Abstract
Background
On July 30, 2021, the administration of a third (booster) dose of the BNT162b2 messenger RNA vaccine (Pfizer–BioNTech) was approved in Israel for persons who were 60 years of age or older and who had received a second dose of vaccine at least 5 months earlier. Data are needed regarding the effect of the booster dose on the rate of confirmed coronavirus 2019 disease (Covid-19) and the rate of severe illness.
Methods
We extracted data for the period from July 30 through August 31, 2021, from the Israeli Ministry of Health database regarding 1,137,804 persons who were 60 years of age or older and had been fully vaccinated (i.e., had received two doses of BNT162b2) at least 5 months earlier. In the primary analysis, we compared the rate of confirmed Covid-19 and the rate of severe illness between those who had received a booster injection at least 12 days earlier (booster group) and those who had not received a booster injection (nonbooster group). In a secondary analysis, we evaluated the rate of infection 4 to 6 days after the booster dose as compared with the rate at least 12 days after the booster. In all the analyses, we used Poisson regression after adjusting for possible confounding factors.
Results
At least 12 days after the booster dose, the rate of confirmed infection was lower in the booster group than in the nonbooster group by a factor of 11.3 (95% confidence interval [CI], 10.4 to 12.3); the rate of severe illness was lower by a factor of 19.5 (95% CI, 12.9 to 29.5). In a secondary analysis, the rate of confirmed infection at least 12 days after vaccination was lower than the rate after 4 to 6 days by a factor of 5.4 (95% CI, 4.8 to 6.1).
Conclusions
In this study involving participants who were 60 years of age or older and had received two doses of the BNT162b2 vaccine at least 5 months earlier, we found that the rates of confirmed Covid-19 and severe illness were substantially lower among those who received a booster (third) dose of the BNT162b2 vaccine.
A combination of rapid development of effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and their deployment in the general population has proved to be a highly successful strategy for reducing both viral transmission and disease burden. In Israel, the early initiation of a nationwide campaign resulted in the full vaccination (i.e., receipt of two vaccine doses) in more than half the population by the end of March 2021.1 Consequently, the incidence of coronavirus 2019 disease (Covid-19) dropped from approximately 900 cases per million per day in mid-January 2021 to fewer than 2 cases per million per day by June 2021.1 Nevertheless, the emergence of new variants of concern (specifically, the delta variant) has led to a recent resurgence in both confirmed infection and severe illness.2 In early June, fewer than 20 cases of Covid-19 were confirmed on polymerase-chain-reaction (PCR) assay per day, and approximately half of those cases were diagnosed in persons who were returning from abroad. At that time, the number of active severe cases reached approximately 20. By the end of August, more than 10,000 PCR-confirmed cases were being detected daily, and more than 600 persons with severe cases were hospitalized.
Several causes are possible for the high levels of transmission of the delta variant, including the increased infectiousness of the variant,3 waning vaccine-elicited immunity,2,4 and heightened immune evasion by the variant.5 Of these causes, the latter two directly contribute to a decrease in vaccine efficacy. An analysis of the Israeli data with respect to the outbreak of the delta variant indicated a high degree of waning immunity.2,4
In an effort to address the challenge presented by the delta variant and to reduce the load on the health care system, Israeli authorities approved the administration of a booster dose, first to high-risk populations, on July 12, 2021, and then to persons who were 60 years of age or older, on July 30, 2021. Initial studies have suggested that a BNT162b2 booster dose increases the antibody neutralization level by a factor of approximately 10, on average, as compared with the level after a second dose.6 It is thought that an increased neutralization titer could lead to increased protection against infection and severe illness.7 However, in terms of real-world effectiveness, the size of such an effect remains unclear. Here, we used initial data from the Israeli Ministry of Health database to evaluate the rates of confirmed infection and severe illness among participants who were 60 years of age or older and who had received a third booster dose (booster group) as compared with those who had received only two vaccine doses. We used the data to quantify by how much the booster dose reduced the rates of confirmed SARS-CoV-2 infection and severe Covid-19 illness.
