Abstract
Introduction:
The objective of this study was to use the American College of Surgeons’ National Cancer Database (NCDB) to examine the association between primary treatment and overall survival (OS) among patients with locoregionally advanced hypopharyngeal cancer.
Methods:
6,055 adult patients diagnosed between 2004–2015 with stage III or IV, M0, hypopharyngeal squamous cell carcinoma were identified within the NCDB. Patients who received primary chemoradiation (CRT) were compared to those that received surgery with adjuvant radiation or chemoradiation (S+Adj). OS was compared between treatment groups using Kaplan-Meier analyses, propensity score adjustment, and Cox regression analyses.
Results:
The median survival was 22.7 months (IQR 11.0–49.0). The S+Adj group had a significantly higher comorbidity score, higher grade disease, and more advanced stage disease than the CRT group. S+Adj was associated with significantly improved survival when compared to CRT (p<0.0001). A propensity score adjusting for facility type, facility location, care at multiple facilities, histology, and T stage was developed. S+Adj was associated with longer survival (HR: 0.72, 95% CI: 0.64–0.80) when compared to CRT in a multivariable Cox regression analysis (adjusting for age, race and ethnicity, insurance status, a comorbidity index, diagnosis year, treatment delay, N stage, and the propensity score). S+Adj was associated with significantly improved survival among those with T2 disease (p=0.02), T3 disease (p=0.02), and T4 disease (p<0.0001) in sensitivity analyses examining these subcohorts independently.
Conclusions:
Among patients with advanced hypopharyngeal cancer reported in NCDB, treatment with S+Adj was associated with longer survival compared to those treated with primary CRT.
Keywords: Hypopharynx, Squamous Cell Carcinoma of the Head and Neck, Hypopharyngeal Neoplasms, Head and Neck Cancer
Introduction
Treatment of hypopharyngeal squamous cell carcinoma (HSCC) is guided by several landmark randomized controlled clinical trials (RCT). The Veteran’s Affairs (VA) Trial1 demonstrated that for locoregionally advanced laryngeal squamous cell carcinoma (LSCC), organ preservation with induction chemotherapy (IC) followed by radiation therapy (RT) did not have significantly different survival outcomes when compared to total laryngectomy (TL) followed by RT. Subsequent larynx trials established that concurrent chemoradiation therapy (CRT) was superior to induction chemotherapy followed by radiation therapy, which has been confirmed by large meta-analyses2. Because of the limited number of trials conducted on HSCC patients, findings of these larynx trials have been extrapolated to HSCC patients and helped to shape treatment approaches. Though limited, a few trials have been conducted exclusively on HSCC patients. These have shown no survival differences between CRT and surgery3–6. Most notably the European Organization for Research and Treatment of Cancer Trial #24891 (EORTC 24891) compared outcomes among patients with HSCC managed with IC+RT or IC+surgery compared to surgery+RT and found initial survival differences with those in the experimental arm surviving longer3. Long-term follow-up of that cohort found no survival differences, and the CRT group had a functional laryngeal preservation rate of only 8.7% at 10 years4. Though these RCTs demonstrated no survival differences, subsequent observational studies suggest that organ-preservation with CRT may come at a survival cost7 with only a subset of hypopharyngeal patients able to achieve long-term laryngeal preservation.
While the cohorts in these large RCTs included a subset of subjects with HSCC, they were primarily composed of subjects with LSCC. There are key differences between HSCC and LSCC that may impact treatment selection and outcome. Most HSCC is diagnosed as stage IV disease (59.1%), while the rate is much lower in LSCC (26.8%)8. The 5-year survival rate for HSCC is a dismal 33% while 62% of patients with LSCC survive at 5 years8. Studies of large databases and retrospective studies of hypopharyngeal cancer have suggested a survival benefit to surgery with adjuvant RT or adjuvant CRT (S+Adj)9–12. These facts suggest further investigation into appropriate patient selection for CRT or S+Adj is warranted among patients with HSCC.
The National Cancer Database (NCDB)13 is a national database maintained by the Commission on Cancer of the American College of Surgeons and the American Cancer Society, capturing an estimated 70% of all newly diagnosed cancers in the United States. The NCDB has been previously used to investigate factors associated with survival in locoregionally advanced HSCC. Kuo et al.10 investigated initial treatment and survival in HSCC patients diagnosed between 2003–2006 and found a longer survival among those that received surgery and adjuvant therapies after adjustments for age, sex, race, insurance, Charlson-Deyo comorbidity score, and staging characteristics. Others have found associations with insurance status14,15, treatment delays16,17, as well as HPV status18 and survival among subjects with HSCC in NCDB. Though many have used NCDB to investigate hypopharyngeal cancer, to our knowledge, our study is the first to focus on the sequence of treatments received, and thus compare surgical and non-surgical standard approaches for treating HSCC.
Recently, we utilized the Surveillance, Epidemiology and End-Results (SEER) database, a large population-based database managed by the National Cancer Institute, to explore the association between initial treatment modality (CRT vs. S+Adj) and disease-specific survival (DSS) and overall survival (OS)12. We found that S+Adj was associated with longer survival compared to those treated with CRT alone12. Based on those findings, we hypothesized that this survival advantage may be in part due to appropriate intensification of adjuvant therapies based on pathologic staging and characterization. Compared to SEER, the NCDB database contains information from approximately 3 times more patients19, includes subjects’ Charlson-Deyo comorbidity score20, more detailed insurance variables, and more details regarding treatment (including days between diagnosis and treatment initiation, and radiation dosing). The objective of this study was to use data from the NCDB to validate our previous findings, testing the hypothesis that S+Adj would be associated with longer survival as compared to CRT.
