This cohort study assesses the potential association of ocrelizumab, a B-cell–depleting agent, with the T-cell and antibody SARS-CoV-2 response following vaccination with the messenger RNA vaccine BNT162b2 in patients with multiple sclerosis.
Key Points
Question
Do patients with multiple sclerosis treated with the B-cell–depleting agent ocrelizumab develop T-cell and humoral responses to the SARS-CoV-2 messenger RNA vaccine?
Findings
In this cohort study of 112 participants, those treated with ocrelizumab developed lower serology response compared with untreated patients and healthy controls but showed preserved T-cell response to the SARS-CoV-2 vaccine compared with healthy controls.
Meaning
In this study, preserved vaccine-specific T-cell responses in patients with multiple sclerosis treated with ocrelizumab are reassuring and will help to develop therapeutic strategies in patients with multiple sclerosis during the COVID-19 pandemic.
Abstract
Importance
B-cell–depleting therapies may affect the development of a protective immune response following vaccination. Understanding the ability to develop vaccine-specific immunity to COVID-19 in patients with multiple sclerosis (MS) treated with B-cell–depleting therapy is of importance for clinical decisions.
Objective
To assess SARS-CoV-2 vaccine-specific humoral and cellular responses in patients treated with ocrelizumab compared with healthy controls.
Design, Setting, and Participants
This single-center study performed at Hadassah Medical Center in Jerusalem, Israel, included patients with MS treated with ocrelizumab, healthy controls, and untreated patients with MS. Vaccination occurred between December 2020 and April 2021. Participants donated blood 2 to 4 and 2 to 8 weeks after the second vaccine dose for antibody and T-cell assessments, respectively.
Exposures
All participants received 2 doses of BNT162b2 vaccine (Pfizer/BioNTech) and completed the study.
Main Outcomes and Measures
Proportion of patients treated with ocrelizumab with SARS-CoV-2–specific serology and/or T-cell responses following vaccination. All participants underwent SARS-CoV-2 antibody testing; 29 patients treated with ocrelizumab and 15 healthy controls had evaluation of SARS-CoV-2–specific T-cell responses.
Results
Of 112 participants, 49 (43.8%) had MS and were treated with ocrelizumab (33 [67.3%] female; mean [SD] age, 47.9 [13.3] years), 23 (20.5%) had MS and were not treated with disease-modifying therapies (18 [78.3%] female; mean [SD] age, 49 [13.4] years), and 40 (35.7%) were healthy controls (25 [62.5%] female; mean [SD] age, 45.3 [16] years). Twenty-six of 29 patients (89.7%) treated with ocrelizumab and 15 of 15 healthy controls (100%) had SARS-CoV-2–specific T cells following vaccination at similar levels (mean [SD], 15.4 [7.6] and 14.3 [6.3] spot-forming cells, respectively). Mean antibody titers and positive serology rate were lower in the group of patients treated with ocrelizumab (mean [SD] antibody titers and positive serology rate, 26.2 [49.2] and 376.5 [907.6] AU/mL; 10 of 40 [25%] and 20 of 49 [40.8%] for S1/S2 and receptor-binding domain, respectively) compared with healthy controls (mean [SD] antibody titers and positive serology rate, 283 [100] and 12 712 [9114] AU/mL; 100% S1/S2 and receptor-binding domain) and untreated patients (mean [SD] antibody titers and positive serology rate, 288.3 [113.8] and 10 877 [9476] AU/mL; 100% S1/S2 and receptor-binding domain), with positive association to time from ocrelizumab infusion (S1/S2: r = 0.7, P < .001; receptor-binding domain: r = 0.4, P = .04).
Conclusion and Relevance
In this study, patients with MS who were treated with ocrelizumab generated comparable SARS-CoV-2–specific T-cell responses with healthy controls and had lower antibody response following vaccination. Given the potential role of T cells in protection from severe disease, this is reassuring and will help physicians develop consensus guidelines regarding MS treatment in the era of the COVID-19 pandemic.
Introduction
The SARS-CoV-2 pandemic has presented a clinical concern for patients with multiple sclerosis (MS), whose mainstay treatment are immunosuppressive/immunomodulatory disease-modifying therapies.1 Most approved vaccines, including the messenger RNA vaccines, induce robust humoral and cellular immune responses against the virus spike protein2,3; however, it is still unknown whether SARS-CoV-2 vaccines confer sufficient protection in patients with MS treated with disease-modifying therapies.