Methods
Study Population
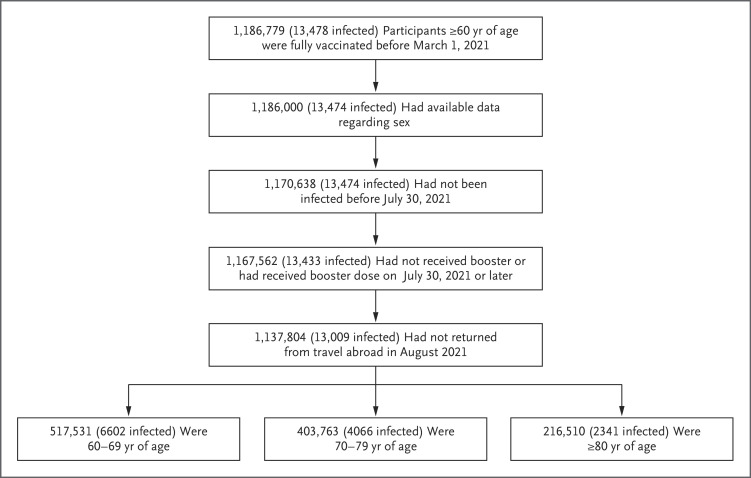
Our analysis was based on medical data from the Ministry of Health database that were extracted on September 2, 2021. At that time, a total of 1,186,779 Israeli residents who were 60 years of age or older had been fully vaccinated (i.e., received two doses of BNT162b2) at least 5 months earlier (i.e., before March 1, 2021) and were alive on July 30, 2021. We excluded from the analysis participants who had missing data regarding sex; were abroad in August 2021; had received a diagnosis of PCR-positive Covid-19 before July 30, 2021; had received a booster dose before July 30, 2021; or had been fully vaccinated before January 16, 2021. A total of 1,137,804 participants met the inclusion criteria for the analysis (Figure 1).
Figure 1. Study Population.
The participants in the study included persons who were 60 years of age or older and who had been fully vaccinated before March 1, 2021, had available data regarding sex, had no documented positive result on polymerase-chain-reaction assay for SARS-CoV-2 before July 30, 2021, and had not returned from travel abroad in August 2021. The number of confirmed infections in each population is shown in parentheses.
The data included vaccination dates (first, second, and third doses); information regarding PCR testing (sampling dates and results); the date of any Covid-19 hospitalization (if relevant); demographic variables, such as age, sex, and demographic group (general Jewish, Arab, or ultra-Orthodox Jewish population), as determined by the participant’s statistical area of residence (similar to a census block)8; and clinical status (mild or severe disease). Severe disease was defined as a resting respiratory rate of more than 30 breaths per minute, an oxygen saturation of less than 94% while breathing ambient air, or a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen of less than 300.9
Study Design
Our study period started at the beginning of the booster vaccination campaign on July 30, 2021. The end dates were chosen as August 31, 2021, for confirmed infection and August 26, 2021, for severe illness. The selection of dates was designed to minimize the effects of missing outcome data owing to delays in the reporting of test results and to the development of severe illness. The protection gained by the booster shot was not expected to reach its maximal capacity immediately after vaccination but rather to build up during the subsequent week.10,11 At the same time, during the first days after vaccination, substantial behavioral changes in the booster-vaccinated population are possible (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). One such potential change is increased avoidance of exposure to excess risk until the booster dose becomes effective. Another potential change is a reduced incidence of testing for Covid-19 around the time of receipt of the booster (Fig. S2). Thus, it is preferable to assess the effect of the booster only after a sufficient period has passed since its administration.
We considered 12 days as the interval between the administration of a booster dose and its likely effect on the observed number of confirmed infections. The choice of the interval of at least 12 days after booster vaccination as the cutoff was scientifically justified from an immunologic perspective, since studies have shown that after the booster dose, neutralization levels increase only after several days.6 In addition, when confirmed infection (i.e., positivity on PCR assay) is used as an outcome, a delay occurs between the date of infection and the date of PCR testing. For symptomatic cases, it is likely that infection occurs on average 5 to 6 days before testing, similar to the incubation period for Covid-19.12,13 Thus, our chosen interval of 12 days included 7 days until an effective buildup of antibodies after vaccination plus 5 days of delay in the detection of infection.