Methods
Inclusion/Exclusion Criteria—
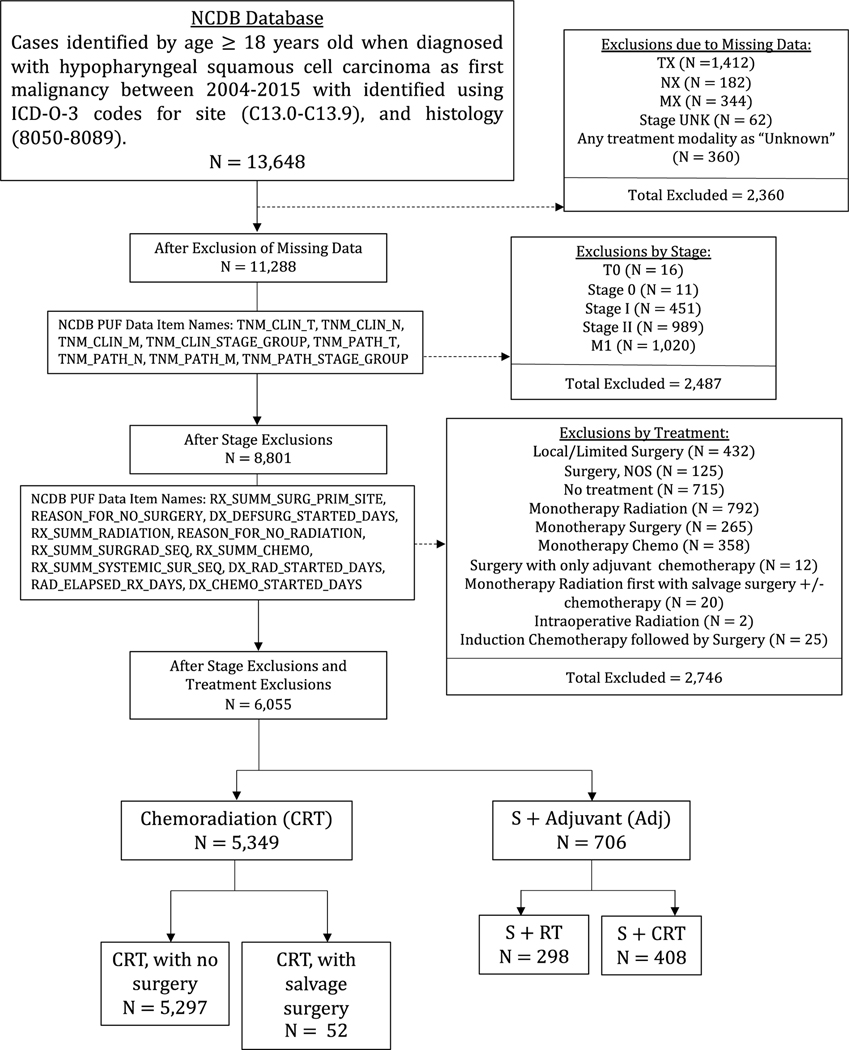
Data was collected on neoplasms of the hypopharynx (International Classification of Diseases for Oncology, Third Edition17, site codes C13.0-C13.9) within the NCDB diagnosed between 2004–2015. To be included, neoplasms had to be the subject’s first malignancy and histologically confirmed squamous cell carcinoma (ICD- O-3 histology codes 8050 to 8089)21. Staging criteria were defined using the American Joint Committee on Cancer 6th or 7th edition staging manual22,23 depending on year of diagnosis. Since there were no major changes between these guidelines, staging was not converted to a single staging system. Cases were excluded if they were overall stage I or II, had distant metastases (M1), or were missing data. Details of inclusion and exclusion criteria24 can be found in Figure 1.
Figure 1.
CONSORT diagram for the study cohort: selection of subjects with overall stage III or stage IV hypopharyngeal squamous cell carcinoma diagnosed between 2005 and 2015 in the National Cancer Database (NCDB).
Treatment Groups—
Treatment groups were defined using the variables for surgical procedure, radiation therapy, and chemotherapy. Subjects were included in the CRT group if they received both chemotherapy (CT) and RT with either no surgery or surgery after CRT. Subjects were included in the surgery with adjuvant therapy group (S+Adj) if they received surgery prior to receiving adjuvant RT (S+RT) or adjuvant CRT (S+CRT). Since these groups represent the standard of care treatment approaches, all other regimens were excluded (Figure 1).
Sequence of CT and RT among those treated with CRT was evaluated. The CRT group was divided into those that received induction chemotherapy with RT and those that received concomitant CRT. Similar to other studies using NCDB data11, IC was defined as CT that began prior to RT, and concomitant CRT was defined as CT beginning during RT.
Other Variables—
Insurance status was divided into three categories for inclusion in multivariable models: uninsured, insured with private insurance/Medicare, and insured with Medicaid. Delay in treatment was defined as greater than 60 days from diagnosis to initiation of curative intent treatment based on previously defined cut-offs16,25.
Statistical Analyses–
Analyses were carried out using STATA SE, version 15.1 (stata.com). Bivariate associations between the primary treatment groups were tested using Kruskal-Wallis tests and Chi-square tests.
Univariate Survival Analyses–
OS was calculated using vital status and follow-up time from diagnosis date. Kaplan-Meier (KM) survival analyses and log-rank tests were used to assess for the association between treatment and OS. Univariate Cox regression analyses were performed to select potential variables for consideration in the multivariable model.
Propensity Score Analysis—
Propensity scores (PS) are used to adjust for potential sources of selection bias in observational studies and to isolate treatment effects from the effect of patient traits on treatment selection. A multivariable logistic model describing the probability of receiving curative intent surgery as compared to CRT was built using a backwards-stepwise approach with an exclusion criterion of p-value for Wald statistic of ≥0.05 and p-value for likelihood ratio test ≥0.05. Excluded variables were re-assessed within the PS model for their ability to reduce standardized differences between the treatment groups. Maximum standardized differences for each variable within the quintiles of PS was defined as 25%. After the iterative model building process, the final propensity score included variables for facility type, geographic region, treatment at multiple locations, histology, and T stage and resulted in improved balance of the covariates (Supplemental Table 1).
Multivariable Survival Analyses with Propensity Score Adjustment—
A multivariable model was used to adjust for patient and disease characteristics which may confound results but were not included in the propensity score. The propensity score was included a priori, and a backwards stepwise approach was built using all variables found to be significant in univariate analyses. Variables were excluded using an exclusion criterion of p-value ≥0.05 on likelihood ratio tests. First-order interactions and the proportional hazards assumption of the final model were assessed.
