Abstract
Type 1 diabetes is an insulin-dependent, autoimmune disease where the pancreatic β cells are destroyed resulting in hyperglycemia. This multifactorial disease involves multiple environmental and genetic factors, and has no clear etiology. Accumulating evidence suggests that early signaling defects within the β cells may promote a change in the local immune milieu leading to autoimmunity. Therefore, many studies have been focused on intrinsic β-cell mechanisms that aid in the restoration of cellular homeostasis under environmental conditions that cause dysfunction. One of these intrinsic mechanisms to promote homeostasis is autophagy, defects which are clearly linked with β-cell dysfunction in the context of type 2 diabetes. Recent studies have now also pointed towards β-cell autophagy defects in the context of type 1 diabetes. In this perspectives review, we will discuss the evidence supporting a role for β-cell autophagy in the pathogenesis of type 1 diabetes, including a potential role for unconventional secretion of autophagosomes/lysosomes in the changing dialogue between the β cell and immune cells.
Keywords: autophagy, β cell, lysosome, type 1 diabetes
INTRODUCTION
Type 1 diabetes (T1D) is a chronic disease characterized by immune-mediated destruction of the insulin producing pancreatic β cells. Individuals with a genetic predisposition are at higher risk of disease development. However, the progression to disease cannot be attributed solely to genetic predisposition and widespread evidence suggests the involvement of environmental factors such as viral infection (1). This multifactorial effect triggers an autoimmune attack of the β cells and hyperglycemia due to loss of insulin from the β cells. However, despite many advances in our understanding of disease pathogenesis, the trigger leading to T1D development is still unknown.
Under conditions that are known to disrupt β-cell homeostasis, such as hyperglycemia or the accumulation of reactive oxygen species (ROS), the β cells are under increased demand to produce insulin and/or antioxidant proteins which can lead to increased protein load, causing endoplasmic reticulum (ER) stress (2, 3). In addition, accumulating ROS can cause damage to both proteins and organelles, making it increasingly difficult for the cell to ramp up these adaptive stress response mechanisms. Endogenous processes that contribute to mitigation of these cellular stressors to promote the restoration of homeostasis are thus critical for cell survival.
The importance of autophagy in maintaining β-cell homeostasis and promoting cell survival in this context has been reviewed in depth (4–6). However, nearly all studies of β-cell autophagy in the literature have been focused on dysfunctional autophagy in the context of type 2 diabetes (T2D). Very few studies address autophagy or selective forms of autophagy in the setting of T1D. Autophagy is an evolutionarily conserved process that functions in all cells to facilitate the degradation and recycling of cellular materials under a variety of conditions, including in response to ER stress. There are several known mechanisms by which cargo can be taken up by the lysosome and degraded by lysosomal hydrolases (Fig. 1), including macroautophagy, where cargo encapsulated in double membraned autophagosomes is transferred to the lysosome; crinophagy, where secretory granules directly fuse with lysosomes; microautophagy, where cytoplasmic materials are taken up directly by the lysosome; and chaperone mediated autophagy, where substrates containing the motif “KFERQ” are translocated across the lysosomal membrane with the help of chaperones (7). All of the above -phagic degradation processes appear to be critical for β-cell survival and function (4). There is also a clear role for autophagy in the cellular response to viral infection (8) and the proinflammatory cytokines that may be released during viral infection (9). It is therefore reasonable to propose that autophagy may play a critical role in the restoration of β-cell homeostasis under these conditions.
Figure 1.

Types of autophagy. A: macroautophagy: excess or damaged cellular components are encompassed by a double-membraned autophagosome which fuses with the acidic lysosome to form an autolysosome. The autophagosome contents are then degraded and recycled. B: crinophagy: secretory granules fuse directly with the lysosome and the granule contents are degraded and recycled. C: chaperone-mediated autophagy (CMA): proteins containing the “KFERQ” motif are brought to the lysosome via chaperones and are degraded with the assistance of the 70 KDa heat shock cognate protein, Hsc70, and LAMP-2A. D: microautophagy: a nonselective mechanism of autophagy where cytosolic components are directly taken up by the lysosome for degradation. Figure created with Biorender.com.
