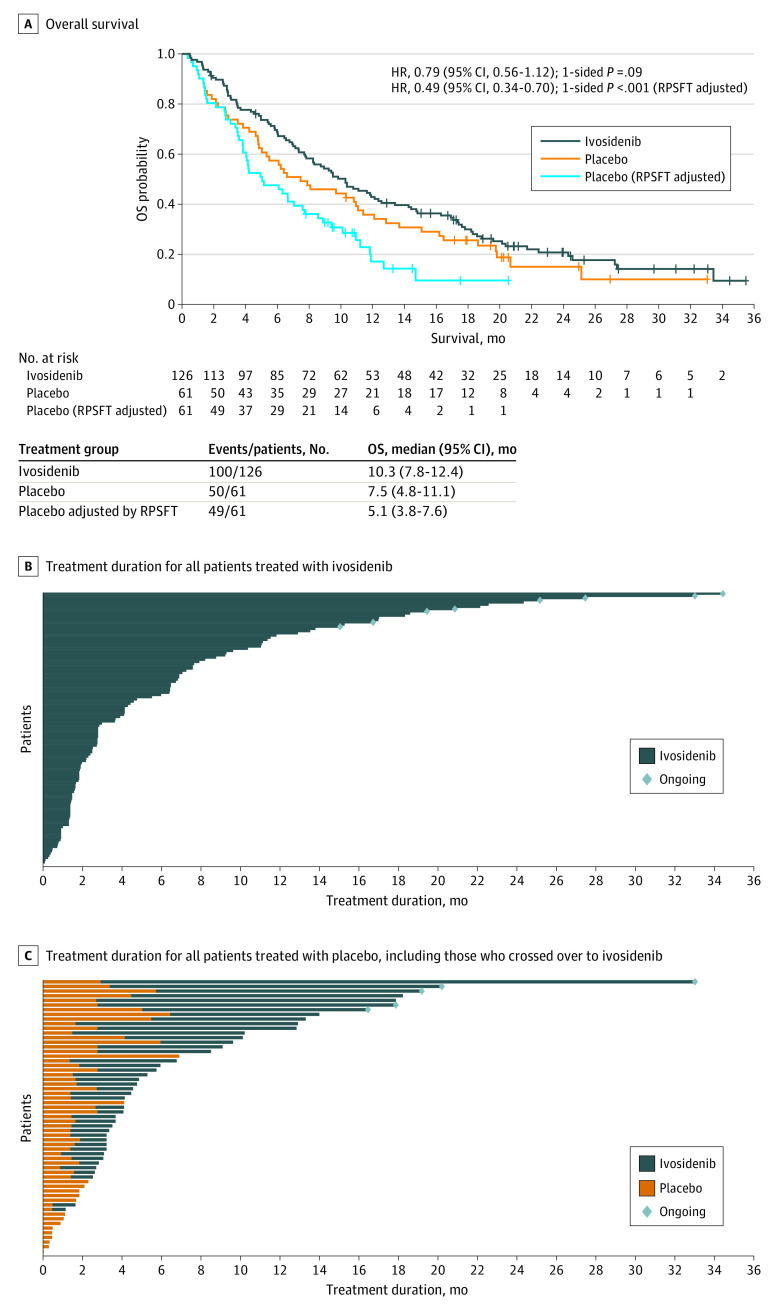
Based on 150 OS events (100 for ivosidenib [79%] and 50 for placebo [82%]), the median OS was 10.3 months (95% CI, 7.8-12.4 months) with ivosidenib and 7.5 months (95% CI, 4.8-11.1 months) with placebo (HR, 0.79 [95% CI, 0.56-1.12]; 1-sided P = .09) (Figure 2A).30 The RPSFT-adjusted median OS was 5.1 months (95% CI, 3.8-7.6 months) with placebo (HR, 0.49 [95% CI, 0.34-0.70]; 1-sided P < .001). The 12-month survival rate was 43% (95% CI, 34%-51%) for the ivosidenib group vs 36% (95% CI, 24%-48%) for the placebo group. Overall survival benefit by subgroup is reported in the eResults and eFigure 1 in Supplement 2. The OS data were mature; 37 patients were censored (26 of 126 patients [21%] in the ivosidenib group and 11 of 61 patients [18%] in the placebo group). The maximum treatment duration with ivosidenib was 34.4 months (range, 0.1-34.4 months) vs 6.9 months (range, 0-6.9 months) with placebo. The median treatment duration was 2.8 months (range, 0.1-34.4 months) for the ivosidenib group (n = 123) and 1.6 months (range, 0-6.9 months) for the placebo group (n = 59) (Figure 2B and C). The median treatment duration for the ivosidenib group after 43 patients crossed over from the placebo group was 2.7 months (range, 0.3-29.8 months) (Figure 2C). Treatment duration appeared to be longer for patients with plasma D-2-hydroxyglutarate levels below 100 ng/mL after 1 cycle of ivosidenib treatment (eMethods and eFigure 2 in Supplement 2). A total of 25 of 166 patients (15%), including 6 patients who crossed over from the placebo group, remained in the ivosidenib group for at least 1 year.
