Abstract
Adiponectin, an adipokine that circulates as multiple multimeric complexes at high levels in serum, has antidiabetic, anti-inflammatory, antiatherogenic, and cardioprotective properties. Understanding the mechanisms regulating adiponectin’s physiological effects is likely to provide critical insight into the development of adiponectin-based therapeutics to treat various metabolic-related diseases. In this review, we summarize our current understanding on adiponectin action in its various target tissues and in cellular models. We also focus on recent advances in two particular regulatory aspects; namely, the regulation of adiponectin gene expression, multimerization, and secretion, as well as extravasation of circulating adiponectin to the interstitial space and its degradation. Finally, we discuss some potential therapeutic approaches using adiponectin as a target and the current challenges facing adiponectin-based therapeutic interventions.
Keywords: adiponectin, diabetes, cardiovascular, synthesis, endothelial
Adiponectin Overview
Circulating Adiponectin Correlation with Disease States
The adipose tissue secretes a variety of peptide hormones, collectively called adipokines. In 1995 leptin was the first adipokine discovered, which was shown to be a critical regulator of appetite and, thus, energy intake (1). Following this, many other adipokines, and indeed organokines, were discovered, and in this review, we will focus specifically on adiponectin (Ad) (2, 3). Adiponectin was discovered between 1995 and 1996 by four independent groups using different approaches (4–7). Over the last 20 years, an impressive amount of literature has highlighted multiple intriguing aspects of the molecule’s structure and function (3, 8–10).
Adiponectin circulates in high levels in both rodents and humans (2–20µg/ml) and is found in multiple multimeric complexes, including hexameric and trimeric forms (11, 12). Various studies have demonstrated that adiponectin has a range of physiological properties, such as insulin-sensitizing, anti-atherogenic, and anti-inflammatory (13). Furthermore, chronic inflammation of metabolic disorders such as Type 2 diabetes, obesity, and cardiovascular disease, are correlated with lower levels of circulating adiponectin (14–17). When it comes to inflammation, adiponectin acts as a double-edged sword, with both anti-inflammatory and proinflammatory properties. On one side, adiponectin plays an anti-inflammatory role in diseases, such as obesity, Type 2 diabetes, and atherosclerosis (13). On the other hand, adiponectin acts as a proinflammatory agent for the development of rheumatoid arthritis, chronic kidney disease, and bowel disease (18). Recently, adiponectin has also been shown to play an important role in promoting longevity and prolonging lifespan (19–21).
Therefore, the establishment of these strong correlations with diseases indicates that understanding adiponectin physiological functions in various metabolic tissues will allow further comprehension of the disease pathogenesis and allow opportunities for therapeutic intervention in their progression and prevention.
Insight on Adiponectin Action Derived from Animal Models
To further understand adiponectin metabolic effects, various animal models have been established investigating the downstream effects of adiponectin signaling and function in different tissues. For instance, Ad-knockout (KO) mice present a fairly normal phenotype until challenged with a high-fat diet, and later develop metabolic perturbations, including insulin resistance (22). Additionally, adiponectin receptors (AdipoR) AdipoR1 and AdipoR2 knockout mice present increased lipid accumulation in various tissues, indicating the important relevance of the main mediators of adiponectin action in the body (23, 24).
Adiponectin has been extensively documented to mediate various cardioprotective effects (25), many mediated via AMP-activated protein kinase (AMPK) (26–28). For instance, cardiac remodeling and dysfunction in obesity and diabetes are associated with altered levels of adiponectin (25). Botta et al. (29) have demonstrated that adiponectin protects from high-fat diet (HFD)-induced cardiac insulin resistance. In Ad-KO mice fed with an HFD, it was observed that they presented insulin resistance and cardiomyocyte lipotoxicity, which was reversed by replenishing normal circulating adiponectin levels, which led to activation of AMPK signaling (29).
Among the various important remodeling events that characterize heart failure, autophagy is of growing interest. Early studies in genetic mouse models of autophagy deficiency demonstrated that decreased autophagic flux induction was positively correlated with the development of heart failure (30, 31). Interestingly, in an Ad-KO mouse subjected to aortic banding to induce pressure overload (PO) and heart failure, it was observed that Ad-KO animals exhibited less PO-induced cardiac autophagy compared to wild type (32). Additionally, it was evident the mitochondrial dysfunction was exacerbated in Ad-KO mice. This phenomenon may result in a lack of proper mitophagy in Ad-KO mice, which would usually clear out damaged mitochondria. Therefore, adiponectin may directly stimulate autophagy flux. Without adiponectin and in response to PO in aged Ad-KO mice, the cellular events that contribute to the development of cardiac dysfunction may be exacerbated (32).
Adiponectin is also expressed in muscle, and its intramyocellular localization, especially in the glycolytic type II fibers, is positively correlated with increased intramyocellular accumulation (33). In an Ad-KO mouse model, it was observed that lack of adiponectin resulted in contractile dysfunction and phenotypical changes in the skeletal muscle (34). Furthermore, male Ad-KO mice exposed to a HFD and treated with and without adiponectin injections presented muscle insulin resistance (35). However, adiponectin treatment reversed HFD-induced insulin resistance and defects in insulin signaling. Additionally, adiponectin replenishing also improved pathological mitochondrial morphology changes induced by HFD in the muscle (35). In a muscle-specific AdipoR1-knockout (muscle-R1KO) mouse model, Iwabu et al. (36) also demonstrated that muscle-R1KO animals presented lower AMPK phosphorylation concurrent with significant high plasma glucose and insulin levels, suggestive of insulin resistance.
Similar to the effects observed in the heart, one of the molecular mechanisms proposed by which adiponectin promotes antidiabetic properties is through promotion of skeletal muscle autophagy and antioxidant potential to reduce insulin resistance caused by HFD. Muscle samples were analyzed from an Ad-KO mouse model, where administration of recombinant adiponectin alleviated the HFD-induced insulin resistance effect. Moreover, HFD-induced autophagy flux was observed in wild-type mice, but not in knockout mice, suggesting that the autophagic flux activation in muscle is also dependent on adiponectin (37).
In the liver, adiponectin increases insulin sensitivity and promotes antidiabetic effects. In an Ad-KO mouse model fed with HFD, animals presented hepatic lipid accumulation and insulin resistance. These effects were attenuated by adiponectin supplementation, which also contributed to enhancing fatty acid oxidation (38). Finally, it has also been observed that lack of adiponectin contributes to endothelial activation and exacerbation of sepsis-related mortality. Sepsis has multiple contributing factors and often results in death (39). Therefore, adiponectin deficiency may predispose patients to sepsis-related complications in late stages of obesity, diabetes, and insulin resistance.
Direct Effects of Adiponectin on Cellular Models
Adiponectin mediates several tissue-specific signaling pathways. Some of the major direct effects of adiponectin on cells are targeted to the adipose tissue, skeletal muscle, vascular endothelium, macrophages, and insulin-sensitizing actions (13). The primary mechanism of action of adiponectin is via membrane receptors binding, such as AdipoR1, AdipoR2, and T-cadherin (40, 41). To induce a response in any target tissue, adiponectin must first exit circulation and cross the endothelial barrier (42). The two central adiponectin receptors are the AdipoR1 isoform, which is mostly found in skeletal muscle, and AdipoR2 isoform, found in the liver (23, 43).
Alterations in adiponectin expression have been associated with a positive correlation between receptor expression and insulin resistance (44). A crucial downstream signaling effector of adiponectin is AMPK (41). For instance, AdipoR1 KO mice presented glucose tolerance and impaired AMPK activation (24), while AdipoR2 KO mice showed better glucose tolerance and lipid metabolization (45). The main mechanism of adiponectin-induced glucose uptake in skeletal muscle has been highly elucidated (46). Briefly, adiponectin binds to cell surface AdipoRs and, subsequently, binds to the adaptor protein (APPL1), resulting in activation of AMPK and translocation of glucose transporter-four (GLUT4) to the plasma membrane (47, 48). Adiponectin can also activate insulin receptor substrate-one (IRS1) phosphorylation, and downstream PI3K-AKT axis, to promote cell survival in muscle and hepatocytes following stress (49). It also mediates activation of various pathways that contribute to increased fatty acid oxidation, including PPAR-α, AMPK, and p38 MAPK (50, 51).
On adipose tissue, adiponectin function is mostly related to the control of the adipose endocrine system. Adiponectin represses secretion of leptin and proinflammatory cytokines, such as IL-6 and TNF-α via AMPK and NF-κB signalling, therefore reducing its own expression level (52, 53). Similarly, on macrophages, adiponectin has anti-inflammatory effects by repressing M1 macrophage (proinflammatory) activation, and favors activation of M2 macrophage (anti-inflammatory) (54). Furthermore, in human macrophages, adiponectin also activates anti-inflammatory factors (IL-10) and decreases proinflammatory ones (IFN-γ, IL-6, and TNF-α) (55). Finally, vascular protection and endothelium relaxation are promoted by nitric oxide (NO) production activated by AMPK-endothelial nitric oxide synthase (eNOS) (56). In addition to that, adiponectin contributes to NO production and suppression of ROS and NF-κB (57). Therefore, adiponectin regulates vascular homeostasis on endothelial cells via activation of cAMP-PKA and AMPK signalling.
As noted, there is a controversy as to whether adiponectin acts as an anti- or proinflammatory mediator (54, 58–67). Some studies report that adiponectin functions as an anti-inflammatory mediator during the progression of metabolic diseases (62, 67). In agreement with these reports, extensive evidence shows that adiponectin acts as anti-inflammatory mediator through the regulation of M1 and M2 macrophage proliferation, plasticity and polarization (55, 63, 68–71). In contrast, other studies found that adiponectin promotes an inflammatory response by inhibiting group two innate lymphoid cell (ILC2) function, activating NF-κB and inducing inflammatory cytokines IL-1 and IL-6 under certain circumstances (64, 72, 73). The regulatory effect of adiponectin on innate immunity under physiological conditions may emerge as an important determinant of metabolic adaption. During the development of obesity, the decreased levels of adiponectin by obesity in circulation favor its anti-inflammatory action and subsequent insulin-sensitizing effect. Whereas adiponectin behaves as an initial proinflammatory factor in response to LPS and helps to desensitize macrophages to further proinflammatory stimuli (64, 72, 74). In agreement with this, adiponectin suppresses cold stress-induced ILC2 activation and type 2 inflammatory response through an AMPK-mediated feedback inhibition of IL-33 signaling, acting as a molecular brake on thermogenesis (73). A better understanding of the physiological role of adiponectin in the regulation of innate immunity may provide a basis for the development of adiponectin-based therapeutic strategies (54).