Multivariable Survival Analyses with Propensity Matching—
The final multivariable model was also assessed in a subset of the cohort which was built using 1:1 nearest neighbor propensity score matching (N=1404) (Supplemental Table 2). Given that these results were consistent with the results seen in the entire population, here we describe the results of the multivariable model built using the entire cohort for ease of interpretation.
Sensitivity Analyses:
Stratification by T Stage— Since T stage is one of the most important factors in determining treatment approach, we stratified our cohort by T stage and repeated Kaplan Meier analyses and re-assessed Cox regression models within each sub-cohort.
Stratification by N Stage—We repeated analyses after stratified our cohort by N stage and repeated Kaplan Meier analyses and re-assessed Cox regression models within each sub-cohort.
Surgery Only Subjects—To approximate an intention to treat analysis, Kaplan Meier analyses and Cox regression models were re-assessed with subjects receiving surgery alone included in the S+Adj group.
Partial Pharyngectomy Subjects—In the primary analyses, subjects that received curative intent limited/partial pharyngectomies were included in the S+Adj group. In order to eliminate potentially misclassified subjects, results were re-assessed with only those receiving a total pharyngectomy and/or a total laryngectomy (PL).
Re-assessment using Alternate Survival Time—Survival time may be affected by any delay in treatment or prolonged treatment course. As a sensitivity analysis, survival time was calculated from the end of radiation therapy until last follow-up date or death. This allowed for re-assessment of Kaplan-Meier analyses to assess if the treatment effects were attributable to delays in treatment or prolonged treatment course.
Results
The cohort was composed of 6,055 subjects with 5,349 (88%) subjects in the CRT group and 706 (12%) subjects in the S+Adj group. The cohort had a mean age of 61.13 (SD ±10.00) and was predominantly male (81.4%), of non-Hispanic white race (75.8%), and insured (92.8%). Most subjects were insured through Medicare (37.3%) followed by private insurance (35.8%) and Medicaid (16.7%). The S+Adj subjects lived further from the hospital, had a higher Charlson-Deyo Comorbidity score, and had a higher proportion with high-grade tumors compared to the CRT group (each p<0.05). The S+Adj group contained a higher proportion of patients with T4 disease (54.0%) compared to the CRT group (27.1%) and had a higher proportion of patients with N2 disease (63.9% vs 57.2%), while the CRT group carried a higher percentage with N3 disease (8.4% vs 4.6%). The groups differed in overall stage (p<0.0001). The CRT group had a significantly higher percentage of patients with stage III (25.5% vs. 15.3%), stage IVA (57.3% vs. 7.8%), and stage IVNOS disease (1.6% vs. 0%). The S+Adj group had a higher proportion with stage IVB disease (76.9% vs 15.6%). S+Adj had a longer median survival than CRT (27.3 months vs 22.0 months, p<0.001). All of the selected cohort were treated according to NCCN treatment guidelines24 (100%). Cohort and treatment group characteristics are detailed in Table 1.
Table 1.
Characteristics of selected cohort of patients from the National Cancer Database (NCDB)* with stage III or IV (M0) hypopharyngeal squamous cell carcinoma (SCC) diagnosed between 2004 and 2015.
| Characteristics† | CRT | S+Adj | Total | P-Value‡ | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N = 5,349 | N = 706 | N = 6,055 | |||||
| Age | 60 | (54–68) | 60 | (54–66) | 60 | (54–68) | 0.030 |
| Sex | 0.35 | ||||||
| Female | 1,002 | (18.7%) | 122 | (17.3%) | 1,124 | (18.6%) | |
| Male | 4,347 | (81.3%) | 584 | (82.7%) | 4,931 | (81.4%) | |
| Race/Ethnicity§ | 0.50 | ||||||
| NHW | 3,835 | (75.6%) | 513 | (77.3%) | 4,348 | (75.8%) | |
| NHB | 847 | (16.7%) | 109 | (16.4%) | 956 | (16.7%) | |
| NHO | 142 | (2.8%) | 18 | (2.7%) | 160 | (2.8%) | |
| Hispanic | 250 | (4.9%) | 24 | (3.6%) | 274 | (4.8%) | |
| Distance to Hospital, miles | 9 | (3.9–21.9) | 15.3 | (5.6–45.5) | 9.5 | (4.0–23.9) | <0.001 |
| Insurance | 0.15 | ||||||
| Not Insured | 382 | (7.4%) | 40 | (5.9%) | 422 | (7.2%) | |
| Private Insurance | 1,845 | (35.7%) | 252 | (37.0%) | 2,097 | (35.8%) | |
| Medicaid | 846 | (16.4%) | 132 | (19.4%) | 978 | (16.7%) | |
| Medicare | 1,947 | (37.6%) | 239 | (35.1%) | 2,186 | (37.3%) | |
| Other Government | 154 | (3.0%) | 18 | (2.6%) | 172 | (2.9%) | |
