Abstract
Effector T cells comprise the cellular arm of the adaptive immune system and are essential for mounting immune responses against pathogens and cancer. To reach effector status, costimulation through CD28 is required. Here, we report that sialic acid-containing glycans on the surface of both T cells and APCs are alternative ligands of CD28 that compete with binding to its well-documented activatory ligand CD80 on the APC, resulting in attenuated costimulation. Removal of sialic acids enhances antigen-mediated activation of naïve T cells and also increases the revival of effector T cells made hypofunctional or exhausted via chronic viral infection. This occurs through a mechanism that is synergistic with antibody blockade of the inhibitory PD-1 axis. These results reveal a previously unrecognized role of sialic acid ligands in attenuation of CD28-mediated costimulation of T cells.
Short abstract
T cells require multiple signals to activate, including costimulation via CD28. We report that sialoglycans are alternative ligands for CD28 and attenuate costimulation by competing with CD80.
Introduction
T cell-mediated immunity is central to host defense against pathogens, progression of autoimmunity, and elimination of cancer cells.1 To fully activate, T cells must receive two signals from an antigen presenting cell (APC) via a cell–cell interface called the immunological synapse (IS).2 The first signal is antigen-specific and is initiated by the T cell receptor (TCR) recognizing an antigenic peptide displayed on a major histocompatibility complex (MHC) on an APC.3 The second, “costimulatory” signal is antigen-independent and mediated by CD28 on T cells, a receptor recruited to the IS by its protein ligands CD80/CD86, expressed on the APC.4,5 Both signals are required for naïve T cells to differentiate into functional effector cells. In contrast to these activatory signals, T cell activation and continued function can be suppressed by immunological “checkpoint” receptors such as PD-1 and CTLA-4. These inhibitory receptors on T cells are similarly recruited to the IS if their cognate ligands are expressed on the APC, leading to functional “exhaustion” of T cells.6 Under these circumstances, while presented antigens are still recognized by the TCR, full activation cannot be achieved.7 Blocking checkpoint receptors using therapeutic antibodies has led to transformational developments in treatment of refractory cancers via functional revival of cancer-specific effector T cells from hypofunctional and exhausted phenotypes.8 The success of these approaches has created enormous interest in understanding the detailed mechanisms that regulate activation of naïve and effector T cells.
Motivated by recent reports of the sialic acid-binding immunoglobulin-like receptors (Siglecs) as immunological checkpoints,9−11 we were drawn to work dating back nearly 40 years that showed T cell activation was enhanced by enzymatic removal of sialic acids from the surface of T cells and/or APCs.12−17 Using an antigen-independent system, these studies showed that T cell activation could be significantly enhanced by prior treatment of syngeneic B cells with sialidases.14 While these observations were suggestive of a role of sialic acids in costimulation, the mechanism has remained elusive. Here, we show that sialic acids are alternative ligands of CD28 that compete for binding to CD80. Thus, by destroying sialic acid ligands, CD28 is better able to engage CD80, accounting for increased costimulation of T cells. Further, we find that removal of sialic acid ligands leads to dramatic revival of hypofunctional and exhausted PD-1+ T cells and that this enhancement is synergistic with blockade of the PD-1 checkpoint pathway.
Results and Discussion
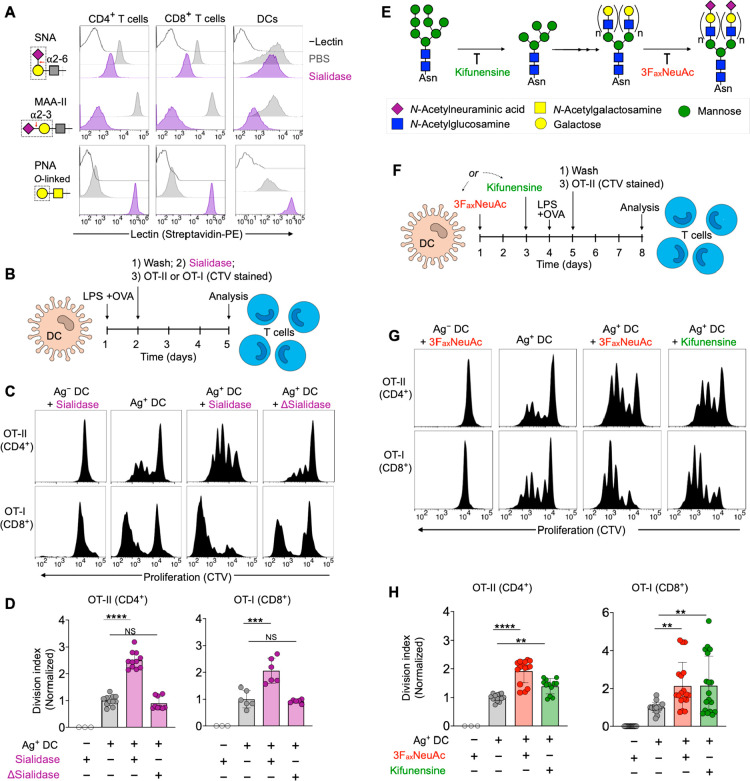
To further assess the impact of sialic acids on antigen-specific T cell activation, we used chicken ovalbumin (OVA)-specific CD4+ and CD8+ T cells (OT-I and OT-II cells, respectively) in combination with bone marrow-derived dendritic cells (DCs) matured with bacterial lipopolysaccharide (LPS). Treatment of T cells and DCs with sialidase from Vibrio cholerae efficiently removed cell surface sialic acids from the most common NeuAcα2-3Gal and NeuAcα2-6Gal linkages found on glycoproteins of T cells and DCs, as detected by fluorescent lectin staining (Figure 1A). In the context of T cell activation, sialidase treatment of cocultures containing OVA-presenting DCs and OT-I or OT-II cells led to a significant enhancement in proliferation of both T cell types, as measured by dilution of the proliferation reporter dye CellTrace Violet (CTV) (Figure 1B–D). This effect was conserved under treatment with an alternative sialidase from Streptococcus pneumoniae and when alternatively matured DCs or splenocytes were substituted as APCs (Figure S1). We observed no effect when heat-denatured enzyme (Δ) was used, confirming the dependence on specific sialidase activity.