In this study, we assessed the potential association of ocrelizumab, a B-cell–depleting agent, with the T-cell and antibody SARS-CoV-2 response following vaccination with the messenger RNA vaccine BNT162b2.
Methods
Participants and Setting
This single-center study was performed at Hadassah Medical Center in Jerusalem, Israel, and was approved by the Hadassah Medical Organization Ethics Committee. Participants were vaccinated between December 2020 and April 2021 and donated blood 2 to 4 and 2 to 8 weeks following their second vaccine dose of BNT162b2 vaccine (Pfizer/BioNTech) for antibody and T-cell assessments, respectively. Five healthy controls who were not vaccinated also participated in the study. All participants provided written informed consent (975-20 HMO). Data on race and ethnicity were not collected. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
SARS-CoV-2 IgG
Serology response was measured using Liaison SARS-CoV-2 S1/S2 IgG (DiaSorin) and spike receptor-binding domain (RBD) Architect SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics) with a positive response defined by IgG titer of 19 or more or 50 or more arbitrary units (AU) per mL, respectively. The serology tests were performed on serum samples 2 to 4 weeks after the second vaccine dose and in 8 patients treated with ocrelizumab also 8 weeks after the second vaccine dose.
Interferon γ Enzyme-Linked Immunospot
T-cell immune response to SARS-CoV-2 was assessed by detecting interferon γ using T-SPOT Discovery SARS-CoV-2 (Oxford Immunotec), a modified enzyme-linked immunospot technology, IVD CE–marked assay, using freshly isolated peripheral blood mononuclear cells. Peripheral blood mononuclear cells, isolated 3 to 5 hours from blood drawn, were stimulated with (1) a panel of SARS-CoV-2 spike peptides, (2) nucleocapsid peptides, (3) positive control (phytohemagglutinin), and (4) negative control (medium) and incubated for 20 hours according to manufacturer instructions. Results are presented as the number of spot-forming cells (SFCs) per 250 000 cells. A positive response was defined as SFC of 6 or more. T-cell analysis was performed from April 1, 2021, to eligible patients 2 to 8 weeks after the second vaccine dose.
Statistical Analysis
Statistical analyses were performed using 1-way analysis of variance, t test, Pearson correlation coefficient, and χ2. The results are presented as mean (SD). Two-sided P values are statistically significant at less than .05.
Results
Participants
Of 72 patients with MS, 49 (68.1%) were treated with ocrelizumab and 23 (31.9%) were untreated for at least 6 months before vaccination. Of 49 patients treated with ocrelizumab, 33 (67.3%) were female, 16 (32.6%) were male, the mean (SD) age was 47.9 (13.3) years, the mean (SD) Expanded Disability Status Scale score was 4.4 (1.9), the mean (SD) disease duration was 11.7 (8.6) years, and the mean (SD) duration of treatment was 19.9 (1.9) months. In that group, 23 individuals had relapsing MS and 26 had progressive MS. Of 23 patients who were untreated before vaccination, 18 (78.3%) were female, 5 (21.7%) were male, the mean (SD) age was 49 (13.4) years, the mean (SD) Expanded Disability Status Scale score was 2 (2), and the mean (SD) disease duration was 10.7 (9.9) years. Forty healthy controls (25 [62.5%] female; 15 [37.5%] male; mean [SD] age, 45.3 [16] years) also participated.
SARS-CoV-2 Messenger RNA Vaccine Antibody Response
To assess vaccine antibody responses to SARS-CoV-2 spike protein, we evaluated S1/S2 IgG and RBD IgG titers at baseline and 2 to 4 weeks after the second vaccine dose. Prevaccination antibody titers were negative in all participants.
The mean IgG levels and response rate were significantly lower in patients treated with ocrelizumab (mean [SD], 26.2 [49.2] and 376.5 [907.6] AU/mL; 10 of 40 [25%] and 20 of 49 [40.8%] for S1/S2 and RBD, respectively) compared with healthy controls (mean [SD], 283 [100] and 12 712 [9114] AU/mL; 100% S1/S2 and RBD) and untreated patients (mean [SD], 288.3 [113.8] and 10 877 [9476] AU/mL; 100% S1/S2 and RBD) as shown in Figure 1A-B. None of the 8 weeks’ postvaccination samples from 8 patients treated with ocrelizumab reached a positive serology threshold (Figure 1C).
Figure 1. SARS-CoV-2 Messenger RNA Vaccine Antibody Response in Patients With Multiple Sclerosis Treated With Ocrelizumab.
