Abstract
Objectives
To examine the accuracy and cost-effectiveness of various chronic obstructive pulmonary disease (COPD) screening tests and combinations within a Chinese primary care population.
Design
Screening test accuracy study.
Setting
Urban and rural community health centres in four municipalities of China: Beijing (north), Chengdu (southwest), Guangzhou (south) and Shenyang (northeast).
Participants
Community residents aged 40 years and above who attended community health centres for any reason were invited to participate. 2445 participants (mean age 59.8 (SD 9.6) years, 39.1% (n=956) male) completed the study (February–December 2019), 68.9% (n=1684) were never-smokers and 3.6% (n=88) had an existing COPD diagnosis. 13.7% (n=333) of participants had spirometry-confirmed airflow obstruction.
Interventions
Participants completed six index tests (screening questionnaires (COPD Diagnostic Questionnaire, COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE), Chinese Symptom-Based Questionnaire (C-SBQ), COPD-SQ), microspirometry (COPD-6), peak flow (model of peak flow meters used in the study (USPE)) and the reference test (ndd Easy On-PC).
Primary and secondary outcomes
Cases were defined as those with forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) below the lower limit of normal (LLN-GLI) on the reference test. Performance of individual screening tests and their combinations was evaluated, with cost-effectiveness analyses providing cost per additional true case detected.
Results
Airflow measurement devices (sensitivities 64.9% (95% CI 59.5% to 70.0%) and 67.3% (95% CI 61.9% to 72.3%), specificities 89.7% (95% CI 88.4% to 91.0%) and 82.6% (95% CI 80.9% to 84.2%) for microspirometry and peak flow, respectively) generally performed better than questionnaires, the most accurate of which was C-SBQ (sensitivity 63.1% (95% CI 57.6% to 68.3%) specificity 74.2% (95% CI 72.3% to 76.1%)). The combination of C-SBQ and microspirometry used in parallel maximised sensitivity (81.4%) (95% CI 76.8% to 85.4%) and had specificity of 68.0% (95% CI 66.0% to 70.0%), with an incremental cost-effectiveness ratio of £64.20 (CNY385) per additional case detected compared with peak flow.
Conclusions
Simple screening tests to identify undiagnosed COPD within the primary care setting in China is possible, and a combination of C-SBQ and microspirometry is the most sensitive and cost-effective. Further work is required to explore optimal cut-points and effectiveness of programme implementation.
Trial registration number
ISRCTN13357135.
Keywords: general medicine (see internal medicine), respiratory medicine (see thoracic medicine), chronic airways disease
Strengths and limitations of this study.
Defining airflow obstruction according to the lower limit of normal increased the likelihood that identified cases were true chronic obstructive pulmonary disease.
Recruiting participants from both urban and rural community hospitals maximised the generalisability of our findings to primary care patients.
This study did not explore optimal cut-points for index tests, thus, further work is required.
While the study was conducted in four geographically disparate municipalities, our findings may not be generalisable to all adults ≥40 years old in China.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common long-term condition characterised by persistent respiratory symptoms and airflow limitation.1 Nearly one-third of the 3.2 million annual global deaths from COPD are from China2 3 where COPD ranks among the top three leading causes of death with associated direct medical costs of 118% of local average annual income.4 COPD develops slowly, resulting in delays in symptom recognition and high rates of underdiagnosis. Ninety per cent of the estimated 100 million people with COPD in China are undiagnosed; slightly higher than the 60%–80% underdiagnosis rate worldwide.5–9 Symptom reporting and recognition are lower in China, with 60% of diagnosed patients not reporting symptoms such as cough, expectoration and wheeze.10
While COPD screening programmes are not currently endorsed in the USA and UK,11–13 considering the high proportion and heavy burden of undiagnosed disease,4 early identification is being prioritised in China. National policies recommend screening for undiagnosed COPD14, but do not specify which screening tests to use. Furthermore, though spirometry is required for clinical diagnosis,1 it is not widely available in primary care settings in China. Therefore, screening could reduce the numbers needing spirometry referral.
Globally, various COPD screening tools have been developed, including questionnaires and airflow measurement devices.15–17 However, accuracy studies were mainly conducted in Western countries and have not been validated in a Chinese population where the distribution and underlying causes of undiagnosed COPD may differ. Furthermore, the majority of Chinese studies have used secondary or tertiary care COPD populations rather than people from community settings.18 19 Finally, the cost-effectiveness of different screening tests have not been previously estimated in China; a crucial consideration given the high prevalence of COPD in this middle-income country.
We examined the accuracy and cost-effectiveness of various screening tests and combinations within a Chinese primary care population.
Methods
Study design and participants
We conducted a cross-sectional, multicentre study to evaluate the accuracy and cost-effectiveness of various COPD screening tests and test combinations in primary care in China. Full details of participant recruitment and study assessments are described in the published protocol.20
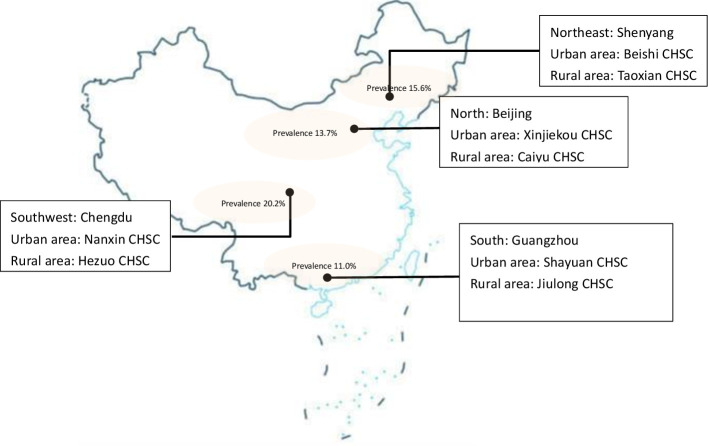
Participants were recruited from one urban and one rural community health centre (CHC) in each of four municipalities: Beijing (North China), Chengdu (southwest China), Guangzhou (south China) and Shenyang (northeast China) (figure 1). Between February and December 2019, community-dwelling residents aged 40 years and above who attended CHCs for any reason were invited to participate, either directly by the attending clinician, or through poster or social media (WeChat) advertisements. Participants who were unable to give informed consent, had contraindications for spirometry or unable to perform the test for other reasons were excluded.
Figure 1.
Map of breathe Well-China research sites. CHSC, Community Health Service Center.