Figure 1.
Desialylation enhances antigen-dependent activation of T cells. (A) Lectin staining of T cells (from a mixture of splenocytes) and bone marrow-derived dendritic cells from WT C57BL/6J mice before (PBS) and after treatment with sialidase from V. cholerae (55 mU). The red arrow indicates site of action of sialidase, and the dashed box shows the lectin recognition motif. SNA = lectin from Sambucus nigra. MAA-II = lectin from Maackia amurensis. PNA = peanut agglutinin. (B) Antigen-specific T cell proliferation assay setup. (C) T cell proliferation histograms (dilution of CTV) for cocultures in the presence of sialidase from V. cholerae. DC to T cell ratio was 1:2. Δ = heat inactivated. (D) Quantification of T cell proliferation data from C (n ≥ 3). (E) Schematic of glycosylation pathways inhibited by 3FaxNeuAc and kifunensine. See Figure 2D for pictogram definitions of monosaccharides. (F) Proliferation of T cells using DCs selectively desialylated via pretreatment with 3FaxNeuAc or kifunensine. (G) T cell proliferation histograms for cocultures as set up in F. DC to T cell ratio was 1:2. (H) Quantification of T cell proliferation data from G (n ≥ 3). Mean ± SD (D, H). **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, NS = not significant (D, H). One-way ANOVA followed by Tukey’s multiple comparisons test (D, H). Normalized division index corresponds to T cell division index for sialidase-treated cultures divided by the division index for the corresponding PBS-treated control. T cell proliferation data is pooled from at least three separate experiments. Gating strategies: CD4+ T cells, viability dye– CD4+ (TCR Vα2+ for OT-II); CD8+ T cells, viability dye– CD8+ (TCR Vα2+ for OT-I); DCs, viability dye– CD11c+ MHC II+.
To assess the impact of selective desialylation on APCs, we cultured DCs in the presence of two specific inhibitors of glycosylation, kifunensine or 2,4,7,8,9-pentaacetyl-3Fax-Neu5Ac-CO2Me (3FaxNeuAc).18 Kifunensine acts early in N-linked glycan maturation, preventing further processing of precursor high-mannose type glycans (and thus subsequent addition of sialic acids as terminal sugar residues).19 In contrast, 3FaxNeuAc directly prevents sialic acid transfer through inhibition of sialyltransferases (Figure 1E).18−20 Both kifunensine and 3FaxNeuAc were able to reduce DC sialylation on the time scale of T cell activation assays, albeit to a lesser extent than constant exposure to sialidase (Figure S2A–C). Nonetheless, DCs treated with either inhibitor were significantly more potent in activating normally sialylated CD4+ and CD8+ T cells as compared to untreated controls, suggesting that sialic acids on N-linked glycans within the glycocalyx of DCs suppress activation of T cells (Figure 1F–H). To determine if this effect was dependent on IS formation with the APC or was mediated by secreted soluble factors, we conducted experiments where T cells and DCs were physically separated using a transwell system. Neither kifunensine nor 3FaxNeuAc-desialylated DCs showed any ability to enhance T cell activation over PBS controls when physically separated from untreated T cells, and expression of both activatory/inhibitory receptors on the DCs remained unchanged (Figure S1G,H and Figure S2D,E). Taken together, these data suggested that sialic acids on N-linked glycans negatively impact signaling between T cells and APCs and that sialic acids of APCs can contribute to attenuation of antigen-mediated T cell activation through direct interference at the IS.