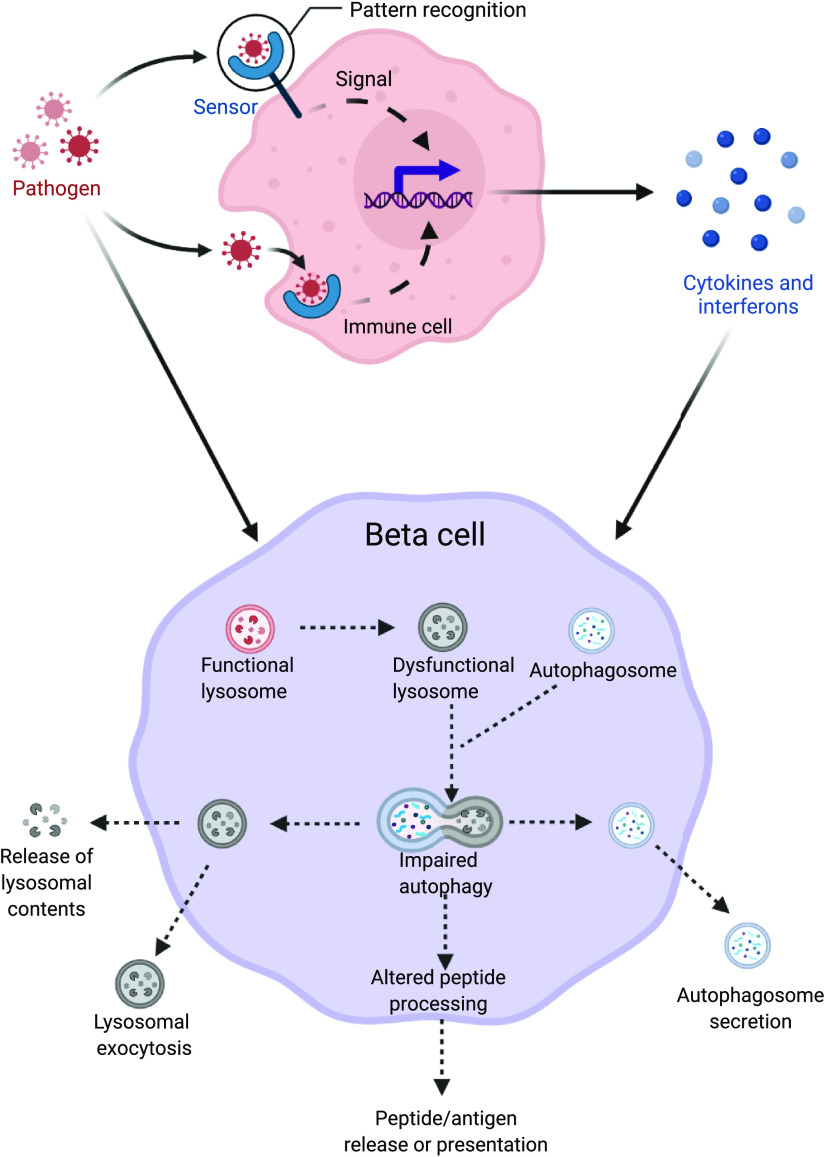
Indeed, we have recently demonstrated a loss of β-cell autophagy in the context of human T1D. Perhaps more importantly, we observed evidence of dysfunctional autophagy components in the β cells of autoantibody positive organ donors, prompting the suggestion that defects in this critical adaptive response mechanism may play a causative role in early β-cell dysfunction during T1D pathogenesis (10). In this perspectives review, we will explore the mechanistic evidence supporting the hypothesis that islet autophagy defects contribute to β-cell dysfunction and autoimmunity in T1D pathogenesis (Fig. 2).
Figure 2.
Schematic diagram showing potential mechanisms of lysosome dysfunction in the β cells induced by early life viral infections or proinflammatory cytokines. This defect in lysosomes could lead to altered peptide processing and presentation or exocytosis of autophagosomes/lysosomes from the β cells, thus potentially eliciting an immune attack in the pathogenesis of type 1 diabetes. Figure created with Biorender.com.