| Charlson-Deyo Score | 0.001 | ||||||
| 0 | 4,183 | (78.2%) | 510 | (72.2%) | 4,693 | (77.5%) | |
| 1 | 871 | (16.3%) | 157 | (22.2%) | 1,02 8 | (17.0%) | |
| 2 | 217 | (4.1%) | 30 | (4.2%) | 247 | (4.1%) | |
| >=3 | 78 | (1.5%) | 9 | (1.3%) | 87 | (1.4%) | |
| Primary Site‖ | 0.010 | ||||||
| Piriform Sinus | 2,754 | (51.5%) | 413 | (58.5%) | 3,167 | (52.3%) | |
| Post-Cricoid Region | 92 | (1.7%) | 10 | (1.4%) | 102 | (1.7%) | |
| AE Fold | 328 | (6.1%) | 29 | (4.1%) | 357 | (5.9%) | |
| Posterior Wall | 301 | (5.6%) | 31 | (4.4%) | 332 | (5.5%) | |
| OL | 246 | (4.6%) | 34 | (4.8%) | 280 | (4.6%) | |
| NOS | 1,628 | (30.4%) | 189 | (26.8%) | 1,817 | (30.0%) | |
| HPV¶ | 0.90 | ||||||
| HPV− | 711 | (74.0%) | 108 | (74.5%) | 819 | (74.1%) | |
| HPV+ | 250 | (26.0%) | 37 | (25.5%) | 287 | (25.9%) | |
| Grade# | 0.002 | ||||||
| I | 194 | (4.9%) | 15 | (2.2%) | 209 | (4.5%) | |
| II | 2,189 | (55.7%) | 368 | (54.7%) | 2,557 | (55.6%) | |
| III | 1,510 | (38.4%) | 278 | (41.3%) | 1,788 | (38.9%) | |
| IV | 35 | (0.9%) | 12 | (1.8%) | 47 | (1.0%) | |
| T Stage | <0.001 | ||||||
| T1 | 536 | (10.0%) | 44 | (6.2%) | 580 | (9.6%) | |
| T2 | 1,627 | (30.4%) | 101 | (14.3%) | 1,728 | (28.5%) | |
| T3 | 1,741 | (32.5%) | 186 | (26.3%) | 1,927 | (31.8%) | |
| T4 | 1,445 | (27.0%) | 375 | (53.1%) | 1,820 | (30.1%) | |
| N Stage | <0.001 | ||||||
| N0 | 749 | (14.0%) | 113 | (16.0%) | 862 | (14.2%) | |
| N1 | 1,104 | (20.6%) | 122 | (17.3%) | 1,226 | (20.2%) | |
| N2 | 3,076 | (57.5%) | 466 | (66.0%) | 3,542 | (58.5%) | |
| N3 | 420 | (7.9%) | 5 | (0.7%) | 425 | (7.0%) | |
| Summary Stage | <0.001 | ||||||
| III | 1,364 | (25.5%) | 108 | (15.3%) | 1,472 | (24.3%) | |
| IVA | 3,064 | (57.3%) | 55 | (7.8%) | 3,11 9 | (51.5%) | |
| IVB | 834 | (15.6%) | 543 | (76.9%) | 1,377 | (22.7%) | |
| IVNOS | 87 | (1.6%) | 0 | (0.0%) | 87 | (1.4%) | |
| Survival Time, months | 22.0 5 | (10.7–48.5) | 27. 3 | (13.5–55.7) | 22.7 | (11.0–49.0) | <0.001 |
Subjects were included in the chemotherapy and radiation group (CRT) if they had received primary radiation and chemotherapy. Subjects were included in the surgery with adjuvant therapy group (S+Adj) if they received primary surgery with either adjuvant radiation or adjuvant radiation and chemotherapy.
Plus-minus values are mean ± standard deviation for normally distributed continuous variables. Values for non-normal continuous variables are reported as median (interquartile range). Frequencies are reported as number (proportion of overall group within column).
The continuous variables all had non-normal distribution and were assessed with Kruskall-Wallis test. Chi-Square tests were used to assess categorical variables.
Abbreviations: NHW—Non-Hispanic ethnicity, white race; NHB—Non-Hispanic ethnicity, black race; NHO—Non-Hispanic ethnicity, other race; H—Hispanic ethnicity, all races
Abbreviations: AE Fold—Aryepiglottic fold; OL—Overlapping lesion of the hypopharynx; NOS—Hypopharynx, not otherwise specified
HPV Status was only available for the 1,123 subjects those diagnosed between 2010–2015.
Grade was missing for 1,298 subjects (34 of these were in the S+Adj group and 1,264 were in the CRT group).
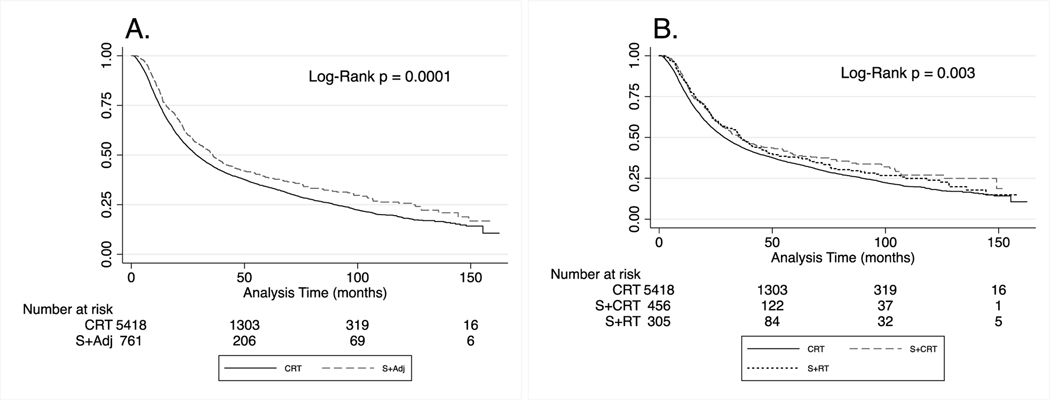
Unadjusted OS was significantly longer for the S+Adj group compared to the CRT group (p=0.0001, Figure 2A). When re-assessed with S+Adj stratified into S+RT and S+CRT, both S+RT and S+CRT were associated with longer OS compared to CRT (p=0.003, Figure 2.B.). There was no significant difference observed between S+RT and S+CRT.
Figure 2.
Survival of patients with stage III and stage IV hypopharyngeal squamous cell carcinoma diagnosed between 2005 and 2015 in the National Cancer Database. Patients are grouped by recorded treatment received. (A) Overall survival (OS). (B) OS with S + Adj split into surgery with adjuvant radiation therapy (S + RT) and surgery with adjuvant chemoradiation therapy (S + CRT).
Univariable Cox regression analyses found that treatment at more than one CoC center, age, race/ethnicity, Charlson-Deyo comorbidity score, insurance status, diagnosis year, histology, extracapsular extension, HPV status, site of cancer, T stage, N stage, and delay in treatment were all associated with significantly different survival between treatment groups (likelihood ratio test p<0.05 for all). Treatment with S+Adj was associated with an 18% reduction in hazard of death in univariable Cox regression analysis (95% CI: 0.74–0.90; Supplemental Table 2).