To estimate the reduction in the rates of confirmed infection and severe disease among booster recipients, we analyzed data on the rate of confirmed infection and on the rate of severe illness among fully vaccinated participants who had received the booster dose (booster group) and those who had received only two vaccine doses (nonbooster group). The membership in these groups was dynamic, since participants who were initially included in the nonbooster group left it after receipt of the booster dose and subsequently were included in the booster group 12 days later, provided that they did not have confirmed infection during the interim period (Fig. S3).
In each group, we calculated the rate of both confirmed infection and severe illness per person-days at risk. In the booster group, we considered that days at risk started 12 days after receipt of the third dose and ended either at the time of the occurrence of a study outcome or at the end of the study period. In the nonbooster group, days at risk started 12 days after the beginning of the study period (August 10, 2021) and ended at time of the occurrence of a study outcome, at the end of the study period, or at the time of receipt of a booster dose. The time of onset of severe Covid-19 was considered to be the date of the confirmed infection. In order to minimize the problem of censoring, the rate of severe illness was calculated on the basis of cases that had been confirmed on or before August 26, 2021. This schedule was adopted to allow for a week of follow-up (until the date when we extracted the data) for determining whether severe illness had developed. The study protocol is available at NEJM.org.
Oversight
The study was approved by the institutional review board of the Sheba Medical Center. All the authors contributed to the writing and critical review of the manuscript, approved the final version, and made the decision to submit the manuscript for publication. The Israeli Ministry of Health and Pfizer have a data-sharing agreement, but only the final results of this study were shared.
Statistical Analysis
We performed Poisson regression to estimate the rate of a specific outcome, using the function for fitting generalized linear models (glm) in R statistical software.14 These analyses were adjusted for the following covariates: age (60 to 69 years, 70 to 79 years, and ≥80 years), sex, demographic group (general Jewish, Arab, or ultra-Orthodox Jewish population),8 and the date of the second vaccine dose (in half-month intervals). We included the date of the second dose as a covariate to account for the waning effect of the earlier vaccination and for the likely early administration of vaccine in high-risk groups.2 Since the overall rate of both confirmed infection and severe illness increased exponentially during the study period, days at the beginning of the study period had lower exposure risk than days at the end. To account for growing exposure risk, we included the calendar date as an additional covariate. After accounting for these covariates, we used the study group (booster or nonbooster) as a factor in the regression model and estimated its effect on rate. We estimated the rate ratio comparing the nonbooster group with the booster group, a measure that is similar to relative risk. For reporting uncertainty around our estimate, we took the exponent of the 95% confidence interval for the regression coefficient without adjustment for multiplicity. We also used the results of the model to calculate the average between-group difference in the rates of confirmed infection and severe illness.15
In a secondary analysis, we compared infection rates before and after the booster dose became effective. Specifically, we repeated the Poisson regression analysis described above but compared the rate of confirmed infection between 4 and 6 days after the booster dose with the rate at least 12 days after the booster dose. Our hypothesis was that the booster dose was not yet effective during the former period.10 This analysis compares different periods after booster vaccination among persons who received the booster dose and may reduce selection bias. However, booster recipients might have undergone less frequent PCR testing and behaved more cautiously with regard to virus exposure soon after receiving the booster dose (Fig. S2). Thus, we hypothesize that the rate ratio could be underestimated in this analysis.
To further examine the reduction in the rate of confirmed infection as a function of the interval since receipt of the booster, we fitted a Poisson regression that includes days 1 to 32 after the booster dose as separate factors in the model. The period before receipt of the booster dose was used as the reference category. This analysis was similar to the Poisson modeling described above and produced rates for different days after the booster vaccination.
To test for different possible biases, we performed several sensitivity analyses. First, we analyzed the data using alternative statistical methods relying on matching and weighting. These analyses are described in detail in the Methods section in the Supplementary Appendix. Second, we tested the effect of a specific study period by splitting the data into different study periods and performing the same analysis on each. Third, we performed the same analyses using data only from the general Jewish population, since the participants in that cohort dominated the booster-vaccinated population.