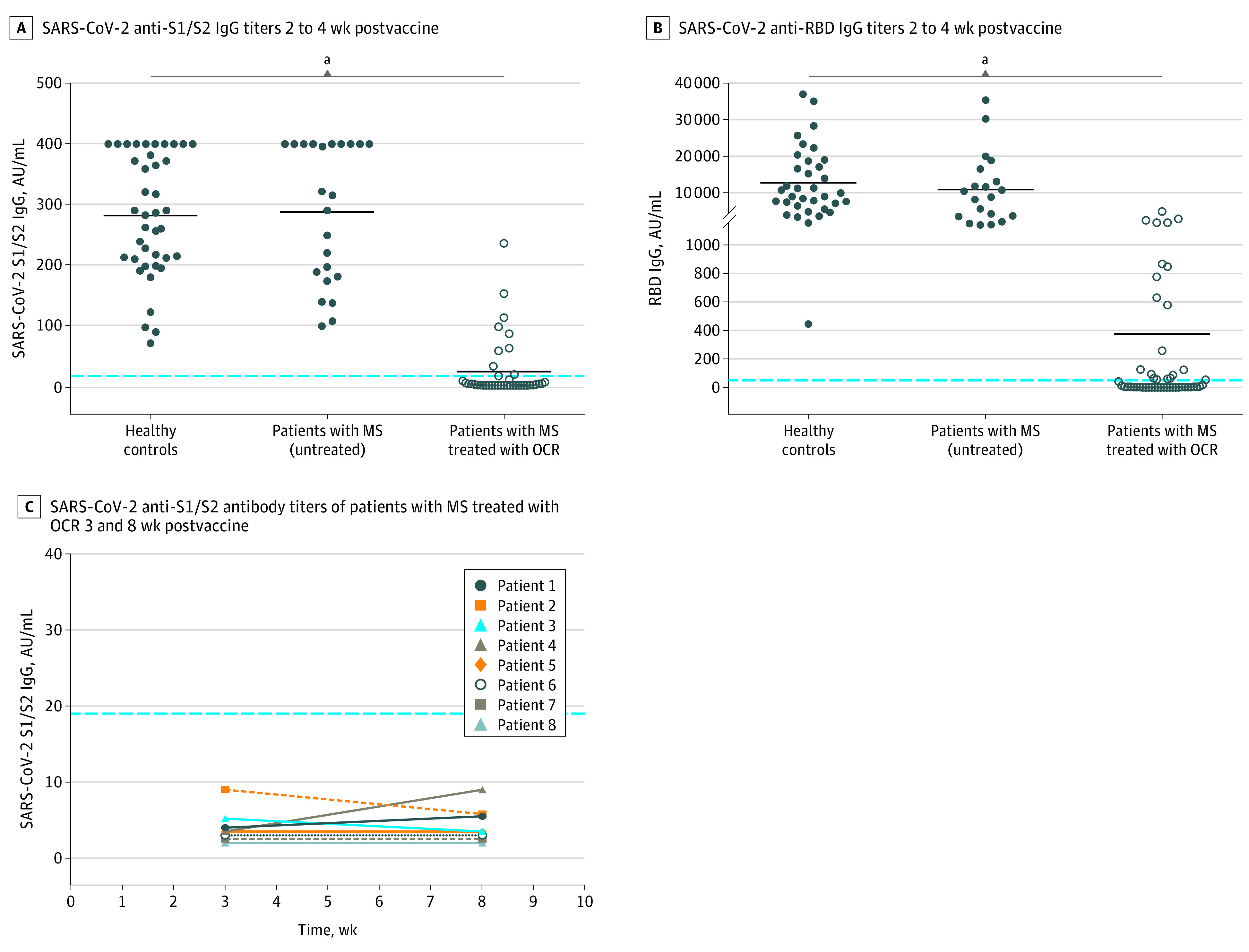
Serology response to SARS-CoV-2 messenger RNA vaccine. A, SARS-CoV-2 anti-S1/S2 IgG titers 2 to 4 weeks postvaccine in healthy controls (n = 40): mean (SD), 283 (100) AU/mL; patients with multiple sclerosis (MS) not treated (n = 23): mean (SD), 288.3 (113.8) AU/mL; patients with MS treated with ocrelizumab (OCR) (n = 40): mean (SD), 26.2 (49.2) AU/mL (P < .001). B, SARS-CoV-2 anti–receptor-binding domain (RBD) IgG titers 2 to 4 weeks after vaccination in healthy controls (n = 35): mean (SD), 12 712 (9114) AU/mL; patients with MS not treated (n = 20): mean (SD), 10 877 (9476); and patients treated with OCR (n = 49): mean (SD), 376.5 (907.6) AU/mL. The dotted line indicates positive threshold (≥19 and ≥50 AU/mL in the Liaison and Architect assay, respectively). Horizontal bars indicate the mean. C, SARS-CoV-2 anti-S1/S2 antibody titers of patients with MS treated with OCR (n = 8) at 2 time points (3 and 8 weeks after vaccination). AU indicates arbitrary units.