Eligible participants provided informed consent at the start of the assessment visit, prior to height and weight measurement and completion of all index and reference tests. Participants also completed a study questionnaire concerning demographics, smoking status, exposures, medical diagnoses, respiratory symptoms21 and quality of life.22 Data were entered into a secure online Research Electronic Data Capture database.23 24
Participants with airflow obstruction on the reference test were offered health education, smoking cessation advice, influenza vaccination and inhalers if relevant, or referred to tertiary hospitals for further treatment including pharmacotherapy or pulmonary rehabilitation.
Study assessment
Index tests
The six index tests included four screening questionnaires: COPD Diagnostic Questionnaire (CDQ, cut-point ≥20),16 25 COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) (cut-point ≥2),26 COPD Screening Questionnaire (COPD-SQ, cut-point ≥16)19 and the Chinese Symptom-Based Questionnaire (C-SBQ, cut-point ≥17)18 and two airflow measurement devices: microspirometry (Vitalograph COPD-6, cut-point for positive test forced expiratory volume in one second (FEV1)/FEV6<0.78),27 28 peak flow (model of peak flow meters used in the study (USPE), cut-point <350 L/min men, <250 L/min women).26 Questionnaires were selected to maximise symptom capture and minimise item duplication, while allowing comparison of the most relevant questionnaires (online supplemental appendix 1). Previously defined cut-points were used to identify participants at risk of COPD.
bmjopen-2021-051811supp001.pdf (224.8KB, pdf)
Trained researchers provided instructions before participants performed three prebronchodilator manoeuvres with each airflow measurement device. The order of administering peak flow or microspirometry alternated by participant, and the best forced expiratory volume in one second (FEV1) and FEV6 measure for each device were used for analyses, irrespective of which attempt they came from.
Participants completed the four screening questionnaires immediately after administration of a bronchodilator (400 ug, Salbutamol). Questionnaires were intended to be self-completed, although researchers were available to assist if needed.
Reference test
The reference test was quality diagnostic spirometry (ndd Easy On-PC), performed 20–60 min after bronchodilation. Spirometry was administered by a second researcher not involved in the index tests and blind to their results. Participants performed a minimum of 3 blows, and a maximum of 6, until repeatability within 100mls or 5%.29 Flow volume curves were classified according to the American thoracic Society/European Respiratory Society (ERS)29 criteria. Tests with at least three curves meeting these criteria, were ‘good’. ‘Acceptable’ tests contained at least one curve which concurred with the criteria, allowing accurate assessment of forced expiratory volume in one second (FEV1). If accurate assessment was not possible the curves were classified as ‘unacceptable’, and the test was excluded from analysis. All traces were over-read for quality by one of three independent respiratory experts and graded according to standard criteria,29 without knowledge of the index test results.
Airflow obstruction was defined as post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio below the lower limit of normal (LLN) using the Global Lung Initiative (GLI) equations.
Sample size
The Alonzo method30 for paired test accuracy studies was used to calculate the sample size. Assuming independence of tests and prevalence of 12%, we required 1622 participants to detect a difference in sensitivity of 10% (95% vs 85%16 26 31 32 for the comparison of CAPTURE and peak flow for example) with 90% power. With lower test sensitivity (90% vs 80%) 2279 participants are needed to detect this difference with 90% power.
Statistical analysis
The diagnostic performance of each index test was investigated by presenting 2×2 tables and calculating the sensitivity, specificity, positive predictive value and negative predictive value with 95% CIs. Comparative test accuracy was assessed by calculating the difference in sensitivity and specificity, presenting 95% CIs and using McNemar’s test.
The primary analysis compared the sensitivity and specificity between the CAPTURE screening questionnaire and the peak flow meter. The comparison was specified a priori as CAPTURE was rigorously developed, accounted for exposures other than smoking and was intended for use in conjunction with peak flow. Secondary analyses evaluated the comparative performance of all other individual index tests, as well as plausible combination test strategies. Test strategies were formed using two predetermined combinations for appropriate pairs of individual index tests (questionnaires and lung function tests); first, to maximise sensitivity, where a participant with a positive result for either index test would be positive for the strategy (parallel testing strategy) and second, to maximise specificity, where a participant would need a positive result on both index tests to be positive for the strategy (serial testing strategy).
All analyses were conducted in Stata V.15.
Economic analysis
We conducted a cost-effectiveness analysis to calculate the cost per additional case detected for all tests and combination strategies. The strategies were ordered by the number of true cases detected, from least to greatest and the principle of dominance was applied to eliminate redundant strategies (where they were more costly and less effective). Each test was then compared with the next best alternative. For the purpose of this paper, the individual index tests and the combination strategy with the highest sensitivity were compared.
The unit costs and quantity of any equipment, medication and consumables required, staff time (and salary costs) to deliver each individual test and use of facilities were determined to calculate the healthcare costs of delivering each screening test/strategy. Each individual test was timed at a sample of assessment clinics to estimate an overall mean time and range for each test. Equipment costs were depreciated (at 3.5% a year) over the estimated lifespan of the equipment (ranging from 1 to 6 years). Cost per patient visit was calculated assuming the equipment would be used for 12 000 patients per clinic per year. It was also assumed that positive cases would be confirmed with quality diagnostic spirometry (assuming 4000 patients/year). Costs were calculated in UK£ for a price year of 2019, and converted to Chinese Yuan (¥) using Purchasing Power Parities33 with a conversion rate of 6.0 (online supplemental appendix 2).
bmjopen-2021-051811supp002.pdf (39.6KB, pdf)
The paper follows the Standards for Reporting Diagnostic accuracy studies (STARD) guidance34 for reporting studies of diagnostic accuracy.
Results
Sample
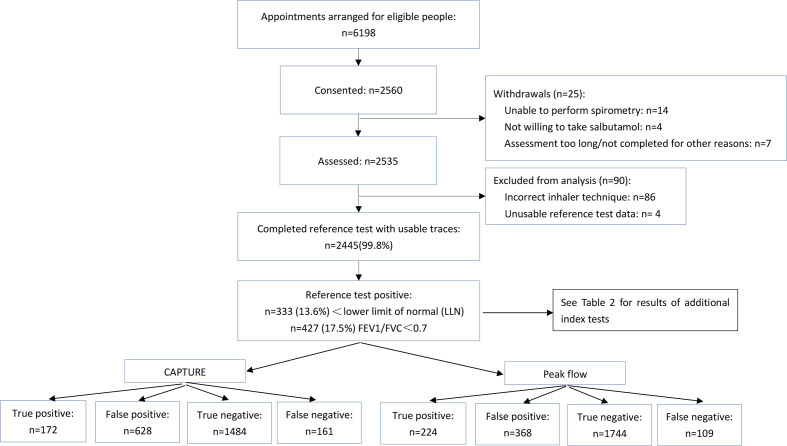
We invited 6198 eligible people to the study. A total of 2560 (41.3%) consented, of whom 25 withdrew and 90 were excluded from analysis (86 because of incorrect inhaler technique, four had unusable spirometry data). A total of 2445 participants with complete data on all index and reference test were included in the final analysis (figure 2). Approximately two-thirds (68.0%) were recruited through their attending clinician, 24.5% via advertisements and 7.5% through word of mouth.