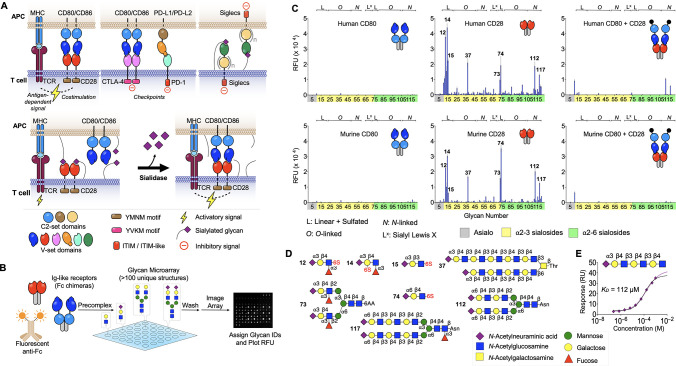
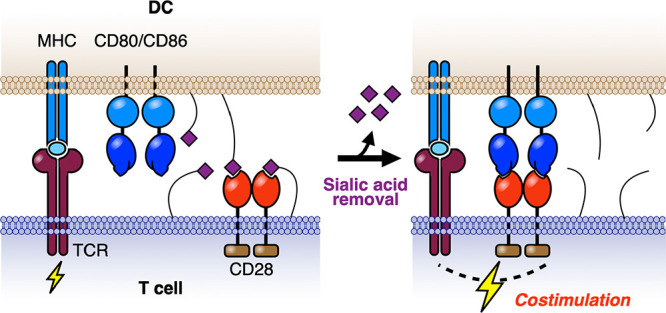
The costimulatory receptor CD28, its ligands, CD80/CD86, and the inhibitory receptors PD-1/PD-L1/PD-L2/CTLA-4 are all immunoglobulin (Ig)-like cell surface receptors. We therefore considered the possibility that one or more of these proteins might directly bind sialic acids as ligands, similar to the sialic acid-binding immunoglobulin-like lectin (Siglec) family of receptors, which are also members of the Ig-like superfamily.21−26 Indeed, like the Siglecs, these proteins all possess an N-terminal V-set Ig-like domain and share significant sequence and structural homology with the sialic acid ligand-binding region present in each Siglec (Figure 2A, top and Figure S3A,B).21−26 To evaluate potential sialoside-binding activity, we employed recombinant chimeras comprising N-terminal V-set Ig-like domains (and C2-set domains where appropriate) fused to the Fc-domain of human IgG. These were applied to glycan arrays that contained a diverse library of glycans capped with sialic acids including intact N-linked and O-linked glycans, as well as fragments representing terminal sequences commonly found in glycoprotein and glycolipid glycans. Representative glycans without terminal sialic acids were included as controls (Figure 2B, Table S1).27 Importantly, human and murine CD28-Fc exhibited strikingly similar binding profiles to sialoglycans on the array, including several N-linked and O-linked glycans with the sequence NeuAcα2-3/6Galβ1-4GlcNAc, and to shorter fragments with an additional sulfate on the Gal or GlcNAc (Figure 2C,D). Quantitative binding to sialosides was also evaluated via surface plasmon resonance (SPR), revealing that recombinant monomeric CD28 binds to a representative glycan (#19, see Table S1) with KD = 112 μM (Figure 2E). This value is comparable to affinities of members of the Siglec family of sialic acid-binding proteins that range from ∼KD/IC50 of 0.3 μM (Siglec-4) to 4.5 mM (CD33/Siglec-3) and is most similar to human CD22/Siglec-2 (KD ∼ 110 μM).28 None of the other Fc-chimeras examined for PD-1, PD-L1, PD-L2, CDLA-4, CD80, or CD86 displayed significant binding to the array (Figure 2C and Figure S3C). Importantly, when murine and human CD28-Fc were precomplexed with their respective CD80 ligands prior to exposure to the array, binding to sialic acids was blocked (Figure 2C). These data reveal that sialylated glycans are alternative ligands for both human and murine CD28 and that binding to CD80 appears competitive with binding to sialosides (Figure 2A, bottom).
Figure 2.
CD28 binds sialylated glycans. (A) Survey of Ig-like receptors at the IS (top). Competition between sialic acid ligands of CD28 and CD80 at a normally sialylated IS and increased costimulatory interactions as a result of treatment with sialidase (bottom). (B) Glycan array screening workflow for IS receptors. (C) Glycan array binding data for human and mouse CD80, CD28, and both receptors precomplexed. Compound IDs for top hits are indicated on the plots for CD28 (n = 4 for each peak). (D) Structures of glycan ligands for CD28. (E) Steady state binding of α2-3 sialyl-diLacNAc to surface immobilized CD28-GFP measured via SPR. The black line represents steady-state fit. Mean ± SD (C).