Summary of Adiponectin’s Principal Physiological Effects
Adiponectin has been demonstrated by various studies to promote antidiabetic effects. The majority of adiponectin in circulation is derived from adipocytes. However, other tissues, such as skeletal muscle, also produce adiponectin. Most adipokines present a proinflammatory action, such as tumor necrosis factor-α, adipocyte fatty acid-binding protein and lipocalin-2, but contrary to all other adipokines, adiponectin promotes anti-inflammatory effects, is antidiabetic and cardioprotective, and plays an important role in cancer (75–77).
The metabolic effects of adiponectin have been well characterized in skeletal muscle and liver, and other observations also indicate similar effects in the heart, where it enhances glucose uptake and fatty acid metabolism (78). The majority of adiponectin action in all target tissues has been shown to be regulated by AMPK, which plays an important role in regulating a wide range of physiological effects of adiponectin on glucose metabolism (79). Furthermore, exercise has been shown to be highly effective as an insulin sensitizer to promote glucose uptake in skeletal muscle, increase fatty acid oxidation, and reduce obesity (80). Exercise may also facilitate adiponectin action by upregulating AdipoRs expression, therefore, increasing the glucose-sensitizing effects (81, 82). A recent human study in obese and prediabetic patients demonstrated that intensive interval training could elevate circulating adiponectin levels, which may be clinically relevant in aiding to reduce the cardiometabolic risk (83).
Besides its physiological relevance in the muscle, heart, and liver, adiponectin also plays a role in respiratory complications (75). Decreased serum adiponectin levels have been correlated with deficient lung function and chronic obstructive pulmonary disease (COPD) (84). Additionally, recent studies have also shown a positive correlation between obesity and pulmonary hypertension (85). However, the mechanisms by which obesity drives pulmonary hypertension are still very complex and not well understood. It is well known that adiponectin plays a pleiotropic effect on inflammation and cell proliferation (86), representing a potential protective function on the pulmonary vasculature. Therefore, adiponectin could potentially become a therapeutic target for pulmonary hypertension treatment.
On the basis of all the physiological effects of adiponectin in various tissues, it is evident that it would make an attractive therapy option to treat various metabolic diseases. Currently, AdipoRon, a synthetic analog of endogenous adiponectin, presents great pharmacological properties mimicking adiponectin effects, which can bind and activate adipoR1 and adipoR2 receptors, contributing to the treatment of various metabolic diseases, including obesity, Type 2 diabetes, and cancer (87). Other therapies are also under current investigation that may be effective in increasing the adiponectin effect in the body; it has been extensively reviewed by Liu et al. (9).
Mechanisms Regulating Adiponectin’s Physiological Effects
Gene Expression and Secretion
Adiponectin is mainly produced from mature adipocytes in white adipose tissue. Originally thought to be exclusively expressed by adipose tissue; currently it is well established that adiponectin is also produced and secreted by numerous cell types, including skeletal and cardiac muscles (34, 88–91). It circulates in the plasma at very high concentrations (2–20 μg/mL), constituting approximately 0.01% to 0.05% of total serum proteins (92, 93). Recent studies have shown that adiponectin is secreted predominantly during the day, following a circadian-rhythm pattern. Even though the impact of the circadian rhythm on adiponectin expression is not yet well understood, some metabolic diseases, including obesity and insulin resistance, have been linked to disrupted circadian rhythm (33). Mice with high-fat diet-induced insulin resistance had significantly lower expression of adiponectin (94).
The regulation of adiponectin gene expression, specifically, is highly regulated by different transcription factors (97). Some of the transcription factors with active binding sites on both human and mouse adiponectin promoter include the peroxisome proliferator‐activated receptor (PPAR)‐response elements, C/EBP sites, FOXO sterol regulatory elements (SREs), and E‐box (98). The major positive regulator of adiponectin expression is PPARγ, mainly expressed in adipose tissue, in abundance (99, 100). Activation of PPARγ by antidiabetic drugs thiazolidinedione can stimulate adiponectin expression and secretion in adipose tissue of both humans and rodents (101). In contrast, the negative regulators of adiponectin expression levels are the inflammatory and obesity mediators known to induce insulin resistance, such as reactive oxygen species (ROS), TNF-α, and IL-6 (102, 103). Moreover, in vitro studies have demonstrated that adiponectin secretion in 3T3-L1 adipocytes is regulated by endothelin-1, an important vasoconstrictive agent that is increased in diabetes and obesity disease states (104). Therefore, various factors may be involved in the regulation of adiponectin gene expression and secretion, including vascularly derived factors, which represents an area to be further explored.
Recent studies have brought to attention the role of adiponectin gene DNA methylation in gestational diabetes (GDM). Children exposed to GDM are at a higher risk of becoming glucose intolerant later in life, among other metabolic complications (105–107). Early-life alterations of DNA methylation leads to disrupted adiponectin gene (ADIPOQ) expression, which was associated with alterations in maternal glucose levels, leading to hyperglycemia (108, 109). Although the precise molecular mechanism of how this happens is not really well known, epigenetics alterations around ADIPOQ are highly suggestive of being one possible mechanism involved in the metabolic perturbations observed in the offspring of GDM women (110). Furthermore, recent evidence shows that other chronic conditions during pregnancy, such as obstructive sleep apnoea, was associated with endothelial dysfunction, reduced perivascular adiponectin, and epigenetic modifications on the adiponectin gene promotor of adult male offspring (111).
Adiponectin exists as a full-length protein of 30 kDa, circulating in serum as three major isoforms: trimer, hexamer, and high-molecular weight (HMW) multimer (92). Different adiponectin multimers have been shown to exert distinct biological properties in their various target tissues (112). The HMW adiponectin is the major active form mediating its insulin-sensitizing effects, whereas the central action of adiponectin is attributed primarily to the hexameric and trimeric multimers (51, 113–118). A distinction in circulating levels of adiponectin has been observed in both humans and rodents, where total and HMW adiponectin is higher in females than in males. This is possibly because HMW adiponectin production is inhibited by testosterone in males (92, 119, 120). Also, the globular domain of adiponectin (∼18–25 kDa), which can be produced via the action of neutrophil elastase (121), has been shown to similarly present significant metabolic effects in various tissues (121). Many factors that regulate adiponectin gene expression and secretion can directly influence plasma levels of adiponectin, via the regulatory mechanisms at the levels of transcriptional, translational, and posttranslational modifications.
Posttranslational Modification and Multimerization
The selective reduction of the HMW adiponectin concentrations (or impaired adiponectin multimerization) was found to be associated with various metabolic diseases, such as obesity, insulin resistance, Type 2 diabetes, and arteriosclerosis (11, 113, 122–124). An increase in the HMW form, rather than the total level of adiponectin, was found to be associated with insulin-sensitizing effect of thiazolidinediones (TZDs) in both mice and human diabetic patients (124). Moreover, increasing the HMW form of adiponectin by fat-specific overexpression of DsbA-L-protected mice from diet-induced insulin resistance (125). In fact, it has been suggested that reduced levels of the HMW form rather than total levels of adiponectin could be a superior biomarker for insulin resistance, the metabolic syndrome, and Type 2 diabetes (113, 126).
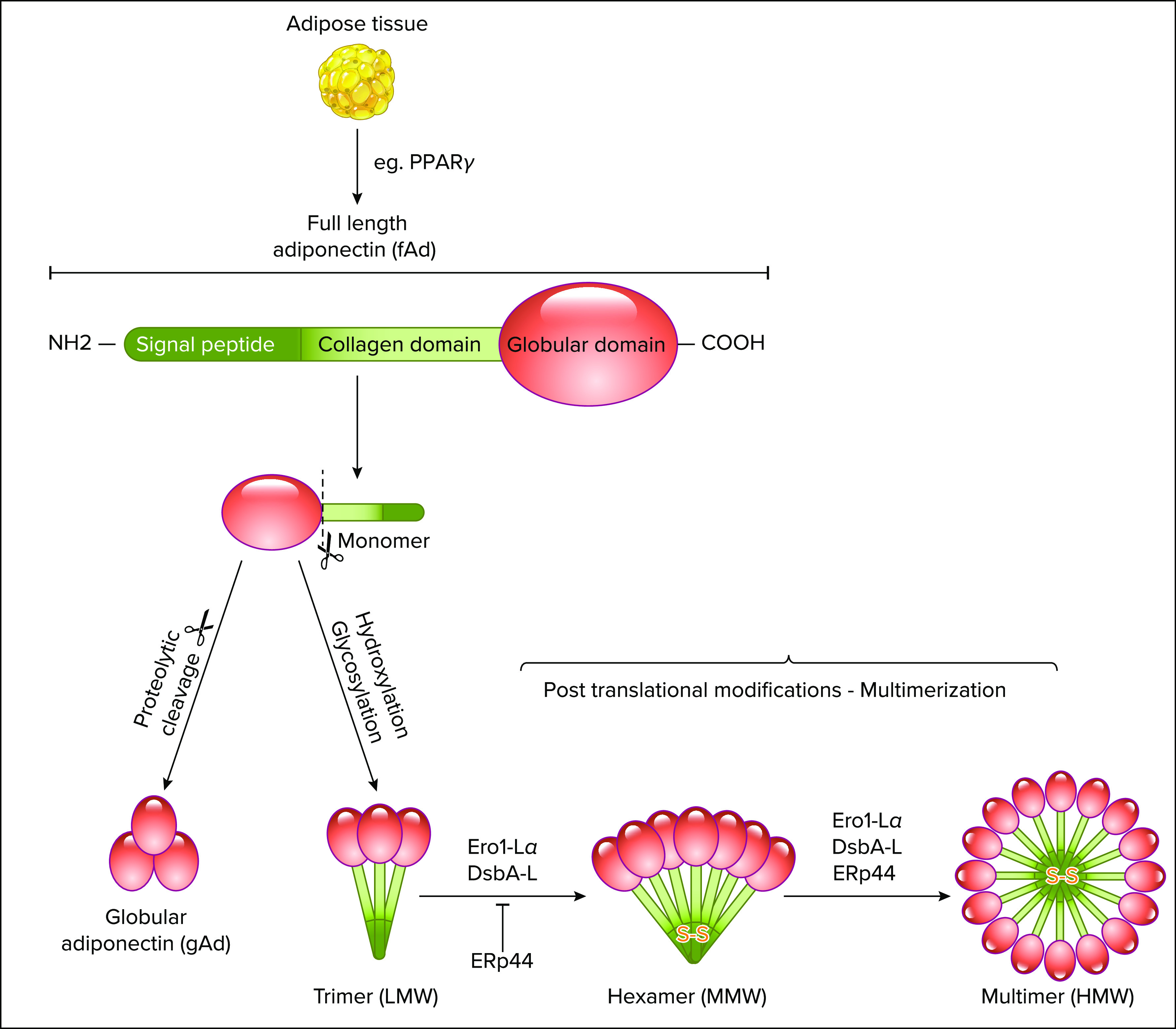
The biosynthesis of the adiponectin multimers is a complex process involving extensive posttranslational modifications, as shown in FIGURE 1 (129). There is a conserved cysteine residue at the NH2 terminus (Cys36 in human and Cys39 in mouse) and the formation of an intermolecular disulfide bond via this residue is essential for adiponectin multimerization and secretion (127). Cys39 was also identified as a key site of succination of adiponectin, which blocks adiponectin multimerization and may contribute to the decrease in plasma adiponectin in diabetes (128). In addition to disulfide bond formation, hydroxylation and glycosylation of several conserved lysine residues in the collagenous domain of adiponectin are necessary for the intracellular assembly and stabilization of its high-order multimeric structures (12, 129, 130). There are four conserved lysine residues in the collagenous domain of adiponectin (Lys65, Lys68, Lys77, and Lys101 for human adiponectin) and hydroxylation, and subsequent glycosylation at these sites are required for intracellular assembly of the trimer of adiponectin into the HMW multimer (12, 129). Whitehead and colleagues (131) identified three additional hydroxylations on Pro71, Pro76, and Pro95 in human adiponectin, including Pro71, Pro76, and Pro95, and demonstrate that inhibition of proline hydroxylation results in a more severe impairment of adiponectin multimerization .