Results
Study Population
We compared the characteristics of the participants in the two groups according to person-days at risk, since the primary analysis was adjusted for person-days at risk and the participants contributed days to both groups, as specified in the study design (Table 1). The table summarizes data only for person-days used in the main analysis. The nonbooster group included approximately 5.2 million person-days (4.6 million for the analysis of severe illness), with 4439 confirmed infections and 294 cases of severe illness. The booster group included approximately 10.6 million person-days (6.3 million for the analysis of severe illness), with 934 confirmed infections and 29 cases of severe illness. The booster group, as compared with the nonbooster group, had more men (49% vs. 42%), more participants from the general Jewish population (92% vs. 81%), more participants who were 70 years of age or older (58% vs. 46%), and more participants who had received vaccination in January 2021 (74% vs. 38%). The estimates of rate ratios have been adjusted to account for these substantial between-group differences.
Table 1. Demographic and Clinical Characteristics of the Study Population at Baseline.*.
| Characteristic | Nonbooster Group | Booster Group | ||||
|---|---|---|---|---|---|---|
| Confirmed Infection | Severe Illness | Confirmed Infection | Severe Illness | |||
| % person-days at risk | no. of cases | no. of cases | % person-days at risk | no. of cases | no. of cases | |
| Sex | ||||||
| Female | 57.8 | 2414 | 116 | 51.2 | 402 | 11 |
| Male | 42.2 | 2025 | 178 | 48.8 | 532 | 18 |
| Age distribution | ||||||
| 60–69 yr | 53.6 | 2522 | 73 | 41.7 | 373 | 5 |
| 70–79 yr | 28.8 | 1206 | 91 | 38.7 | 323 | 5 |
| ≥80 yr | 17.6 | 711 | 130 | 19.6 | 238 | 19 |
| Population | ||||||
| General Jewish | 81.4 | 3752 | 254 | 92.4 | 851 | 26 |
| Arab | 14.0 | 335 | 25 | 4.1 | 36 | 2 |
| Ultra-Orthodox Jewish | 4.6 | 352 | 15 | 3.5 | 47 | 1 |
| Vaccination period in 2021 | ||||||
| January 16–31 | 38.2 | 1882 | 139 | 74.0 | 663 | 22 |
| February 1–15 | 46.6 | 1972 | 128 | 23.6 | 248 | 7 |
| February 16–28 | 15.2 | 585 | 27 | 2.4 | 23 | 0 |
Since the two study groups had dynamic membership and participants could contribute data to both groups, the table presents the percentage of person-days at risk rather than the number of individual participants. Person-days and events that are presented are for the main analysis and cover the study period from July 30 to August 31, 2021, for confirmed infection and from July 30 to August 26, 2021, for severe illness. In the nonbooster group, the number of person-days at risk was 5,193,825 for confirmed infection and 4,574,439 for severe illness. In the booster group, the number of person-days at risk was 10,603,410 for confirmed infection and 6,265,361 for severe illness.
Effect of the Booster Dose
The results of the full Poisson regression analysis for confirmed infection and severe illness are provided in Tables S1 and S2, respectively, and are summarized in Table 2. The rate of confirmed infection was lower in the booster group than in the nonbooster group by a factor of 11.3 (95% confidence interval [CI], 10.4 to 12.3). The absolute between-group difference in the rate of confirmed infection was 86.6 infections per 100,000 person-days. The rate of severe illness was lower in the booster group than in the nonbooster group by a factor of 19.5 (95% CI, 12.9 to 29.5). The absolute between-group difference in the rate of severe illness was 7.5 cases per 100,000 person-days. In the secondary analysis, the rate of confirmed infection at least 12 days after receipt of the vaccine was substantially lower than the rate 4 to 6 days after receipt (rate ratio, 5.4; 95% CI, 4.8 to 6.1).
Table 2. Primary Outcomes of Confirmed Infection and Severe Illness.*.
| Outcome | Nonbooster Group | Booster Group | Adjusted Rate Ratio (95% CI)† |
|---|---|---|---|
| Confirmed infection | 11.3 (10.4–12.3) | ||
| No. of cases | 4439 | 934 | |
| No. of person-days at risk | 5,193,825 | 10,603,410 | |
| Severe illness | 19.5 (12.9–29.5) | ||
| No. of cases | 294 | 29 | |
| No. of person-days at risk | 4,574,439 | 6,265,361 |
Listed are the results of the Poisson regression analysis in participants who received a booster vaccine and in those who did not receive a booster. The booster group includes data that were obtained at least 12 days after receipt of the booster dose.