Figure 2. Overall Survival and Treatment Duration in the Intent-to-Treat Population.
A, Overall survival in the intent-to-treat population. Reproduced from Zhu.30 B, Median treatment duration with ivosidenib (n = 123), 2.8 months (range, 0.1-34.4 months). C, All patients treated with placebo are shown in orange (n = 59); those who crossed over to ivosidenib are shown in dark blue (n = 43). Median treatment duration with placebo, 1.6 months (range, 0-6.9 months); median treatment duration with ivosidenib after crossover, 2.7 months (range, 0.3-29.8 months). HR indicates hazard ratio; OS, overall survival; and RPSFT, rank-preserving structural failure time. Crosses indicate censoring.
aPatients without documentation of death at the data cutoff date were censored at the date the patient was last known to be alive or the data cutoff date, whichever was earlier.
Safety
The most common all-grade TEAE in both treatment groups before crossover was nausea (51 of 123 patients [42%] who received ivosidenib and 17 of 59 patients [29%] who received placebo) (eTable 1 in Supplement 2). The most common grade 3 or higher TEAE reported in both treatment groups was ascites (11 patients [9%] who received ivosidenib and 4 patients [7%] who received placebo) (eTable 2 in Supplement 2). Other most common grade 3 or higher TEAEs (≥5%) with ivosidenib vs placebo were anemia (8 patients [7%] vs 0 patients), increased blood bilirubin level (7 patients [6%] vs 1 patient [2%]), and hyponatremia (7 patients [6%] vs 6 patients [10%]).
Six patients (5%) receiving ivosidenib experienced a TEAE leading to death, none of which were assessed by the investigator as being associated with treatment, and were considered to be complications associated with the underlying disease or comorbid conditions. Serious TEAEs were reported for 42 patients (34%) receiving ivosidenib and were considered associated with treatment for 3 patients (2%) (grade 4 hyperbilirubinemia, grade 3 cholestatic jaundice, grade 2 prolonged QT interval on electrocardiogram, and grade 3 pleural effusion; hyperbilirubinemia and cholestatic jaundice were observed in the same patient). These patients were the same 3 reported previously.19 Serious TEAEs were reported for 14 patients (24%) receiving placebo; none were associated with treatment.
Prolonged QT interval on electrocardiogram, a TEAE of special interest, was reported for 12 patients (10%) receiving ivosidenib and 2 patients (3%) receiving placebo. Treatment-emergent adverse events requiring a dose reduction and interruption were uncommon, with 5 patients (4%) in the ivosidenib group requiring a dose reduction vs none in the placebo group. Treatment-emergent adverse events leading to study drug discontinuation occurred for 9 patients (7%) in the ivosidenib group vs 5 patients (8%) in the placebo group.