Figure 2.
Study flow chart.
The mean age of participants was 59.8 (SD 9.6), 39.1% (n=956) were male, two-thirds (n=1684, 68.9%) were never smokers and over half lived in an urban area (1338, 54.7%). 46.7% had no diagnosed conditions (n=1142); the most common diagnosed condition was hypertension (n=842, 34.4%), 3.6% (n=88) had an existing COPD diagnosis and 8.4% (n=205) had an existing chronic bronchitis/emphysema diagnosis (table 1). 99.8% of participants had an acceptable usable spirometry (with 63.3% (n=1547) defined as good). 13.6% (n=333) of participants had spirometry-confirmed airflow obstruction using the LLN criteria, of whom 175 (52.5%) had moderate to severe obstruction, that is, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II or above.1 Those with airflow obstruction were older (63.5 vs 69.2 years) and more likely to be male (59.8% vs 35.8%), have a positive smoking history (55.5% vs 27.3%) and childhood respiratory infections (14.7% vs 7.8%) compared with those without airflow obstruction. Respiratory symptoms of wheeze, productive cough or breathlessness (Modified British Medical Research Council (mMRC) ≥2) were reported by 52.9% of those with airflow obstruction (66.3% of those who were GOLD stage II or above), and 25.1% of those without. Among participants with no previously reported COPD diagnosis, the prevalence of obstruction was 9.9% (n=218), of whom 89 (40.8%) were GOLD stage II or above. Using the forced expiratory volume in one second (FEV1)/forced vital capacity (FVC)<0.7 criteria,1 17.4% (n=425) of all participants had airflow obstruction.
Table 1.
Characteristics of study participants
| Characteristic | Total sample (n=2445) |
Reference test positive (n=333) |
Reference test negative (n=2112) |
| Male sex, n (%) | 956 (39.1%) | 199 (59.8%) | 757 (35.8%) |
| Age in years; mean (SD) | 59.8 (9.6) | 63.5 (8.9) | 59.2 (9.6) |
| BMI; mean (SD) | 24.9 (3.5) | 24.3 (3.4) | 25.0 (3.4) |
| Education, n (%) | |||
| High school or below | 1879 (76.9) | 277 (83.2%) | 1602 (75.9%) |
| Above high school | 566 (23.1) | 56 (16.8%) | 510 (24.1%) |
| Employment status, n (%) | |||
| Employed | 674 (27.6%) | 54 (16.2%) | 620 (29.4%) |
| Unemployed | 665 (27.2%) | 98 (29.4%) | 567 (26.9%) |
| Retired | 1106 (45.2%) | 181 (54.4%) | 925 (43.8%) |
| Living in urban areas; n (%) | 1338 (54.7%) | 174 (52.3%) | 1164 (55.1%) |
| Smoking status, n (%) | |||
| Current smoker | 472 (19.3%) | 113 (33.9%) | 359 (17.0%) |
| Ex-smoker | 289 (11.8%) | 72 (21.6%) | 217 (10.3%) |
| Never smoker | 1684 (68.9%) | 148 (44.5%) | 1536 (72.7%) |
| Male | -- | 27 (18.2%) | -- |
| Female | -- | 121 (81.8%) | -- |
| Pack years; mean (SD) | 9.0 (17.8) | 18.0 (21.0) | 7.6 (16.8) |
| Health in general, n (%) | |||
| Very good-good | 1255 (51.3%) | 127 (38.1%) | 1128 (53.4%) |
| Fair-very bad | 1190 (48.7%) | 206 (61.9%) | 984 (46.6%) |
| Diagnosed conditions, n (%) | |||
| COPD | 88 (3.6%) | 64 (19.2%) | 24 (1.1%) |
| Chronic bronchitis/emphysema | 205 (8.4%) | 93 (27.9%) | 112 (5.3%) |
| Asthma | 105 (4.3%) | 48 (14.4%) | 57 (2.7%) |
| Tuberculosis | 41 (1.7%) | 12 (3.6%) | 29 (1.4%) |
| Hypertension | 842 (34.4%) | 119 (35.7%) | 723 (34.2%) |
| Diabetes mellitus | 330 (13.5%) | 43 (12.9%) | 287 (13.6%) |
| Heart disease | 274 (11.2%) | 43 (12.9%) | 231 (10.9%) |
| Other | 269 (11.0%) | 31 (9.3%) | 238 (11.3%) |
| None of the above | 1142 (46.7%) | 106 (31.8%) | 1036 (49.1%) |
| Symptoms, n (%) | |||
| At least occasional wheeze | 322 (13.2) | 110 (33.0) | 212 (10.0) |
| Productive cough | 457 (18.7) | 117 (35.1) | 340 (16.1) |
| mMRC, n (%) | |||
| Grade 0–1 | 2222 (90.9%) | 257 (77.2%) | 1965 (93.0%) |
| Grade 2–4 | 223 (9.1%) | 76 (22.8%) | 147 (7.0%) |
| CAT, mean (SD) | 6.1 (5.4%) | 8.9 (6.9%) | 5.6 (4.9%) |
| Bronchitis, pneumonia or severe whooping cough in childhood | 169 (6.9%) | 38 (11.4%) | 131 (6.2%) |
| Tuberculosis in childhood | 45 (1.8%) | 11 (3.3%) | 34 (1.6%) |
| Exposure to pollutants*, n (%) | |||
| Current/past exposure | 2256 (92.3%) | 307 (92.2%) | 1949 (92.3%) |
| Never | 189 (7.7%) | 26 (7.8%) | 163 (7.7%) |
| Year(s) of exposure, mean (SD) | 8.9 (6.4) | 9.1 (6.6) | 8.8 (6.4) |
| GOLD stage if <LLN, n (%) | |||
| I (FEV1 ≥80% predicted) | -- | 158 (47. 5%) | -- |
| II (FEV1 50%–79% predicted) | -- | 137 (41.1%) | -- |
| III (FEV1 30%–49% predicted) | -- | 33 (9.9%) | -- |
| IV (FEV1 <30% predicted) | -- | 5 (1.5%) | -- |
*Cooking fumes, biomass smoking, gas, steams, dust.
