Abstract
Aim:
Investigate real-world outcomes and healthcare utilization of patients with glioblastoma multiforme (GBM) related to O6-methylguanine DNA methyltransferase (MGMT) promoter testing and methylation.
Patients & methods:
US Oncology Network data were analyzed for patients receiving first-line (1L) treatment for GBM.
Results:
Most patients received 1L radiation with temozolomide. Unadjusted median overall survival (OS) was higher in tested versus untested (median:18.1 vs 11.8 months) and in methylated versus unmethylated (median: 25.5 vs 12.4 months). Untested status, unmethylated MGMT and older age were associated with reduced OS and longer 1L treatment with increased OS. Similar findings were observed for progression-free survival. Utilization was similar between cohorts.
Conclusion:
In community oncology practices, MGMT methylation and testing were predictive of better survival in GBM.
Keywords: : glioblastoma, MGMT methylation, MGMT testing, real-world evidence, survival
Lay abstract
We studied the characteristics and survival of patients with newly diagnosed glioblastoma multiforme (GBM) in community-based oncology practices. These patients had received temozolomide and radiotherapy with surgery, which is the standard of care for GBM. We were interested in how patient survival was related to methylation of the O6-methylguanine DNA methyltransferase (MGMT) promoter. The study showed that patients with methylated versus unmethylated MGMT GBM survived longer. However, patients who were tested for methylation, whether MGMT was methylated or not, also survived longer. This may be because patients who get tested also get better care in general.
Graphical abstract
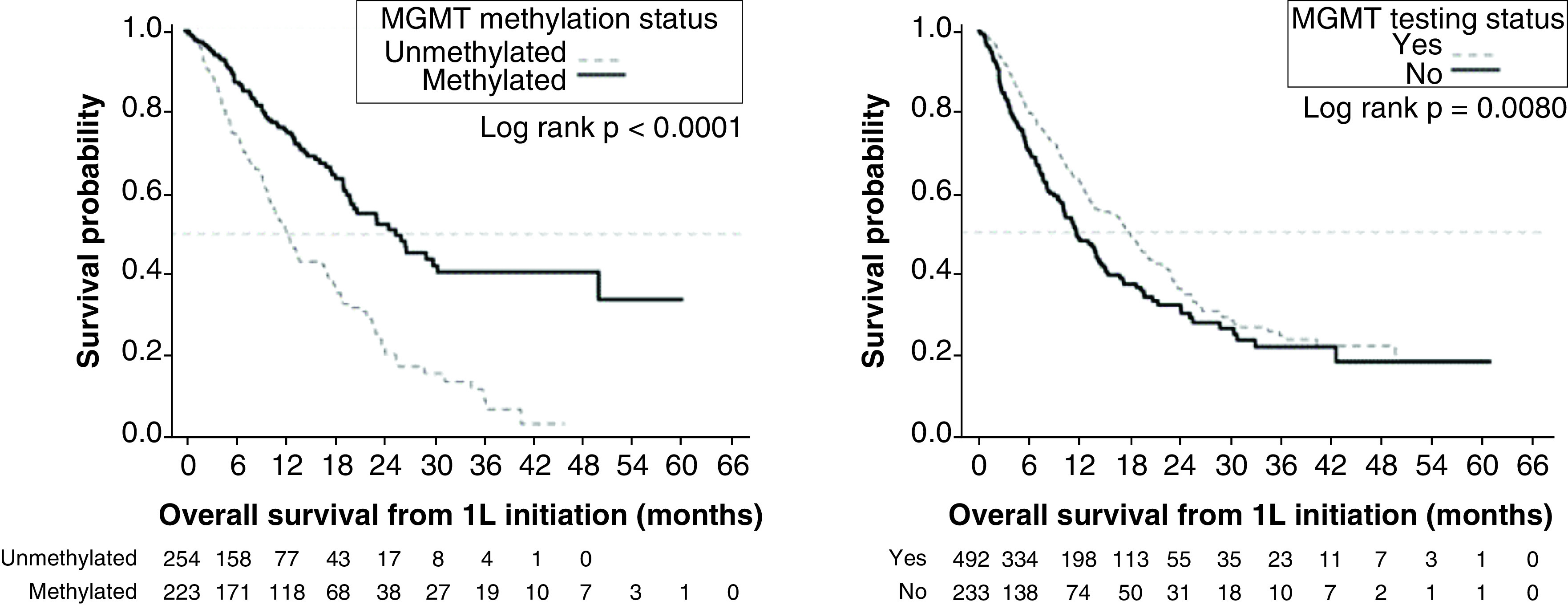
Kaplan–Meier analysis showed that patients with methylated MGMT GBM had better overall survival compared with unmethylated MGMT (median: 25.5 vs 12.4 months). However, so did patients who were tested for MGMT methylation compared with those untested for MGMT methylation (median: 18.1 vs 11.8 months).
Introduction/background & objective
Glioblastoma multiforme (GBM) is a malignant brain tumor with poor prognosis. It accounts for 48.3% of malignant brain and central nervous system tumors in the USA [1]. The average annual age-adjusted incidence rate in the US over the period 2012–2016 was 11,833 patients per 100,000; the 5-year survival rate following diagnosis was 6.8% [1,2]. GBM can progress quickly, leading to rapid clinical decline and death within 15 months of diagnosis [3].
The National Comprehensive Cancer Network guidelines recommend maximal surgical resection followed by radiotherapy (RT) with concomitant and adjuvant chemotherapy with temozolomide (TMZ) [4]. TMZ was approved by the US FDA in 2005 for the treatment of patients with newly diagnosed GBM, to be administered concurrently with RT and then as adjuvant treatment [5]. This treatment is associated with improved outcomes compared with RT alone.
Patients with methylated O6-methylguanine-DNA methyltransferase (MGMT) promoters have impaired DNA repair, which augments the mechanism of action of TMZ-based chemotherapy and improves survival compared with patients with unmethylated MGMT, both in clinical trials and real-world studies [6]. According to real-world studies, approximately 35–50% of all patients with GBM test positive for MGMT methylation [7,8].
In a randomized Phase III trial by the European Organisation for Research and Treatment of Cancer (EORTC)/National Cancer Institute of Canada Clinical Trials Group (NCIC), MGMT methylation was an independent prognostic factor for improved survival among patients treated with TMZ and RT or with RT alone; among those with methylation receiving TMZ + RT, median overall survival (OS) was 21.7 versus 15.3 months among those receiving RT alone [9]. In a 5-year analysis of data from the EORTC/NCIC trial, median OS for the methylated (n = 46) and unmethylated (n = 60) cohorts were 23.4 and 12.6 months, respectively [10].
As for real-world studies, a retrospective study of four hospitals in Italy found that response to treatment, OS and progression-free survival (PFS) correlated positively with methylated MGMT according to both univariate and multivariate analyses [11]. Similarly, a multivariate analysis in a single-center retrospective study in Tunisia demonstrated a positive association of recurrence-free survival with MGMT methylation (p = 0.003) [7].
Most published data in patients with GBM are from clinical trials or hospital settings outside the USA. These results may not adequately model outcomes in US community oncology practices. There is a need for research describing patient characteristics, treatment patterns and outcomes in patients with GBM using real-world data from that setting.
We performed a retrospective cohort study to describe baseline demographic and clinical characteristics, treatment patterns, OS, and PFS among patients with newly diagnosed GBM receiving first-line (1L) treatment stratified by MGMT methylation status (tested vs untested and methylated vs unmethylated) in community-based oncology practices in the USA.
Materials & methods
Study design & data sources
This was a retrospective observational study of adult patients newly diagnosed with GBM receiving 1L treatment from community-based practices in the US Oncology Network. The US Oncology Network utilizes iKnowMed™ (iKM) electronic health record (EHR) and is affiliated with over 2000 providers in 800 sites of care across 40 states in the USA, representing approximately 1.2 million patients treated annually. Data were obtained via programmatic EHR database abstraction and supplemented with chart review.
Patients were identified who met the eligibility criteria from 1 January 2013 to 30 June 2018, with the potential of 3 months of follow up through 30 September 2018 (study period; Figure 1). Patients were followed from the date of initial GBM diagnosis through the end of the study period, date of last visit or date of death, whichever occurred first. The index date was defined as the date of initial GBM biopsy or surgical resection recorded in the iKM database. Diagnosis of GBM was determined through a review of the iKM EHR discrete diagnosis and histology fields, which are populated during the routine course of care. GBM diagnosis was confirmed during chart review through review of pathology reports.
Figure 1. . Study design.