In the multivariable model of OS with adjustment for propensity score, S+Adj was associated with an independent 27% reduction in hazard of all-cause mortality as compared to CRT after adjustments for age, sex, race and ethnicity, insurance through Medicaid, comorbidity score, year of diagnosis, and N stage (HR: 0.72; 95% CI: 0.64–0.80; p<0.001; Table 2). When the model was re-developed with S+Adj stratified into S+RT and S+CRT, both S+RT and S+CRT were associated with significantly longer OS after adjustments for other model components (S+RT HR: 0.74; 95% CI: 0.63–0.87; p=0.001; S+CRT HR: 0.72; 95% CI: 0.63–0.83; p<0.001; Supplemental Table 3).
Table 2.
Results of multivariable Cox regression of overall survival in selected cohort of patients from the National Cancer Database (NCDB) with stage III or IV (M0) hypopharyngeal squamous cell carcinoma including propensity score adjustment.
| Overall Survival | |||
|---|---|---|---|
|
| |||
| HR | 95% CI | P-Value | |
| Age | 1.02 | 1.02 to 1.03 | <0.001 |
| Gender | |||
| Female | 1 | (Reference) | |
| Male | 0.96 | 0.88 to 1.05 | 0.396 |
| Race | |||
| Non-Hispanic, White | 1 | (Reference) | |
| Non-Hispanic, Black | 1.13 | 1.03 to 1.23 | 0.012 |
| Non-Hispanic, Other | 0.85 | 0.68 to 1.06 | 0.145 |
| Hispanic, all Races | 0.97 | 0.82 to 1.14 | 0.694 |
| Insurance | |||
| Private Insurance/Medicare | 1 | (Reference) | |
| Uninsured | 1.36 | 1.19 to 1.56 | <0.001 |
| Medicaid | 1.45 | 1.32 to 1.60 | <0.001 |
| Charlson-Deyo Comorbidity Score | |||
| 0 | 1 | (Reference) | |
| 1 | 1.13 | 1.04 to 1.24 | 0.005 |
| 2 | 1.17 | 0.99 to 1.37 | 0.063 |
| >3 | 1.45 | 1.11 to 1.89 | 0.006 |
| Year of Diagnosis | |||
| 2004 | 1 | (Reference) | |
| 2005 | 0.84 | 0.72 to 0.99 | 0.034 |
| 2006 | 0.86 | 0.74 to 1.01 | 0.065 |
| 2007 | 0.86 | 0.73 to 1.01 | 0.058 |
| 2008 | 0.88 | 0.76 to 1.03 | 0.110 |
| 2009 | 0.85 | 0.72 to 0.99 | 0.034 |
| 2010 | 0.90 | 0.77 to 1.05 | 0.182 |
| 2011 | 0.77 | 0.66 to 0.91 | 0.002 |
| 2012 | 0.75 | 0.64 to 0.88 | <0.001 |
| 2013 | 0.68 | 0.58 to 0.80 | <0.001 |
| 2014 | 0.73 | 0.61 to 0.86 | <0.001 |
| 2015 | 0.66 | 0.55 to 0.80 | <0.001 |
| Treatment Delay* | 1.09 | 0.99 to 1.19 | 0.065 |
| N Stage | |||
| N0 | 1 | (Reference) | |
| N1 | 0.95 | 0.84 to 1.07 | 0.401 |
| N2 | 1.13 | 1.02 to 1.25 | 0.016 |
| N3 | 1.67 | 1.44 to 1.94 | <0.001 |
| Propensity Score† | 7.91 | 4.86 to 12.86 | <0.001 |
| Treatment‡ | |||
| CRT | 1 | (Reference) | |
| S+Adj | 0.72 | 0.64 to 0.80 | <0.001 |
Delay in treatment was defined as 60 days or longer from diagnosis to treatment based on the results published by Liao et al. 2019.
Propensity score included adjustments for facility type, geographic region, treatment at multiple locations, histology, and T stage.
Subjects were included in the chemotherapy and radiation group (CRT) if they had received primary radiation and chemotherapy. Subjects were included in the surgery with adjuvant therapy group (S+Adj) if they received primary surgery with either adjuvant radiation or adjuvant radiation and chemotherapy.
The final multivariable model was also assessed in a subset of the cohort which was built using 1:1 nearest neighbor propensity score matching. These results were consistent with the findings in the model with adjustments for propensity score and showed a 45% reduction in the hazard of all-cause mortality with S+Adj compared to CRT (HR: 0.58 to 0.75, p<0.001, N=1404, Supplemental Table 2).