BMI, body mass index; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; GOLD, global initiative for chronic obstructive lung disease; LLN, lower limit of normal; mMRC, modified medical research council dyspnea scale.
Performance of individual tests and screening strategies
Among the screening questionnaires, the C-SBQ had the highest sensitivity in detecting airflow obstruction at 63.1% (95% CI 57.6% to 68.3%), CAPTURE the lowest sensitivity (51.7% (95% CI 46.1% to 57.1%), with CDQ (55.0% (95% CI 49.4% to 60.4%)) similar to COPD-SQ (55.3% (95% CI 49.7% to 60.7%)). The CDQ had the highest specificity (78.6% (95% CI 76.8% to 80.4%)). CAPTURE compared with CDQ had the most obvious difference in specificity of 8.4% (−10.7% to -6.0%; p<0.001) ((tables 2–4)).
Table 2.
Accuracy of index tests and strategies
| Part 1 | Part 2 | Strategy type | TP | FP | TN | FN | Sensitivity% (95% CI) |
Specificity% (95% CI) |
PPV% (95% CI) |
NPV% (95% CI) |
| CAPTURE | n/a | Individual | 172 | 628 | 1484 | 161 | 51.7 (46.1 to 57.1) |
70.3 (68.3 to 72.2) |
21.5 (18.7 to 24.5) |
90.2 (88.7 to 91.6) |
| CDQ | n/a | Individual | 183 | 451 | 1661 | 150 | 55.0 (49.4 to 60.4) |
78.6 (76.8 to 80.4) |
28.9 (25.4 to 32.6) |
91.7 (90.4 to 92.9) |
| C-SBQ | n/a | Individual | 210 | 545 | 1567 | 123 | 63.1 (57.6 to 68.3) |
74.2 (72.3 to 76.1) |
27.8 (24.6 to 31.2) |
92.7 (91.4 to 3.9) |
| COPD-SQ | n/a | Individual | 184 | 479 | 1633 | 149 | 55.3 (49.7 to 60.7) |
77.3 (75.5 to 79.1) |
27.8 (24.4 to 31.3) |
91.6 (90.3 to 92.9) |
| Peak flow | n/a | Individual | 224 | 368 | 1744 | 109 | 67.3 (61.9 to 72.3) |
82.6 (80.9 to 84.2) |
37.8 (33.9 to 41.9) |
94.1 (92.9 to 95.1) |
| Microspirometry | n/a | Individual | 216 | 217 | 1895 | 117 | 64.9 (59.5 to 70.0) |
89.7 (88.4 to 91.0) |
49.9 (45.1 to 54.7) |
94.2 (93.1 to 95.2) |
| CAPTURE | Peak flow | Parallel (OR) |
257 | 863 | 1249 | 76 | 77.2 (72.3 to 81.6) |
59.1 (57.0 to 61.2) |
22.9 (20.5 to 25.5) |
94.3 (92.9 to 95.5) |
| CDQ | Peak flow | Parallel (OR) |
259 | 663 | 1449 | 74 | 77.8 (72.9 to 82.1) |
68.6 (66.6 to 70.6) |
28.1 (25.2 to 31.1) |
95.1 (93.9 to 96.2) |
| C-SBQ | Peak flow | Parallel (OR) |
268 | 729 | 1383 | 65 | 80.5 (75.8 to 84.6) |
65.5 (63.4 to 67.5) |
26.9 (24.2 to 29.7) |
95.5 (94.3 to 96.5) |
| COPD-SQ | Peak flow | Parallel (OR) |
259 | 687 | 1425 | 74 | 77.8 (72.9 to 82.1) |
67.5 (65.4 to 69.5) |
27.4 (24.6 to 30.3) |
95.1 (93.8 to 96.1) |
| CAPTURE | Microspirometry | Parallel (OR) |
262 | 764 | 1348 | 71 | 78.7 (73.9 to 83.0) |
63.8 (61.7 to 65.9) |
25.5 (22.9 to 28.3) |
95.0 (93.7 to 96.1) |
| CDQ | Microspirometry | Parallel (OR) |
261 | 585 | 1527 | 72 | 78.4 (73.6 to 82.7) |
72.3 (70.3 to 74.2) |
30.9 (2.8 to 34.1) |
95.5 (94.4 to 96.5) |
| C-SBQ | Microspirometry | Parallel (OR) |
271 | 675 | 1437 | 62 | 81.4 (76.8 to 85.4) |
68.0 (66.0 to 70.0) |
28.6 (25.8 to 31.6) |
95.9 (94.7 to 96.8) |
| COPD-SQ | Microspirometry | Parallel (OR) |
262 | 620 | 1492 | 71 | 78.7 (73.9 to 83.0) |
70.6 (68.7 to 72.6) |
29.7 (26.7 to 32.8) |
95.5 (94.3 to 96.4) |
| CAPTURE | Peak flow | Serial (AND) |
139 | 133 | 1979 | 194 | 41.7 (36.4 to 47.2) |
93.7 (92.6 to 94.7) |
51.1 (45 to 57.2) |
91.1 (89.8 to 92.2) |
| CDQ | Peak flow | Serial (AND) |
148 | 156 | 1956 | 185 | 44.4 (39.0 to 50.0) |
92.6 (91.4 to 93.7) |
48.7 (42.9 to 54.5) |
91.4 (90.1 to 92.5) |
| C-SBQ | Peak flow | Serial (AND) |
166 | 184 | 1928 | 167 | 49.8 (44.4 to 55.4) |
91.3 (90.0 to 92.5) |
47.4 (42.1 to 52.8) |
92 (90.8 to 93.2) |
| COPD-SQ | Peak flow | Serial (AND) |
149 | 160 | 1952 | 184 | 44.7 (39.3 to 50.3) |
92.4 (91.2 to 93.5) |
48.2 (42.5 to 53.9) |
91.4 (90.1 to 92.5) |
| CAPTURE | Microspirometry | Serial (AND) |
126 | 81 | 2031 | 207 | 37.8 (32.6 to 43.3) |
96.2 (95.3 to 96.9) |
60.9 (53.9 to 67.6) |
90.8 (89.5 to 91.9) |
| CDQ | Microspirometry | Serial (AND) |
138 | 83 | 2029 | 195 | 41.4 (36.1 to 46.9) |
96.1 (95.2 to 96.9) |
62.4 (55.7 to 68.8) |
91.2 (90.0 to 92.4) |
| C-SBQ | Microspirometry | Serial (AND) |
155 | 87 | 2025 | 178 | 46.5 (41.1 to 52.1) |
95.9 (94.9 to 96.7) |
64.0 (57.7 to 70.1) |
91.9 (90.7 to 93) |
| COPD-SQ | Microspirometry | Serial (AND) |
138 | 76 | 2036 | 195 | 41.4 (36.1 to 46.9) |
96.4 (95.5 to 97.2) |
64.5 (57.7 to 70.9) |
91.3 (90.0 to 92.4) |
Serial = positive on BOTH tests required for screen positivity; Parallel = positive on EITHER test required for screen positivity.