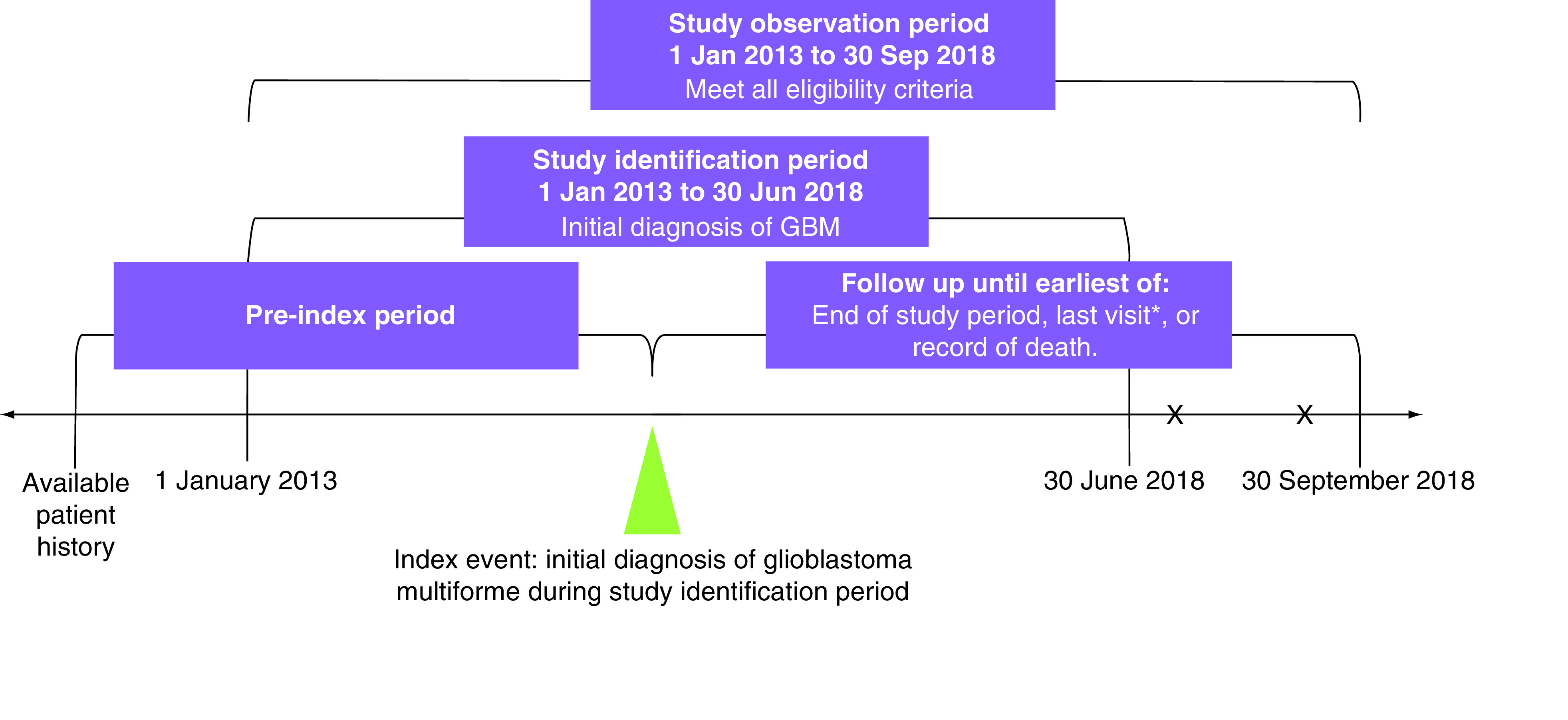
*Last physical encounter.
GBM: Glioblastoma multiforme; X: Additional visit or record of death.
Eligible patients had to be ≥18 years of age at diagnosis of GBM and with ≥two visits within the US Oncology Network during the study period. Patients were excluded if, during the study period, they had been enrolled in clinical trials or if they had another documented primary cancer diagnosis.
Outcomes of interest
Baseline demographic and clinical characteristics, treatment patterns, outcomes (OS and PFS), healthcare resource utilization (HCRU; hospitalizations and emergency department [ED] visits) and 1L treatment-attributable adverse events (AE) were evaluated. Patient characteristics and outcomes were reported for the overall study population and were compared between the cohorts of patients tested versus untested for MGMT methylation status and the cohorts of patients with methylated versus unmethylated MGMT.
Treatment patterns assessed included treatment regimens, treatment sequences, duration of treatment (DOT), and rates of treatment discontinuation. DOT was defined as the interval between the date of initiation of a drug or regimen and the date of the last administration, including treatment holidays or other breaks of 180 consecutive days or less, as recorded in the iKM EHR. Reasons for treatment discontinuation were extracted from chart review.
OS was defined as the interval between the index date and the death date as recorded in the Social Security Death Index or the iKM EHR. PFS was estimated as the interval between the start date of systemic treatment (i.e., immunotherapy or chemotherapy) and the earliest date of progression or death as recorded in the Social Security Death Index or the iKM EHR.
OS and PFS were assessed for the overall study population and compared by MGMT methylation status (tested vs untested and methylated vs unmethylated). In addition, OS and PFS were compared by presence or absence of baseline steroid use within each of these cohorts. Steroid use was defined as the use of dexamethasone >3 mg/day or prednisone >20 mg/day at the time of 1L treatment initiation. Lower doses of steroid use or no steroid use at 1L treatment initiation were considered to indicate substantial recovery after surgery.
AE explicitly attributed to 1L treatment by providers were captured during chart review. The incidence rates of treatment-related AE were assessed for the overall study population and were also compared by MGMT methylation status (tested vs untested and methylated vs unmethylated). In addition, within the cohorts, incidence rates of treatment-related AE were compared by presence or absence of baseline steroid use within the overall study population.
HCRU was measured in terms of number of hospitalizations and ED visits and length of stay across the different cohorts (methylated, unmethylated or untested). Records of hospitalizations and ED visits due to any cause were captured through chart review.
Statistical analysis
Demographic, clinical, and treatment characteristics were reported using descriptive statistics and then stratified by MGMT methylation cohort (tested vs untested and methylated vs unmethylated) and steroid usage. Associations between categorical variables were measured with chi-squared or Fisher’s exact tests, and between continuous variables using analysis of variance/t-tests or Kruskal–Wallis tests.
The Kaplan–Meier (KM) method was used to evaluate the time-to-event end points (i.e., OS and PFS) from 1L treatment initiation and to generate event curves. Data of patients who did not experience an event were censored on the study end date or the last visit date available in the database, whichever occurred first.
Patients in each MGMT methylation cohort (tested vs untested and methylated vs unmethylated) were matched by clinical characteristics using propensity score matching. Propensity scores were obtained using logistic regression and matched using a greedy algorithm with an 8 to 1 digit match [12]. Multivariable Cox regression models were used to compare outcomes in the propensity score-matched cohorts after adjusting for these covariates: 1L and 2L treatment, age, sex, race, ethnicity, practice region (South, Midwest, Northeast, West), surgery type (resection or biopsy only), steroid use prior to 1L treatment, time from diagnosis to 1L treatment and duration of 1L treatment.
Results
There were 2773 adult patients with a diagnosis of GBM with at least two visits within the US Oncology Network receiving care utilizing the full EHR capacities of iKM at time of treatment. Among these, 265 were excluded due to other cancer diagnoses, 235 due to clinical trial enrollment, and 127 who were duplicates or whose data were not accessible for research. The data from the 2146 patients who met the study eligibility criteria were evaluated through chart review. During chart review, patient eligibility was confirmed and followed by assignment to one of the MGMT methylation cohorts: untested, methylated, unmethylated and indeterminate.
Since the number of patients with methylated MGMT was lower than the unmethylated and untested cohorts, chart reviews continued until all patients (n = 229) with methylated MGMT were identified. At that point, chart reviews were stopped. The full sample comprised 750 patients assessed through chart review, in order to obtain similar sample sizes in the untested, methylated and unmethylated cohorts. The final sample numbers were 504 tested for MGMT methylation (229 methylated, 260 unmethylated and 15 indeterminate) and 246 untested.
Demographics & clinical characteristics
Among the overall study population (Tables 1 & 2), more than half were aged younger than 65 years, more than half were male, and most were white and/or not Hispanic or Latino. More than half of the overall patients were treated in the South, followed by the West. A higher proportion of patients with methylated MGMT were female (47.2 vs 37.7%; Table 2). The proportion of patients tested versus not tested for MGMT methylation status (Table 1) differed significantly by region (p < 0.0001); a higher proportion of patients treated were tested versus not tested in the Northeast (14.1 vs 2.0%, respectively), whereas the opposite was true in the South (50.0 vs 57.7%, respectively). With regards to ethnicity (p = 0.0079 for overall distribution), 5.2 and 11.4% in the tested and untested respectively identified as Hispanic/Latino although 21.2 and 11.8%, respectively, had unknown ethnicity. More than half of the overall patients had Eastern Cooperative Oncology Group performance status 0–1 at diagnosis (0 = fully active, 1 = restricted in physically strenuous activity, 2 = ambulatory and capable of self-care but unable to work, 3 = capable only of limited self-care, 4 = completely disabled and 5 = dead; Tables 1 & 2).