T1D GENETIC SUSCEPTIBILITY LOCI WITH ROLES IN AUTOPHAGY
T1D is a multifactorial disease of polygenic inheritance. A number of T1D susceptibility genes have been identified (11), and several are known to play critical roles in autophagic degradation pathways. For example, the E3 ligase, CLEC16A, plays a crucial role in the selective degradation of mitochondria by autophagy (a process known as mitophagy) and also in insulin secretion in rodent β cells (12). Soleimanpour et al. (13) found that expression of Clec16a was controlled by the critical β-cell transcription factor, PDX1, via its interacting partner NRDP1. In this study, knockdown of PDX1 impaired the fusion of mitochondria-loaded autophagosomes to lysosomes, leading to dysregulation of mitochondrial turnover. Sidarala et al. (14) subsequently found that adenoviral overexpression of CLEC16A could protect human β cells against cytokine-induced apoptosis, thus supporting the critical role of CLEC16A in the protective process of mitophagy. Another T1D susceptibility locus, cathepsin H (CTSH), is a lysosomal cysteine protease involved in the terminal degradation stage of autophagy (15–17). The CTSH susceptibility allele in intron 1 (rs3825932) is associated with accelerated disease progression in newly diagnosed diabetes and reduced β-cell function in healthy humans (17). Finally, two other T1D susceptibility loci, PTPN2 and PTPN22 have roles in β-cell destruction and rapid disease progression, respectively (18). One of the lesser known functions of both of these tyrosine phosphatases is their involvement in the formation of autophagosomes in the context of bacterial infection (19) and NLRP3 inflammasome activation (20). Collectively, these data suggest a variety of potential routes leading to autophagy dysfunction due to inheritance of T1D susceptibility alleles.
EARLY LIFE VIRAL INFECTIONS AND THE ROLE FOR AN AUTOPHAGIC RESPONSE
Autophagy plays a key role in the removal of invading pathogens, including infections by enterovirus (21). β Cells express the coxsackie and adenovirus receptor (CAR), potentially facilitating enteroviral infection after exposure (22). Increased expression of CAR has been observed in the pancreatic islets of organ donors with T1D, as well as autoantibody positive donors when compared with nondiabetic control subjects (23). Increased enteroviral RNA has also been observed in sera of human subjects with T1D (24). In addition, enterovirus-positive islets were not only found in donors with T1D (25–27), but were also detected in newly diagnosed living patients with T1D (28).
It has been suggested that viral infections may be an early trigger for a cascade of events leading to β-cell destruction and T1D (29), thus the adaptive response to this potential trigger could very well dictate the functional outcome in T1D pathogenesis. Wernersson et al. (8) recently showed that enteroviruses impair the later stages of autophagic degradation without modulating the expression of autophagy genes in human islets. Under normal conditions, β cells appear to be more adept at antiviral response than α cells (30), possibly due to the presence of more efficient cell-specific antiviral mechanisms. Several of these findings have been extensively reviewed (1, 31–33) and suggest a putative role of early life viral infections leading to T1D development. The most recent large-scale study however, upon assessment of fecal samples in children, found that long term enterovirus B infection could be associated with islet autoimmunity but not T1D (34). In addition, this study identified that children carrying minor allele for rs6517774 in the CXADR gene (which encodes the Coxsackievirus and adenovirus receptor) had increased T1D risk (34). These data suggest that islet autoimmunity leading to T1D development can be attributed to a combinatorial effect of different viral infections coupled to genetic susceptibility. A definitive role for autophagy in this context, however, has yet to be defined.
IMPAIRMENT OF β CELL -PHAGIC DEGRADATION IN T1D
Although the role of autophagy has been studied extensively in β cells in the context of T2D, significantly less is known about β-cell autophagy in the context of T1D pathogenesis. A recent study by Lambelet et al. (9) analyzing the effects of cytokines, such as those that might be elevated in T1D, in the context of β cell and islet autophagy showed that autophagic flux was impaired in response to cytokine exposure, leading to increased ER stress and eventual cell death mediated via lysosome membrane permeabilization. This suggests a potential role for defective autophagy in the in vivo T1D environment. Indeed, we recently observed that autophagy is impaired in islets of diabetic NOD mice and in the residual proinsulin-positive β cells of human organ donors with T1D (10). We also observed an impairment in the related -phagic degradation process of crinophagy in the residual β cells of human donors with T1D. Perhaps most intriguingly, we observed an increase in autophagosomes as well as telolysosomes (lysosomes containing oxidized proteins and lipids) in the β cells of Aab+ individuals (10), suggesting that autophagic flux may be defective before overt hyperglycemia in the pathogenesis of diabetes and therefore could potentially play a role in disease development.