FIGURE 1.
Adiponectin synthesis and multimerizationAdiponectin exists as a full-length protein of 30 kDa, circulating in serum as three major isoforms: trimer, hexamer, and high-molecular weight (HMW) multimer. Adiponectin is mostly secreted by the adipose tissue, highly regulated by the PPARγ signaling pathway. Hydroxylation and subsequent glycosylation of lysine residues in the collagenous domain of adiponectin are required for intracellular assembly of the trimer of adiponectin into the HMW multimer. Additionally, the multimerization and secretion of adiponectin are controlled by molecular chaperones in the ER (endoplasmic reticulum), including ERp44 (ER protein of 44 kDa), Ero1-Lα (ER oxidoreductase 1-Lα) and DsbA-L. The ERp44 forms a mixed disulfide bond (S-S) with adiponectin at the NH2-terminal region and inhibits the secretion of adiponectin multimers. In contrast, Ero1-Lα enhances the secretion of HMW adiponectin through releasing adiponectin trapped by ERp44 and by transferring its own disulfide bond to protein disulfide-isomerase, allowing disulfide bond formation and adiponectin multimerization. Lastly, DsbA-L also play a role in specifically recognizing the LMW and MMW, and enhancing HMW assembly. This figure was created using Servier Medical Art (available at https://smart.servier.com/) and PAGES software.
The multimerization and secretion of adiponectin are also tightly controlled by a pair of molecular chaperones in the endoplasmic reticulum (ER), including ERp44 (ER protein of 44 kDa), Ero1-Lα (ER oxidoreductase 1-Lα), and DsbA-L, all of which are induced during adipogenesis, as shown in FIGURE 1 (125, 132, 138). ERp44 forms a mixed-disulfide bond with adiponectin through the cysteine residue (Cys36 in human and Cys39 in mouse) at the NH2-terminal region and inhibits the secretion of adiponectin multimers through a thiol-mediated retention (132). In contrast, Ero1-Lα enhances the secretion of HMW adiponectin through releasing adiponectin trapped by ERp44 (133, 132, 138). Ero1-Lα also participates in adiponectin maturation by transferring its own disulfide bond to protein disulfide-isomerase (PDI) that is essential for the intermolecular disulfide bond formation and adiponectin multimerization (133). On the other hand, ERp44 was found to specifically recognizes the LMW and MMW in the cis-Golgi and enhance HMW assembly by regulating the population of adiponectin intermediates with appropriate oxidative state (134). Unlike Ero1-Lα and ERp44, DsbA-L does not contain CXXX motif, whereas it serves as an adiponectin interactive protein that selectively promotes adiponectin multimerization in adipocytes (125). In addition, DsbA-L alleviates ER stress-induced adiponectin downregulation via an autophagy-dependent mechanism and protects against obesity and ER stress-mediated suppression of adiponectin multimerization (135, 136). In support of this, the spliced form of XBP1s (X-box-binding protein 1), a key transcription factor of the ER stress response, promotes adiponectin multimerization through a direct regulation of the expression of several ER chaperones, including PDIA6 (protein disulfide isomerase family A, member 6) ERp44, and DsbA-L (137). The PPARγ (peroxisome-proliferator-activated receptor γ) agonists TZDs selectively enhance the secretion of HMW adiponectin through upregulation of Ero1-Lα and DsbA-L (125, 138). These data provide evidence for a link between impaired adiponectin multimerization and the causes of a diabetic phenotype in humans and suggest that not only total concentrations, but also multimer distribution, should always be a consideration in the interpretation of plasma adiponectin levels in health, as well as various disease states (139).
From the ER, adiponectin is engaged in the secretion pathway and undergoes multimerization into trimer, hexamer, and HMW adiponectin. Two pathways, multimerization and secretion, are coupled through shared components such as ER chaperones Ero1-Lα, ERp44, and DsbA-L (125, 132, 138). ERp44 is mainly localized in the ER-Golgi intermediate compartment/cis-Golgi region and retains adiponectin in the early secretory compartment (132, 133). Whereas Ero1-Lα is able to facilitate the secretion of HMW adiponectin through releasing adiponectin trapped by ERp44 (132, 138). Another chaperone DsbA-L promotes the secretion of HMW adiponectin through induction of adiponectin multimerization in adipocytes (125). However, what other factors are involved in adiponectin secretion is poorly understood. Future studies are also urgently needed to elucidate the precise mechanisms underlying the secretion of various multimeric forms of adiponectin given its promising therapeutic potential.
Adiponectin receptors, AdipoR1 and AdipoR2, have been found to bind to the globular form, as well as the full-length adiponectin, rapidly inducing intracellular signaling cascades (23, 48, 49). Despite the most active form of adiponectin, how HMW adiponectin functions in its target cells remains largely unknown. T-cadherin was identified as a selective receptor for the HMW adiponectin that potentiates adiponectin signaling (140). However, T-cadherin lacks the intracellular structural domain and appears to serve as a binding protein of adiponectin (141–143). The intracellular signaling events upon the binding of HMW adiponectin to T-cadherin remain to be defined.
Extravasation of Adiponectin from Vasculature to Interstitial Space
Structure and function of endothelium.
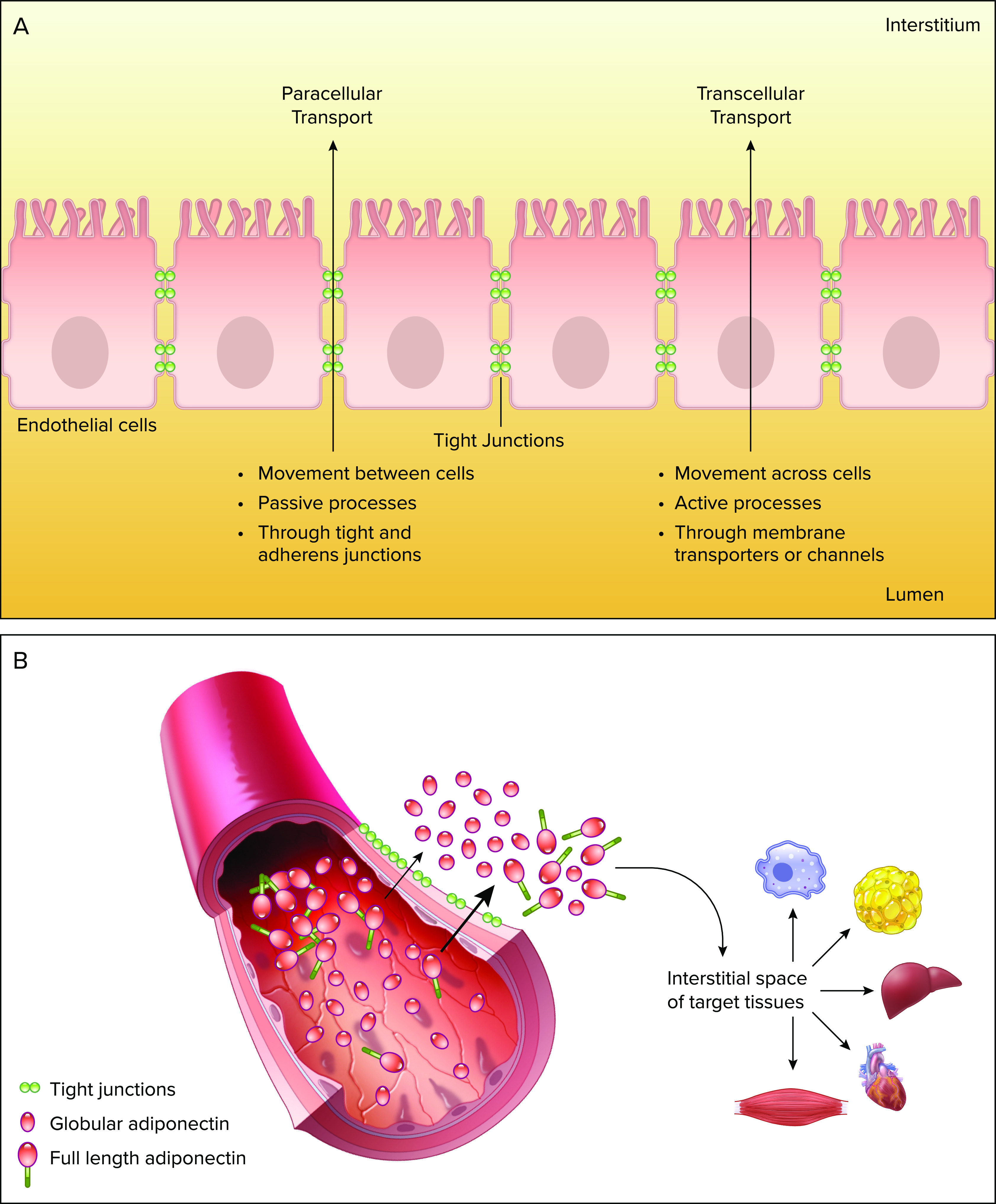
The endothelium consists of a monolayer of endothelial cells that act as an important barrier regulating various signaling molecules between the vascular lumen and the interstitial space (144). It also plays a range of roles, including maintenance of extracellular matrix, regulation of immune response, and regulation of cellular growth (144). The endothelial permeability can be regulated via two distinct mechanisms, transcellular and paracellular (FIGURE 2A). Transcellular pathway allows solutes to be transported actively across the endothelium. It is driven by active processes involving transport proteins, pumps, and receptors. Alternatively, the paracellular pathway allows solutes to move between adjacent endothelial cells (42). The regulatory mechanism of the paracellular movement requires the involvement of tight and adherent junctions, which have been shown to be essential in regulating hormone transport (145). An interesting distinction between both transport methods is that in the transcellular movement, larger macromolecules are carried across the endothelium via vesicle trafficking, while the paracellular route restricts the passage of large solutes, FIGURE 2B (146).