Table 1. . Baseline demographic and clinical characteristics for patients with glioblastoma multiforme by MGMT promoter testing status.
| Variable | MGMT methylation testing status | |||
|---|---|---|---|---|
| Overall | No | Yes | p-value | |
| Total patient count (n) | 750 | 246 | 504 | |
| Age at diagnosis (years) | 0.2227 | |||
| Patients with available data (n) | 750 | 246 | 504 | |
| Mean, years (SD) | 62.1 (12.4) | 62.9 (12.2) | 61.6 (12.5) | |
| Median, years (min, max) | 63.4 (20.1, 89.6) | 63.8 (22.1, 87.4) | 63.2 (20.1, 89.6) | |
| Age group, n (%) | 0.5595 | |||
| <65 years | 418 (55.7) | 131 (53.3) | 287 (56.9) | |
| ≥65–75 years | 228 (30.4) | 77 (31.3) | 151 (30.0) | |
| >75 years | 104 (13.9) | 38 (15.4) | 66 (13.1) | |
| Sex, n (%) | 0.8779 | |||
| Female | 317 (42.3) | 103 (41.9) | 214 (42.5) | |
| Male | 433 (57.7) | 143 (58.1) | 290 (57.5) | |
| Race, n (%) | 0.8901 | |||
| Caucasian | 597 (79.6) | 209 (85.0) | 388 (77.0) | |
| Black or African–American | 17 (2.3) | 6 (2.4) | 11 (2.2) | |
| Asian | 13 (1.7) | 3 (1.2) | 10 (2.0) | |
| American–Indian or Alaskan native | 3 (0.4) | 1 (0.4) | 2 (0.4) | |
| Unknown | 120 (16.0) | 27 (11.0) | 93 (18.5) | |
| Ethnicity, n (%) | 0.0079 | |||
| Hispanic or Latino | 54 (7.2) | 28 (11.4) | 26 (5.2) | |
| Not Hispanic or Latino | 560 (74.7) | 189 (76.8) | 371 (73.6) | |
| Unknown | 136 (18.1) | 29 (11.8) | 107 (21.2) | |
| Practice region, n (%) | <0.0001 | |||
| South | 394 (52.5) | 142 (57.7) | 252 (50.0) | |
| West | 231 (30.8) | 81 (32.9) | 150 (29.8) | |
| Northeast | 76 (10.1) | 5 (2.0) | 71 (14.1) | |
| Midwest | 49 (6.5) | 18 (7.3) | 31 (6.2) | |
| ECOG PS at diagnosis, n (%) | 0.1798 | |||
| 0 | 44 (5.9) | 14 (5.7) | 30 (6.0) | |
| 1 | 397 (52.9) | 125 (50.8) | 272 (54.0) | |
| 2 | 128 (17.1) | 49 (19.9) | 79 (15.7) | |
| ≥3 | 29 (3.9) | 14 (5.7) | 15 (3.0) | |
| Unknown | 152 (20.3) | 44 (17.9) | 108 (21.4) | |
| Surgery type, n (%) | 0.0008 | |||
| Resection >90% | 15 (2.0) | 5 (2.0) | 10 (2.0) | |
| Resection <90% (not a biopsy) | 15 (2.0) | 2 (0.8) | 13 (2.6) | |
| Resection NOS | 603 (80.4) | 184 (74.8) | 419 (83.1) | |
| Biopsy | 114 (15.2) | 52 (21.1) | 62 (12.3) | |
| No GBM-related surgery | 3 (0.4) | 3 (1.2) | 0 (0.0) | |
| Resection vs biopsy, n (%) | 114 (15.3) | 52 (21.4) | 62 (12.3) | 0.0002 |
| Steroid use prior to 1L treatment, n (%) | 400 (53.3) | 136 (55.3) | 264 (52.4) | 0.5166 |
Bold numbers are p-values indicating significant difference.
1L: First line; ECOG PS: Eastern Cooperative Oncology Group performance status; GBM: Glioblastoma; MGMT: O6-methylguanine-DNA methyltransferase; NOS: Not otherwise specified; SD: Standard deviation.
Table 2. . Baseline demographic and clinical characteristics for patients with glioblastoma multiforme by MGMT promoter methylation status.
| Variable | MGMT methylation status† | |||
|---|---|---|---|---|
| Overall | Methylated | Unmethylated | p-value | |
| Total patient count (n) | 489 | 229 | 260 | |
| Age at diagnosis (years) | 0.9180 | |||
| Patients with available data (n) | 489 | 229 | 260 | |
| Mean, years (SD) | 61.5 (12.6) | 61.5 (13.0) | 61.4 (12.2) | |
| Median, years (min, max) | 62.8 (20.1, 89.6) | 62.1 (26.7, 89.6) | 63.1 (20.1, 85.9) | |
| Age group, n (%) | 0.9547 | |||
| <65 years | 282 (57.7) | 132 (57.6) | 150 (57.7) | |
| ≥65–75 years | 143 (29.2) | 66 (28.8) | 77 (29.6) | |
| >75 years | 64 (13.1) | 31 (13.5) | 33 (12.7) | |
| Sex, n (%) | 0.0343 | |||
| Female | 206 (42.1) | 108 (47.2) | 98 (37.7) | |
| Male | 283 (57.9) | 121 (52.8) | 162 (62.3) | |
| Race, n (%) | 0.0499 | |||
| Caucasian | 376 (76.9) | 176 (76.9) | 200 (76.9) | |
| Black or African–American | 11 (2.2) | 7 (3.1) | 4 (1.5) | |
| Asian | 10 (2.0) | 8 (3.5) | 2 (0.8) | |
| American–Indian or Alaskan native | 2 (0.4) | 2 (0.9) | 0 (0.0) | |
| Unknown | 90 (18.4) | 36 (15.7) | 54 (20.8) | |
| Ethnicity, n (%) | 0.7574 | |||
| Hispanic or Latino | 26 (5.3) | 11 (4.8) | 15 (5.8) | |
| Not Hispanic or Latino | 361 (73.8) | 164 (71.6) | 197 (75.8) | |
| Unknown | 102 (20.9) | 54 (23.6) | 48 (18.5) | |
| Practice region, n (%) | 0.9683 | |||
| South | 243 (49.7) | 112 (48.9) | 131 (50.4) | |
| West | 146 (29.9) | 68 (29.7) | 78 (30.0) | |
| Northeast | 71 (14.5) | 35 (15.3) | 36 (13.8) | |
| Midwest | 29 (5.9) | 14 (6.1) | 15 (5.8) | |
| ECOG PS at diagnosis, n (%) | 0.4814 | |||
| 0 | 30 (6.1) | 14 (6.1) | 16 (6.2) | |
| 1 | 261 (53.4) | 124 (54.1) | 137 (52.7) | |
| 2 | 75 (15.3) | 28 (12.2) | 47 (18.1) | |
| ≥3 | 15 (3.1) | 7 (3.1) | 8 (3.1) | |
| Unknown | 108 (22.1) | 56 (24.5) | 52 (20.0) | |
| Surgery type, n (%) | 0.9088 | |||
| Resection >90% | 10 (2.0) | 5 (2.2) | 5 (1.9) | |
| Resection <90% (not a biopsy) | 13 (2.7) | 5 (2.2) | 8 (3.1) | |
| Resection NOS | 405 (82.8) | 189 (82.5) | 216 (83.1) | |
| Biopsy | 61 (12.5) | 30 (13.1) | 31 (11.9) | |
| No GBM-related surgery | 428 (87.5) | 199 (86.9) | 229 (88.1) | 0.6942 |
| Resection v biopsy, n (%) | 254 (51.9) | 125 (54.6) | 129 (49.6) | 0.2380 |
| Steroid use prior to 1L treatment, n (%) | 489 | 229 | 260 | |
Patients with MGMT untested/indeterminate methylation status are not included.
Bold numbers are p-values indicating significant difference.
1L: First line; ECOG PS: Eastern Cooperative Oncology Group performance status; GBM: Glioblastoma; MGMT: O6-methylguanine-DNA methyltransferase; NOS: Not otherwise specified; SD: Standard deviation.
Patient characteristics were mostly balanced between the tested and untested cohorts (Table 1) with some exceptions. Surgery type was significantly different between the testing cohorts (p < 0.0008 overall). For example, more tested patients underwent resection (87.7 vs 78.0%), while more untested patients underwent biopsy only as opposed to resection (21.1 vs 12.3%). Patient characteristics were also balanced between the MGMT methylated and unmethylated cohorts (Table 2), except the proportion of female patients was significantly greater in the MGMT-methylated cohort compared with the unmethylated cohort (p = 0.03), and the distribution of race was significantly different (p = 0.05). Types of surgery were similar between the cohorts (Table 1).
Treatment patterns & sequences
Among the overall study population, the most common 1L treatment was concurrent RT and TMZ (90.1%) (Table 3). Among patients receiving 1L treatment, 304 (42%) went on to 2L treatment. The most common 2L treatment was bevacizumab (31%). Significantly more patients in the methylated cohort received 2L bevacizumab (p = 0.0087) compared with the unmethylated cohort.