CAPTURE, COPD assessment in primary care to identify undiagnosed respiratory disease and exacerbation risk; CDQ, COPD Diagnostic Questionnaire; COPD, chronic obstructive pulmonary disease; C-SBQ, Chinese Symptom-Based Questionnaire; FN, false negative; FP, false positive; n/a, not applicable’; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
Table 3.
Comparative sensitivity for individual tests
| Individual test | CAPTURE (95% CI, p value) |
CDQ (95% CI, p value) |
C-SBQ (95% CI, p value) |
COPD-SQ (95% CI, p value) |
Peak flow (95% CI, p value) |
Microspirometry (95% CI, p value) |
| CAPTURE | −3.3 (−9.6 to 2.9; 0.3245) | −11.4 (−16.9 to 5.9; <0.0001) | −3.6 (−9.6 to 2.5; 0.2615) | −15.6 (−22.1 to −9.1; <0.0001) | −13.2 (−20.2 to −6.2; 0.0002) | |
| CDQ | −8.1 (−12.6 to −3.6; 0.0003) | −0.3 (−5.3 to 4.7; 1.0000) | −12.3 (−18.7 to −6.0; 0.0001) | −9.9 (−16.7 to −3.2; 0.0037) | ||
| C-SBQ | 7.8 (3.2 to 12.4; 0.0007) | −4.2 (−10.4 to 2.0; 0.1978) | −1.8 (−8.4 to 4.8; 0.6427) | |||
| COPD-SQ | −12.0 (−18.3 to −5.7; 0.0002) | −9.6 (−16.4 to −2.8; 0.0052) | ||||
| Peak flow | 2.4 (−4.1 to 8.9; 0.5047) | |||||
| Microspirometry |
Values indicate the difference in sensitivity (with 95% CI and p values), comparing index tests in the column against index tests in the row. For example, sensitivity for CAPTURE is 3.3% lower than for CDQ (95% CI −9.6 to 2.9; 0.3245).
CAPTURE, COPD assessment in primary care to identify undiagnosed respiratory disease and exacerbation risk; CDQ, COPD Diagnostic Questionnaire; COPD, chronic obstructive pulmonary disease; C-SBQ, Chinese Symptom-Based Questionnaire.
Table 4.
Comparative specificity for individual tests
| Individual test | CAPTURE (95% CI, p value) |
CDQ (95% CI, p value) |
C-SBQ (95% CI, p value) |
COPD-SQ (95% CI, p value) |
Peak flow (95% CI, p value) |
Microspirometry (95% CI, p value) |
| CAPTURE | −8.4 (−10.7 to −6.0; <0.0001) | −3.9 (−6.2 to −1.6; 0.0008) | −7.1 (−9.3 to −4.8; <0.0001) | −12.3 (−14.8 to −9.8; <0.0001) | −19.5 (−21.8 to −17.1; <0.0001) | |
| CDQ | 4.5 (3.0 to 5.9; <0.0001) | 1.3 (−0.4 to 3.0; 0.1335) | −3.9 (−6.1 to −1.8; 0.0003) | −11.1 (−13.2 to −9.0; <0.0001) | ||
| C-SBQ | −3.1 (−4.8 to −1.5; 0.0002) | −8.4 (−10.6 to −6.2; <0.0001) | −15.5 (−17.7 to −13.3; <0.0001) | |||
| COPD-SQ | −5.3 (−7.4 to −3.1; <0.0001) | −12.4 (−14.6 to −10.3; <0.0001) | ||||
| Peak flow | −7.1 (−9.1 to −5.2; <0.0001) | |||||
| Microspirometry |
Values indicate the difference in specificity (with 95% CI and p values), comparing index tests in the column against index tests in the row. For example, specificity for CAPTURE is 8.4% lower than for CDQ (95% CI −10.7 to 6.0; <0.0001).
CAPTURE, COPD assessment in primary care to identify undiagnosed respiratory disease and exacerbation risk; CDQ, COPD Diagnostic Questionnaire; COPD, chronic obstructive pulmonary disease; C-SBQ, Chinese Symptom-Based Questionnaire.
Both peak flow and microspirometry devices had higher sensitivity and specificity compared with all questionnaires (tables 3 and 4). Peak flow had the highest sensitivity (67.3%) and microspirometry the highest specificity (89.7%) (tables 3 and 4).
Of the combined screening strategies, C-SBQ combined with airflow measurement devices in parallel (ie, recorded as screen-positive if either test was positive) had the best performance, with sensitivities of 80.5%–81.4% and specificities of 65.5%–68%. Parallel strategies (requiring either test to be positive) optimised sensitivity and serial strategies (requiring both tests to be positive) optimised specificity. Taking CAPTURE and peak flow as an example, a parallel combination had sensitivity of 77.2% compared with 41.7% in serial combination, while the specificity significantly increased from 59.1% to 93.7% (table 2).
Overall, test performance was slightly higher when screening questionnaires were combined with microspirometry rather than peak flow. Strategies including CAPTURE performed less well compared with those based on other questionnaires. Parallel strategies including the C-SBQ had the highest sensitivities, whereas those based on the CDQ had the highest specificity (tables 2 and 3). Full comparisons of serial and parallel strategies are described in online supplemental appendix 3.
bmjopen-2021-051811supp003.pdf (114.4KB, pdf)
Cost-effectiveness of preferred screening tests
Analysis of the C-SBQ parallel strategies revealed that the most costly strategy was the combination of C-SBQ and microspirometry, but this also detected the most true cases (table 5). The C-SBQ alone was dominated by microspirometry (more costly, less effective). The incremental cost-effectiveness ratio for C-SBQ and microspirometry (vs peak flow) was greatest at £64.20 (CNY 385.20), but could be considered cost-effective if the threshold willingness to pay for an additional true case detected in China is at least CNY 385.