aValues were significant (P < .001).
We observed a positive association between SARS-CoV-2 IgG levels and time from last ocrelizumab treatment to vaccination (S1/S2: r = 0.7, P < .001; RBD: r = 0.4, P = .04; eFigure 1 in the Supplement). Patients who were vaccinated 5 months or more following the last ocrelizumab dose had a significantly increased likelihood for a positive serologic response compared with patients who were vaccinated earlier (14 of 23 [60.9%] vs 6 of 26 [23.1%]; χ2 = 7.2; P = .007).
No correlation was found between antibody levels (S1/S2 and RBD) and lymphocyte counts (r = 0.11 and r = 0.31, respectively; P ≥ .06), disease duration (r = 0.13 and r = 0.14, respectively; P ≥ .30), and number of ocrelizumab infusions (r = 0.007 and r = −0.22, respectively; P ≥ .10). In the entire cohort, there was a correlation between age and antibody levels (r = −0.3 and r = −0.12 for S1/S2 and RBD, respectively; P ≤ .04) (eFigure 2 in the Supplement).
SARS-CoV-2–Specific T-Cell Responses Following Vaccination
We evaluated T-cell responses 2 to 8 weeks following the second dose of vaccine using direct ex-vivo interferon γ enzyme-linked immunospot. Freshly isolated peripheral blood mononuclear cells of 29 patients treated with ocrelizumab and 15 healthy controls were stimulated with a panel of SARS-CoV-2 spike and nucleocapsid peptides and the magnitude of specific T-cell responses was determined. We detected positive SARS-CoV-2–specific T-cell responses in 26 of 29 patients treated with ocrelizumab (89.7%), in all the 15 vaccinated healthy controls, and in none of the unvaccinated controls (Figure 2A). The mean number of responding T cells in vaccinated patients with MS treated with ocrelizumab was similar to healthy controls (mean [SD], 15.36 [7.6] vs 14.33 [6.25] SFCs; 95% CI, −3.5 to 5.6; P = .65). No difference was detected between patients treated with ocrelizumab with positive and negative serology response (mean [SD], 14.5 [7.4] and 15.9 [7.9] SFCs; 95% CI, −4.6 to 7.4; P = .64; Figure 2B). No correlation was found between T cells and antibody levels (S1/S2: r = −0.08, P = .60; RBD: r = −0.03, P = .80). No response to the nucleocapsid peptides was seen in any of the participants, indicating absence of previous SARS-CoV-2 infection.
Figure 2. SARS-CoV-2 Spike-Specific T-Cell Response Following Vaccination.

Postvaccination T-cell response to SARS-CoV-2 spike protein peptides. Wells stimulated with SARS-CoV-2 spike peptides as measured by an interferon γ enzyme-linked immunospot. Each participant’s peripheral blood mononuclear cells are placed into 4 wells (250 000 cells per well) where they are exposed to nil control and 2 separate panels of SARS-CoV-2 antigens containing overlapping peptides spanning sequences derived from spike and nucleocapsid proteins and a phytohemagglutinin control. A, SARS-CoV-2–specific T-cell response of healthy controls (n = 15) and patients with multiple sclerosis (MS) treated with ocrelizumab (OCR) (n = 29) postvaccination and 5 healthy controls prevaccine as measured by T-SPOT (Oxford Immunotec). Spot-forming cells (SFCs) per 250 000 cells for each participant represent the number of T cells specific to spike SARS-CoV-2. B, T-cell response postvaccination of patients with MS treated with OCR who had positive SARS-CoV-2 IgG (IgG+) or negative SARS-CoV-2 (IgG−). Ten of 11 patients with MS treated with OCR who had positive serology response were also positive for T-cell response (mean [SD] SFC, 14.5 [7.4]). Sixteen of 18 patients with negative serology response had positive specific T-cell response (mean [SD], 15.9 [7.9]). The dotted line indicates positive threshold (SFC, ≥6). Horizontal bars indicate the mean.