Table 3. . Treatment characteristics for patients with glioblastoma multiforme by MGMT promoter testing status and by MGMT promoter methylation status.
| Variable | MGMT methylation testing status | MGMT methylation status† | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | No | Yes | p-value | Overall | Methylated | Unmethylated | p-value | |
| Total patient count | 750 | 246 | 504 | 489 | 229 | 260 | ||
| 1L treatment, n (%) | 725 (96.7) | 233 (94.7) | 492 (97.6) | 0.7425 | 477 (97.5) | 223 (97.4) | 254 (97.7) | 0.2580 |
| Radiation concurrent with TMZ | 676 (90.1) | 215 (87.4) | 461 (91.5) | 447 (91.4) | 209 (91.3) | 238 (91.5) | ||
| Radiation sequential (radiation followed by TMZ) | 11 (1.5) | 5 (2.0) | 6 (1.2) | 6 (1.2) | 3 (1.3) | 3 (1.2) | ||
| Radiation only | 15 (2.0) | 6 (2.4) | 9 (1.8) | 9 (1.8) | 2 (0.9) | 7 (2.7) | ||
| TMZ only | 16 (2.1) | 4 (1.6) | 12 (2.4) | 11 (2.2) | 8 (3.5) | 3 (1.2) | ||
| Bevacizumab | 4 (0.5) | 2 (0.8) | 2 (0.4) | 2 (0.4) | 1 (0.4) | 1 (0.4) | ||
| Optune (tumor treating field) | 1 (0.1) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.00) | 1 (0.4) | ||
| Palliative radiation | 2 (0.3) | 1 (0.4) | 1 (0.2) | 1 (0.2) | 0 (0.00) | 1 (0.4) | ||
| No 1L treatment | 25 (3.3) | 13 (5.3) | 12 (2.4) | 12 (2.5) | 6 (2.6) | 6 (2.3) | ||
| Time from diagnosis to 1L treatment initiation (weeks) | 0.3120 | 0.8541 | ||||||
| Patients with available data | 725 | 233 | 492 | 477 | 223 | 254 | ||
| Mean (SD) | 5.2 (4.4) | 5.2 (4.2) | 5.2 (4.5) | 5.3 (4.5) | 5.5 (5.6) | 5.1 (3.2) | ||
| Median (min, max) | 4.4 (0.7, 78.1) | 4.3 (1.1, 45.9) | 4.4 (0.7, 78.1) | 4.6 (0.7, 78.1) | 4.4 (0.7, 78.1) | 4.6 (0.7, 40.1) | ||
| Duration of 1L treatment (weeks) | 0.0333 | <0.0001 | ||||||
| Patients with available data | 725 | 233 | 492 | 477 | 223 | 254 | ||
| Mean (SD) | 26.8 (26.8) | 26.0 (30.0) | 27.1 (25.1) | 27.5 (25.3) | 32.9 (29.5) | 22.7 (19.7) | ||
| Median (min, max) | 19.3 (0.1, 216.9) | 16.1 (0.1, 186.0) | 20.8 (0.1, 216.9) | 21.3 (0.1, 216.9) | 24.9 (1.1, 216.9) | 18.4 (0.1, 118.3) | ||
| Reason for 1L treatment discontinuation, n (%) | ||||||||
| Progression | 248 (34.2) | 88 (37.8) | 160 (32.5) | 0.1642 | 157 (32.9) | 58 (26.0) | 99 (39.0) | 0.0106 |
| Toxicity | 47 (6.5) | 11 (4.7) | 36 (7.3) | 0.1849 | 34 (7.1) | 17 (7.6) | 17 (6.7) | 0.9026 |
| Decline in performance status | 21 (2.9) | 6 (2.6) | 15 (3.0) | 0.7225 | 13 (2.7) | 6 (2.7) | 7 (2.8) | 0.9746 |
| Financial/insurance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0000 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0000 |
| Completed planned treatment | 174 (24.0) | 54 (23.2) | 120 (24.4) | 0.7207 | 116 (24.3) | 60 (26.9) | 56 (22.0) | 0.4556 |
| Hospice | 77 (10.6) | 35 (15.0) | 42 (8.5) | 0.0081 | 41 (8.6) | 14 (6.3) | 27 (10.6) | 0.2332 |
| Death | 51 (7.0) | 22 (9.4) | 29 (5.9) | 0.0811 | 28 (5.9) | 11 (4.9) | 17 (6.7) | 0.6993 |
| Patient preference | 17 (2.3) | 7 (3.0) | 10 (2.0) | 0.4194 | 10 (2.1) | 6 (2.7) | 4 (1.6) | 0.7513 |
| Physician preference | 20 (2.8) | 3 (1.3) | 17 (3.5) | 0.1429 | 17 (3.6) | 9 (4.0) | 8 (3.2) | 0.8517 |
| Other | 100 (13.8) | 25 (10.7) | 75 (15.2) | 0.0997 | 73 (15.3) | 48 (21.5) | 25 (9.8) | 0.0019 |
| Not documented | 12 (1.7) | 2 (0.9) | 10 (2.0) | 0.3555 | 9 (1.9) | 5 (2.2) | 4 (1.6) | 0.8858 |
| Patients initiating 2L treatment, n (% of patients receiving 1L treatment) | 304 (41.9) | 95 (40.8) | 209 (42.5) | 205 (43.0) | 98 (43.9) | 117 (46.1) | ||
| Surgery | 63 (8.7) | 12 (5.2) | 51 (10.4) | 0.0199 | 51 (10.4) | 24 (10.5) | 27 (10.4) | 0.9724 |
| Radiation | 43 (5.9) | 10 (4.3) | 33 (6.7) | 0.1985 | 33 (6.7) | 15 (6.6) | 18 (6.9) | 0.8697 |
| Optune | 24 (3.3) | 5 (2.1) | 19 (3.9) | 0.2278 | 19 (3.9) | 7 (3.1) | 12 (4.6) | 0.3735 |
| Systemic treatment, n (%) | 283 (39.0) | 89 (38.2) | 194 (39.4) | 0.7505 | 190 (38.9) | 81 (35.4) | 109 (41.9) | 0.1380 |
| Bevacizumab | 223 (30.8) | 72 (30.9) | 151 (30.7) | 0.9543 | 148 (30.3) | 56 (24.5) | 92 (35.4) | 0.0087 |
| Carboplatin | 2 (0.3) | 1 (0.4) | 1 (0.2) | 0.5398 | 1 (0.2) | 0 (0.00) | 1 (0.4) | 1.0000 |
| Carmustine | 1 (0.1) | 0 (0.0) | 1 (0.2) | 1.0000 | 1 (0.2) | 1 (0.4) | 0 (0.00) | 0.4683 |
| Cyclophosphamide | 1 (0.1) | 1 (0.4) | 0 (0.0) | 0.3214 | 16 (3.3) | 5 (2.2) | 11 (4.2) | 0.2041 |
| Irinotecan | 29 (4.0) | 12 (5.2) | 17 (3.5) | 0.2768 | 14 (2.9) | 5 (2.2) | 9 (3.5) | 0.3977 |
| Lomustine | 16 (2.2) | 2 (0.9) | 14 (2.8) | 0.1078 | 71 (14.5) | 38 (16.6) | 33 (12.7) | 0.2217 |
| TMZ | 100 (13.8) | 28 (12.0) | 72 (14.6) | 0.3399 | 7 (1.4) | 3 (1.3) | 4 (1.5) | 1.0000 |
| Other | 8 (1.1) | 1 (0.4) | 7 (1.4) | 0.4475 | 489 | 229 | 260 | |
| Common 1L-2L treatment sequences | ||||||||
| Total patient count | 725 | 233 | 492 | 477 | 223 | 254 | ||
| 1L-2L treatment sequence | 0.0024 | 0.0363 | ||||||
| Radiation concurrent with TMZ || Bevacizumab | 89 (12.3) | 38 (16.3) | 51 (10.4) | 49 (10.3) | 15 (6.7) | 34 (13.4) | ||
| Radiation concurrent with TMZ || None | 338 (46.6) | 119 (51.1) | 219 (44.5) | 210 (44.0) | 107 (48.0) | 103 (40.6) | ||
| Other | 298 (41.1) | 76 (32.6) | 222 (45.1) | 218 (45.7) | 101 (45.3) | 117 (46.1) | ||
Patients with MGMT untested/indeterminate methylation status are not included.
Bold numbers are p-values that indicate statistical significance.
1L: First line; 2L: Second line; GBM: Glioblastoma; MGMT: O6-methylguanine-DNA methyltransferase; SD: Standard deviation; TMZ: Temozolomide.
DOT was estimated for all patients and for each MGMT methylation cohort: tested versus untested and methylated versus unmethylated. The median DOT for the combined tested and untested cohorts was 20.9 weeks (95% CI: 18.3–23.0), and the KM analysis showed that the DOT curves were similar between the tested versus untested cohorts (Supplementary Figure 1A). The median DOT for the combined methylated and unmethylated cohorts was 23.0 weeks (95% CI: 20.1–24.7), but unlike with the tested and untested cohorts, the DOT curves were significantly different between the methylated and unmethylated cohorts (log rank p < 0.0001), and the median DOT for the respective cohorts were 30.3 weeks (95% CI: 23.4–33.3) and 19.3 weeks (95% CI: 14.9–23.0). (Supplementary Figure 1B).
Progression and completed planned treatment were the most common reasons for 1L treatment discontinuation in the overall study population, regardless of testing or methylation status. However, the proportion of patients in the methylated cohort that discontinued 1L treatment due to progression was significantly lower compared with the unmethylated cohort (p = 0.01) (Table 3).