PUTATIVE CROSS TALK OF β-CELL AUTOPHAGY AND IMMUNE CELLS
A number of studies in recent years highlight the potential role of autophagy in both MHC class I and MHC class II peptide presentation during T1D pathogenesis. For example, following IFNα exposure, islets exhibit an increase in cell surface MHC-I presentation (35, 36). Intracellular peptides are typically presented at the cell surface by MHC-I molecules on antigen presenting cells, which are recognized by cytotoxic CD8+ T-cells, thus triggering an adaptive immune response. Interestingly, autophagy-deficient antigen presenting cells, specifically dendritic cells, have also been shown to elicit increased expression of MHC-I leading to CD8+ T cell activation [37–39]. Thus, considering our observation of impaired β-cell autophagy (10) and previous observations of elevated MHC-I presentation (35, 40) in the context of T1D, studies of autophagy and MHC-I presentation in this context would likely yield additional insights into the pathogenesis of T1D. In addition to elevated MHC-I presentation, Russell et al. (41) also recently demonstrated the heterogeneous presence of MHC-II on β cells of human organ donors with T1D, thereby suggesting direct communication of β cells with CD4+ T cells. Interestingly, highly antigenic chimeric epitopes can be formed by transpeptidation in β-cell secretory granule proteins through the action of lysosomal cathepsin L, and it was found that these neoepitopes could be presented by MHC-II to activate diabetogenic CD4+ T cells (42). To further support the role of autophagic processes in islet autoimmunity specifically, Vomund et al. (43) identified the presence of β-cell specific immunogenic epitopes, derived from insulin peptides found in the crinophagic bodies, in blood leukocytes of NOD mice. Together, these studies support a crucial role of the lysosome and lysosomal enzymes in driving autoimmunity. Indeed, the putative relevance of defective β-cell autophagy in antigen processing and presentation was recently reviewed (44). One key point that remains difficult to parse out is whether autophagy defects in the β cell, or complementary defects in the immune cells play a greater role in the cascade of events leading to T1D. Given the non-cell-selective nature of defects in T1D-linked autophagic components, and the systemic reliance on autophagy for cellular homeostasis and stress response, additional mechanistic studies will be required to answer this question.
ASSESSING THE ROLE OF LYSOSOMES IN T1D PATHOGENESIS
A converging point for all -phagic degradation processes is the lysosome. Lysosomes are multifunctional organelles that not only degrade and recycle, but are also involved in extracellular secretory pathways. Thus, lysosome dysfunction can be extremely damaging to both the intrinsic function of a cell, and to cell-cell communication. It is suggested that oxidative stress can lead to accumulation of oxidized proteins and lipids in tertiary lysosomes (45) (also referred as telolysosomes or lipofuscin bodies). These structures have been found to accumulate within aging β cells (46). Interestingly, we observed an increased number of telolysosomes with a nitrogen-rich corona in the β cells of autoantibody positive donors (10), further supporting a possible defect in β-cell lysosomes before clinical hyperglycemia.
A key component of a lysosome’s degradative function is the maintenance of an acidic lumen through the action of lysosomal acid hydrolases. Many of the lysosomal acid hydrolases have a mannose-6-phosphate (M6P) tag and are trafficked to the lysosome via recognition by either the cation-dependent or cation-independent mannose-6-phosphate receptor (M6PR) from the Golgi (47). The majority of these lysosomal proteins are transported to the lysosomes via the cation-independent M6PR (CI-M6PR), deficiency of which has been shown to enhance the susceptibility of the β cells to palmitate-induced damage (48) presumably due to ineffective acidification of the lysosome lumen. In support of this, deletion of Atp6ap2, a vacuolar ATPase, impairs β-cell lysosomal acidity leading to defective insulin secretion and compromised autophagic flux (49). Reacidification of lysosomes using nanoparticles can restore β-cell function and mitochondrial turnover in the context of T2D (50), suggesting that defects in lysosome acidity can directly impair β-cell function in diabetes pathogenesis. To our knowledge, there are currently no published studies in a T1D model to determine if stimulating β-cell lysosome function can prevent apoptosis and diabetes development.
Typically, acidic, and autophagy-ready lysosomes are localized to the perinuclear region and have a low pH, whereas lysosomes that have a high pH are often located near the periphery of the cell (51, 52). Peripheral lysosomes are less likely to be involved in the degradation process. Instead, these high pH lysosomes have been shown to participate in lysosomal exocytosis (53), where the lysosomes are secreted from cells to maintain cellular health. This occurs via activation of the lysosome transcription factor TFEB that leads to activation of the lysosome ion channel, mucolipin1, and peripheral lysosome accumulation (54). Unconventional secretion of autophagy components can also occur in a process called “secretory autophagy” when peptides lacking an N-terminal secretion signal are released to the extracellular environment, and is often triggered by cellular stress (55). The best example relevant to inflammatory disease, is the release of endosomal/early lysosomal vesicles containing proIL1-β from monocytes (56, 57). Though this is a relatively unexplored area in β-cell biology, especially in the context of T1D, it has been hypothesized that release of partially digested peptides such as insulin could play a role in the stimulation of autoimmune attack (58).