FIGURE 2.
Adiponectin extravasation into the circulationA: paracellular and transcellular movement. B: adiponectin extravasation from circulation into interstitial space on target tissue. Process mediated through paracellular movement across the endothelium. Decreased tightness of junctions allows higher amounts of full-length adiponectin extravasation into the interstitial space. This figure was created using Servier Medical Art (available at https://smart.servier.com/) and PAGES software.
Role of transcellular/paracellular transport.
An extensive literature currently indicates that endothelium not only plays a massive role in cardiovascular disease but also diabetes (144, 147–149). Interestingly, exocytosis of adiponectin from circulation has been shown to promote protective effects (3). Adiponectin must leave circulation into the interstitial space on target tissues in order to act, where vascular permeability may highly contribute to the regulation of adiponectin action, as illustrated in FIGURE 2B (150). Recent studies from Sweeney’s group demonstrated an underappreciated and novel link between vascular permeability and adiponectin flux during hyperglycemia or diabetes. In both in vivo and in vitro approaches, a correlation was observed between the tightness of an endothelial barrier upon hyperglycemic treatment. Reduction in tight junction proteins, such as claudin-7, significantly contributed to increased permeability in diabetic mice, allowing an increased flux of both low- and high-molecular weight proteins (150). Studies also suggest that another key player in adiponectin translocation may be due to AdipoR-mediated endocytosis (151). However, Yoon et al. (150) demonstrated that there were no significant changes in the levels of both AdipoR1 and AdipoR2 in the heart, following hyperglycemia.
Furthermore, altered transendothelial glucocorticoids are also great mediators of endothelium permeability (152). Recently, Sweeney’s group demonstrated that glucocorticoids may impair adiponectin action in target tissues by reducing the transendothelial flux of adiponectin across endothelial monolayers in vivo and in vitro (153). For instance, the adiponectin flux across the endothelial monolayers was associated with reduced claudin-10 mRNA levels in skeletal muscle of glucocorticoid-induced diabetic rats (153). Therefore, these all indicate that paracellular transport is a major mechanism in which glucocorticoid-mediated tightening can reduce adiponectin flux across endothelial monolayer cells. As a consequence, decreased adiponectin action due to reduced extravasation from circulation to interstitial space can contribute to a diabetic phenotype followed by glucocorticoid exposure.
Thus, adiponectin plays an essential role in vascular physiology by mediating cross-talk among endothelial cells, smooth muscle cells, leukocytes, and platelets (93). The endothelium-derived nitric oxide (NO) is an important factor promoter of vasodilation and inhibition of inflammation (144). More importantly, reduced NO is an early event in the atherosclerosis process; however, adiponectin acts via AdipoR1 and AdipoR2 to potentiate NO production via the AMPK signaling pathway (154). Endothelial dysfunction, characterized by impaired NO production, has been highly investigated and associated with not only cardiovascular disease, but also insulin resistance (155) and diabetes (148, 151). However, adiponectin can attenuate these metabolic defects via the cAMP/PKA pathway and inhibit proinflammatory actions via endothelial ROS inhibition (156). Currently, some antidiabetic drugs have been shown to protect the endothelium. For instance, there is strong evidence that metformin exerts a beneficial effect on endothelial function, as well as glucagon-like peptide 1 (GLP-1) agonists, and SGLT2 inhibitors (144, 157, 158).
Improving adiponectin access to interstitial space.
One possible mechanism of adiponectin action is primarily via binding of the membrane receptors, such as AdipoR1, AdipoR2, and T-cadherin. Alternatively, vascular permeability is another critical factor in determining adiponectin action (42). To promote an effect in a target tissue, adiponectin must first exit circulation into the interstitial space by crossing the endothelial barrier. Paracellular transport is one of the major determinants of adiponectin flux across the endothelium (FIGURE 2). However, little is known about its mechanisms, and alterations in the paracellular transport have been associated with endothelial dysfunction in diabetes.
The endothelial barrier that controls the adiponectin movement is cell and tissue specific. Previously, Dang et al. (153) demonstrated that glucocorticoids may impair adiponectin action in target tissues by reducing the transendothelial flux of adiponectin across endothelial monolayers in vivo and in vitro. However, in a more recent study, Yoon et al. (150) for the first time tracked adiponectin biodistribution via fluorescence rhodamine-labeled full-length adiponectin in streptozotocin-induced diabetic mice, where hyperglycemia increased vascular flux of adiponectin. Surprisingly, only a few studies have investigated adiponectin interstitial space levels, with two of them suggesting that exercise may contribute to increased interstitial adiponectin (159, 160).
Receptor Expression and Localization
AdipoR1 and AdipoR2 are widely expressed in various metabolic tissues, such as adipose tissue, liver, skeletal muscle, and the cardiovascular, as well as in the reproductive tract and brain. The AdipoR1 isoform is mostly found in skeletal muscle and AdipoR2 isoform in the liver (23, 43). The difference between the two is that AdipoR1 is a receptor for globular adiponectin, while AdipoR2 is a receptor for full-length adiponectin (23). Both receptors possess very similar homology at the protein level and contain seven transmembrane domains; however, structurally and functionally distinct from G protein-coupled receptors (8, 23). AdipoR1 and R2 contain a zinc-binding catalytic site and are closely located to the inner surface of the plasma membrane (161). The interaction between the ligand and receptor takes place between the globular domain of adiponectin and the extracellular surface of the receptors (23). Once adiponectin binds to the zinc‐binding motif of AdipoR1 and R2, a series of downstream signaling events are initiated in many target tissues, including skeletal muscle, liver, heart, kidney, and pancreas (162). Some of the major signals activated are AMPK, p38 mitogen-activated protein kinase (p38 MAPK), and higher activity of peroxisome proliferator-activated receptor alpha (PPARα) ligand, playing an important role in glucose and fatty acid metabolisms (23). These signals are regulated by the leucine zipper motif (APPL1), an adaptor protein discovered in 2006, which directly binds to intracellular regions of AdipoR1 and AdipoR2 (48). Another key regulator of adiponectin function is the T-cadherin receptors, discovered in 2004 (140). Even though T-cadherin receptor does not have a membrane or intracellular domain, it plays an important role in binding adiponectin and promoting adiponectin signaling in both smooth muscle and endothelial cells (140).
Degradation of Adiponectin
Adiponectin biosynthesis is well understood; however, its fate, once in circulation, is less well established. A decrease in plasma adiponectin levels is often associated with obesity; however, the underlying mechanisms remain unclear. In 2009, Shrerer’s group showed the clearance rate of adiponectin for the first time. The rate varies among different isoforms of adiponectin. For instance, HMW adiponectin is cleared the slowest and trimer the fastest (163). Also, the main site of clearance is the liver, while the degradation of final products is excreted by the kidney (163). Various studies have shown that reduced endogenous adiponectin levels might not be only a result of upstream defects at the exocytotic machinery. Defective adiponectin synthesis or a posttranslational modification could also sequester adiponectin to the endoplasmic reticulum (ER) to be degraded (7, 132, 134, 164).
In 3T3-L1 adipocytes, it was found that ER stress promotes autophagy-dependent adiponectin degradation, providing a mechanism underlying obesity-induced adiponectin downregulation (135). Even though inhibition of autophagy prevented adiponectin degradation, it did not rescue insulin sensitivity. Perhaps that is because of an adaptive role of autophagy during ER stress-induced insulin resistance (135). Conversely, our group extensively reported that autophagy is an important cellular mechanism induced by adiponectin (32, 37, 165). Recently, we demonstrated that adiponectin directly stimulated autophagy in skeletal muscle cells via an AMPK-dependent signaling mechanism and alleviated insulin resistance (37, 166). Gu and colleagues proposed an alternative signaling pathway that, once blunted, leads to adiponectin degradation. Rodents exposed to HFD developed obesity and a decline in plasma adiponectin, which was associated with suppression of ERK1/1 activation in adipocytes. Moreover, inhibition of the MEK/ERK1/2 pathway in adipocytes significantly decreased intracellular and secretory adiponectin levels, suggesting that it may promote adiponectin degradation (167).
Capitalizing on Knowledge of Adiponectin Action for Therapeutics
Recombinant Adiponectin
Adiponectin is primarily produced in adipocytes as a monomer and posttranslationally modified into diverse multimers. The recombinant adiponectin produced by Escherichia coli, on the other hand, is composed of only monomeric adiponectin, meaning that multimer formation requires posttranslational processing by mammalian adipocytes (8). The multimeric complexes highly contribute to the various biological effects of adiponectin (92, 127). In healthy individuals, the plasma concentration of adiponectin is usually high (∼5–30 μg/mL); however, in the case of obesity, the level of blood adiponectin is often lower, contributing to increase insulin resistance and Type 2 diabetes (168). Supplementation with recombinant adiponectin has been shown to improve insulin resistance, protect diabetic phenotype or metabolic challenges in both in vivo and in vitro studies (35, 169–171). However, there is controversy over whether recombinant adiponectin may be really effective in lowering blood glucose in diabetic animal models (172). Even though adiponectin has been suggested as a potent target for developing a therapeutic agent for treating metabolic diseases, such as diabetes, the preparation of recombinant adiponectin has been a challenge due to posttranslational modifications. As a result, currently, no drug targeting AdipoRs has yet been approved.
Adiponectin Peptides
AdipoR agonists, for a long time, have been the focus of research and development programs in cardiovascular and metabolic diseases (10, 165). Designing adiponectin agonists that activate adiponectin receptor-mediated downstream signaling is highly desired; however, it poses great challenges in the production of biologically active adiponectin and optimization of the right dose and via of administration. Since the discovery of AdipoR1 and AdipoR2 by Kadowaki’s group (23), various small molecules or peptides have been discovered (10). Recently, an adiponectin-mimetic peptide, ADP355 (174) and a small molecule, AdipoRon (173), have been developed as agonists to AdipoRs.
To date, ADP355 is the first synthetic adiponectin-mimetic peptide composed of non-natural amino acids (174, 175). Initially intended for development for cancer treatment, ADP355 presents the ability to activate the AdipoRs-mediated AMPK pathway, which represents an opportunity for the use of synthetic peptides in developing agonists against AdipoRs. On the basis of the success of the first generation of AdipoR agonist ADP355, Otvos et al. (175) developed a second generation of the peptide, the dimeric ADP399. The improved ADP399 had a 20-fold increase in activity in comparison to the monomeric peptide ADP355 (175). Moreover, to counteract ADP355 and ADP399, a novel octapeptide (ADP400) was developed after several screenings (10, 175). ADP400 antagonizes endogenous adiponectin and acts as an inverse agonist. Therefore, at a similar dose as the agonist ADP399, the ADP400 peptide showed greater antagonist activity, representing an important target as a validation tool to further study adiponectin functions (175).