OS & PFS by MGMT methylation testing & methylation status
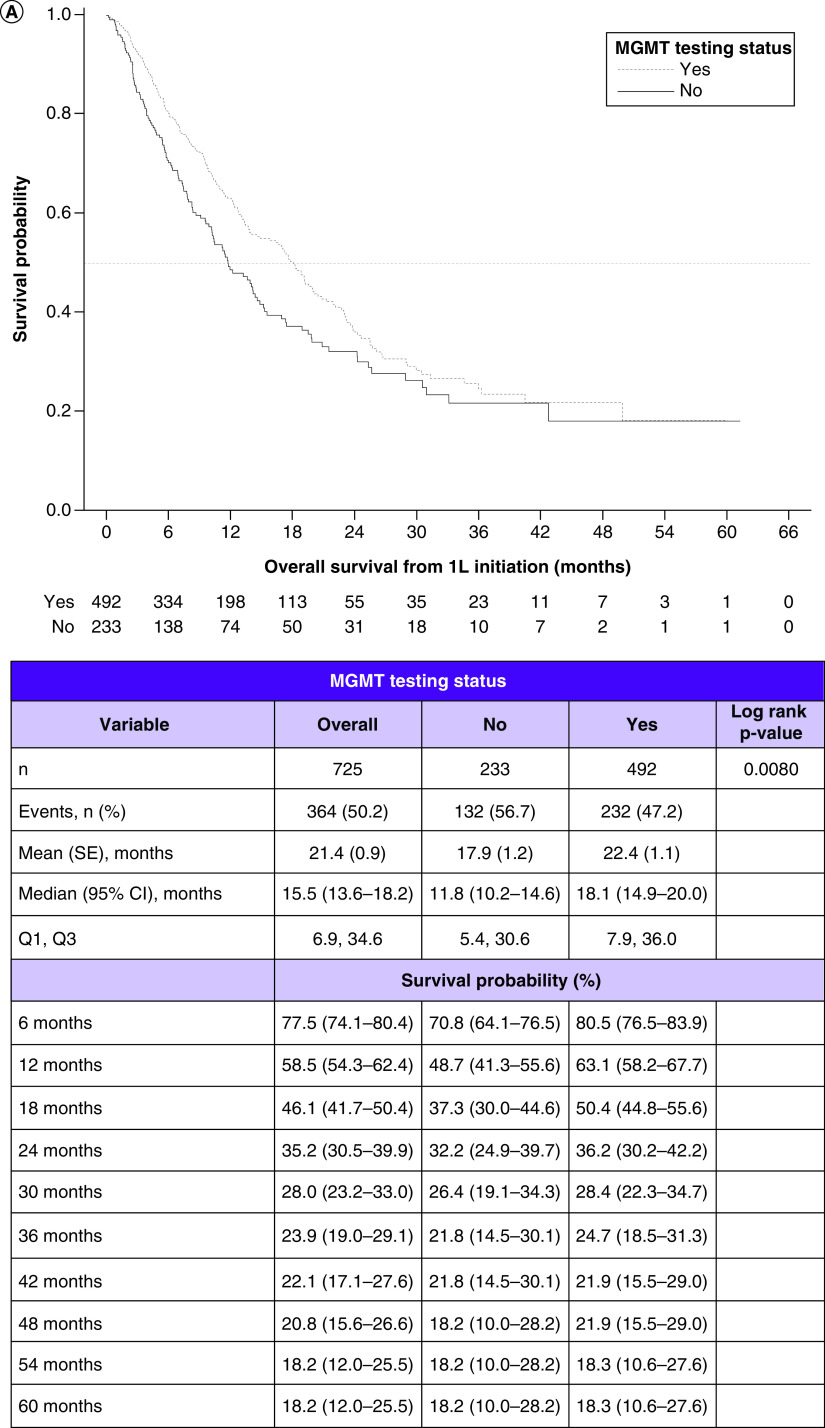
Among the combined MGMT tested and untested cohorts, the median OS was 15.5 months (95% CI: 13.6–18.2). In the tested and untested cohorts, they were 18.1 months (95% CI: 14.9–20.0) and 11.8 months (95% CI: 10.2–14.6), respectively. Comparing the KM curves, OS was significantly longer in the tested versus untested cohort (log rank p = 0.0080) (Figure 2A).
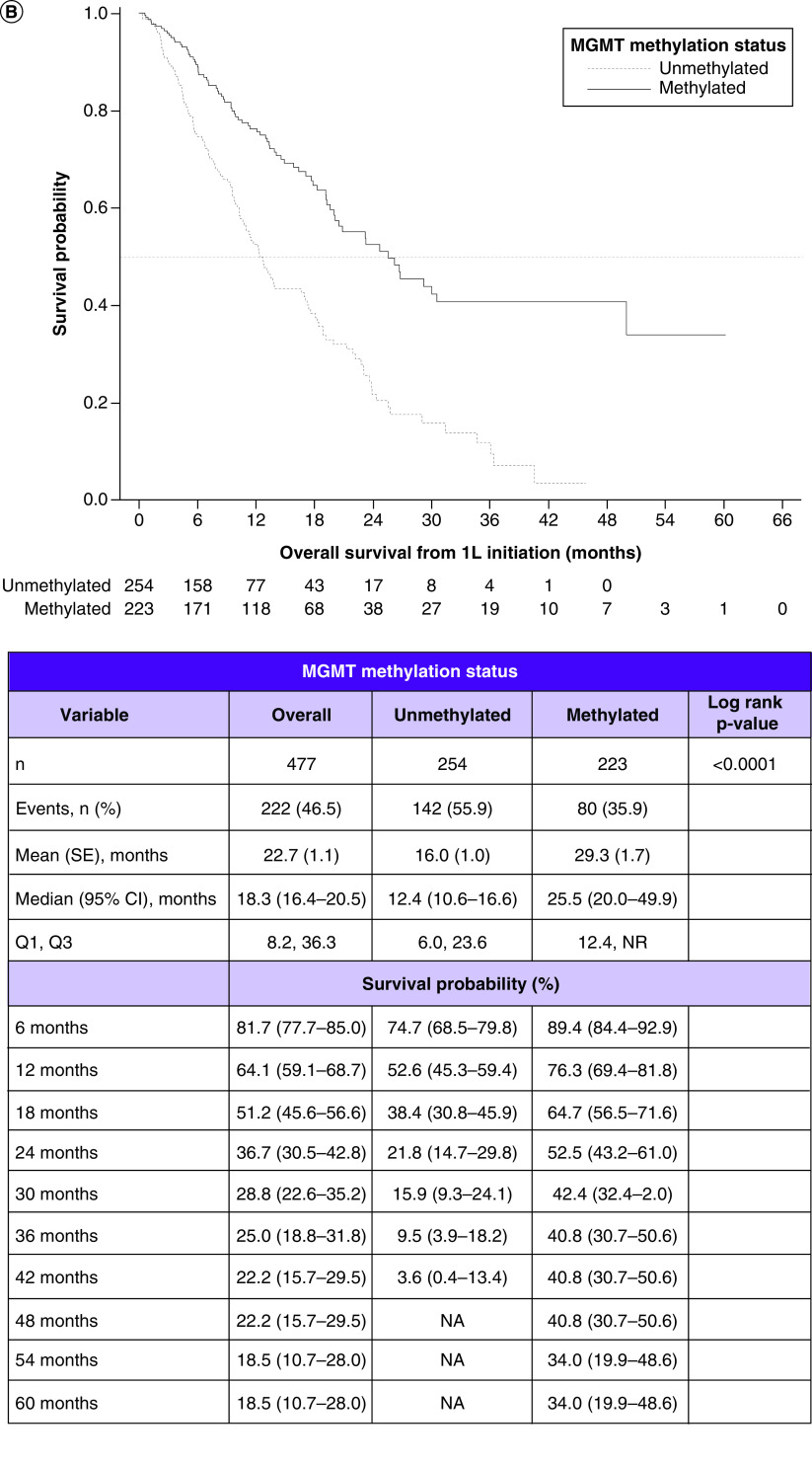
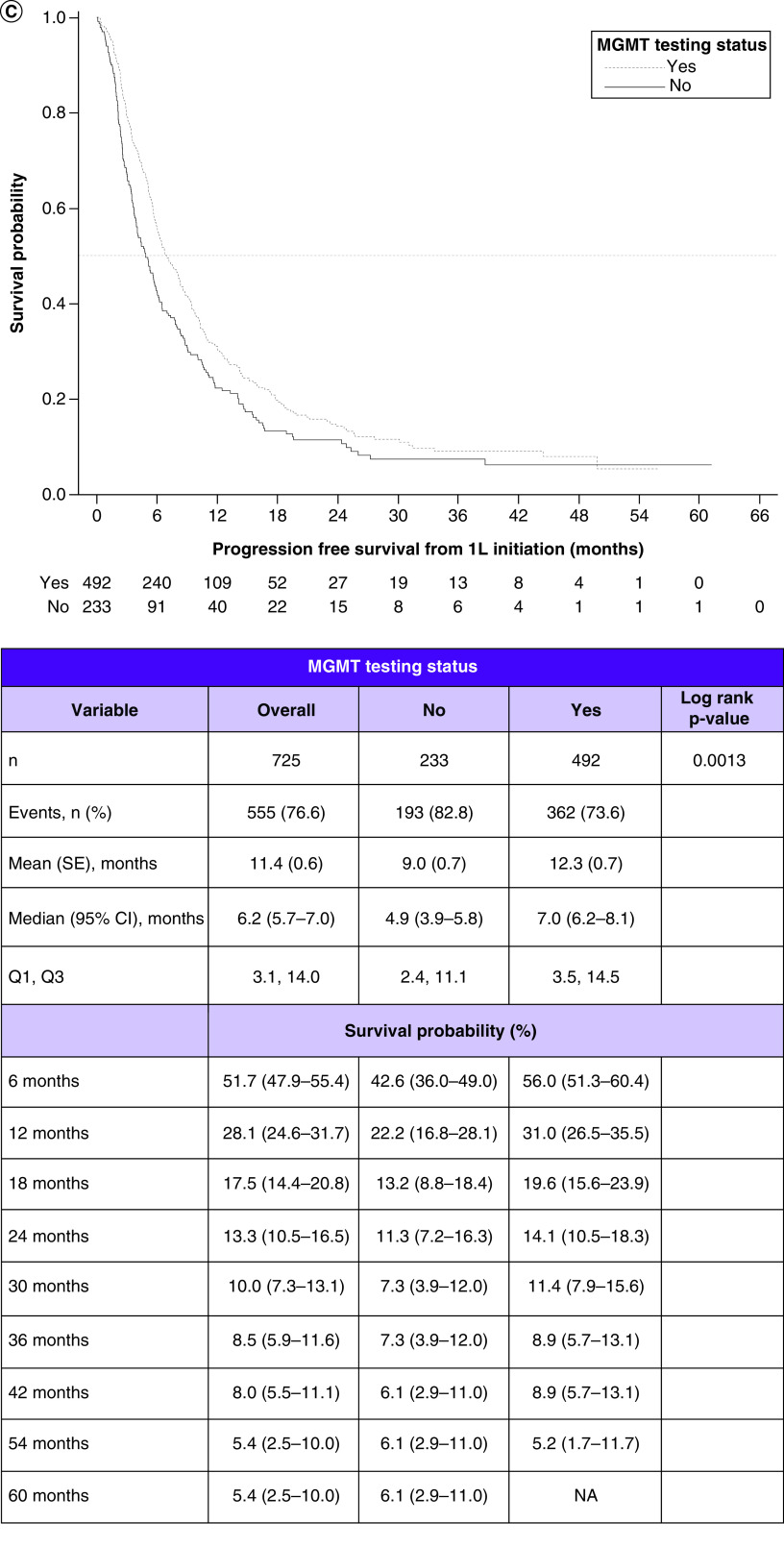
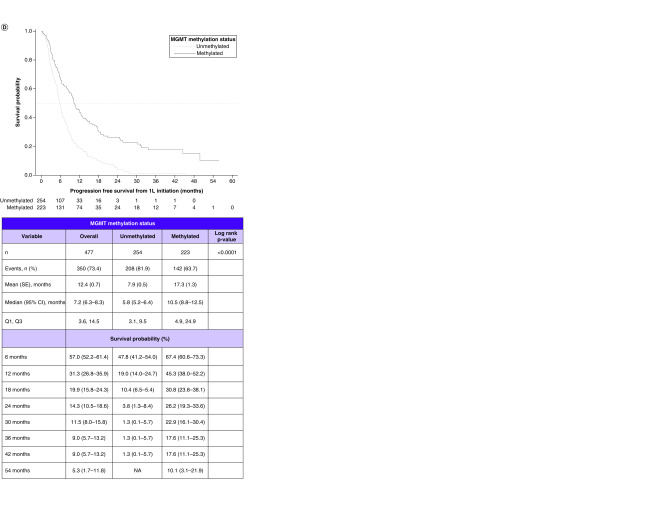
Figure 2. . Survival outcomes in patients with glioblastoma multiforme and their relationship with methyltransferase promoter testing status and with methyltransferase promoter methylation status.
(A) Kaplan–Meier curves for overall survival by testing status. (B) Overall survival by methylation status. (C) Progression-free survival by testing status. (D) Progression-free survival by methylation status.
1L: First line; MGMT: O6-methylguanine-DNA methyltransferase; NA: Not available; SE: Standard error.
Among the combined MGMT methylated and unmethylated cohorts, the median OS was 18.3 months (95% CI: 16.4–20.5). In the methylated and unmethylated cohorts, they were 25.5 months (95% CI: 20.0–49.9) and 12.4 months (95% CI: 10.6–16.6), respectively. Comparing the KM curves, OS was significantly longer in the methylated versus unmethylated cohort (log rank p < 0.0001) (Figure 2B).
Among the combined MGMT tested and untested cohorts, the median PFS was 6.2 months (95% CI: 5.7–7.0). In the tested and untested cohorts, they were 7.0 months (95% CI: 6.2–8.1) and 4.9 months (95% CI: 3.9–5.8), respectively. Comparing the KM curves, PFS was significantly longer in the tested versus untested cohort (log rank p = 0.0013) (Figure 2C).
Among the combined MGMT methylated and unmethylated cohorts, the median PFS was 7.2 months (95% CI, 6.3, 8.3). In the individual methylated and unmethylated cohorts, they were 10.5 months (95% CI: 8.8–12.5) and 5.8 months (95% CI: 5.2–6.4), respectively. Comparing the KM curves, PFS was significantly longer in the methylated versus unmethylated cohort (log rank p < 0.0001) (Figure 2D).
OS & PFS by steroid use prior to 1L treatment
KM analyses were conducted to assess the relationship of steroid use prior to 1L treatment initiation with OS and PFS.
In the MGMT untested cohort, comparison of KM curves indicated that patients not receiving steroids prior to 1L treatment initiation had longer OS compared with those receiving steroids (log rank p = 0.0158: Supplementary Figure 2A & B). Within the methylated cohort and within the unmethylated cohort, there was no difference in OS among those receiving and not receiving steroids prior to 1L treatment (methylated: Supplementary Figure 2C & D; unmethylated: Supplementary Figure 2E & F).
In the MGMT-untested cohort, comparison of KM curves indicated that patients not receiving steroids prior to 1L treatment initiation had longer PFS compared with those receiving steroids (log rank p = 0.0218, Supplementary Figure 3A & B). Within the methylated cohort and within the unmethylated cohort, there was no difference in PFS among those receiving versus not receiving steroids prior to 1L treatment (methylated: Supplementary Figure 3C & D; unmethylated: Supplementary Figure 3E & F).