CONCLUSIONS
Although 100 years has passed since the discovery of insulin, treatment with insulin infusions still stands as the primary care for individuals with T1D. Previous literature has suggested a critical role for autophagy in β-cell homeostasis, where dysfunctional autophagy contributes to impairment of β-cell function and apoptosis. New evidence supports this, indicating loss of autophagy in the context of T1D where the lysosome appears to be a key player in this dysfunction. A number of potential mechanisms could lead to autoimmunity relating back to dysfunctional lysosomes (Fig. 2). Therefore, we suggest that molecular targeting of the lysosome may be a viable approach to aid in restoring β-cell homeostasis for T1D prevention. Furthermore, we propose that selective stimulation of autophagy and/or lysosome function may yield a similar restoration of β-cell function and homeostasis in the early prediabetic phase.
GRANTS
This work was supported by the Showalter Trust and NIDDK Grants R03DK115990, R03DK127766, and R01DK124380 to A.K. Linnemann. C. Muralidharan is supported by a Cagiantas Scholarship from the Graduate Division at IU School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.L. conceived and designed research; C.M. prepared figures; C.M. and A.K.L. drafted manuscript; C.M. and A.K.L. edited and revised manuscript; C.M. and A.K.L. approved final version of manuscript.
REFERENCES
- 1.Craig ME, Kim KW, Isaacs SR, Penno MA, Hamilton-Williams EE, Couper JJ, Rawlinson WD. Early-life factors contributing to type 1 diabetes. Diabetologia 62: 1823–1834, 2019. doi: 10.1007/s00125-019-4942-x. [DOI] [PubMed] [Google Scholar]
- 2.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 26: 501–518, 2017. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsholme P, Keane KN, Carlessi R, Cruzat V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. Am J Physiol Cell Physiol 317: C420–C433, 2019. doi: 10.1152/ajpcell.00141.2019. [DOI] [PubMed] [Google Scholar]
- 4.Marasco MR, Linnemann AK. β-cell autophagy in diabetes pathogenesis. Endocrinology 159: 2127–2141, 2018. doi: 10.1210/en.2017-03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivot K, Pasquier A, Goginashvili A, Ricci R. Breaking bad and breaking good: β-cell autophagy pathways in diabetes. J Mol Biol 432: 1494–1513, 2020. doi: 10.1016/j.jmb.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, Kim J, Park K, Lee M-S. β-cell autophagy: Mechanism and role in β-cell dysfunction. Mol Metab 27S: S92–S103, 2019. doi: 10.1016/j.molmet.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20: 460–473, 2014. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wernersson A, Sarmiento L, Cowan E, Fex M, Cilio CM. Human enteroviral infection impairs autophagy in clonal INS(832/13) cells and human pancreatic islet cells. Diabetologia 63: 2372–2384, 2020. doi: 10.1007/s00125-020-05219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambelet M, Terra LF, Fukaya M, Meyerovich K, Labriola L, Cardozo AK, Allagnat F. Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis. Cell Death Dis 9: 96, 2018. doi: 10.1038/s41419-017-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muralidharan C, Conteh AM, Marasco MR, Crowder JJ, Kuipers J, de Boer P, Linnemann AK. Pancreatic beta cell autophagy is impaired in type 1 diabetes. Diabetologia. 64: 865–877, 2021. doi: 10.1007/s00125-021-05387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Rodríguez M, Pérez-González B, Pasquali L. The β-cell genomic landscape in T1D: implications for disease pathogenesis. Curr Diab Rep 21: 1, 2021. doi: 10.1007/s11892-020-01370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soleimanpour SA, Gupta A, Bakay M, Ferrari AM, Groff DN, Fadista J, Spruce LA, Kushner JA, Groop L, Seeholzer SH, Kaufman BA, Hakonarson H, Stoffers DA. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell 157: 1577–1590, 2014. doi: 10.1016/j.cell.