Although the adiponectin- and AdipoR1-bound structure is not available, the extracellular COOH-terminal domain has been identified to be critical for adiponectin binding to AdipoR1 (24).
Recently, Kim’s group (168) identified the ligand-binding regions in AdipoR1 and designed peptides that stimulate interaction in those regions. After several rounds of designing and screening, the BHD1028 was identified as the most potent peptide in activating AMPK (160). Even though further clinical studies are needed to validate its efficacy, BHD1028 already presents a potential drug candidate for treating diabetes and metabolic diseases. Finally, another peptide Pep70 has also been ultimately identified as an inhibitor of fibrotic responses and as a potential AdipoR1 agonist (176). However, it was concluded that more studies with Pep70 are necessary to achieve a higher level of bioactivity.
Adiponectin Gene Therapy
As indicated above, in Type 2 diabetes patients’ adiponectin levels are reduced. A study was designed to investigate whether an injection of plasmid DNA-encoding adiponectin promotes any therapeutic effects on increasing adiponectin levels (177). In a nonobese Type 2 diabetes mouse model, it was observed that they significantly had higher blood adiponectin levels and decreased glucose concentrations following adiponectin injections (177). Adiponectin adenoviral injections also decreased blood pressure levels and increased circulating adiponectin in obese hypertense mice and salt-fed Ad- KO mice (57). Furthermore, Dyck’s group concluded that injectable adiponectin gene therapy could reduce fat and improve insulin sensitivity in HFD-treated mice (178).
One of the most attractive forms of gene delivery is through synthetic polymer vectors because of its relative safety and practicality. The downside is its low efficiency; for this reason, successful applications have focused on therapy with genes that at low concentrations promote biological effects (179). As adiponectin is a highly abundant circulating protein, it represents a great candidate for this nonviral gene delivery. Banerjee et al. (180) recently developed a gene-based targeted nanoparticle formulation capable of improving insulin sensitivity in Type 2 diabetes by stimulating adiponectin production in adipose tissue and increasing its circulation levels in the body for an extended period. In their work, they used free amino groups on chitosan-oleic acid polymer conjugated to adipose homing peptide to enhance its internalization into adipose tissue. This delivery approach has been previously described and validated by various studies (181–183). Adiponectin gene therapy could be a clinically useful tool for the treatment of Type 2 obesity and endothelial dysfunction. However, more studies are needed as efficiency, stability, specificity, safety, and convenience are key factors that require careful consideration before the clinical application of any gene delivery system.
Inducing Adiponectin Expression/Secretion
Overcoming the reduced availability of circulating adiponectin levels in various disease states requires interventions that enhance adiponectin receptor expression. The expression of adiponectin receptors can be increased by lifestyle modification, such as diet, exercise, and pharmacological therapy (9). In obese patients, weight loss induced by hypocaloric diet and physical exercise promoted an increase in adiponectin levels in circulation, enhancing glucose utilization, lipid oxidation, and energy expenditure (82, 184–186). Both human and animal studies demonstrate that intense exercise training potentiates the increase in AdipoR1 in skeletal muscle (82, 187). Furthermore, in rodent models of Duchenne muscular dystrophy, moderate exercise plays a significant role in improving muscle performance and increasing serum adiponectin. Moreover, serum adiponectin could be used as a biomarker for the benefits of exercise in Duchenne muscular dystrophy (188). Collectively, these studies support the idea that consistent weight loss and lifestyle intervention, such as exercise, contribute to elevating adiponectin levels.
Thiazolidinediones (TZD), an antidiabetic drug, is also a great regulator of adiponectin expression. The major mechanism of TZD- induced adiponectin expression is through activation of proliferator-activated receptor gamma (PPARγ). In human and animal studies of subjects treated with TZD presented increased adiponectin levels (189–191), concurrent with improvement in insulin sensitivity (192). However, the major challenge in relying on TZD as an agent to increase adiponectin levels is its adverse effects, including but not limited to, heart failure and cardiac ischemia (193–195). Moreover, incretin hormones, such as GLP-1 analogs, which are used to increase incretin availability, also upregulate adiponectin expression both in vitro (196) and in clinical trials (197). Evidence from human studies demonstrates that by targeting the renin-angiotensin-aldosterone system through angiotensin-converting enzyme inhibitor (ACEi) and angiotensin blockers (ARBs) significantly increased adiponectin levels and enhanced adipogenesis via PPARγ activation (198). Altogether, there are multiple pathways that regulate adiponectin expression in circulation via stimulation of gene expression of adiponectin or adipogenesis and insulin sensitivity-enhancing mechanisms.
In addition to its effects on adiponectin expression and adipogenesis, PPARγ pathway also plays a critical role in regulating adiponectin multimerization and secretion. TZD treatment upregulated expression levels of ER chaperones Ero1-Lα and DsbA-L and, subsequently, promoted HMW adiponectin formation and release in adipocytes (125, 138). Consistent with this, pioglitazone intervention led to increased levels of all adiponectin multimers, with the largest fold increases in nonamer (9mer) and dodecamer (12mer) levels despite a disproportionate increase in the amount of octadecamer (18mer), in diabetic patients (199). On the other hand, the endoplasmic reticulum (ER) stress appears to play a causative role in obesity-induced adiponectin downregulation and -impaired adiponectin multimerization (135, 136). In support of this, weight reduction by dietary intervention or inhibiting ER stress using pharmaceutical approach resulted in a relative increase of HMW adiponectin in the circulation of humans and rodents, respectively (136, 200). In addition, the inter-trimer disulfide bond formation is essential for adiponectin multimerization and favors an oxidizing redox environment with sufficient amounts of redox couples for disulfide rearrangement (201). Therefore, redox environment in adipocytes is a potential target for future treatments against Type 2 diabetes (201). This is supported by the evidence that mitochondria dysfunction-induced ROS overproduction is associated with decreased levels of the HMW adiponectin in adipose tissue of caveolin-1 knockout mice (102). However, whether the redox environment is able to promote adiponectin multimerization and secretion in vivo necessitates future clarification.
Conclusions
Here, we propose that the transendothelial movement of adiponectin may be highly regulated by paracellular transport. However, very little is known about studies examining the paracellular movement of adiponectin in diabetes target tissues, such as muscle and liver. Adiponectin plays an important role in vasculature. It is well established that it acts as a vasodilator inducing eNOS activation and endothelial NO production and protects the endothelium from oxidative stress. Furthermore, it mediates the activation of anti-inflammatory and proinflammatory markers. A few studies investigating the role of adiponectin in the paracellular movement concluded that hyperglycemia significantly regulated tight junctions, which contributed to increase adiponectin flux in STZD diabetes-induced mice. Alternatively, glucocorticoid-mediated tightening of endothelial barrier reduced the flux of adiponectin across the endothelial monolayers. Further investigations from the therapeutic perspective are needed, as this may represent a possible mechanism of adiponectin physiological actions.
In summary, even though much is known about general vascular functions of adiponectin, most evidence is generated from genetically modified rodent models, and not from humans. Notably, human vascular physiology and structure are quite different from animals. Therefore, further studies are needed, including larger animals, in order to provide a deeper understanding and to confirm vascular functional properties of adiponectin.
Acknowledgments
We thank Samantha Chow for reading and editing the manuscript.
Related work in the corresponding authors lab is funded by National Science and Engineering Research Council of Canada, the Canadian Institutes of Health Research and Heart & Stroke Foundation of Canada. Related work is also funded by National Institute of Diabetes and Digestive and Kidney Diseases (R01 Award DK-110439 to M.L.).
No conflicts of interest, financial or otherwise, are declared by the authors.
S.C.d.S.R. prepared figures; S.C.d.S.R., M.L., and G.S. drafted manuscript; M.L. and G.S. edited and revised manuscript; S.C.d.S.R., M.L., and G.S. approved final version of manuscript.