Quality of Life
The numbers of patients with available EORTC QLQ-C30 and EORTC QLQ-BIL21 assessments at baseline, day 1 of cycle 2, and day 1 of cycle 3 are provided in eTable 3 in Supplement 2 and missing data are described in the eResults in Supplement 2. Ivosidenib preserved QLQ-C30 physical functioning (where a higher score denotes better functioning), whereas patients receiving placebo experienced declines from baseline at day 1 of cycle 2 and day 1 of cycle 3 (eFigures 3 and 4 in Supplement 2). At day 1 of cycle 2, the least-squares mean (SE) change from baseline was –2.4 (1.8) for ivosidenib vs –13.3 (3.0) for placebo, with a least-squares mean difference in change from baseline for ivosidenib vs placebo of 11.0 (95% CI, 4.2-17.7; 2-sided P = .002) (eTable 4 in Supplement 2). The decline in physical functioning at day 1 of cycle 2 was clinically meaningful only in the placebo group, based on the threshold estimated using anchor-based methods described previously.19 At day 1 of cycle 3, the least-squares mean (SE) change from baseline was –0.2 (1.9) for ivosidenib vs –12.6 (3.9) for placebo, with a least-squares mean difference in change from baseline for ivosidenib vs placebo of 12.3 (95% CI, 3.9-20.8; 2-sided P = .004) (eTable 5 in Supplement 2). Ivosidenib was favored on the QLQ-C30 pain subscale (where a higher score denotes worse symptoms) at day 1 of cycle 2 (least-squares mean difference in change from baseline for ivosidenib vs placebo, –10.4 [95% CI, –20.2 to –0.5]; 2-sided P = .04). Neither group was favored on other prespecified subscales (QLQ-C30 appetite loss and QLQ-BIL21 pain and eating). For the exploratory QOL analyses, P ≤ .05 was considered to indicate a difference between groups. At day 1 of cycle 2, ivosidenib was favored for all other subscales in which differences were observed, including QLQ-C30 emotional functioning, cognitive functioning, and dyspnea and QLQ-BIL21 anxiety and tiredness (eFigure 3 in Supplement 2). At day 1 of cycle 3, the difference in the QLQ-C30 emotional functioning subscale persisted, favoring ivosidenib (eFigure 4 in Supplement 2). Findings for the PGI-C and EQ-5D-5L assessments were reported previously.19,31
Baseline Covariant Analyses
All screened patients underwent a determination of variant IDH1 status and identification of covariants in archival formalin-fixed paraffin-embedded samples using a 52-gene next-generation sequencing panel (Oncomine Focus Assay). Tumor tissue specimens were collected from 0.3 months up to 7.5 years before randomization (median, 3.7 months). The covariants identified among the 187 enrolled patients are shown in eTable 6 in Supplement 2. The most frequent oncogenic covariants found in this data set were PI3KCA (n = 20 [11%]), KRAS (n = 14 [8%]), BRAF (n = 8 [4%]), and FGFR2 (n = 8 [4%]). These findings are consistent with covariant analyses reported previously in a phase 1 study of ivosidenib.18 All detected FGFR2 covariants were short variants; the common intrahepatic cholangiocarcinoma FGFR2 fusion partner genes (BICC1, MGEA5, and TACC3) are not specifically targeted in the Oncomine Focus Assay panel. No significant association was observed between baseline covariants in any single gene and OS, PFS, or treatment duration (eFigure 5 in Supplement 2).