To further assess the impact of sialic acids on CD28:CD80 interactions, we measured direct binding of CD28-Fc to DCs and CD80-Fc to T cells, with and without prior sialidase treatment (Figure 3). Binding of CD28-Fc to desialylated DCs was significantly enhanced compared to untreated cells and could be blocked in the presence of anti-CD80, demonstrating that sialic acids can act in “trans” on the DC to reduce binding of CD28 to CD80 (Figure 3A,B). Similarly, binding of CD80-Fc to either CD4+ or CD8+ T cell populations was dramatically enhanced by sialidase treatment (Figure 3C,D). These results suggest that sialic acid-containing glycans present on the surface of T cells may also act in “cis” as competitive ligands, sequestering available CD28 and thus inhibiting binding to CD80 (Figure 3C). In this context, it is notable that the sialic acid content in the lymphocyte glycocalyx is >100 mM,29 far above the likely KD for binding of most sialic acid ligands to CD28 (Figure 2E). We note that the rCD28-Fc and rCD80-Fc constructs used in these studies were expressed in Chinese Hamster Ovary cells and are thus sialylated themselves (e.g., mCD80 has five potential glycosylation sites). The fact that we observed increased binding of these sialylated proteins following desialylation of only DC or T cell surfaces demonstrates that sialic acids on these recombinant proteins do not impair binding and suggests that sialic acid-containing glycans on other glycoproteins contribute in part or predominantly to the inhibition of CD28:CD80 binding. Consistent with this conclusion are reports that the glycosylation sites on the Ig domain of CD80 that binds to CD28 are on the opposite face to the CD28 binding site, thus precluding direct interactions between the glycans and CD28.30 For CD28, deletion of all N-linked glycosylation, constituting 40% of the mass of CD28, resulted in a recombinant CD28 that exhibited modestly increased binding to CD80, suggesting that sialic acids on these glycans might contribute in part to inhibition of CD28:CD80 interactions.31 Although sialic acid ligands on the T cell block CD80-Fc binding, the sialoside:CD28-Fc interaction is not sufficiently avid to support binding of CD28-Fc to T cells alone, with staining appearing identical to sialidase-treated controls (Figure S2F). Taken together, these results frame a role for sialic acids in attenuating T cell costimulation since binding of CD28 to CD80 is required for productive costimulation4,5 (Figure 2A).
Figure 3.
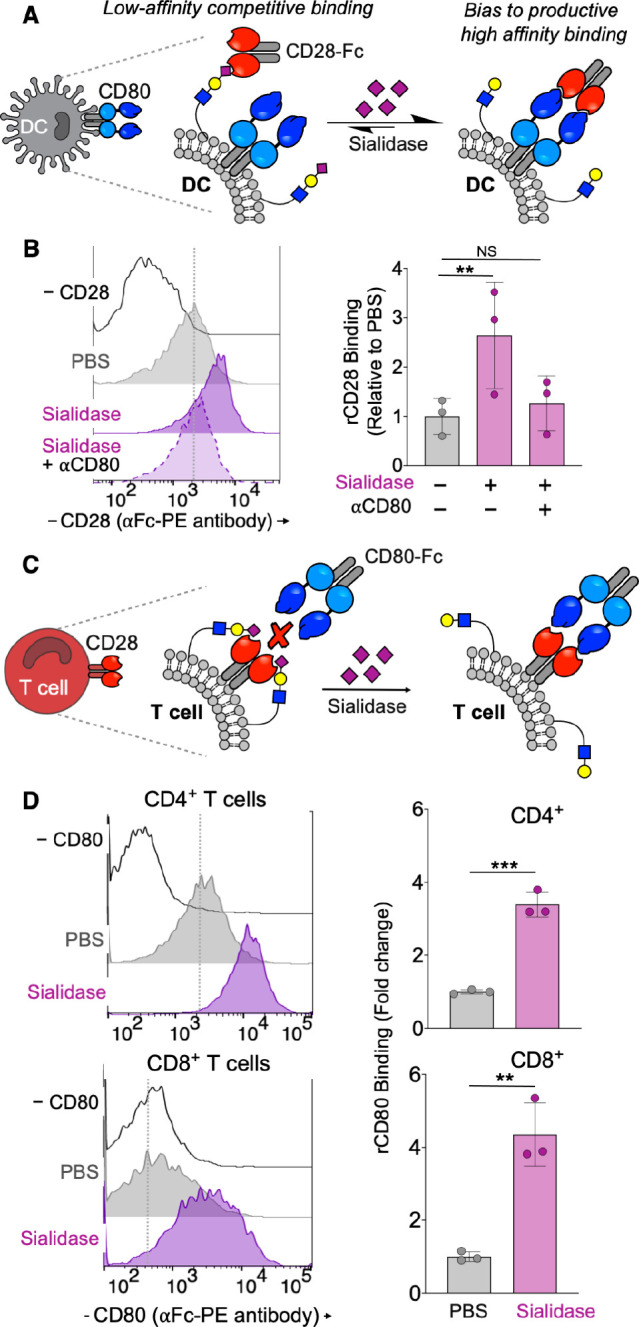
Sialylated glycans on T cells and DCs impair CD28 binding to CD80. (A) Schematic of the impact of DC desialylation to costimulatory synapse formation. (B) Staining of bone marrow-derived DCs (WT C57BL/6J donor) with recombinant CD28-Fc. Sialidase from V. cholerae (55 mU) was used to desialylate DCs. (C) Schematic of the impact of T cell desialylation to costimulatory synapse formation. (D) Staining of splenic T cells from WT C57BL/6J mice with recombinant CD80-Fc. Sialidase from V. cholerae (55 mU) was used to desialylate T cells. Mean ± SD (B, D). **P ≤ 0.01, ***P ≤ 0.001, NS = not significant (B, D). One-way ANOVA followed by Tukey’s multiple comparisons test as a paired analysis (B, D). Gating strategies: DCs, viability dye– CD11c+ MHC II+; CD4+ T cells, viability dye– CD3+ CD4+; CD8+ T cells, viability dye– CD3+ CD8+.