OS & PFS: multivariable Cox regression analyses
Factors associated with OS were examined in propensity score-matched MGMT methylation tested versus untested patients. The following were significantly associated with reduced OS: longer time from diagnosis to 1L treatment initiation (hazard ratio [HR]: 1.030; 95% CI: 1.002–1.060; p = 0.0361), not being tested (HR: 1.495; 95% CI: 1.140–1.960; p = 0.0036), age 65–75 years (HR: 1.469; 95% CI: 1.072–2.013; p = 0.0168), age older than 75 years (HR: 2.596; 95% CI: 1.775–3.797; p < 0.0001) and higher ECOG (2+) at diagnosis (HR: 2.049; 95% CI: 1.457–2.883; p < 0.0001). Longer duration of 1L treatment was significantly associated with longer OS (HR: 0.953; 95% CI: 0.944–0.962; p < 0.0001), as was lack of steroid use (HR: 0.729; 95% CI: 0.553–0.947; p = 0.0250).
Factors associated with OS were examined in propensity score-matched MGMT-methylated and -unmethylated patients. Factors that were significantly associated with reduced OS were unmethylated status (HR: 1.978; 95% CI: 1.401–2.793; p = 0.0001), age >75 years (HR: 4.273; 95% CI: 2.604–7.013; p < 0.0001), age 65 to 75 years (HR: 2.049; 95% CI: 1.370–3.067; p = 0.0005) and practice regions Northeast (HR: 2.029; 95% CI: 1.149–3.583; p = 0.0148) and West (HR: 1.816; 95% CI: 1.224–2.694; p = 0.0030). Longer DOT (HR: 0.966; 95% CI: 0.956–0.976; p < 0.0001) was associated with longer OS.
Factors associated with PFS were examined in propensity score-matched MGMT methylation tested versus untested patients. Factors that were significantly associated with shorter PFS were not being tested (HR 1.686; 95% CI: 1.350–2.106; p < 0.0001) and age older than 75 years (HR: 1.495; 95% CI: 1.082–2.064; p = 0.0147). Factors that were associated with longer PFS were longer duration of 1L treatment (HR: 0.948; 95% CI: 0.941–0.955; p < 0.0001) and no steroid use (HR: 0.736; 95% CI: 0.586–0.924; p = 0.0083).
Factors associated with PFS were examined in propensity-score–matched MGMT-methylated and -unmethylated cohorts. Factors that were associated with reduced PFS were unmethylated status (HR: 1.577; 95% CI: 1.215–2.046; p = 0.0006) and age >75 years (HR: 1.950; 95% CI: 1.314–2.895; p = 0.0009). Factors that were associated with longer PFS were duration of 1L treatment (HR: 0.955; 95% CI: 0.947–0.963; p < 0.0001) and practice region Midwest (HR: 0.393; 95% CI: 0.210–0.736; p = 0.0035).
Safety outcomes
Incidence rates of AE explicitly attributed to 1L treatment by providers were measured and stratified according to MGMT methylation tested/not tested and MGMT methylated/not methylated. A significantly higher proportion of the tested cohort had constipation, skin irritation, and fatigue, and a significantly lower proportion had ‘other AE.’ A significantly higher proportion of the methylated cohort had thrombocytopenia, gastrointestinal AE, fatigue and ‘other AE.’ A significantly higher proportion of the unmethylated cohort had dermatologic AE, rash and fever (Supplementary Table 1).
All types of AE related to 1L treatment were similar between patients with a history of steroid use and those without, except a lower proportion of patients with a history of steroid use had headache (5.3%, n = 19 vs 10.8%, n = 42, respectively; p = 0.0361).