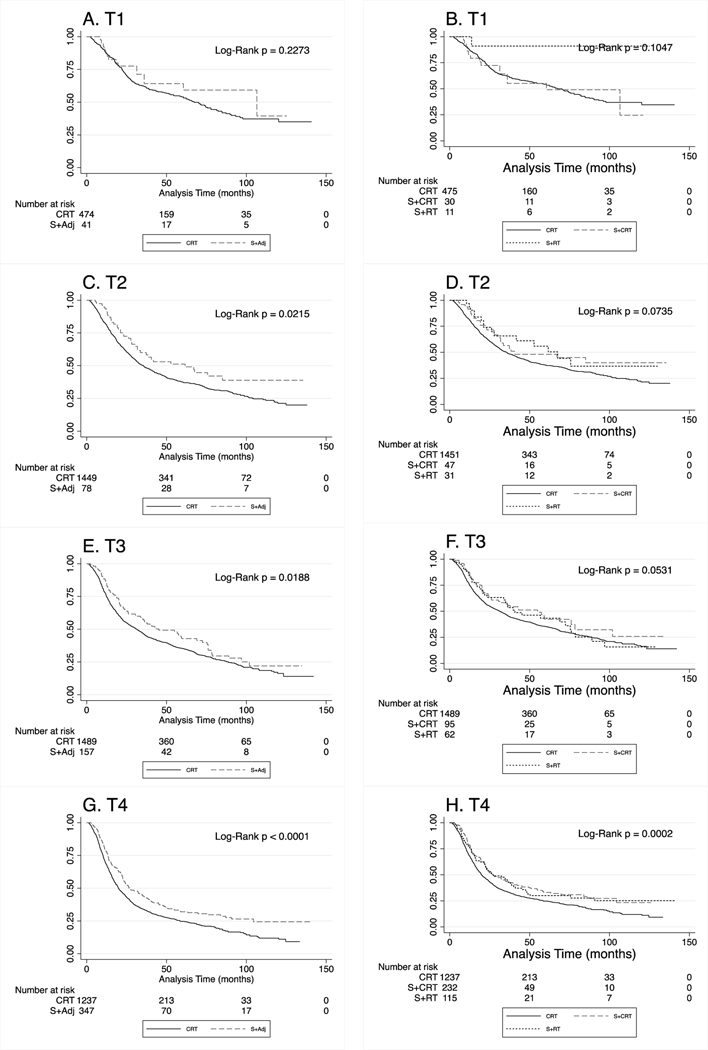
After stratification by T stage, Kaplan-Meier analyses were reconsidered. S+Adj was associated with longer OS in T2, T3 and T4 disease (log rank p<0.05 for all, Figure 3). In multivariable models stratified by T-stage, S+Adj remained independently associated with a reduction in hazard of all-cause mortality in models in the T2 disease, T3 disease, and T4 disease subcohorts after adjustments for age, sex, race and ethnicity, insurance through Medicaid, year of diagnosis, N stage and the propensity score. Among patients with T2 disease, S+Adj was associated with a 36% reduction in hazard of death (HR: 0.64; 95% CI: 0.48–0.86; p=0.003; Supplemental Table 4). In T3 disease, S+Adj was associated with a 21% reduction in hazard of death (HR: 0.79; 95% CI: 0.64–0.97; p=0.025), and in T4 disease, S+Adj was associated with a 25% reduction in hazard of death (HR: 0.75; 95% CI: 0.65–0.87; p<0.001; Supplemental Table 4).
Figure 3.
Kaplan-Meier survival analysis stratified by American Joint Committee on Cancer (AJCC) tumor (T) staging among cohort of subjects from the National Cancer Database (NCDB) with overall stage III and stage IV hypopharyngeal squamous cell carcinoma diagnosed between 2005 and 2015. Chemoradiation (CRT) was defined as primary radiation and chemotherapy. S + Adj was defined as primary surgery with adjuvant CRT or adjuvant radiation therapy. (A) Demonstrates overall survival (OS) among subjects with T1 disease. (B) Demonstrates OS among those with T1 disease with S+Adj stratified into surgery with adjuvant radiation therapy (S+RT) and surgery with adjuvant chemoradiation therapy (S+CRT). (C) OS among patients with T2 disease. (D) OS among patients with T2 disease, where S+Adj was stratified by S+RT vs. S+CRT. (E) OS among patients with T3 disease. (F) OS among patients with T3 disease with S+Adj stratified into S+RT and S+CRT groups. (G) OS among patients with T4 disease. (H) OS among patients with T4 disease with S+Adj stratified into S+RT and S+CRT groups.
After stratification by N stage, Kaplan-Meier analyses of unadjusted overall survival found S+Adj was associated with longer OS in N0, N1, and N2 disease (log rank p<0.05 for all, Supplemental Figure 1). In multivariable models stratified by N-stage, S+Adj remained independently associated with a reduction in hazard of all-cause mortality (Supplemental Table 5). For patients with N0 disease, S+Adj was associated with a 36% reduction in hazard of death (HR: 0.64; 95% CI: 0.47–0.86; p=0.003). In N1 disease, S+Adj was associated with a 38% reduction in hazard of death (HR: 0.62; 95% CI: 0.46–0.82; p=0.001) and in N2 disease, S+Adj was associated with a 24% reduction in hazard of death (HR: 0.76; 95% CI: 0.66–0.88; p<0.001). In N3 disease, S+Adj was associated with a non-significant 60% reduction in hazard of death (HR: 0.40; 95% CI: 0.12–1.30; p=0.127).
The cohort was re-evaluated including subjects who received surgery alone in the S+Adj group to evaluate for potential selection bias potentially caused by patients that died prior to receiving planned adjuvant therapy. In this analysis, unadjusted OS was not significantly different between S+Adj and CRT (p=0.11; Supplemental Figure 2). However, in the multivariable model of OS with surgery-only subjects included in the S+Adj group, S+Adj remained significantly associated with longer survival, with a 26% reduction in hazard of all-cause mortality as compared to CRT after adjustments for other model components (HR: 0.74; 95% CI: 0.65–0.83; p<0.001; Supplemental Table 6).
There was potential selection bias if patients who received limited surgery were actually diagnostic procedures that were mis-coded as surgical procedures, however when partial laryngectomies were excluded, the results did not change substantially. OS remained longer among those receiving S+Adj (p=0.04; Supplemental Figure 3). After adjustment in multivariable Cox regression analysis of OS with partial laryngectomy/pharyngectomy subjects excluded, S+Adj was associated with an independent 26% reduction in hazard of all-cause mortality as compared to CRT after adjustments for other model components (HR: 0.74; 95% CI: 0.66–0.87; p<0.001; Supplemental Table 7).
Kaplan-Meier analysis comparing subjects receiving induction CT with RT to those that received concurrent CRT found no difference in overall survival (p=0.54, Supplemental Figure 4). Kaplan-Meier curves were re-assessed using an alternate calculation of survival time, from end of radiation therapy until last follow-up or death. S+Adj remained associated with a longer survival as compared to CRT (p=0.0065, Supplemental Figure 5).
Discussion
In this study, we used the NCDB to compare OS between the two standard treatment approaches for locoregionally advanced HSCC, carefully defining treatment groups with consideration for treatment sequence. We defined our cohort to include only advanced stage disease patients managed with standard of care treatment according to NCCN guidelines24. We found that first-line surgical intervention with adjuvant therapy was associated with a significant increase in OS as compared to CRT after adjustment for key confounders. We found that unadjusted OS was significantly longer among those managed with S+Adj compared to CRT, despite S+Adj being associated with more advanced disease characteristics.