Discussion
To our knowledge, this is the first randomized phase 3 trial demonstrating the clinical benefit of targeting the IDH1 variant for patients with advanced cholangiocarcinoma with IDH1 mutation or any other solid tumor with IDH1 mutation. Ivosidenib demonstrated a 63% reduction in the risk of progression or death (HR, 0.37 [95% CI, 0.25-0.54]; 1-sided P < .001) compared with placebo among patients previously treated with chemotherapy.19 In a previous report, the IDH1-variant allele fraction from plasma circulating tumor DNA and the plasma D-2-hydroxyglutarate levels were suppressed by ivosidenib,32 further supporting the antitumor and pharmacodynamic effect of the drug. The robust improvement in PFS led to the addition of ivosidenib to contemporary treatment guidelines as a subsequent-line treatment option for patients with cholangiocarcinoma with IDH1 mutation after disease progression: the NCCN Clinical Practice Guidelines in Oncology (see Additional Information in the end matter),33 the Thésaurus National de Cancérologie Digestive guidelines (France),34 and the Associazione Italiana de Oncologia Medica guidelines (Italy).35
To some extent, the population in this trial represents a real-world population in that patients receiving second-line and third-line treatment were included and there were no exclusions for comorbid conditions, such as ascites, pleural effusions, or biliary stents. In fact, more than 90% of patients had metastatic disease at baseline, and approximately 25% had baseline ascites. Progression-free survival among patients with advanced biliary cancer receiving second-line chemotherapy is approximately 2 to 3 months,11,36,37 and chemotherapy may lead to cumulative toxic effects. Ivosidenib provides an alternative therapeutic option for patients in need of new noncytotoxic treatments that can target tumors, delay progression, preserve QOL, and potentially extend survival.
In this final analysis, ivosidenib numerically improved OS, despite a high rate of crossover from the placebo group (70%), and this improvement was further supported by the difference in OS vs placebo when adjusted for crossover (HR, 0.49 [95% CI, 0.34-0.70]; 1-sided P < .001). The median OS of 10.3 months compares favorably with the published literature on chemotherapy and other targeted agents for patients with advanced biliary tract cancer, for whom the median OS is approximately 6 months.5,36,38,39 The 12-month survival rate was 43% for the ivosidenib group. In addition, QOL results tended to favor ivosidenib, with preservation of domains including physical and emotional functioning relative to worsening for patients in the placebo group through day 1 of cycle 3. The presence of comutations at baseline has been investigated in this data set to identify potential genes or biological pathways that may be associated with overall response with ivosidenib. The most common oncogenic comutations identified in this study were PI3KCA, KRAS, BRAF, and FGFR2, consistent with a previous report on ivosidenib.18 No significant association was found between comutations in any single gene and OS, PFS, or treatment duration in this large data set—an important factor for this type of analysis—of patients with cholangiocarcinoma with IDH1 mutation. These findings suggest that rational treatment combinations for this patient population may include PI3K-targeting agents but not agents targeting neurotrophic tyrosine receptor kinase fusions.
Limitations
Although the findings reported here herald a paradigm shift in the treatment of cholangiocarcinoma, the study has some limitations. It is very likely that the treatment effect estimate on the primary analysis of OS was confounded by the allowance of crossover. The option for crossover was included in the study design based on feedback from patient advocacy groups and clinicians. This study design feature supported the accrual of this rare biomarker-selected patient population. Moreover, analyses of the QOL data were limited by small sample sizes at day 1 of cycle 2 and day 1 of cycle 3 owing, in part, to rapid progression and subsequent withdrawal from the study, which is typical of this disease. Last, a limitation of the comutation analysis was the lack of on-study biopsies to understand mechanisms of resistance. Additional translational studies are under way to assess the relapse mechanisms using circulating tumor DNA sequencing.
Conclusions
Taken together, the efficacy data and tolerable safety profile, as well as supportive QOL data, demonstrate the clinical benefit of ivosidenib compared with placebo for patients with this aggressive disease in which there is an unmet need for new therapies.
Trial Protocol
eMethods.
eResults.
eReferences.