References
- 1.Wolf G. Leptin: the weight-reducing plasma protein encoded by the obese gene. Nutr Rev 54: 91–93, 2009. doi: 10.1111/j.1753-4887.1996.tb03878.x. [DOI] [PubMed] [Google Scholar]
- 2.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556, 2004. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 3.Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis 292: 1–9, 2020. doi: 10.1016/j.atherosclerosis.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703, 1996. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221: 286–289, 1996. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 6.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 120: 803–812, 1996. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 7.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 8.Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol 8: 1031–1063, 2018. doi: 10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Vu V, Sweeney G. Examining the potential of developing and implementing use of adiponectin-targeted therapeutics for metabolic and cardiovascular diseases. Front Endocrinol 10: 842, 2019. doi: 10.3389/fendo.2019.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otvos L. Potential adiponectin receptor response modifier therapeutics. Front Endocrinol 10, 539, 2019. doi: 10.3389/fendo.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Lu G, Wong WPS, Vliegenthart JFG, Gerwig GJ, Lam KSL, Cooper GJS, Xu A. Proteomic and functional characterization of endogenous adiponectin purified from fetal bovine serum Proteomics 4: 3933–3942, 2004. doi: 10.1002/pmic.200400826. [DOI] [PubMed] [Google Scholar]
- 13.Choi HM, Doss HM, Kim KS. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int J Mol Sci 21, 2020. doi: 10.3390/ijms21041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djoussé L, Wilk JB, Hanson NQ, Glynn RJ, Tsai MY, Gaziano JM. Association between adiponectin and heart failure risk in the physicians’ health study. Obesity 21: 831–834, 2013. doi: 10.1002/oby.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasbarrino K, Gorgui J, Nauche B, Côté R, Daskalopoulou SS. Circulating adiponectin and carotid intima-media thickness: a systematic review and meta-analysis. Metabolism 65: 968–986, 2016. doi: 10.1016/j.metabol.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Luo M, Oza-Frank R, Narayan KMV, Gokulakrishnan K, Mohan V. Serum total adiponectin is associated with impaired glucose tolerance in Asian Indian females but not in males. J Diabetes Sci Technol 4: 645–651, 2010. doi: 10.1177/193229681000400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 1271: 37–43, 2012. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol 121: 326–330, 2008. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-L, Tao J, Zhao P-J, Tang W, Xu J-P, Zhang K-Q, Zou C-G. Adiponectin receptor PAQR-2 signaling senses low temperature to promote C. elegans longevity by regulating autophagy. Nat Commun 10: 2602, 2019. doi: 10.1038/s41467-019-10475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada-Iwabu M, Yamauchi M, Kadowaki T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech Dis 1: 1–6, 2015. doi: 10.1038/npjamd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab 17: 185–196, 2013. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH-J, Paul A, Chan L. Increased β-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277: 34658–34661, 2002. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13: 332–339, 2007. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 25.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 88: 389–419, 2008. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res 67: 705–713, 2005. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10: 1384–1389, 2004. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botta A, Liu Y, Wannaiampikul S, Tungtrongchitr R, Dadson K, Park T-S, Sweeney G. An adiponectin-S1P axis protects against lipid induced insulin resistance and cardiomyocyte cell death via reduction of oxidative stress. Nutr Metab 16, 2019. doi: 10.1186/s12986-019-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 31.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6: 600–606, 2010. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 32.Jahng JWS, Turdi S, Kovacevic V, Dadson K, Li R-K, Sweeney G. Pressure overload-induced cardiac dysfunction in aged male adiponectin knockout mice is associated with autophagy deficiency. Endocrinology 156: 2667–2677, 2015. doi: 10.1210/en.2015-1162. [DOI] [PubMed] [Google Scholar]
- 33.Krause MP, Milne KJ, Hawke TJ. Adiponectin—consideration for its role in skeletal muscle health. Int J Mol Sci 20: 1528, 2019. doi: 10.3390/ijms20071528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol 295: C203–C212, 2008. doi: 10.1152/ajpcell.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Turdi S, Park T, Morris NJ, Deshaies Y, Xu A, Sweeney G. Adiponectin corrects high-fat diet–induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes 62: 743–752, 2013. doi: 10.2337/db12-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464: 1313–1319, 2010. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 64: 36–48, 2015. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Sen S, Wannaiampikul S, Palanivel R, Hoo RLC, Isserlin R, Bader GD, Tungtrongchitr R, Deshaies Y, Xu A, Sweeney G. Metabolomic profiling in liver of adiponectin-knockout mice uncovers lysophospholipid metabolism as an important target of adiponectin action. Biochem J 469: 71–82, 2015. doi: 10.1042/BJ20141455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teoh H, Quan A, Bang KWA, Wang G, Lovren F, Vu V, Haitsma JJ, Szmitko PE, Al-Omran M, Wang C-H, Gupta M, Peterson MD, Zhang H, Chan L, Freedman J, Sweeney G, Verma S. Adiponectin deficiency promotes endothelial activation and profoundly exacerbates sepsis-related mortality. Am J Physiol Endocrinol Metab 295: E658–E664, 2008. doi: 10.1152/ajpendo.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab 28: 15–23, 2014. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Yoon N, Dang TQ, Chasiotis H, Kelly SP, Sweeney G. Altered transendothelial transport of hormones as a contributor to diabetes. Diabetes Metab J 38: 92–99, 2014. doi: 10.4093/dmj.2014.38.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang X, Palanivel R, Zhou X, Liu Y, Xu A, Wang Y, Sweeney G. Hyperglycemia- and hyperinsulinemia-induced alteration of adiponectin receptor expression and adiponectin effects in L6 myoblasts. J Mol Endocrinol 35: 465–476, 2005. doi: 10.4093/dmj.2014.38.2.92. [DOI] [PubMed] [Google Scholar]
- 44.Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S, Berria R, Belfort R, DeFronzo RA, Mandarino LJ, Ravussin E. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia 47: 816–820, 2004. doi: 10.1007/s00125-004-1359-x. [DOI] [PubMed] [Google Scholar]
- 45.Bjursell M, Ahnmark A, Bohlooly-Y M, William-Olsson L, Rhedin M, Peng X-R, Ploj K, Gerdin A-K, Arnerup G, Elmgren A, Berg A-L, Oscarsson J, Lindén D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56: 583–593, 2007. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 46.Marette A, Liu Y, Sweeney G. Skeletal muscle glucose metabolism and inflammation in the development of the metabolic syndrome. Rev Endocr Metab Disord 15: 299–305, 2014. doi: 10.1007/s11154-014-9296-6. [DOI] [PubMed] [Google Scholar]
- 47.Cheng KKY, Lam KSL, Wang B, Xu A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract Res Clin Endocrinol Metab 28: 3–13, 2014. doi: 10.1016/j.beem.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim R-Y, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 49.Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol 8: 101–109, 2016. doi: 10.1093/jmcb/mjw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 98: 2005–2010, 2001. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 52.Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJG, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-κB/PTEN suppression. J Biol Chem 283: 24889–24898, 2008. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 105: 804–809, 2002. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 54.Luo Y, Liu M. Adiponectin: a versatile player of innate immunity. J Mol Cell Biol 8: 120–128, 2016. doi: 10.1093/jmcb/mjw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323: 630–635, 2004. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 56.Deng G, Long Y, Yu Y-R, Li M-R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK–eNOS pathway. Int J Obes 34: 165–171, 2010. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 57.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47: 1108–1116, 2006. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 58.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab 13: 401–412, 2011. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem 287: 36896–36904, 2012. doi: 10.1074/jbc.M112.409516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esmaili S, Xu A, George J. The multifaceted and controversial immunometabolic actions of adiponectin. Trends Endocrinol Metab 25: 444–451, 2014. doi: 10.1016/j.tem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109: 2046–2049, 2004. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 62.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 63.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285: 6153–6160, 2010. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park P-H, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem 282: 21695–21703, 2007. doi: 10.1074/jbc.M701419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang C-H, Chiu Y-C, Tan T-W, Yang R-S, Fu W-M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-κB pathway. J Immunol 179: 5483–5492, 2007. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- 66.Tsao T-S, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem 277: 29359–29362, 2002. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 67.Xu A, Wang Y, Keshaw H, Xu LY, Lam KSL, Cooper GJS. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003. doi: 10.1172/JCI200317797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 288: R1220–R1225, 2005. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 69.Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KSL, Xu A. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab 22: 279–290, 2015. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316: 924–929, 2004. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 71.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96: 1723–1732, 2000. doi: 10.1182/blood.V96.5.1723. [DOI] [PubMed] [Google Scholar]
- 72.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Adiponectin induces TNF-α and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun 335: 1254–1263, 2005. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Luo Y, Luo L, Wu D, Ding X, Zheng H, Wu H, Liu B, Yang X, Silva F, Wang C, Zhang X, Zheng X, Chen J, Brigman J, Mandell M, Zhou Z, Liu F, Yang XO, Liu M. Adiponectin restrains ILC2 activation by AMPK-mediated feedback inhibition of IL-33 signaling. J Exp Med 218: e20191054, 2021. doi: 10.1084/jem.20191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Peripheral factors in the metabolic syndrome: the pivotal role of adiponectin. Ann N Y Acad Sci 1083: 185–195, 2006. doi: 10.1196/annals.1367.013. [DOI] [PubMed] [Google Scholar]
- 75.Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol 2: 62, 2011. doi: 10.3389/fendo.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev 33: 547–594, 2012. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hui X, Lam KSL, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol 165: 574–590, 2012. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopaschuk GD. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes 32: S29–S35, 2008. doi: 10.1038/ijo.2008.120. [DOI] [PubMed] [Google Scholar]
- 79.Pandey GK, Vadivel S, Raghavan S, Mohan V, Balasubramanyam M, Gokulakrishnan K. High molecular weight adiponectin reduces glucolipotoxicity-induced inflammation and improves lipid metabolism and insulin sensitivity via APPL1-AMPK-GLUT4 regulation in 3T3-L1 adipocytes. Atherosclerosis 288: 67–75, 2019. doi: 10.1016/j.atherosclerosis.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic β cells and adipocytes. Best Pract Res Clin Endocrinol Metab 28: 43–58, 2014. doi: 10.1016/j.beem.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab 95: 911–919, 2010. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- 82.Vu V, Riddell MC, Sweeney G. Circulating adiponectin and adiponectin receptor expression in skeletal muscle: effects of exercise. Diabetes Metab Res Rev 23: 600–611, 2007. doi: 10.1002/dmrr.778. [DOI] [PubMed] [Google Scholar]