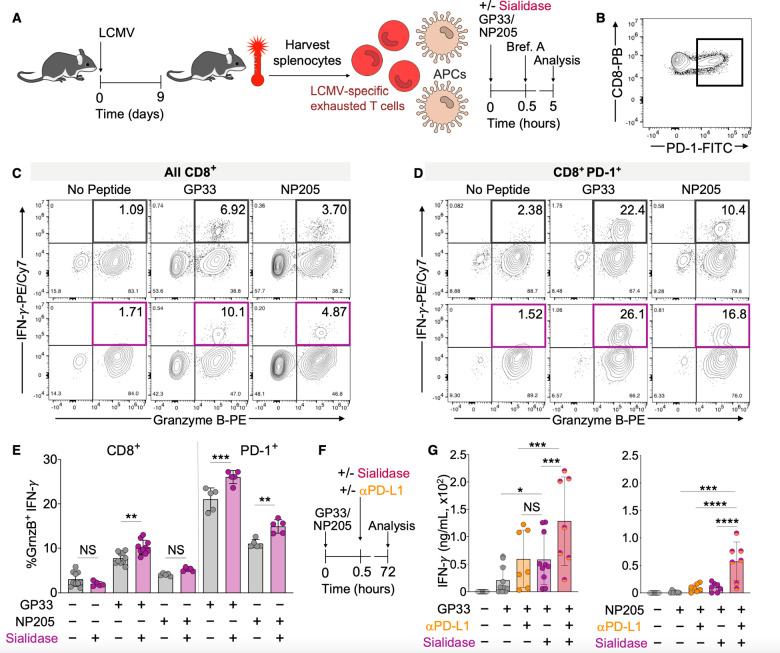
Since costimulation through CD28 is key to reviving hypofunctional and exhausted T cells through checkpoint blockade therapy,32 we reasoned that the efficacy of checkpoint blockade would be increased following removal of sialic acids. To test this, we used leukocytes from mice infected with lymphocytic choriomeningitis virus (LCMV, clone 13) as a source of hypofunctional or exhausted (PD-1+) polyclonal CD8+ T cells with defined antigen specificity (Figure 4A,B).33 Splenocytes from these animals were cultured ex vivo for 5 h in the presence of viral peptide antigens (glycoprotein33 or 276 (GP33 or GP276) or nucleoprotein205 (NP205)) and/or sialidase (Figure 4A). Under these conditions, sialidase treatment increased the percentage of functional CD8+ T cells over GP33 alone, as defined by increased expression of both granzyme B (GrnzB) and interferon-γ (IFN-γ) (Figure 4C,E). Importantly, we observed that potentially hypofunctional (PD-1+) CD8+ T cells also exhibited increased activation when treated with sialidase and restimulated with either GP33 or NP205 (Figure 4C,E). No significant activation was observed in the absence of antigen with or without sialidase. To place these findings into context with direct blockade of the PD-1 axis, we performed additional restimulation assays with anti-PD-L1 (αPD-L1) antibody blockade and/or sialidase at an extended time point (72 h) (Figure 4F and Figure S4A). We chose αPD-L1 to facilitate comparisons with previous studies using similar systems.32,34 In these experiments, functional revival of antigen stimulated splenocytes from mice infected with LCMV for 10 or 25 days (hypofunctional and exhausted T cells, respectively) was increased in the presence of sialidase as compared to antigen alone, with an effect comparable to αPD-L1 treatment (Figure 4G and Figure S4B). Importantly, combination treatment with both αPD-L1 and sialidase resulted in the strongest and most significant reactivation of CD8+ T cells for all antigens tested (Figure 4G and Figure S4B). These results are consistent with our expectation that enhanced costimulation as enabled by enzymatic desialylation can enhance the efficacy of checkpoint blockade strategies.
Figure 4.