eFigure 1. Overall Survival in the Intent-to-Treat Population: Forest Plot by Subgroup
eFigure 2. Plasma D-2-Hydroxyglutarate (2-HG) Levels Following Ivosidenib Treatment
eFigure 3. Mixed-Effect Model With Repeated Measurements Least Squares Mean Differences of Ivosidenib vs Placebo Before Crossover for EORTC QLQ-C30 and EORTC QLQ-BIL21 Change Scores Between Arms at Cycle 2 Day 1
eFigure 4. Mixed-Effect Model With Repeated Measurements Least Squares Mean Differences of Ivosidenib vs Placebo Before Crossover for EORTC QLQ-C30 and EORTC QLQ-BIL21 Change Scores Between Arms at Cycle 3 Day 1
eFigure 5. Baseline Comutation Data in Tumor Tissue
eTable 1. Treatment-Emergent Adverse Events by Grade, Including Crossover Patients
eTable 2. Summary of Grade 3 or Higher TEAEs, Including Crossover Patients
eTable 3. Summary of Quality of Life Assessment Completion
eTable 4. EORTC QLQ-C30 and QLQ-BIL21 Prespecified Subscale Score Changes From Baseline at Cycle 2 Day 1 From Mixed-Effect Modelling for Ivosidenib vs Placebo Before Crossover
eTable 5. EORTC QLQ-C30 and QLQ-BIL21 Prespecified Subscale Score Changes From Baseline at Cycle 3 Day 1 From Mixed-Effect Modelling for Ivosidenib vs Placebo Before Crossover
eTable 6. Gene Comutation Frequency at Baseline
Data Sharing Statement
References
- 1.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594-599. doi: 10.1634/theoncologist.2015-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-588. doi: 10.1038/s41575-020-0310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 4.Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: an update. World J Gastrointest Oncol. 2013;5(7):171-176. doi: 10.4251/wjgo.v5.i7.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamarca A, Palmer DH, Wasan HS, et al. ABC-06: a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol. 2019;37(15)(suppl):abstract 4003. doi: 10.1200/JCO.2019.37.15_suppl.4003 [DOI] [Google Scholar]
- 6.Plentz RR, Malek NP. Systemic therapy of cholangiocarcinoma. Visc Med. 2016;32(6):427-430. doi: 10.1159/000453084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeva J, Sangro B, Salati M, et al. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39(suppl 1):123-142. doi: 10.1111/liv.14100 [DOI] [PubMed] [Google Scholar]
- 8.Merck & Co Inc. Highlights of prescribing information: KEYTRUDA (pembrolizumab). Accessed February 10, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s084lbl.pdf
- 9.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi: 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doebele RC, Drilon A, Paz-Ares L, et al. ; trial investigators . Entrectinib in patients with advanced or metastatic NTRK fusion–positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi: 10.1016/S1470-2045(19)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Incyte Corporation . Highlights of prescribing information: pemazyre (pemigatinib). Accessed February 10, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213736s000lbl.pdf
- 13.Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671-684. doi: 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol. 2019;10(4):751-765. doi: 10.21037/jgo.2019.03.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha SK, Parachoniak CA, Ghanta KS, et al. Mutant IDH inhibits HNF4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513(7516):110-114. doi: 10.1038/nature13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popovici-Muller J, Lemieux RM, Artin E, et al. Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9(4):300-305. doi: 10.1021/acsmedchemlett.7b00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agios Pharmaceuticals Inc. Highlights of prescribing information: TIBSOVO (ivosidenib). Accessed August 5, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211192s001lbl.pdf
- 18.Lowery MA, Burris HA III, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol. 2019;4(9):711-720. doi: 10.1016/S2468-1253(19)30189-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796-807. doi: 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 23.Friend E, Yadegarfar G, Byrne C, et al. ; EORTC Quality of Life Group . Development of a questionnaire (EORTC module) to measure quality of life in patients with cholangiocarcinoma and gallbladder cancer, the EORTC QLQ-BIL21. Br J Cancer. 2011;104(4):587-592. doi: 10.1038/sj.bjc.6606086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. [Google Scholar]