HCRU (hospitalizations, length of hospital stay, and ED visits) were similar between the MGMT-tested and -untested cohorts (Supplementary Table 2). Overall, there was a median of 1.0 hospitalization per patient with a median length of stay of 4.0 days, and a median of 1.0 ED visit per patient. HCRU was similar between the MGMT-methylated and -unmethylated cohorts.
Discussion
The results of our study show superior survival outcomes in patients with GBM and methylated MGMT compared with unmethylated MGMT. Notably, the methylated and unmethylated cohorts had similar 1L treatment regimen patterns, while at the same time the methylated cohort was less likely to discontinue 1L treatment due to disease progression. However, HCRU was similar between the methylated and unmethylated cohorts.
The demographics of the study population are comparable with clinical trial populations and those in real-world studies [13–16]. Median and mean ages ranged from 55 to 65 years and 58–61% were male. Patients with methylated MGMT were more likely to be female. However, a higher proportion of the tested cohort was in the Northeast and a lower proportion in the South, suggesting that MGMT testing may differ regionally by practice. A study of the National Cancer Data Base (NCDB; n = 12,725 patients diagnosed with GBM from 2010 to 2012 treated with adjuvant chemotherapy) found similar results. A significantly higher proportion of patients with methylated MGMT were female. Treatment at hospitals in the northeastern USA compared with the western USA was associated with a 55% higher likelihood of having been tested, although they also observed a 20% higher likelihood of having been tested with hospitals in the South. The NCDB study also found that patients treated at academic centers were also more likely to be tested compared with nonacademic centers (OR: 1.97, p < 0.001), and that increasing age, no resection and subtotal resection compared with gross total resection were associated with a lower likelihood of MGMT testing [17]. In our study, more patients who had been tested underwent resection (87.7 vs 78.0%), and more untested patients underwent a biopsy without a resection (21.4 vs 12.3%; p = 0.0002). In our study, there was a significant difference between tested and untested in terms of ethnicity; a higher proportion of study patients identifying as Hispanic/Latino had not been tested (11.4 vs 5.2%). However, our study did not show sex or race as significantly associated with testing status, similar to the NCDB study.
Median 1L DOT in our study was similar to that in another real-world study. Median 1L DOT in our study was 4.9 months, which is comparable to Norden et al.: median 1L DOT of 5.1 months for those who received TMZ + RT and 1.7 months for RT alone [14]. In our study, median 1L DOT was significantly longer among the methylated cohort compared with the unmethylated cohort.
The most common reasons for 1L treatment discontinuation were disease progression (32.9%) and completion of planned treatment (24.3%). These results were similar to clinical trial data: disease progression was also the most common reason for treatment discontinuation in Stupp et al. (39%) [15]. Disease progression as reason for 1L treatment discontinuation was observed at a significantly greater frequency among the MGMT unmethylated cohort, which may be expected given the significantly shorter PFS observed among those in the unmethylated cohort in this study and in other studies [7,11]. At the same time, there was no significant differences in reasons for 1L treatment discontinuation between the tested and untested cohorts.
Comparisons of OS by methylation status in our study were comparable to those in clinical trials and in real-world studies [7–11]. In our study, log-rank analysis showed that OS and PFS for the methylated cohort were significantly longer compared with the unmethylated cohort. The NCDB study showed that among 12,725 patients receiving adjuvant chemoradiation for GBM diagnosed from 2010 to 2012, the median OS for patients with methylated MGMT was significantly longer than for those with unmethylated MGMT (20 vs 15 months; p < 0.001) [17].
In our study, the tested cohort had significantly longer OS and PFS. Methylation testing may be a surrogate for more comprehensive cancer care, which could account for the improved outcomes. In the NCDB analysis, the median OS for patients with methylated MGMT was significantly longer than for those with unknown MGMT status (20 vs 14.6 months; p < 0.001). At the same time, there was no difference between those with untested and those with unmethylated MGMT (14.6 vs 15.0 months) [17]. It was noted in the NCDB study that patients treated at academic centers were more than twice as likely to have been tested compared with nonacademic centers, which may reflect more comprehensive care at academic centers. Even so, the NCDB study showed that among the 12,725 patients receiving adjuvant chemoradiation for GBM diagnosed from 2010 to 2012, 86.9% had records of MGMT methylation status unknown or not tested [17].
TMZ and RT are associated with improved survival outcomes for those with methylated MGMT GBM. Guidelines also indicate that because TMZ may be less beneficial in patients with newly diagnosed GBM with unmethylated MGMT, RT alone is an option for those patients [4]. However, the TMZ and RT combination is also used among patients with unmethylated MGMT GBM, owing to the lack of treatment options, which underscores the need for further research [4,13,14,17]. In fact, MGMT methylation testing is not always conducted in community oncology settings, as treatment strategies are similar between patients with methylated versus unmethylated MGMT [4]. So far, novel therapies have been associated with, at best, modest improvements in survival in GBM in both methylated and unmethylated study populations [4,18–20]. Increasing the rate of MGMT testing will not only result in better and more appropriate care for patients with methylated MGMT but will also help support research into new therapies for patients with unmethylated MGMT [6,19].
Steroid use prior to 1L treatment initiation was also associated with significantly shorter OS and PFS among the MGMT untested cohort. A meta-analysis of patients with GBM (22 publications, n = 8752) also showed that steroid treatment was associated with shorter OS (HR: 1.54; 95% CI: 1.37–1.75; p < 0.01) and PFS (HR: 1.28; 95% CI: 1.1–1.49; p < 0.01) [21]. The difference in survival outcomes may be due to the fact that patients with more severe GBM are more likely to receive steroid treatment, which is the standard of care for decreasing brain edema and for alleviating symptoms such as pain and loss of appetite [22]. As for methylation status, there were no significant differences in OS or PFS when comparing steroid use with non-use within the methylated cohort or when comparing steroid use with non-use within the unmethylated cohort in this study. However, results of our study may be difficult to interpret, as comparable analyses examining the relationship of MGMT methylation status with steroid use and outcomes have not been reported, to our knowledge. Furthermore, our study captured steroid use at only one point during treatment – initiation of 1L – whereas patients with GBM often receive steroid administration at several timepoints in their course of care.
Some of the treatment-related AEs in this study were similar to those in clinical trials. In this study, 59.0% had AEs, among whom 32% had nervous system disorders, 16.7% had hematologic AEs, 13.1% had thrombocytopenia, 4.1% had neutropenia, 4.0% had leukopenia and 1.1% had infection-related AEs (Supplementary Table 1). Stupp et al. in the EORTC-NCIC trial comparing patients receiving TMZ as concomitant versus adjuvant therapy (n = 284) found that 16% had grade 3 or 4 hematologic toxic effects: 7% had leukopenia, 7% had neutropenia, 12% had thrombocytopenia and 1% had anemia. Moderate-to-severe fatigue was observed in 33%, severe infections in 4.2% and thromboembolic events in 4.2% [15]. In a randomized trial comparing TMZ and TTFields with TMZ alone, Stupp et al. found that among those treated with TMZ alone (n = 229), 44% had at least one grade 3 or 4 event: 20% had nervous system disorders, 11% had blood and lymphatic system disorders, 5% had thrombocytopenia and 5% had infections. However, 6% in that trial had asthenia, fatigue and gait disturbance, whereas our study had more patients with fatigue (25.8%) [23]. Differences in the patient populations may account for the differences in AE frequencies.
When comparing our study with real-world studies, Desjardins et al. found that among patients who received RT concurrent with TMZ and bevacizumab, 55% had grade 3 AE and 9% grade 4 AE; the most frequently reported grades 3 and 4 AE were white blood cell count decreased (16%), fatigue (14%) and neutrophil count decreased (12%). In a retrospective multicenter observational study of 750 patients receiving RT and concurrent TMZ followed by adjuvant TMZ for GBM, grades 3 and 4 hematologic toxicity events were observed in 8.4% during the concurrent TMZ period and 10.2% during adjuvant treatment [24].
Regarding HCRU, in the overall study population there was a median of 1 hospitalization per patient (range: 1.0–8.0), a median of 1 ED visit per patient (range: 1.0–13.0), a median length of stay of 4.0 days (range: 0.0–68.0) and a mean length of stay of 6.7 days (standard deviation: 7.9). These utilizations were similar between the MGMT-untested and -tested cohorts (Supplementary Table 2), and between the MGMT-methylated and -unmethylated cohorts. Norden et al. observed that patients had a mean length of stay of 5.6 days in the 6 months and 4.2 days in the period 7–12 months following initiation of 1L therapy, and 5.4 and 4.8 days, respectively, following initiation of 2L therapy [14]. However, length of stay in our study was measured from the index date (date of new GBM diagnosis) rather than initiation of 1L treatment, which limits comparison to Norden et al.
Strengths & limitations
Conclusions about the study results must be drawn in the context of the strengths and limitations of the data source and study design. First, as a retrospective, observational EHR study, limitations include potential missing or incomplete data. Data on services provided outside of US Oncology Network practices would not be available, and therefore the relationship of all patient outcomes with all patient characteristics cannot be determined. EHR data are recorded for clinical care, not for research, which may result in data errors of omission and commission. Because of this, certain variables of interest for this study, such as type of resection, were not usually available for the entire study population. For example, >90% resection, which indicates better prognosis, is difficult to determine and is often not reported in patient charts. Second, even after propensity score matching, there were significant differences between the tested and untested cohorts in ethnicity, practice region and type of surgery, and between the methylated and unmethylated cohorts in sex and race. However, the multivariate Cox analysis showed that none of these factors had a significant relationship with OS or PFS. Third, steroid use was assessed only at one time point (i.e., after surgery prior to 1L treatment initiation) using a dose cutoff of >3 mg/day for dexamethasone and >20 mg/day for prednisone. Lower doses were not assessed. Last, generalizability of this study to other real-world populations may be limited due to the location distribution of US Oncology Network practices and their use of evidence-based guidelines. However, use of the EHR data represents usual care in a large network of community oncology practices. Therefore, these data can be used to report real-world findings that are more representative of typical patients with GBM.