Sialidase enhances revival of hypofunctional T cells. (A) Generation of polyclonal hypofunctional LCMV-specific CD8+ T cells from WT C57BL6/J mice. Animals were infected with LCMV (2 × 106 pfu, clone 13). Spleens were harvested on day 9 postinfection, and splenocytes (containing a mixture of leukocytes including T cells and APCs) were cultured in the presence of GP33 or NP205 LCMV peptide antigens and/or sialidase from V. cholerae (55 mU). Brefeldin A (Brf. A) was added at 0.5 h, and cytokine production in CD8+ T cells was assessed at 5 h via flow cytometry. (B) Representative PD-1 expression on CD8+ T cells from LCMV infected mice. Parent gate: viability dye– CD3+. (C) Representative density maps of activated (GranzymeB+ IFN-γ+) LCMV antigen-specific polyclonal CD8+ T cells. Parent gate: viability dye– CD3+ CD8+. (D) Representative density maps of activated PD-1+ (GranzymeB+ IFN-γ+) LCMV antigen-specific polyclonal CD8+ T cells. Parent gate: viability dye– CD3+ CD8+ PD-1+. (E) Quantification of the percentage of activated CD8+ T cells from C and D (n ≥ 5). (F) Assay workflow for longer term (72 h) activation of T cells made hypofunctional via chronic LCMV infection as in A. (G) Quantification of antigen-induced IFN-γ production by polyclonal CD8+ T cells from LCMV infected mice as in A and F. IFN-γ was quantified via ELISA after 72 h ex vivo stimulation with antigen and anti-mouse PD-L1 (25 μg/mL) and/or sialidase from V. cholerae (55 mU) (n ≥ 4). Mean ± SD (E, G). *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, NS = not significant (E, G). One-way ANOVA followed by Tukey’s multiple comparisons test as a paired analysis (E, G).
Discussion
Costimulation through CD28 is an indispensable signal required for full activation of naïve and effector T cells—a role that has been recognized for over 30 years.35 Here we have shown that CD28 recognizes sialic acid-containing glycans as ligands and that CD28-glycan complexes have a reduced capacity to interface with canonical activatory ligands expressed by APCs. We propose that sialic acid-mediated attenuation of CD28:CD80 interactions provides a mechanistic basis for the decades-old observation that sialidase treatment of T cells or APCs enhances T cell activation. The implications of this finding naturally extend to activation of T cells in other contexts. For example, we and others have shown that sialic acid-containing glycans on T cells are dynamically remodeled during differentiation and activation as a result of altered expression of sialyltransferase and neuraminidase genes.36,37 When placed into context with this work, these observations suggest that such remodeling is a biologically authentic step in T cell activation and could tune the amount of CD28 available for costimulation through altered expression of sialic acid ligands in cis. Similarly, various APCs (e.g., DCs, B cells, cancer cells, etc.) have cell-type-specific glycosylation with unique compositions of sialic acid-containing glycans that could act to attenuate costimulation in trans. Therapeutic precedence for roles of sialic acids in T cell attack of tumor cells has been obtained by genetic impairment of tumor cell sialylation38 or intratumor injection with a potent sialyltransferase inhibitor39 resulting in enhanced T cell-mediated suppression of tumor growth. Similarly, the Bertozzi group found that a sialidase–antibody conjugate targeting tumor cells to destroy ligands for inhibitory Siglecs did indeed suppress tumor growth.10,11 While the mechanism(s) for suppressed tumor growth and the cell types involved have not yet been fully illuminated, we suggest that removal of sialic acids on tumor cells could also enhance CD28-mediated costimulation of T cells as a result of increased engagement with B7 ligands on the tumor cell. Our discovery that sialidase can enhance reactivation of hypofunctional and exhausted T cells and that this effect is synergistic with blockade of the PD-1 axis is also potentially relevant to these and other disease models where PD-1 is expressed on tumor-infiltrating lymphocytes.40 Thus, approaches to reduce the sialic acid content of T cells or APCs may have value in settings requiring enhanced costimulation via CD28 for generation of effector or revival of hypofunctional T cells, especially in scenarios where blockade of the PD-1 axis alone is insufficient.
Quantification and Statistical Analysis
All statistical parameters were calculated via one-way ANOVA in Prism v.9 (GraphPad). Division indices were calculated using FlowJo v.10 (BD).
Acknowledgments
The authors thank Cory Rillahan for preparing 3FaxNeuAc, Charli Worth and Ryan McBride for assistance with acquiring glycan microarray data, Britni Arlian Cruz and Jasmine Stamps for genotyping mice, Alan Saluk and Brian Seegers for assistance with flow cytometry, and Haissi Cui and Corwin Nycholat for helpful discussions. This work was supported by NIH grants Al050143 to J.C.P. and Al123210 to J.R.T. L.J.E. is grateful for postdoctoral fellowship support from the Natural Sciences and Engineering Research Council of Canada (fellowship no. 502448-2017).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00525.
Author Contributions
L.J.E. and J.C.P. designed the study. L.J.E., A.J.T., V.F.V., C.K., and J.L.W. executed experiments. J.R.T. advised on LCMV-related experiments. The manuscript was written by L.J.E. and J.C.P. with input from all authors.