Table 5.
Per patient cost, effectiveness and cost-effectiveness of selected screening strategies
| Strategy | Cost per test UK£ (CNY) |
Difference in cost UK£ (CNY) |
True cases detected | Difference in true cases detected | ICER UK£ (CNY) per additional true case detected |
| C-SBQ | 2.22 (13.30) | – | 0.0858 | – | Dominated by microspirometry |
| Microspirometry | 1.60 (9.60) | −0.62 (−3.70) | 0.0883 | 0.0025 | 18.13 (108.78) vs no screening* |
| Peak flow | 1.71 (10.25) | 0.11 (0.64) | 0.0915 | 0.0057 | 32.89 (197.36) vs microspirometry |
| C-SBQ and microspirometry | 3.43 (20.59) | 1.72 (10.35) | 0.1184 | 0.0269 | 64.20 (385.20) vs peak flow |
*Due to the symptom-based question being excluded from the analysis, the next option is compared with no screening.
C-SBQ, Chinese Symptom-Based Questionnaire; ICER, incremental cost-effectiveness ratio.
Discussion
This is the first study assessing the accuracy of individual screening tools and their combinations to identify undiagnosed COPD within Chinese community populations. We showed that the combination of a simple questionnaire and airflow measurement device could adequately identify adults requiring diagnostic spirometry. Our overall findings were consistent with a meta-analysis of studies from other countries35 that airflow measurement devices were more accurate than questionnaires, and that combinations of screening tests improved ability to detect COPD in primary care. Within single test strategies, microspirometry had the best performance (sensitivity 64.9%, specificity 89.7%). For combination strategies, the C-SBQ and microspirometry used in parallel, maximised sensitivity (81.4%) with reasonable specificity (68%) and would be deemed cost-effective if the Chinese health service was willing to pay ≥CNY385 per additional case detected.
C-SBQ had the highest sensitivity of all screening questionnaires in our study, with comparable specificity. However, accuracy of the C-SBQ was worse than reported in the validation paper of the Chinese tool, with lower sensitivity (63.1% vs 82.5%) but slightly higher specificity (74.2% vs 72.9%). The observed discrepancy may be due to differences in the spectrum of clinical characteristics36 (community sample rather than tertiary care population in previous study) and airflow obstruction criteria used (we used the LLN rather than the GOLD criteria).
Inclusion of the C-SBQ and the CDQ from which it was derived allowed direct comparison of the two measures, confirming that C-SBQ was more accurate for use in Chinese community populations when prioritising sensitivity (sensitivity 63.1% vs 55.0% with slightly lower specificity 74.2% vs 78.6%).
Direct comparison between our findings and those of previous studies was limited by differences in populations and pretest probabilities. COPD among never smokers is more common in China than in western countries and we included never smokers in this study to maximise the range of potential COPD risk factors represented for example, environmental exposures such as dust, biomass fumes and passive smoking, as well as active smoking. Inevitably this contributed to the lower test performance observed. Furthermore, the CAPTURE questionnaire was originally designed to detect more severe COPD. The different case definition in our study therefore precludes direct comparison with previous studies (we plan to report accuracy for detecting more severe clinically significant COPD in a future publication).
Our test accuracy study has highlighted the strengths of different screening tests, which can be used to evaluate future screening programmes. We recruited a large number of participants from urban and rural settings in four geographically diverse municipalities in China, and the proportion of never smokers in our sample (68.9%) was similar to that found in a recent nationally representative cross-sectional study in China (71.4%).;0 which included a younger population (age 20+). We demonstrated that lung function tests and diagnosis of COPD can be implemented by general practitioners (GPs) and nurses after a structured training course with regular quality over reading and feedback, as evidenced by 99% usable spirometry and consistently good quality spirometry in most GP sites. The fully paired study design enabled us to compare the accuracy of multiple index tests and strategies. Alternating the order of peak flow and microspirometry tests during assessments decreased the potential training effect that could have been introduced when conducting consecutive lung function tests in a research context.
We defined the reference test as airflow obstruction regardless of clinical symptoms, to reflect the methods of previous studies and also account for the differing symptom profile reported among Chinese populations, where chronic respiratory symptoms are less recognised. In our study, just over half of those with obstruction were likely to benefit from some treatment due to reported symptoms, and a further quarter of those obstructed would benefit from smoking cessation advice as they had a positive smoking history but no respiratory symptoms.
Accuracy might have differed if the GOLD criteria were used, though unlikely to substantially change the comparative performance of the tests. Defining airflow obstruction according to the LLN criteria increased the likelihood that participants testing positive on study spirometry were true COPD cases, rather than detecting comorbidities with similar clinical presentations such as cardiovascular disease.37 As prebronchodilator spirometry was omitted from the study assessment to minimise participant burden and increase uptake in this large community-based study, we could not assess airflow reversibility.
Our study population included slightly more women than men (60% women). As smoking prevalence is also much lower among women, our study cannot provide an accurate estimate of COPD prevalence. However this should not impact on the estimate of screening test accuracy, which was the primary objective. It was not possible to exclude diagnosed patients with COPD from this study, as Chinese CHCs do not have COPD registers and patients are frequently unaware of their condition. However, as the aim of our study was to determine accuracy of different screening tests by comparing all tests against a reference standard, rather than to evaluate the implementation of a screening programme, inclusion of COPD patients was justified. By including some people with known COPD, we maximised the number of test positives in the study sample.
Although China has recently introduced a national policy of COPD screening, there is no current guidance regarding the tests to use or which test characteristics (ie, sensitivity/specificity) to prioritise. Considering the estimated high prevalence of undiagnosed COPD in China, highly sensitive strategies may be preferred to maximise the number of detected cases, although this would result in large numbers being referred for diagnostic spirometry, many of whom would be false positives. However, the potential inefficiency may be offset by a recent policy to include spirometry in routine primary care health consultations; avoiding the need to refer patients to hospital for diagnostic assessment. While the more sensitive parallel strategies may be preferential in the Chinese healthcare setting, there is a trade-off between sensitivity and specificity according to epidemiology, resources and context; hence, serial strategies may be considered optimal in other settings.
If the strategy of C-SBQ and microspirometry were used in practice and had the same accuracy as reported here, it is likely that true COPD cases who were not detected (false negatives) would have mild disease and would reattend with recurring symptoms, offering further opportunities for referral to diagnostic spirometry.