Our findings here support our previously published findings comparing S+Adj and CRT among subjects with locoregionally advanced HSCC in the SEER database12. Studying SEER, both OS and DSS were significantly longer with S+Adj as compared to CRT after adjustments for confounding variables12. We similarly stratified by T stage and found that S+Adj was associated with longer DSS among those with T1/T2, and in those with T4 disease, while the increase among those with T3 disease was a non-significant. Here, we found that S+Adj was associated with significantly longer OS within the subcohorts of subjects with T2, T3, and T4 disease. The larger sample size available in the NCDB may have enabled detection of a significant difference in the T3 group.
In our study of SEER data, we found that survival was longest among those with S+CRT as compared to those with S+RT12. Here, we found no significant difference in OS between the S+CRT group versus those the S+RT group. This could reflect improved outcomes after appropriate allocation of adjuvant treatment guided by pathologic findings, such as the presence of extracapsular lymph node spread of disease, which is associated with treatment failure and a clear indication for treatment escalation26. This may be due to inherent differences between the SEER database and the NCDB (i.e., different patient populations, treatment centers, etc.). SEER is captured from a smaller number of institutions, while the NCDB reports data from a larger number of Commission on Cancer (CoC) sites19. The difference between S+CRT and S+RT in SEER may be due to differences in the allocation of adjuvant therapies at SEER sites as compared to NCDB sites. The NCDB reports treatment data if received at multiple CoC sites, while SEER only captures data from single institutions. This may have resulted in misclassification in our SEER-based study. There are several advantages of this NCDB-based study over our previous SEER-based study, such as the ability to adjust for patient comorbidities, treatment delays, and a higher degree of treatment detail in the NCDB – allowing for more accurate segregation of our cohort into appropriate treatment groups.
EORTC 24891 found an initial survival advantage for patient with hypopharyngeal SCC managed with IC followed by RT for responders or surgery for non-responders as compared to patients managed with surgery followed by adjuvant RT, but 10-year follow-up found no difference in survival, or progression-free survival3,4. Notably, that study had issues with treatment adherence, whereas in this study we only considered outcomes based on treatments received. The difference in our results maybe in part due to the high rate of cross over observed, highlighting the need for improved methods of allocating patients into their individualized most optimal treatment.
In this study, only 52 CRT patients (0.9%) were reported as having received a salvage surgery after organ-preservation with CRT. This is drastically lower than the expected rate of salvage surgery. Similar to SEER, NCDB collects treatment data on initial planned treatment and doesn’t uniformly collect data on unplanned treatments. Even so, this rate of salvage surgery is lower than the expected rate of early failure rate seen with induction CT plus RT in RTOG 91–11 (4.97%)2. This failure rate is also much lower than the long-term failure rate described among hypopharynx SCC patients in EORTC 248913,4. NCDB describes treatment at multiple CoC institutions, it may miss other treatments received by an individual at a non-CoC site leading to misclassification and may partially explain this difference.
Our results support the findings of other retrospective studies of advanced HSCC. Tassler et al.5 reported a survival advantage with front-line PL as compared to CRT in a retrospective study of 137 subjects at a single institution. Other prior studies have reported a survival advantage with surgery as compared to organ-preservation approaches using data from both NCDB11 and SEER10,12,27. Kim et al.28 reported no survival differences in a study of locoregionally advanced HSCC using SEER data, but notably, they did not consider sequence of treatments nor did they exclude subjects with a past history of other malignancies, both issues that were addressed carefully by these analyses.
Toxicity is expected to be high in this patient population regardless of treatment path29. Toxicity, both early and long term, is not captured and cannot be evaluated using NCDB data. Since outcomes are relatively poor and similar regardless of treatment rendered for patients with advanced hypopharynx cancer, as evidenced by our data, it is important to align treatment selection with patient goals and consideration of the consequences of treatments on quality of life.
This study has limitations inherent to its design and the use of NCDB data. NCDB does not include disease-specific survival measures, nor does it include information about other meaningful endpoints, such as recurrence30, distant metastases, and second primary head and neck cancers. We adjusted for Charlson-Deyo comorbidity score, but this only described the number of chronic comorbidities and does not necessarily represent a subject’s functional status. We could not adjust for several other important variables (i.e., smoking history, operative findings, surgical complications other than death)31. Additionally, the proportion of subjects in the S+Adj group in this study was only 11.8% of the cohort. The large discrepancy in size between the two treatment groups may be due to secular trends in treatment, characteristics of the institutions included in the database, or may represent a source of selection bias in these analyses. The retrospective nature of this study prohibits causal interpretations. Though we used a propensity score to adjust for potential selection bias in this observational study, randomization is the gold standard, and optimal means of adjusting for all potential latent confounders. Despite these limitations, our findings were consistent with our most recent work and with prior studies and suggest that front-line surgery with adjuvant therapy may be optimal for carefully selected patients with advanced HSCC.
Conclusion
There is a role for all treatment modalities in management of hypopharyngeal cancer. The survival difference between surgery and organ-preservation with CRT are not striking but appear consistent. With careful allocation, primary surgery may optimize survival. Though data suggest that the most aggressive approaches lead to optimal survival for patients with HSCC, the overall poor outcomes we report suggest that novel treatments strategies that substantially improve survival while preserving function are warranted.
Supplementary Material
Highlights:
An observational study comparing survival in hypopharynx cancer by treatment.
Treatments compared were chemoradiation and surgery with adjuvant radiation.
Surgery was associated with improved survival when compared to chemoradiation.
Acknowledgements
The authors would like to acknowledge the Office of Medical Student Research and the Clinical Research Training Program at Albert Einstein College of Medicine for their support of Colleen G. Hochfelder’s contribution which was supported by the NIH/NCATS Einstein-Montefiore CTSA grant number KL2TR001071. Thomas J. Ow’s contribution was supported by NIH-NIDCR grant number 1 K23 DE027425–01.