The authors declare the following competing financial interest(s): No financial interests are declared by V.F.V., J.L.W., and J.R.T. L.J.E., A.J.T., C.K., and J.C.P. are listed as coinventors on patent applications covering aspects of this work that have been filed with the United States Patent and Trademark Office and in the event the patents issue may be entitled to a share of royalties received by The Scripps Research Institute during development and potential sales of products covered by those patents.
Notes
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. James C. Paulson (jpaulson@scripps.edu).
Supplementary Material
References
- Mak T. W.; Saunders M. E.. The immune response: basic and clinical principles; Elsevier/Academic: Amsterdam, 2006. [Google Scholar]
- Huppa J. B.; Davis M. M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 2003, 3 (12), 973–83. 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- Rossjohn J.; Gras S.; Miles J. J.; Turner S. J.; Godfrey D. I.; McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015, 33, 169–200. 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- Chen L.; Flies D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13 (4), 227–42. 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. Costimulation and regulation of autoimmunity and tolerance. J. Pediatr. Gastroenterol. Nutr. 2005, 40 (Suppl 1), S20–S21. 10.1097/00005176-200504001-00011. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H.; Pauken K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18 (3), 153–167. 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- Wei S. C.; Duffy C. R.; Allison J. P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discovery 2018, 8 (9), 1069–1086. 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- Seidel J. A.; Otsuka A.; Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.; Paulson J. C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38 (1), 365–395. 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- Xiao H.; Woods E. C.; Vukojicic P.; Bertozzi C. R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (37), 10304–9. 10.1073/pnas.1608069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A.; Stanczak M. A.; Mantuano N. R.; Xiao H.; Pijnenborg J. F. A.; Malaker S. A.; Miller C. L.; Weidenbacher P. A.; Tanzo J. T.; Ahn G. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 2020, 16, 1376. 10.1038/s41589-020-0622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing C.; Chapdelaine J. M. T cells discriminate between Ia antigens expressed on allogeneic accessory cells and B cells: a potential function for carbohydrate side chains on Ia molecules. Proc. Natl. Acad. Sci. U. S. A. 1983, 80 (19), 6000–4. 10.1073/pnas.80.19.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J.; Jenis D. M.; Chesnut R. W.; Grey H. M. Studies on the capacity of intact cells and purified Ia from different B cell sources to function in antigen presentation to T cells. J. Immunol 1988, 140 (2), 388–94. [PubMed] [Google Scholar]
- Bagriacik E. U.; Miller K. S. Cell surface sialic acid and the regulation of immune cell interactions: the neuraminidase effect reconsidered. Glycobiology 1999, 9 (3), 267–75. 10.1093/glycob/9.3.267. [DOI] [PubMed] [Google Scholar]
- Silva M.; Silva Z.; Marques G.; Ferro T.; Goncalves M.; Monteiro M.; van Vliet S. J.; Mohr E.; Lino A. C.; Fernandes A. R.; et al. Sialic acid removal from dendritic cells improves antigen cross-presentation and boosts anti-tumor immune responses. Oncotarget 2106, 7 (27), 41053–41066. 10.18632/oncotarget.9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha A. A.; Berger S. B.; Miller R. A. Enhancement of CD8 T-cell function through modifying surface glycoproteins in young and old mice. Immunology 2006, 119 (2), 187–94. 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasawatari S.; Okamoto Y.; Kumanogoh A.; Toyofuku T. Blockade of N-Glycosylation Promotes Antitumor Immune Response of T Cells. J. Immunol. 2020, 204 (5), 1373–1385. 10.4049/jimmunol.1900937. [DOI] [PubMed] [Google Scholar]
- Rillahan C. D.; Antonopoulos A.; Lefort C. T.; Sonon R.; Azadi P.; Ley K.; Dell A.; Haslam S. M.; Paulson J. C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8 (7), 661–8. 10.1038/nchembio.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D.; Tropea J. E.; Mitchell M.; Kaushal G. P. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J. Biol. Chem. 1990, 265 (26), 15599–605. 10.1016/S0021-9258(18)55439-9. [DOI] [PubMed] [Google Scholar]