While our analyses used recommended cut-points for the index tests, it is important to explore their optimal cut-points when applied in this context, as many tests were developed with alternate purposes and/or populations in mind. Thresholds used to indicate airflow obstruction (either in the screening tests or reference test) may not be valid in the whole Chinese population as adequate reference values for lung function are currently unreliable.
Although we have determined the accuracy of different tests when used for screening Chinese community populations for undiagnosed COPD, we did not evaluate the implementation of a screening programme. A recently published model-based cost-effectiveness analysis from China which used international data on QALYs, demonstrated that use of a screening questionnaire combined with a hand-held spirometer was cost saving compared with no screening, but this did not compare different screening strategies and was not based on data from an implementation trial.38 It is important to undertake a trial to compare the effectiveness and cost-effectiveness of the most efficient screening strategy identified in this study (maximising yield with acceptable false positive rate) against usual care on yield and clinical outcomes. Such a trial would need to assess uptake of screening and incorporate pathways for clinical assessment and subsequent treatment for test positive cases. In our study sample, >75% had potential to benefit; less than half with obstruction had treatable symptoms and a further quarter with obstruction and no symptoms would benefit from smoking cessation advice. We presented cost per additional true case detected, however, no country has, to date, stated a willingness to pay threshold for this outcome. The quality-adjusted life-year (QALY) is a more common metric in health economic analyses, with established cost per QALY thresholds. Although outside the remit of our test accuracy study, future work should attempt to extrapolate cases detected to the management of patients with COPD, to assess the impact on quality of life and survival to allow the calculation of QALYs.
In conclusion, we have demonstrated that within the primary care setting in China, the most efficient screening test strategy was a combination of the C-SBQ and microspirometry where a positive test in either would result in a referral for diagnostic spirometry. Further work is required to explore optimal cut-points and there is a need for a clinical trial to evaluate whether a screening programme using this test combination is clinically and cost-effective.
Supplementary Material
Acknowledgments
The investigator and collaborative team include: R Jordan, P Adab (CIs), Z Pan, AP Dickens, C Chi, X Kong, A Enocson, BG Cooper, KK Cheng, A Sitch, S Jowett, R Adams, J Correia de Sousa, A Farley, N Gale, K Jolly, M Maglakelidze, T Maglakelidze, SM Martins, K Stavrikj, R Stelmach, A Turner and S Williams. We thank the dedicated team of researchers at Department of General Practice, Peking University First Hospital for managing and co-ordinating the project. We are also grateful for support from our Trial Steering Committee (Dr. Semira Manaseki-Holland (Chair), Prof. David Mannino, Mr. Zhiwei Zhang (patient), Dr. Xueying Zhou (clinician), Prof. Chunhua Chi, Dr. Rachel Jordan), and The International Scientific Advisory Committee (Prof. Debbie Jarvis, Dr. Semira Manaseki-Holland, Prof. David Mannino, Prof. Niels Chevannes). We especially want to thank the patients included in the study and the clinicians at all community hospitals for their assistance, without whom this project would not have been possible. The clinicians involved in this study included: Wenyan Duan, Mengmeng Guo, Zhi Jin (Caiyu Community Hospital, Beijing); Yuling Li, Shuo Chen, Kang Rong, Yi Cai, Jing Bai (Xinjiekou Community Hospital, Beijing); Wei Xiong, Honglin Luo, Juan Chen, Na Li (Hezuo Community Hospital, Chengdu); Min Chen, Yaling Luo, Xiaolong Zhou (Nanxin Community Hospital, Chengdu), Shixing Liu, Guoxiong Han, Shuhua Sun, Jian Long Li, Jianlong Cai, Haimei Chen, Yuanping Chen (Shayuan Community Hospital, Guangzhou); Bin Hu, Huilian Xuan, Jieya Zhang, Huanping Chen, Fengqi Liu, Haixia Yin (Jiulong Community Hospital, Guangzhou); Ying Chen, Xinzheng Cui, Yao Chen, Hui Peng, Weihan Jin, Xiaoguang Xu (Beishi Community Hospital, Shenyang); Zhenguo Gao, Zhongning Kan, Lina Song, Yongsheng Tong, Yuxuan Zhang, Shuai Mu, Yang Gao (Taoxian Community Hospital, Shenyang); Zhennan Qi, Hui Pang, Anny Gao, Xue Jin (Peking University First Hospital). We thank the patient advisory group for their useful comments, including Mr. Zhiwei Zhang, Mr. Chen Wang, Mr Baoyin Fang and Mr. Deqian Ma. We thank the support of the Health Committees in Xicheng District, Beijing and in Guangzhou for identifying potential research sites. We would also like to acknowledge Radmila Ristovska (1955-2020), also involved in the initiation of this study. We gratefully acknowledge International Primary Care Respiratory Group (IPCRG) for introducing us to the primary care networks involved in this study and for its continued facilitation of clinical engagement. We obtained appropriate permissions to use the Symptom Based Questionnaire, COPD Screening Questionnaire, COPD Diagnostic Questionnaire and COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE).
Footnotes
Twitter: @BreatheWell_UOB
Contributors: RJ and PA co-led the study design, with contributions and advice from all other authors. CC, XK and KKC contributed to decisions on outcome measures. CC and KKC advised on involving GP practices. BC, APD, AE, RJ and PA advised on lung function testing. BC and AE provided training and oversaw the quality assessment for lung function testing. APD, RJ, AJS and PA designed the testing strategy. AJS and SJ designed the analysis plan and economic evaluations respectively. ZP coordinated the data collection, with support from APD, RJ and PA. ZP conducted the statistical analysis, supported by AJS, SJ and APD. ZP and APD wrote the manuscript with input from all other authors. CC was the local PI and oversaw all activities in China. RA, JC-d-S, AF, NKG, KJ, MM, TM, SM, KS, RS, AMT and SW contributed to the development and oversight of this study. As part of the Breathe Well Global Health Research Group, all authors contributed to and approved the final version.
Funding: This research was funded by the National Institute for Health Research (NIHR) NIHR global group on global COPD in primary care, University of Birmingham, (project reference: 16/137/95) using UK aid from the UK Government to support global health research. This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham.
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care. The funder of the study had no role in study design, data collection, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available upon reasonable request. All data requests should be submitted to authors CC and PA for consideration. Access to anonymised data may be granted following review.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study has been approved by Peking University First Hospital (2018-R-141, PUFH) and University of Birmingham (ERN_18-1177, UoB).