Discussion
In this study, we found that most patients treated with ocrelizumab developed SARS-CoV-2–specific T-cell responses following BNT162b2 vaccination, with similar levels to healthy controls and independent of SARS-CoV-2 IgG titers. A lower percentage of positive SARS-CoV-2 antibody response and lower IgG titers were detected in patients with MS treated with ocrelizumab compared with healthy controls and untreated patients.
Antibodies are believed to be a key component for an effective vaccine to provide protection.4 However, other arms of the immune system may contribute to vaccine efficacy. T cells are critical to generate antibody-producing plasma cells, long-lived memory cells, and for elimination of virus-infected cells. Early and robust T-cell responses have been associated with mild/asymptomatic COVID-19 infection even in the absence of antibodies.5,6,7,8,9 T cells could provide protection from severe disease by limiting viral replication to the upper respiratory tract.10
Robust SARS-CoV-2 T-cell responses and attenuated antibody responses have been reported following COVID-19 infection in patients treated with ocrelizumab.11,12,13 Similarly, we found that most vaccinated patients treated with ocrelizumab developed interferon γ–producing SARS-CoV-2–specific T cells, with levels comparable with healthy controls. Three patients had a T-cell response below the positive cutoff but did not have negative response as the nonvaccinated controls. Possibly, with a more sensitive assay, these patients might show a positive response.
Ocrelizumab depletes circulating B cells within 2 weeks of treatment but spares CD20-negative plasma cells, stem cells, and pro-B cells. As a result, an impairment in the antibody response to nonlive vaccines has been documented14 including for SARS-CoV-2 vaccines,15 similar to our findings. An optimized time for vaccine administration could potentially lead to stronger antibody responses. We found that patients vaccinated 5 or more months after the last dose had a higher probability for positive serology response. The individual serology response might depend on a combination of factors.
Limitations
Limitations of our study include small sample size and short study duration. It is still unclear to what extent individuals who have a negative serology response but do produce vaccine-specific T cells are protected. As vaccine-induced immunity can wane over time, it is important to study the persistence of antibody and T-cells responses.
Conclusions
This single-center study found a preserved vaccine-specific T-cell and decreased humoral response in patients with MS treated with ocrelizumab. T-cell responses were detected in patients with either positive or negative humoral response. Timing of vaccination in relation to last dose of ocrelizumab could improve antibody responses. The emerging role of T cells in protection from severe COVID-19 highlight the importance of vaccination in patients treated with ocrelizumab, as a T-cell response is expected and may confer protection, even in the absence of antibody responses.
eFigure 1. Association between SARS-CoV-2 IgG levels and time from last ocrelizumab treatment to vaccination
eFigure 2. Correlation of post-vaccine IgG titers with age
References
- 1.Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699-708. doi: 10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599. doi: 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 5.Nelde A, Bilich T, Heitmann JS, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol. 2021;22(1):74-85. doi: 10.1038/s41590-020-00808-x [DOI] [PubMed] [Google Scholar]
- 6.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158-168.e14. doi: 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457-462. doi: 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 8.Gallais F, Velay A, Nazon C, et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27(1). doi: 10.3201/eid2701.203611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6):108728. doi: 10.1016/j.celrep.2021.108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861-880. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kempen ZLE, Strijbis EMM, Al MMCT, et al. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol. 2021;78(7):880-882. doi: 10.1001/jamaneurol.2021.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttari F, Bruno A, Dolcetti E, et al. COVID-19 vaccines in multiple sclerosis treated with cladribine or ocrelizumab. Mult Scler Relat Disord. 2021;52:102983. doi: 10.1016/j.msard.2021.102983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kister I, Krogsgaard M, Mulligan MJ, et al. Preliminary results of ongoing, prospective study of antibody and T-cell responses to SARS-CoV-2 in patients with MS on ocrelizumab or other disease-modifying therapies. Neurology. 2021;96(22):e2783-e2784. doi: 10.1212/WNL.0000000000012044 [DOI] [Google Scholar]
- 14.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. doi: 10.1212/WNL.0000000000010380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012835. doi: 10.1177/17562864211012835 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Association between SARS-CoV-2 IgG levels and time from last ocrelizumab treatment to vaccination
eFigure 2. Correlation of post-vaccine IgG titers with age