- Macauley M. S.; Arlian B. M.; Rillahan C. D.; Pang P. C.; Bortell N.; Marcondes M. C.; Haslam S. M.; Dell A.; Paulson J. C. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Biol. Chem. 2014, 289 (51), 35149–58. 10.1074/jbc.M114.606517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley M. S.; Crocker P. R.; Paulson J. C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14 (10), 653–66. 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. J.; Esnouf R. M.; Manso-Sancho R.; Gilbert R. J.; James J. R.; Yu C.; Fennelly J. A.; Vowles C.; Hanke T.; Walse B.; et al. Crystal structure of a soluble CD28-Fab complex. Nat. Immunol. 2005, 6 (3), 271–9. 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- Lazar-Molnar E.; Yan Q.; Cao E.; Ramagopal U.; Nathenson S. G.; Almo S. C. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (30), 10483–8. 10.1073/pnas.0804453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Zang X. Structures of Immune Checkpoints: An Overview on the CD28-B7 Family. Adv. Exp. Med. Biol. 2019, 1172, 63–78. 10.1007/978-981-13-9367-9_3. [DOI] [PubMed] [Google Scholar]
- Stamper C. C.; Zhang Y.; Tobin J. F.; Erbe D. V.; Ikemizu S.; Davis S. J.; Stahl M. L.; Seehra J.; Somers W. S.; Mosyak L. Crystal structure of the B7–1/CTLA-4 complex that inhibits human immune responses. Nature 2001, 410 (6828), 608–11. 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Schwartz J. C.; Almo S. C.; Nathenson S. G. Crystal structure of the receptor-binding domain of human B7–2: insights into organization and signaling. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (5), 2586–91. 10.1073/pnas.252771499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.; de Vries R. P.; Grant O. C.; Thompson A. J.; McBride R.; Tsogtbaatar B.; Lee P. S.; Razi N.; Wilson I. A.; Woods R. J.; et al. Recent H3N2 Viruses Have Evolved Specificity for Extended, Branched Human-type Receptors, Conferring Potential for Increased Avidity. Cell Host Microbe 2017, 21 (1), 23–34. 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O.; Collins B. E.; van den Nieuwenhof I. M.; Crocker P. R.; Paulson J. C. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 2003, 278 (33), 31007–19. 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- Collins B. E.; Blixt O.; DeSieno A. R.; Bovin N.; Marth J. D.; Paulson J. C. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (16), 6104–9. 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach R. J.; Bajorath J.; Naemura J.; Leytze G.; Greene J.; Aruffo A.; Linsley P. S. Both extracellular immunoglobin-like domains of CD80 contain residues critical for binding T cell surface receptors CTLA-4 and CD28. J. Biol. Chem. 1995, 270 (36), 21181–7. 10.1074/jbc.270.36.21181. [DOI] [PubMed] [Google Scholar]
- Ma B. Y.; Mikolajczak S. A.; Yoshida T.; Yoshida R.; Kelvin D. J.; Ochi A. CD28 T cell costimulatory receptor function is negatively regulated by N-linked carbohydrates. Biochem. Biophys. Res. Commun. 2004, 317 (1), 60–7. 10.1016/j.bbrc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Kamphorst A. O.; Wieland A.; Nasti T.; Yang S.; Zhang R.; Barber D. L.; Konieczny B. T.; Daugherty C. Z.; Koenig L.; Yu K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355 (6332), 1423–1427. 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan S. M.; Zajac A. J. Immune Exhaustion: Past Lessons and New Insights from Lymphocytic Choriomeningitis Virus. Viruses 2019, 11 (2), 156. 10.3390/v11020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro B. S.; Zak J.; Zavareh R. B.; Teijaro J. R.; Lairson L. L.; Oldstone M. B. A. Discovery of Small Molecules for the Reversal of T Cell Exhaustion. Cell Rep. 2019, 29 (10), 3293–3302.e3. 10.1016/j.celrep.2019.10.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H.; Ledbetter J. A.; Gillespie M. M.; Lindsten T.; Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol. Cell. Biol. 1987, 7 (12), 4472–81. 10.1128/mcb.7.12.4472-4481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S.; Baum L. G. Sialic acids in T cell development and function. Biochim. Biophys. Acta, Gen. Subj. 2009, 1790 (12), 1599–610. 10.1016/j.bbagen.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Comelli E. M.; Sutton-Smith M.; Yan Q.; Amado M.; Panico M.; Gilmartin T.; Whisenant T.; Lanigan C. M.; Head S. R.; Goldberg D.; et al. Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J. Immunol. 2006, 177 (4), 2431–40. 10.4049/jimmunol.177.4.2431. [DOI] [PubMed] [Google Scholar]
- Perdicchio M.; Cornelissen L. A.; Streng-Ouwehand I.; Engels S.; Verstege M. I.; Boon L.; Geerts D.; van Kooyk Y.; Unger W. W. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 2016, 7 (8), 8771–82. 10.18632/oncotarget.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C.; Boltje T. J.; Balneger N.; Weischer S. M.; Wassink M.; van Gemst J. J.; Bloemendal V. R.; Boon L.; van der Vlag J.; Heise T.; et al. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell-Mediated Tumor Immunity. Cancer Res. 2018, 78 (13), 3574–3588. 10.1158/0008-5472.CAN-17-3376. [DOI] [PubMed] [Google Scholar]
- Kitano A.; Ono M.; Yoshida M.; Noguchi E.; Shimomura A.; Shimoi T.; Kodaira M.; Yunokawa M.; Yonemori K.; Shimizu C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2 (2), e000150 10.1136/esmoopen-2016-000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.