The rate ratio is the estimated factor reduction in the rate in the booster group as compared with the rate in the nonbooster group.
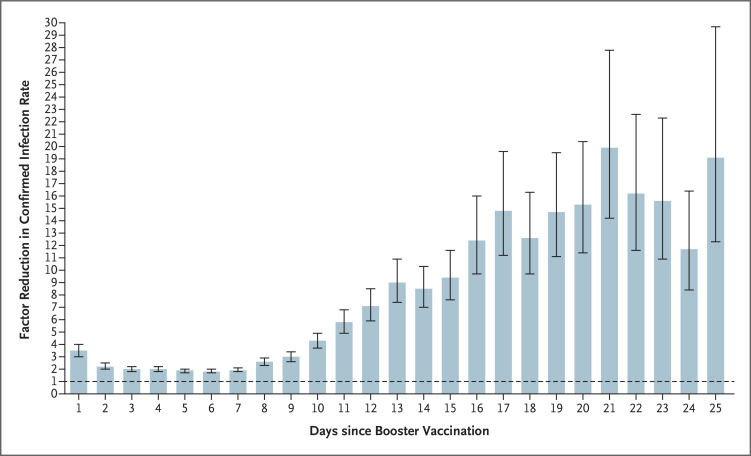
Figure 2 presents the results of the Poisson regression analysis with the number of days after booster vaccination as additional covariates, showing the reduction in the rate of confirmed infection over time as compared with the nonbooster group. On each day during the period from 12 to 25 days after receipt of the booster dose, the booster group had a rate of confirmed infection that was lower than that in the nonbooster group by a factor of 7 to 20, a finding that was similar to the primary results. Immediately after vaccination, the rate of confirmed infection in the booster cohort was lower than that in the nonbooster group, a difference that is probably the result of the aforementioned behavioral changes that often follow vaccination.
Figure 2. Reduction in Rate of Confirmed Infection in Booster Group as Compared with Nonbooster Group.
Shown is the factor reduction in the rate of confirmed infection among participants who received a third (booster) dose of the BNT162b2 vaccine as compared with those who did not receive a booster dose, according to the number of days after the administration of the booster dose. Because of wide confidence intervals, only days 1 through 25 are shown. The dashed horizontal line represents the level at which the booster dose provided no added protection. The 𝙸 bars represent 95% confidence intervals, which have not been corrected for multiplicity.
Sensitivity Analyses
To test the robustness of the results obtained from the primary and secondary analyses, we performed several sensitivity analyses. The rate ratios that were calculated in two different sensitivity analyses were similar to those calculated for the primary analysis (Supplementary Methods section). Figure S4 presents the results of the same analysis that is shown in Figure 2 restricted to participants who received the booster dose at different time periods (August 1 to 4, August 5 to 8, and August 9 to 12). The same U shape for the rate ratio is observed during the three periods, with similar values. Finally, when the primary analysis was restricted to the general Jewish population, the rate ratio was 10.8 (95% CI, 9.9 to 11.7) for confirmed infection and 21.7 (95% CI, 14.1 to 33.6) for severe illness, with corresponding estimates for the average rate differences of 83.4 and 8.2 events, respectively, per 100,000 person-days.