- 83.Heiston EM, Eichner NZ, Gilbertson NM, Malin SK. Exercise improves adiposopathy, insulin sensitivity and metabolic syndrome severity independent of exercise intensity. Exp Physiol 105: 632–640, 2020. doi: 10.1113/EP088158. [DOI] [PubMed] [Google Scholar]
- 84.Thyagarajan B, Jacobs DR, Smith LJ, Kalhan R, Gross MD, Sood A. Serum adiponectin is positively associated with lung function in young adults, independent of obesity: The CARDIA study. Respir Res 11: 176, 2010. doi: 10.1186/1465-9921-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perrotta F, Nigro E, Mollica M, Costigliola A, D’Agnano VDA, Bianco A., Guerra G. Pulmonary hypertension and obesity: focus on adiponectin. Int J Mol Sci 20: 912, 2019. doi: 10.3390/ijms20040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Carvalho MHC, Colaço AL, Fortes ZB. [Cytokines, endothelial dysfunction, and insulin resistance]. Arq Bras Endocrinol 50: 304–312, 2006. doi: 10.1590/S0004-27302006000200016. [DOI] [PubMed] [Google Scholar]
- 87.Bhat IA, Kabeer SW, Reza MI, Mir RH, Dar MO. AdipoRon: a novel insulin sensitizer in various complications and the underlying mechanisms: a review. Curr Mol Pharmacol 13: 94–107, 2020. doi: 10.2174/1874467212666191022102800. [DOI] [PubMed] [Google Scholar]
- 88.Delaigle AM, Senou M, Guiot Y, Many M-C, Brichard SM. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: In vivo and in vitro studies. Diabetologia 49: 1311–1323, 2006. doi: 10.1007/s00125-006-0210-y. [DOI] [PubMed] [Google Scholar]
- 89.Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, Ricks E, Yang Q. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor γ. J Mol Cell Cardiol 43: 73–84, 2007. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo Z, Xia Z, Yuen VG, McNeill JH. Cardiac expression of adiponectin and its receptors in streptozotocin-induced diabetic rats. Metabolism 56: 1363–1371, 2007. doi: 10.1016/j.metabol.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Lan H, Rabaglia ME, Stoehr JP, Nadler ST, Schueler KL, Zou F, Yandell BS, Attie AD. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 52: 688–700, 2003. doi: 10.2337/diabetes.52.3.688. [DOI] [PubMed] [Google Scholar]
- 92.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278: 9073–9085, 2003. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 93.Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M. The role of adiponectin in human vascular physiology. Int J Cardiol 155: 188–193, 2012. doi: 10.1016/j.ijcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 94.Barnea M, Madar Z, Froy O. High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue. Obesity (Silver Spring) 18: 230–238, 2010. doi: 10.1038/oby.2009.276. [DOI] [PubMed] [Google Scholar]
- 95.Kennaway DJ, Owens JA, Voultsios A, Wight N. Adipokines and adipocyte function in Clock mutant mice that retain melatonin rhythmicity. Obesity (Silver Spring) 20: 295–305, 2012. doi: 10.1038/oby.2011.276. [DOI] [PubMed] [Google Scholar]
- 96.Kennaway DJ, Varcoe TJ, Voultsios A, Boden MJ. Global loss of Bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS One 8: e65255, 2013. doi: 10.1371/journal.pone.0065255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 425: 41–52, 2010. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- 98.Moseti D, Regassa A, Kim W-K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci 17, 2016. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR γ2, but not C/EBP alpha-evidence for differential regulation of the αP2 and adiponectin genes. Biochem Biophys Res Commun 308: 933–939, 2003. doi: 10.1016/S0006-291X(03)01518-3. [DOI] [PubMed] [Google Scholar]
- 100.Sharma AM, Staels B. Peroxisome proliferator-activated receptor γ and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 92: 386–395, 2007. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 101.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 51: 2968–2974, 2002. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 102.Asterholm IW, Mundy DI, Weng J, Anderson RGW, Scherer PE. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab 15: 171–185, 2012. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrillo JLM, Campo JOMD, Coronado OG, Gutiérrez PTV, Cordero JFC, Juárez JV. Adipose tissue and inflammation. Curr Opin Pharmacol 37: 35–40, 2017. doi: 10.1016/j.coph.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 104.Clarke KJ, Zhong Q, Schwartz DD, Coleman ES, Kemppainen RJ, Judd RL. Regulation of adiponectin secretion by endothelin-1. Biochem Biophys Res Commun 312: 945–949, 2003. doi: 10.1016/j.bbrc.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 105.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 8: 639–649, 2012. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moyce BL, Dolinsky VW. Maternal β-cell adaptations in pregnancy and placental signalling: implications for gestational diabetes. Int J Mol Sci 19: 3467, 2018. doi: 10.3390/ijms19113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plagemann A. Maternal diabetes and perinatal programming. Early Hum Dev 87: 743–747, 2011. doi: 10.1016/j.earlhumdev.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 108.Bouchard L, Hivert M-F, Guay S-P, St-Pierre J, Perron P, Brisson D. Placental adiponectin gene DNA methylation levels are associated with mothers’ blood glucose concentration. Diabetes 61: 1272–1280, 2012. doi: 10.2337/db11-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ott R, Stupin JH, Melchior K, Schellong K, Ziska T, Dudenhausen JW, Henrich W, Rancourt RC, Plagemann A. Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin Epigenetics 10: 131, 2018. doi: 10.1186/s13148-018-0567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep 5: 278–281, 2005. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 111.Badran M, Yassin BA, Lin DTS, Kobor MS, Ayas N, Laher I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J Physiol 597: 5349–5364, 2019. doi: 10.1113/JP277936. [DOI] [PubMed] [Google Scholar]
- 112.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149: 2270–2282, 2008. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6: 55–68, 2007. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Liu J, Guo M, Zhang D, Cheng S-Y, Liu M, Ding J, Scherer PE, Liu F, Lu X-Y. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci U S A 109: 12248–12253, 2012. doi: 10.1073/pnas.1202835109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med 10: 524–529, 2004. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 117.Tomas E, Tsao T-S, Saha AK, Murrey HE, Zhang CC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A 99: 16309–16313, 2002. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsao T-S, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem 278: 50810–50817, 2003. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 119.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51: 2734–2741, 2002. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 120.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol 153: 409–417, 2005. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 121.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 146: 790–796, 2005. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 122.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes 56: 2174–2177, 2007. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 123.Koenen TB, van Tits LJH, Holewijn S, Lemmers HLM, den Heijer M, Stalenhoef AFH, de Graaf J. Adiponectin multimer distribution in patients with familial combined hyperlipidemia. Biochem Biophys Res Commun 376: 164–168, 2008. doi: 10.1016/j.bbrc.2008.08.111. [DOI] [PubMed] [Google Scholar]
- 124.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 125.Liu M, Zhou L, Xu A, Lam KSL, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A 105: 18302–18307, 2008. .doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang W, Ye DD. The potential of adipokines as biomarkers and therapeutic agents for vascular complications in type 2 diabetes mellitus. Cytokine Growth Factor Rev 48: 32–39, 2019. doi: 10.1016/j.cytogfr.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 127.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. molecular structure and multimer formation of adiponectin. J Biol Chem 278: 40352–40363, 2003. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 128.Frizzell N, Rajesh M, Jepson MJ, Nagai R, Carson JA, Thorpe SR, Baynes JW. Succination of thiol groups in adipose tissue proteins in diabetes: succination inhibits polymerization and secretion of adiponectin. J Biol Chem 284: 25772–25781, 2009. doi: 10.1074/jbc.M109.019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y, Lam KSL, Chan L, Chan KW, Lam JBB, Lam MC, Hoo RCL, Mak WWN, Cooper GJS, Xu A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 281: 16391–16400, 2006. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y, Lam KSL, Yau M, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 409: 623–633, 2008. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 131.Richards AA, Stephens T, Charlton HK, Jones A, Macdonald GA, Prins JB, Whitehead JP. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol 20: 1673–1687, 2006. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 132.Wang ZV, Schraw TD, Kim J-Y, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Bio 27: 3716–3731, 2007. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J 22: 5015–5022, 2003. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hampe L, Radjainia M, Xu C, Harris PWR, Bashiri G, Goldstone DC, Brimble MA, Wang Y, Mitra AK. Regulation and quality control of adiponectin assembly by endoplasmic reticulum chaperone ERp44. J Biol Chem 290: 18111–18123, 2015. doi: 10.1074/jbc.M115.663088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou L, Liu F. Autophagy: roles in obesity-induced ER stress and adiponectin downregulation in adipocytes. Autophagy 6: 1196–1197, 2010. doi: 10.4161/auto.6.8.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes 59: 2809–2816, 2010. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sha H, Yang L, Liu M, Xia S, Liu Y, Liu F, Kersten S, Qi L. Adipocyte spliced form of X-box-binding protein 1 promotes adiponectin multimerization and systemic glucose homeostasis. Diabetes 63: 867–879, 2014. doi: 10.2337/db13-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-Lα. Mol Cell Bio 27: 4698–4707, 2007. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab 28: 25–31, 2014. doi: 10.1016/j.beem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao T-S, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A 101: 10308–10313, 2004. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Denzel MS, Scimia M-C, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest 120: 4342–4352, 2010. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hebbard LW, Garlatti M, Young LJT, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res 68: 1407–1416, 2008. doi: 10.1158/0008-5472.CAN-07-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem 288: 24886–24897, 2013. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Triggle CR, Ding H, Marei I, Anderson TJ, Hollenberg MD. Why the endothelium? The endothelium as a target to reduce diabetes-associated vascular disease. Can J Physiol Pharmacol 98: 415–430, 2020. doi: 10.1139/cjpp-2019-0677. [DOI] [PubMed] [Google Scholar]
- 145.Bruewer M, Nusrat A. Regulation of paracellular transport across tight junctions by the actin cytoskeleton, Landes Bioscience, 2000. –2013. http://www.ncbi.nlm.nih.gov/books/NBK6487/ [12 Mar. 2020]. [Google Scholar]
- 146.Fung KYY, Fairn GD, Lee WL. Transcellular vesicular transport in epithelial and endothelial cells: challenges and opportunities. Traffic 19: 5–18, 2018. doi: 10.1111/tra.12533. [DOI] [PubMed] [Google Scholar]
- 147.Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflügers Arch 459: 977–994, 2010. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- 148.Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes 9: 434–449, 2017. 10.1111/1753-0407.12521 [DOI] [PubMed] [Google Scholar]
- 149.Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes 67: 1729–1741, 2018. doi: 10.2337/dbi17-0044. [DOI] [PubMed] [Google Scholar]
- 150.Yoon N, Dadson K, Dang T, Chu T, Noskovicova N, Hinz B, Raignault A, Thorin E, Kim S, Jeon JS, Jonkman J, McKee TD, Grant J, Peterson JD, Kelly SP, Sweeney G. Tracking adiponectin biodistribution via fluorescence molecular tomography indicates increased vascular permeability after streptozotocin-induced diabetes. Am J Physiol Endocrinol Metab 317: E760–E772, 2019. doi: 10.1152/ajpendo.00564.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ding Q, Wang Z, Chen Y. Endocytosis of adiponectin receptor 1 through a clathrin- and Rab5-dependent pathway. Cell Res 19: 317–327, 2009. doi: 10.1038/cr.2008.299. [DOI] [PubMed] [Google Scholar]