Conclusion
In our study of patients in a large network of community oncology practices, most patients with GBM received treatment that was consistent with National Comprehensive Cancer Network guidelines. This study found that MGMT methylation was predictive of better outcomes, which has also been observed in clinical trials and real-world studies. However, outcomes in patients with GBM continue to be poor, emphasizing the need for more research on novel therapeutic strategies.
Future perspective
TMZ and RT along with resection have been the standard of care for GBM since 2005. However, there is still an unmet need for more effective therapies, either specific to patients with unmethylated MGMT GBM or agnostic in terms of methylation status. While clinical trials of other therapies, such as anti-angiogenic drugs and immunotherapies, have not yet yielded substantial improvements in survival in GBM, efforts are continuing. For example, NCT04396860 is now recruiting patients to investigate the efficacy of immunotherapy compared with standard of care (RT and TMZ) in patients at first diagnosis of unmethylated GBM [25]. In general, research is ongoing to identify and characterize markers in the tumor-immune microenvironment to serve as targets for novel therapies, which include drugs, medical devices, vaccines and immune cell therapy [19].
Summary points.
This retrospective study shows characteristics, clinical outcomes, and utilization associated with usual care of patients with newly diagnosed glioblastoma receiving first-line treatment in a large network of US community-based oncology practices.
Patients received treatments consistent with the National Comprehensive Cancer Network guidelines. At the same time, patients with different O6-methylguanine DNA methyltransferase promoter (MGMT) methylation status still received similar treatments.
MGMT methylation was associated with better overall survival and progression-free survival outcomes.
Testing for MGMT methylation, regardless of the result, was also associated with better survival outcomes.
MGMT methylation was associated with a lower likelihood of disease progression-linked first-line treatment discontinuation.
Rates of hospitalization and emergency department visits were similar regardless of MGMT methylation or testing status.
Acknowledgments
The authors would like to thank L Kaspin-Powell, an employee of Ontada, for medical writing support.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/cns-2021-0007
Author contributions
S Annavarapu, N Robert and T Pham contributed to the study design. T Pham and K Davies conducted the data analyses. S Annavarapu, N Robert, A Gogate and P Singh reviewed and interpreted results. S Annavarapu led development of the manuscript. All authors revised the manuscript.
Financial & competing interests disclosure
This work was supported by Bristol Myers Squibb. S Annavarapu employment: Ontada. A Gogate employment: Bristol Myers Squibb; stock and other ownership interests: Bristol Myers Squibb. T Pham employment: McKesson Specialty Health; stock and other ownership interests: McKesson. K Davies employment: McKesson Specialty Health. P Singh employment: Bristol Myers Squibb; stock and other ownership interests: Bristol Myers Squibb. N Robert employment: Ontada; leadership: Ontada; honoraria: Roche, Bristol Myers Squibb; consulting or advisory role: New Century Health, Bristol Myers Squibb, B Ingelheim; stock and other ownership interests: McKesson. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Since the study did not involve the collection, use or transmittal of individual identifiable data, IRB approval to conduct this study was not required. Therefore, the study protocol received an exception and waiver of informed consent from the US Oncology Institutional Review Board. The security of the data meets the requirements of the Health Insurance Portability and Accountability Act of 1996, and the study adheres to the principles outlined in the Declaration of Helsinki.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Ostrom QT, Cioffi G, Gittleman Het al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro. Oncol. 21(Suppl. 5), v1–v100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao Pet al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro. Oncol. 19(Suppl. 5), v1–v88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A systematic review and meta-analysis presenting trials of bevacizumab, tumor treating fields, and vaccines compared with standard of care for glioblastoma multiforme (GBM). So far, efficacy has not substantially improved with those therapies.
- 3.Marenco-Hillembrand L, Wijesekera O, Suarez-Meade Pet al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J. Neurooncol. 147(2), 297–307 (2020). [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers Version 5.2020. (2021). https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- 5.Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin. Cancer Res. 11(19 Pt 1), 6767–6771 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Mansouri A, Hachem LD, Mansouri Set al. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro. Oncol. 21(2), 167–178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reviews the importance of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in prognosis and treatment of GBM, and explains the need for standardization and increased testing of MGMT methylation to support the development of more appropriate treatment options.
- 7.Limam S, Missaoui N, Abdessayed Net al. Prognostic significance of MGMT methylation and expression of MGMT, P53, EGFR, MDM2 and PTEN in glioblastoma multiforme. Ann. Biol. Clin. (Paris) 77(3), 307–317 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Pinson H, Hallaert G, Van der Meulen Jet al. Weak MGMT gene promoter methylation confers a clinically significant survival benefit in patients with newly diagnosed glioblastoma: a retrospective cohort study. J. Neurooncol. 146(1), 55–62 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Hegi ME, Diserens AC, Gorlia Tet al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352(10), 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Stupp R, Hegi ME, Mason WPet al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10(5), 459–466 (2009). [DOI] [PubMed] [Google Scholar]; •• Follow-up study on the efficacy of the Stupp regimen, now standard of care for GBM.
- 11.Campana D, Walter T, Pusceddu Set al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: an observational retrospective multicenter study. Endocrine 60(3), 490–498 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav. Res. 46(3), 399–424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desjardins A, Herndon JE 2nd, McSherry Fet al. Single-institution retrospective review of patients with recurrent glioblastoma treated with bevacizumab in clinical practice. Health Sci. Rep. 2(4), e114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norden AD, Korytowsky B, You Met al. A real-world claims analysis of costs and patterns of care in treated patients with glioblastoma multiforme in the United States. J. Manag. Care Spec. Pharm. 25(4), 428–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stupp R, Mason WP, van den Bent MJet al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352(10), 987–996 (2005). [DOI] [PubMed] [Google Scholar]; •• Report on the key study used to support radiotherapy and temozolomide as the standard of care for GBM.
- 16.Carter TC, Medina-Flores R, Lawler BE. Glioblastoma treatment with temozolomide and bevacizumab and overall survival in a rural tertiary healthcare practice. Biomed. Res. Int. 2018, 6204676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Youssef I, Osborn VW, Safdieh J, Becker DJ, Schreiber D. The utilization of MGMT promoter methylation testing in United States hospitals for glioblastoma and its impact on prognosis. J. Clin. Neurosci. 51, 85–90 (2018). [DOI] [PubMed] [Google Scholar]; •• This is a very large study showing a very low rate of MGMT methylation testing among nearly 13,000 patients; it underscores the need for increased testing.
- 18.Stupp R, Hegi ME, Gorlia Tet al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, Phase III trial. Lancet Oncol. 15(10), 1100–1108 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Yu MW, Quail DF. Immunotherapy for glioblastoma: current progress and challenge. Front. Immunol. 12, 676301 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A review describing the status of research into immunotherapy treatments for GBM, as many new therapies under investigation involve immunotherapy, but so far efficacy has not substantially improved with those therapies. The review goes into great detail explaining how the characteristics of the tumor-immune microenvironment can interfere with efficacy of immunotherapies.
- 20.Lara-Velazquez M, Shireman JM, Lehrer EJet al. A comparison between chemo-radiotherapy combined with immunotherapy and chemo-radiotherapy alone for the treatment of newly diagnosed glioblastoma: a systematic review and meta-analysis. Front. Oncol. 11, 662302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A systematic review and meta-analysis presenting evidence for efficacy of immunotherapy compared with standard of care for GBM, as many new therapies under investigation involve immunotherapy, but so far efficacy has not substantially improved with those therapies.
- 21.Petrelli F, De Stefani A, Ghidini Aet al. Steroids use and survival in patients with glioblastoma multiforme: a pooled analysis. J. Neurol. 268(2), 440–447 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Zoccarato M, Nardetto L, Basile AM, Giometto B, Zagonel V, Lombardi G. Seizures, edema, thrombosis, and hemorrhages: an update review on the medical management of gliomas. Front. Oncol. 11, 617966 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stupp R, Taillibert S, Kanner Aet al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318(23), 2306–2316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim BS, Seol HJ, Nam DHet al. Concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide for newly diagnosed glioblastoma patients: a retrospective multicenter observation study in Korea. Cancer Res. Treat. 49(1), 193–203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US National Library of Medicine. ClinicalTrials.gov. testing the use of the immunotherapy drugs ipilimumab and nivolumab plus radiation therapy compared to the usual treatment (temozolomide and radiation therapy) for newly diagnosed MGMT unmethylated glioblastoma. ClinicalTrials.gov Identifier: NCT04396860. (2021). https://www.clinicaltrials.gov/ct2/show/NCT04396860; •• NCT04396860 is an example of a study investigating whether immunotherapy could serve as a better treatment for patients with unmethylated MGMT GBM.