- 25.Szende A, Janssen B, Cabases J, eds. Self-Reported Population Health: An International Perspective Based on EQ-5D. Springer; 2014. doi: 10.1007/978-94-007-7596-1 [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE): version 4.03. Accessed August 16, 2021. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 27.Watkins C, Huang X, Latimer N, Tang Y, Wright EJ. Adjusting overall survival for treatment switches: commonly used methods and practical application. Pharm Stat. 2013;12(6):348-357. doi: 10.1002/pst.1602 [DOI] [PubMed] [Google Scholar]
- 28.Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ. Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study. BMC Med Res Methodol. 2011;11:4. doi: 10.1186/1471-2288-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins JM, Tsiatis AA. Correcting for non-compliance in randomized trials using rank preserving structural failure time models. Commun Stat Theory Methods. 1991;20(8):2609-2631. doi: 10.1080/03610929108830654 [DOI] [Google Scholar]
- 30.Zhu AX. Final results from ClarIDHy, a global, phase III, randomised, double-blind study of ivosidenib versus placebo in patients with previously treated cholangiocarcinoma and an isocitrate dehydrogenase 1 (IDH1) mutation. EMJ Oncol. 2021;9(suppl 2):2-5. [Google Scholar]
- 31.Chamberlain CX, Andrae DA, Jiang L, et al. Health-related quality of life in patients treated with ivosidenib for mutant-IDH1 cholangiocarcinoma: results from ClarIDHy. Presented at: Cholangiocarcinoma Foundation Annual Conference; July 22-24, 2020; Virtual. [Google Scholar]
- 32.Aguado-Fraile E, Tassinari A, Ishii Y, et al. Molecular and morphological changes induced by ivosidenib correlate with efficacy in mutant-IDH1 cholangiocarcinoma. Future Oncol. 2021;17(16):2057-2074. doi: 10.2217/fon-2020-1274 [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Hepatobiliary Cancers V.1.2021. Accessed March 11, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1438
- 34.Malka D, Bernardini D, Boudjema K, et al. Cancer des voies biliaires. Thésaurus National de Cancérologie Digestive. Société Nationale Française de Gastro-Entérologie. Accessed March 10, 2021. http://www.tncd.org
- 35.Associazione Italiana de Oncologia Medica. Practice guidelines for biliary tract neoplasms. Accessed March 10, 2021. https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Vie_biliari.pdf
- 36.Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328-2338. doi: 10.1093/annonc/mdu162 [DOI] [PubMed] [Google Scholar]
- 37.Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer. 2015;121(18):3290-3297. doi: 10.1002/cncr.29471 [DOI] [PubMed] [Google Scholar]
- 38.Moik F, Riedl JM, Winder T, et al. Benefit of second-line systemic chemotherapy for advanced biliary tract cancer: a propensity score analysis. Sci Rep. 2019;9(1):5548. doi: 10.1038/s41598-019-42069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying J, Chen J. Combination versus mono-therapy as salvage treatment for advanced biliary tract cancer: a comprehensive meta-analysis of published data. Crit Rev Oncol Hematol. 2019;139:134-142. doi: 10.1016/j.critrevonc.2019.01.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eResults.
eReferences.
eFigure 1. Overall Survival in the Intent-to-Treat Population: Forest Plot by Subgroup
eFigure 2. Plasma D-2-Hydroxyglutarate (2-HG) Levels Following Ivosidenib Treatment
eFigure 3. Mixed-Effect Model With Repeated Measurements Least Squares Mean Differences of Ivosidenib vs Placebo Before Crossover for EORTC QLQ-C30 and EORTC QLQ-BIL21 Change Scores Between Arms at Cycle 2 Day 1
eFigure 4. Mixed-Effect Model With Repeated Measurements Least Squares Mean Differences of Ivosidenib vs Placebo Before Crossover for EORTC QLQ-C30 and EORTC QLQ-BIL21 Change Scores Between Arms at Cycle 3 Day 1
eFigure 5. Baseline Comutation Data in Tumor Tissue
eTable 1. Treatment-Emergent Adverse Events by Grade, Including Crossover Patients
eTable 2. Summary of Grade 3 or Higher TEAEs, Including Crossover Patients
eTable 3. Summary of Quality of Life Assessment Completion
eTable 4. EORTC QLQ-C30 and QLQ-BIL21 Prespecified Subscale Score Changes From Baseline at Cycle 2 Day 1 From Mixed-Effect Modelling for Ivosidenib vs Placebo Before Crossover
eTable 5. EORTC QLQ-C30 and QLQ-BIL21 Prespecified Subscale Score Changes From Baseline at Cycle 3 Day 1 From Mixed-Effect Modelling for Ivosidenib vs Placebo Before Crossover
eTable 6. Gene Comutation Frequency at Baseline
Data Sharing Statement