- 152.Zielińska KA, Van Moortel L, Opdenakker G, De Bosscher K, Van den Steen PE. Endothelial response to glucocorticoids in inflammatory diseases. Front Immunol 7: 592, 2016. doi: 10.3389/fimmu.2016.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dang TQ, Yoon N, Chasiotis H, Dunford EC, Feng Q, He P, Riddell MC, Kelly SP, Sweeney G. Transendothelial movement of adiponectin is restricted by glucocorticoids. J Endocrinol 234: 101–114, 2017. doi: 10.1530/JOE-16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu W, Cheng KKY, Vanhoutte PM, Lam KSL, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci 114: 361–374, 2008. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 155.Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab 89: 2563–2568, 2004. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 156.Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res 78: 376–384, 2008. 10.1093/cvr/cvn034. [DOI] [PubMed] [Google Scholar]
- 157.Triggle CR, Ding H. Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiol 219: 138–151, 2017. doi: 10.1111/apha.12644. [DOI] [PubMed] [Google Scholar]
- 158.Xiong X, Lu W, Qin X, Luo Q, Zhou W. Downregulation of the GLP-1/CREB/adiponectin pathway is partially responsible for diabetes-induced dysregulated vascular tone and VSMC dysfunction. Biomed Pharmacother 127: 110218, 2020. doi: 10.1016/j.biopha.2020.110218. [DOI] [PubMed] [Google Scholar]
- 159.Højbjerre L, Rosenzweig M, Dela F, Bruun JM, Stallknecht B. Acute exercise increases adipose tissue interstitial adiponectin concentration in healthy overweight and lean subjects. Eur J Endocrinol 157: 613–623, 2007. doi: 10.1530/EJE-07-0213. [DOI] [PubMed] [Google Scholar]
- 160.Nielsen NB, Højbjerre L, Sonne MP, Alibegovic AC, Vaag A, Dela F, Stallknecht B. Interstitial concentrations of adipokines in subcutaneous abdominal and femoral adipose tissue. Regul Pept 155: 39–45, 2009. doi: 10.1016/j.regpep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 161.Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, Motoyama K, Ikeda M, Wakiyama M, Terada T, Ohsawa N, Hato M, Ogasawara S, Hino T, Murata T, Iwata S, Hirata K, Kawano Y, Yamamoto M, Kimura-Someya T, Shirouzu M, Yamauchi T, Kadowaki T, Yokoyama S. Crystal structures of the human adiponectin receptors. Nature 520: 312–316, 2015. doi: 10.1038/nature14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo M-S, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17: 55–63, 2011. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Halberg N, Schraw TD, Wang ZV, Kim J-Y, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 58: 1961–1970, 2009. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Phillips SA, Kung J, Ciaraldi TP, Choe C, Christiansen L, Mudaliar S, Henry RR. Selective regulation of cellular and secreted multimeric adiponectin by antidiabetic therapies in humans. Am J Physiol Endocrinol Metab 297: E767–E773, 2009. doi: 10.1152/ajpendo.00378.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu A, Sweeney G. Emerging role of autophagy in mediating widespread actions of ADIPOQ/adiponectin. Autophagy 11: 723–724, 2015. doi: 10.1080/15548627.2015.1034418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ahlstrom P, Rai E, Chakma S, Cho HH, Rengasamy P, Sweeney G. Adiponectin improves insulin sensitivity via activation of autophagic flux. J Mol Endocrinol 59: 339–350, 2017. doi: 10.1530/JME-17-0096. [DOI] [PubMed] [Google Scholar]
- 167.Gu D, Wang Z, Dou X, Zhang X, Li S, Vu L, Yao T, Song Z. Inhibition of ERK1/2 pathway suppresses adiponectin secretion via accelerating protein degradation by ubiquitin-proteasome system: relevance to obesity-related adiponectin decline. Metabolism 62: 1137–1148, 2013. doi: 10.1016/j.metabol.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim S, Lee Y, Kim JW, Son Y-J, Ma MJ, Um J-H, Kim ND, Min SH, Kim DI, Kim BB. Discovery of a novel potent peptide agonist to adiponectin receptor 1. PLoS One 13, e0199256, 2018. doi: 10.1371/journal.pone.0199256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shi W, Guo Z, Ji Y, Feng J. The protective effect of recombinant globular adiponectin on testis by modulating autophagy, endoplasmic reticulum stress and oxidative stress in streptozotocin-induced diabetic mice. Eur J Pharmacol 879: 173132, 2020. doi: 10.1016/j.ejphar.2020.173132. [DOI] [PubMed] [Google Scholar]
- 170.Wang Y, Li Y, Qiao J, Li N, Qiao S. AMPK α1 mediates the protective effect of adiponectin against insulin resistance in INS-1 pancreatic β cells. Cell Biochem Funct 37: 625–632, 2019. doi: 10.1002/cbf.3440. [DOI] [PubMed] [Google Scholar]
- 171.Wang Y, Zhang J, Zhang L, Gao P, Wu X. Adiponectin attenuates high glucose-induced apoptosis through the AMPK/p38 MAPK signaling pathway in NRK-52E cells. PLOS ONE 12: e0178215, 2017. doi: 10.1371/journal.pone.0178215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tullin S, Sams A, Brandt J, Dahl K, Gong W, Jeppesen CB, Krogh TN, Olsen GS, Liu Y, Pedersen AA, Petersen JM, Rolin B, Wahlund P-O, Kalthoff C. Recombinant adiponectin does not lower plasma glucose in animal models of type 2 diabetes. PLoS One 7: e44270, 2012. doi: 10.1371/journal.pone.0044270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503: 493–499, 2013. doi: 10.1371/journal.pone.0044270. [DOI] [PubMed] [Google Scholar]
- 174.Otvos L, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, Cassone M, Wade J, Surmacz E. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. Bio Mol Cell Biotechnol 11: 90, 2011. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Otvos LJ, Knappe D, Hoffmann R, Kovalszky I, Olah J, Hewitson TD, Stawikowska R, Stawikowski M, Cudic P, Lin F, Wade JD, Surmacz E, Lovas S. Development of second generation peptides modulating cellular adiponectin receptor responses. Front Chem 2, 93: 2014. doi: 10.3389/fchem.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma L, Zhang Z, Xue X, Wan Y, Ye B, Lin K. A potent peptide as adiponectin receptor 1 agonist to against fibrosis. J Enzyme Inhib Med Chem 32: 624–631, 2017. doi: 10.1080/14756366.2017.1284067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nan MH, Park J-S, Myung C-S. Construction of adiponectin-encoding plasmid DNA and gene therapy of non-obese type 2 diabetes mellitus. J Drug Target 18: 67–77, 2010. doi: 10.3109/10611860903225719. [DOI] [PubMed] [Google Scholar]
- 178.Kandasamy AD, Sung MM, Boisvenue JJ, Barr AJ, Dyck JRB. Adiponectin gene therapy ameliorates high-fat, high-sucrose diet-induced metabolic perturbations in mice. Nutr Diabetes 2, e45, 2012. doi: 10.1038/nutd.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park JH, Lee M, Kim SW. Non-viral adiponectin gene therapy into obese type 2 diabetic mice ameliorates insulin resistance. J Controlled Release 114: 118–125, 2006. doi: 10.1016/j.jconrel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 180.Banerjee A, Sharma D, Trivedi R, Singh J. Treatment of insulin resistance in obesity-associated type 2 diabetes mellitus through adiponectin gene therapy. Int J Pharm 583: 119357, 2020. doi: 10.1016/j.ijpharm.2020.119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10: 625–632, 2004. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 182.Salameh A, Daquinag AC, Staquicini DI, An Z, Hajjar KA, Pasqualini R, Arap W, Kolonin MG. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 1: e86351, 2016. doi: 10.1172/jci.insight.86351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thovhogi N, Sibuyi NRS, Onani MO, Meyer M, Madiehe AM. Peptide-functionalized quantum dots for potential applications in the imaging and treatment of obesity. Int J Nanomedicine 13: 2551–2559, 2018. doi: 10.2147/IJN.S158687. 10.2217/nnm-2018-0051, 10.2147/IJN.S158687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Corbi G, Polito R, Monaco ML, Cacciatore F, Scioli M, Ferrara N, Daniele A, Nigro E. Adiponectin expression and genotypes in Italian people with severe obesity undergone a hypocaloric diet and physical exercise program. Nutrients 11: 2195, 2019. doi: 10.3390/nu11092195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mohorko N, Černelič-Bizjak M, Poklar-Vatovec T, Grom G, Kenig S, Petelin A, Jenko-Pražnikar Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res 62: 64–77, 2019. 10.1016/j.nutres.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 186.Wu W-C, Inui A, Chen C-Y. Weight loss induced by whole grain-rich diet is through a gut microbiota-independent mechanism. World J Diabetes 11: 26–32, 2020. doi: 10.4239/wjd.v11.i2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Blüher M, Bullen JW, Lee JH, Kralisch S, Fasshauer M, Klöting N, Niebauer J, Schön MR, Williams CJ, Mantzoros CS. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab 91: 2310–2316, 2006. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 188.Zelikovich AS, Quattrocelli M, Salamone IM, Kuntz NL, McNally EM. Moderate exercise improves function and increases adiponectin in the mdx mouse model of muscular dystrophy. Sci Rep 9: 5770, 2019. doi: 10.1038/s41598-019-42203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Espinoza SE, Wang C, Tripathy D, Clement SC, Schwenke DC, Banerji MA, Bray GA, Buchanan TA, Henry RR, Kitabchi AE, Mudaliar S, Stentz FB, Reaven PD, DeFronzo RA, Musi N. Pioglitazone is equally effective for diabetes prevention in older versus younger adults with impaired glucose tolerance. Age 38: 485–493, 2016. doi: 10.1007/s11357-016-9946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Esteghamati A, Azizi R, Ebadi M, Noshad S, Mousavizadeh M, Afarideh M, Nakhjavani M. The comparative effect of pioglitazone and metformin on serum osteoprotegerin, adiponectin and intercellular adhesion molecule concentrations in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Exp Clin Endocrinol Diabetes 123: 289–295, 2015. doi: 10.1055/s-0034-1396864. [DOI] [PubMed] [Google Scholar]
- 191.Tripathy D, Clement SC, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Gastaldelli A, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD, DeFronzo RA. Baseline adiponectin levels do not influence the response to pioglitazone in ACT NOW. Diabetes Care 37: 1706–1711, 2014. doi: 10.2337/dc13-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qian X, Wang H, Yang G, Gao Z, Luo Y, Dong A, Zhang F, Xu M, Liu S, Yang X, Chen Y, Li G. Pioglitazone improved insulin sensitivity and first phase insulin secretion among obese and lean people with diabetes: a multicenter clamp study. Diabetes Ther Res 9: 815–826, 2018. doi: 10.1007/s13300-018-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Riess ML, Elorbany R, Weihrauch D, Stowe DF, Camara AKS. PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts. Cells 9: 252, 2020. doi: 10.3390/cells9010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rizos CV, Elisaf MS, Mikhailidis DP, Liberopoulos EN. How safe is the use of thiazolidinediones in clinical practice? Expert Opin Drug Saf 8: 15–32, 2009. doi: 10.1517/14740330802597821. [DOI] [PubMed] [Google Scholar]
- 195.Xi Y, Zhang Y, Zhu S, Luo Y, Xu P, Huang Z. PPAR-mediated toxicology and applied pharmacology. Cells 9: 352, 2020. doi: 10.3390/cells9020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim Chung LT, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun 390: 613–618, 2009. doi: 10.1016/j.bbrc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 197.Sahebkar A, Ponzo V, Bo S. Effect of ipeptidyl peptidase-4 inhibitors on plasma adiponectin: a systematic review and meta-analysis of randomized controlled trials. Curr Med Chem 23: 1356–1369, 2016. doi: 10.2174/0929867323666160405111354. [DOI] [PubMed] [Google Scholar]
- 198.Tian F, Luo R, Zhao Z, Wu Y, Ban D. Blockade of the RAS increases plasma adiponectin in subjects with metabolic syndrome and enhances differentiation and adiponectin expression of human preadipocytes. Exp Clin Endocrinol Diabetes 118: 258–265, 2009. doi: 10.1055/s-0029-1237706. [DOI] [PubMed] [Google Scholar]
- 199.Mashalidis EH, Briggs DB, Zhou M, Vergara AM, Chhun JJ, Ellsworth RK, Giron RM, Rood J, Bray GA, Smith SR, Wysocki VH, Tsao T-S. High-resolution dentification of human adiponectin oligomers and regulation by pioglitazone in type 2 diabetic patients. Anal Biochem 437: 150–160, 2013. doi: 10.1016/j.ab.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 200.Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, Möhlig M, Pfeiffer AFH, Spranger J. Changes of Adiponectin Oligomer Composition by Moderate Weight Reduction. Diabetes 54: 2712–2719, 2005. doi: 10.2337/diabetes.54.9.2712 [DOI] [PubMed] [Google Scholar]
- 201.Briggs DB, Giron RM, Malinowski PR, Nuñez M, Tsao T-S. Role of redox environment on the oligomerization of higher molecular weight adiponectin. BMC Biochem 12: 24, 2011. doi: 10.1186/1471-2091-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