Funding and Conflict of Interests: Colleen G. Hochfelder’s contributions was supported by NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number KL2TR001071. Thomas J. Ow’s contribution was supported by NIH-NIDCR grant 1 K23 DE027425–01. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685–1690. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre J, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx Preservation in Pyriform Sinus Cancer: Preliminary Results of a European Organization for Research and Treatment of Cancer Phase III Trial. 1996;88(13). [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23(10):2708–2714. doi: 10.1093/annonc/mds065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 Randomized Trial on Larynx Preservation Comparing Sequential vs Alternating Chemotherapy and Radiotherapy. 2009;101(3). doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henriques De Figueiredo B, Fortpied C, Menis J, et al. Long-term update of the 24954 EORTC phase III trial on larynx preservation. Eur J Cancer. 2016;65:109–112. doi: 10.1016/j.ejca.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Sanabria A, Chaves ALF, Kowalski LP, et al. Organ preservation with chemoradiation in advanced laryngeal cancer : The problem of generalizing results from randomized controlled trials. Auris Nasus Larynx. 2017;44:18–25. doi: 10.1016/j.anl.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer Facts & Figures. Atlanta, GA; 2017. doi: 10.1101/gad.1593107 [DOI] [Google Scholar]
- 9.Tassler AB, Gooding WE, Ferris RL. Hypopharyngeal cancer treatment: Does initial surgery confer survival benefit? Head Neck. 2019;41(7):2167–2173. doi: 10.1002/hed.25687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: Temporal trends in the United States. Laryngoscope. 2014;124(9):2064–2069. doi: 10.1002/lary.24651 [DOI] [PubMed] [Google Scholar]
- 11.Kuo P, Sosa JA, Burtness BA, et al. Treatment trends and survival effects of chemotherapy for hypopharyngeal cancer: Analysis of the National Cancer Data Base. Cancer. 2016;122(12):1853–1860. doi: 10.1002/cncr.29962 [DOI] [PubMed] [Google Scholar]
- 12.Hochfelder CG, McGinn AP, Mehta V, Castellucci E, Kabarriti R, Ow TJ. Treatment sequence and survival in locoregionally advanced hypopharyngeal cancer: A surveillance, epidemiology, and end results–based study. Laryngoscope. 2019:1–11. doi: 10.1002/lary.28452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Surgeons. About the National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb/about. Published 2020. Accessed February 23, 2020.
- 14.Sullivan CB, Al-Qurayshi Z, Pagedar NA. Analysis of patients who decline treatment for squamous cell carcinoma of the head and neck: National perspective. Head Neck. 2020;(September 2019):1–10. doi: 10.1002/hed.26040 [DOI] [PubMed] [Google Scholar]
- 15.Amini A, Jones BL, McDermott JD, et al. Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer. 2016;122(10):1533–1543. doi: 10.1002/cncr.29956 [DOI] [PubMed] [Google Scholar]
- 16.Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169–178. doi: 10.1200/JCO.2015.61.5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morse E, Berson E, Fujiwara R, Judson B, Mehra S. Hypopharyngeal Cancer Treatment Delays: Benchmarks and Survival Association. Otolaryngol - Head Neck Surg (United States). 2019;160(2):267–276. doi: 10.1177/0194599818797605 [DOI] [PubMed] [Google Scholar]
- 18.Bates JE, Morris CG, Hitchcock KE, Dziegielewski PT, Mendenhall WM, Amdur RJ. Locally advanced hypopharyngeal and laryngeal cancer: Influence of HPV status. Radiother Oncol. 2019;140:6–9. doi: 10.1016/j.radonc.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 19.Janz TA, Graboyes EM, Nguyen SA, et al. A Comparison of the NCDB and SEER Database for Research Involving Head and Neck Cancer. Otolaryngol Neck Surg. 2018;160(2):284–294. doi: 10.1177/0194599818792205 [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). International Classification of Diseases for Oncology. 3rd ed. (Fritz A, Percy C, Jack A, et al. , eds.). Geneva, Switzerland: World Health Organization; 2013. doi: 10.1016/j.matpr.2017.01.181 [DOI] [Google Scholar]
- 22.American Joint Committee on Cancer. American Joint Committee on Cancer Staging Manual. 6th ed. (Greene FL, Page DL, Fleming ID, et al. , eds.). New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 23.American Joint Committee on Cancer. American Joint Committee on Cancer Staging Manual. 7th ed. (Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds.). New York, NY: Springer; 2011. [Google Scholar]
- 24.National Comprehensive Cancer Network. Head and Neck Cancer (Version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed June 4th, 2021.
- 25.Liao DZ, Schlecht NF, Rosenblatt G, et al. Association of Delayed Time to Treatment Initiation with Overall Survival and Recurrence among Patients with Head and Neck Squamous Cell Carcinoma in an Underserved Urban Population. JAMA Otolaryngol – Head Neck Surg. 2019;145(11):1001–1009. doi: 10.1001/jamaoto.2019.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advances head and neck cancers: A comparative analysis of concurrent postoperative radiation trials of the EORTC (# 22931) AND RTOG (# 9501). Head Neck. 2005;27(October):843–850. doi: 10.1002/hed.20279 [DOI] [PubMed] [Google Scholar]
- 27.Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR. Survival trends in hypopharyngeal cancer: A population-based review. Laryngoscope. 2015;125(3):624–629. doi: 10.1002/lary.24915 [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ. Surgery vs. radiotherapy for locally advanced hypopharyngeal cancer in the contemporary era : A population - based study. Cancer Med. 2018; 7(12):5889–5900. doi: 10.1002/cam4.1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garneau JC, Bakst RL, Miles BA. Hypopharyngeal cancer: A state of the art review. Oral Oncology. 2018; (86):244–250. 10.1016/j.oraloncology.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 30.In H, Bilimoria KY, Stewart AK, et al. Cancer recurrence: An important but missing variable in national cancer registries. Ann Surg Oncol. 2014;21(5):1520–1529. doi: 10.1245/s10434014-3516-x [DOI] [PubMed] [Google Scholar]
- 31.Subbarayan RS, Koester L, Villwock MR, Villwock J. Proliferation and Contributions of National Database Studies in Otolaryngology Literature Published in the United States: 2005–2016. Ann Otol Rhinol Laryngol. 2018;127(9):643–648. doi: 10.1177/0003489418784968 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.