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2018 report). Available: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
- 2.GBD 2015 Chronic Respiratory Disease Collaborators . Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med 2017;5:691–706. 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin P, Wang H, Vos T, et al. A Subnational Analysis of Mortality and Prevalence of COPD in China From 1990 to 2013: Findings From the Global Burden of Disease Study 2013. Chest 2016;150:1269–80. 10.1016/j.chest.2016.08.1474 [DOI] [PubMed] [Google Scholar]
- 4.Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:1353–64. 10.2147/COPD.S161555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bednarek M, Maciejewski J, Wozniak M, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008;63:402–7. 10.1136/thx.2007.085456 [DOI] [PubMed] [Google Scholar]
- 6.Casas Herrera A, Montes de Oca M, López Varela MV, et al. Copd underdiagnosis and misdiagnosis in a high-risk primary care population in four Latin American countries. A key to enhance disease diagnosis: the PUMA study. PLoS One 2016;11:e0152266. 10.1371/journal.pone.0152266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med 2017;5:426–34. 10.1016/S2213-2600(17)30119-4 [DOI] [PubMed] [Google Scholar]
- 8.Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest 2015;148:971–85. 10.1378/chest.14-2535 [DOI] [PubMed] [Google Scholar]
- 9.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753–60. 10.1164/rccm.200612-1749OC [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 11.US Preventive Services Task Force (USPSTF), Siu AL, Bibbins-Domingo K, et al. Screening for chronic obstructive pulmonary disease: US preventive services Task force recommendation statement. JAMA 2016;315:1372–7. 10.1001/jama.2016.2638 [DOI] [PubMed] [Google Scholar]
- 12.UK National Screening Committee . UK national screening Committee. An evaluation of screening for COPD against the National screening Committee criteria, 2013. [Google Scholar]
- 13.Screening for chronic obstructive pulmonary disease (COPD) in the general adult population. external review against programme appraisal criteria for the UK national screening Committee 2018.
- 14.National Health and Family Planning Commission of the People . National Health and Family Planning Commission of the People’s Republic of China. The 13th Five-Year Plan for Healthcare, 2016. Available: http://www.gov.cn/zhengce/content/
- 15.Dickens AP, Fitzmaurice DA, Adab P, et al. Accuracy of vitalograph lung monitor as a screening test for COPD in primary care. NPJ Prim Care Respir Med 2020;30:2. 10.1038/s41533-019-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley AJ, Hasan I, Crockett AJ, et al. Copd diagnostic questionnaire (CDQ) for selecting at-risk patients for spirometry: a cross-sectional study in Australian general practice. NPJ Prim Care Respir Med 2014;24:14024. 10.1038/npjpcrm.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: cross sectional survey. BMJ 2003;327:653–4. 10.1136/bmj.327.7416.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Wang M, Li X, et al. Do symptom-based questions help screen COPD among Chinese populations? Sci Rep 2016;6:30419. 10.1038/srep30419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y-M, Chen S-Y, Tian J, et al. Development and validation of a chronic obstructive pulmonary disease screening questionnaire in China. Int J Tuberc Lung Dis 2013;17:1645–51. 10.5588/ijtld.12.0995 [DOI] [PubMed] [Google Scholar]
- 20.Pan Z, Dickens AP, Chi C, et al. Study to evaluate the effectiveness and cost-effectiveness of different screening strategies for identifying undiagnosed COPD among residents (≥40 years) in four cities in China: protocol for a multicentre cross-sectional study on behalf of the breathe well group. BMJ Open 2020;10:e035738. 10.1136/bmjopen-2019-035738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris BG. Epidemiology standardization project (American thoracic Society). Am Rev Respir Dis 1978;118:1–120. [PubMed] [Google Scholar]
- 22.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J 2009;34:648–54. 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price DB, Tinkelman DG, Halbert RJ, et al. Symptom-Based questionnaire for identifying COPD in smokers. Respiration 2006;73:285–95. 10.1159/000090142 [DOI] [PubMed] [Google Scholar]
- 26.Martinez FJ, Mannino D, Leidy NK, et al. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:748–56. 10.1164/rccm.201603-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frith P, Crockett A, Beilby J, et al. Simplified COPD screening: validation of the PiKo-6® in primary care. Prim Care Respir J 2011;20:190–8. 10.4104/pcrj.2011.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labor M, Vrbica Žarko, Gudelj I, et al. Diagnostic accuracy of a pocket screening spirometer in diagnosing chronic obstructive pulmonary disease in general practice: a cross sectional validation study using tertiary care as a reference. BMC Fam Pract 2016;17:112. 10.1186/s12875-016-0518-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153–61. 10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- 30.Alonzo TA, Pepe MS, Moskowitz CS. Sample size calculations for comparative studies of medical tests for detecting presence of disease. Stat Med 2002;21:835–52. 10.1002/sim.1058 [DOI] [PubMed] [Google Scholar]
- 31.Represas-Represas C, Fernández-Villar A, Ruano-Raviña A, et al. Screening for chronic obstructive pulmonary disease: validity and reliability of a portable device in Non-Specialized healthcare settings. PLoS One 2016;11:e0145571. 10.1371/journal.pone.0145571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Bemt L, Wouters BCW, Grootens J, et al. Diagnostic accuracy of pre-bronchodilator FEV1/FEV6 from microspirometry to detect airflow obstruction in primary care: a randomised cross-sectional study. NPJ Prim Care Respir Med 2014;24:14033. 10.1038/npjpcrm.2014.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.OCED . Purchasing power parities (PPP). Available: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [Accessed 05 Oct 2020].
- 34.Stard 2015: an updated list of essential items for reporting diagnostic accuracy studies. Available: http://www.equator-network.org/reporting-guidelines/stard/ [Accessed 01 Jun 2020].
- 35.Haroon S, Jordan R, Takwoingi Y, et al. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ Open 2015;5:e008133. 10.1136/bmjopen-2015-008133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leeflang MM, Rutjes AW, Reitsma JB, et al. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 2013:18511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med 2015;13:41–8. 10.1370/afm.1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu S, You X, Liu T, et al. Cost-Effectiveness analysis of COPD screening programs in primary care for high-risk patients in China. NPJ Prim Care Respir Med 2021;31:28. 10.1038/s41533-021-00233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051811supp001.pdf (224.8KB, pdf)
bmjopen-2021-051811supp002.pdf (39.6KB, pdf)
bmjopen-2021-051811supp003.pdf (114.4KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available upon reasonable request. All data requests should be submitted to authors CC and PA for consideration. Access to anonymised data may be granted following review.