Discussion
In our study, we found that a booster dose of the BNT162b2 vaccine reduced the rates of both confirmed infection and severe Covid-19 illness in a large Israeli population of participants who were 60 years of age or older. Our findings can be understood through the following example. Suppose, first, that the combined effect of waning immunity and the increased prevalence of the delta variant decreases the efficacy of a vaccine that had been administered 6 months earlier to approximately 50% relative to the susceptibility in an unvaccinated person, as recent reports have suggested.2,16,17 Then suppose that, as suggested by our results, the booster dose reduces the rate of infection for such vaccine recipients by a factor of 10. This would mean that the susceptibility of a person who receives a booster dose would decrease to approximately 5% (i.e., 50% divided by 10) relative to that in an unvaccinated person and would bring the vaccine efficacy among booster recipients to approximately 95%, a value similar to the original vaccine efficacy reported against the alpha variant.9,18
Although our analysis attempted to address possible biases in the source data, such as the effects of confounders and behavioral changes after vaccination, some sources of bias may not have been measured or corrected adequately. These biases include differences between the booster recipients and those who did not receive the booster with respect to care-seeking behaviors and cautiousness, along with differences in coexisting illnesses that are not recorded in the national database. Some of these possible biases are transient and fade with time after the booster vaccination, as schematically shown in Figure S1. This suggests that the real effectiveness of vaccination can be estimated by comparing infection rates before receipt of the booster dose and after a suitable time period (e.g., 12 days) following vaccination. Although independent research is required to fully understand this behavioral model, several indications suggest that our 12-day cutoff is reasonable. First, persons tend to undergo fewer PCR tests on the day of vaccination and during the subsequent few days, which is a potential source for detection bias. Consistent with such behavioral change is the pattern shown in Figure 2, which indicates a large reduction in infection rate on the first day after vaccination, a decrease that is attenuated during the subsequent days before starting to increase as the booster dose becomes effective.
Confounding and detection bias may contribute to the observed reduction in the infection rate. We can put a lower boundary on the effect of the booster dose by focusing on persons who received the booster dose and comparing rates during a period in which the booster effect was expected to be small with rates during a period in which the booster had become effective. We therefore compared the rates at least 12 days after receipt of the booster with rates during days 4 to 6, when the booster effect was expected to be small and behavioral changes after vaccination were less marked. In this secondary analysis, the rate of confirmed infection was lower starting at 12 days after receipt of the booster than during days 4 to 6 after receipt by an estimated factor of 5.4. Even under this conservative analysis, the demonstrated rate reduction highlights the important role that a booster dose could play in mitigating the effects of waning immunity and immune evasion, especially during the emergence of variants of concern, such as the delta variant.
Understanding the protection gained by a booster dose is critical for public health policy. On July 30, 2021, Israel was the first country in the world to make available a third dose of the BNT162b2 vaccine against Covid-19 to all persons who were 60 years of age or older and who had been vaccinated at least 5 months earlier. Since then, Israel has extended the booster program to the entire population. The results of such a policy are important for policymakers in countries that are exploring strategies to mitigate the pandemic. Our findings give clear indications of the effectiveness of a booster dose even against the currently dominant delta variant. Future studies will help determine the long-term effectiveness of the booster dose against current and emerging variants.
Protocol
Supplementary Appendix
Disclosure Forms
This article was published on September 15, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World in Data. 2020. (https://ourworldindata.org/coronavirus).
- 2.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. August 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1). preprint. [DOI] [PMC free article] [PubMed]
- 3.Public Health England. Investigation of SARS-CoV-2 variants of concern: technical briefings. GOV.UK, December 21, 2020. (https://www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201).
- 4.Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2 breakthrough infections to time-from-vaccine: preliminary study. July 31, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.29.21261317v1). preprint. [DOI] [PMC free article] [PubMed]
- 5.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021;397:2331-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfizer quarterly corporate performance — second quarter 2021. Pfizer, July 28, 2021. (https://investors.pfizer.com/events-and-presentations/event-details/2021/Pfizer-Quarterly-Corporate-Performance--Second-Quarter-2021/default.aspx).
- 7.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205-1211. [DOI] [PubMed] [Google Scholar]
- 8.Muhsen K, Na’aminh W, Lapidot Y, et al. A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, February 2020–February 2021. Lancet Reg Health Eur 2021;7:100130-100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med 2021;9:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAloon C, Collins Á, Hunt K, et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open 2020;10(8):e039652-e039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin H, Wong JY, Murphy C, et al. The incubation period distribution of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Infect Dis 2021. June 12 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. R: a language and environment for statistical computing. The R Foundation, 2020. (https://www.r-project.org/).
- 15.Kleinman LC, Norton EC. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res 2009;44:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. August 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v3). preprint.
- 17.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. August 11, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.11.21261885v1). preprint. [DOI] [PubMed]
- 18.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.