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soleimanpour SA, Ferrari AM, Raum JC, Groff DN, Yang J, Kaufman BA, Stoffers DA. Diabetes susceptibility genes Pdx1 and Clec16a function in a pathway regulating mitophagy in β-cells. Diabetes 64: 3475–3484, 2015. doi: 10.2337/db15-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidarala V, Pearson GL, Parekh VS, Thompson B, Christen L, Gingerich MA, Zhu J, Stromer T, Ren J, Reck EC, Chai B, Corbett JA, Mandrup-Poulsen T, Satin LS, Soleimanpour SA. Mitophagy protects β cells from inflammatory damage in diabetes. JCI Insight. 5, 2020. doi: 10.1172/jci.insight.141138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper JD, Smyth DJ, Smiles AM, Plagnol V, Walker NM, Allen JE, Downes K, Barrett JC, Healy BC, Mychaleckyj JC, Warram JH, Todd JA. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet 40: 1399–1401, 2008. doi: 10.1038/ng.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy MVPL, Wang H, Liu S, Bode B, Reed JC, Steed RD, Anderson SW, Steed L, Hopkins D, She JX. Association between type 1 diabetes and GWAS SNPs in the southeast US Caucasian population. Genes Immun 12: 208–212, 2011. doi: 10.1038/gene.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fløyel T, Brorsson C, Nielsen LB, Miani M, Bang-Berthelsen CH, Friedrichsen M, Overgaard AJ, Berchtold LA, Wiberg A, Poulsen P, Hansen L, Rosinger S, Boehm BO, Ram R, Nguyen Q, Mehta M, Morahan G, Concannon P, Bergholdt R, Nielsen JH, Reinheckel T, von Herrath M, Vaag A, Eizirik DL, Mortensen HB, Størling J, Pociot F. CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc Natl Acad Sci USA 111: 10305–10310, 2014. doi: 10.1073/pnas.1402571111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp RC, Abdulrahim M, Naser ES, Naser SA. Genetic variations of PTPN2 and PTPN22: role in the pathogenesis of type 1 diabetes and Crohn’s Disease. Front Cell Infect Microbiol 5, 2015. doi: 10.3389/fcimb.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharl M, Rogler G. The role for protein tyrosine phosphatase nonreceptor type 2 in regulating autophagosome formation. Ann N Y Acad Sci 1257: 93–102, 2012. doi: 10.1111/j.1749-6632.2012.06578.x. [DOI] [PubMed] [Google Scholar]
- 20.Spalinger MR, Lang S, Gottier C, Dai X, Rawlings DJ, Chan AC, Rogler G, Scharl M. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 13: 1590–1601, 2017. doi: 10.1080/15548627.2017.1341453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spalinger MR, Lang S, Vavricka SR, Fried M, Rogler G, Scharl M. Protein tyrosine phosphatase non-receptor type 22 modulates NOD2-induced cytokine release and autophagy. PLoS One 8: e72384, 2013. doi: 10.1371/journal.pone.0072384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ifie E, Russell MA, Dhayal S, Leete P, Sebastiani G, Nigi L, Dotta F, Marjomäki V, Eizirik DL, Morgan NG, Richardson SJ. Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia 61: 2344–2355, 2018. doi: 10.1007/s00125-018-4704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodik M, Anagandula M, Fuxe J, Krogvold L, Dahl-Jørgensen K, Hyöty H, Sarmiento L, Frisk G; POD-V Consortium. Coxsackie–adenovirus receptor expression is enhanced in pancreas from patients with type 1 diabetes. BMJ Open Diabetes Res Care 4: e000219, 2016. doi: 10.1136/bmjdrc-2016-000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oikarinen S, Martiskainen M, Tauriainen S, Huhtala H, Ilonen J, Veijola R, Simell O, Knip M, Hyöty H. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 60: 276–279, 2011. doi: 10.2337/db10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52: 1143–1151, 2009. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 26.Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47: 225–239, 2004. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 27.Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 56: 185–193, 2013. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- 28.Krogvold L, Edwin B, Buanes T, Frisk G, Skog O, Anagandula M, Korsgren O, Undlien D, Eike MC, Richardson SJ, Leete P, Morgan NG, Oikarinen S, Oikarinen M, Laiho JE, Hyöty H, Ludvigsson J, Hanssen KF, Dahl-Jørgensen K. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 64: 1682–1687, 2015. doi: 10.2337/db14-1370. [DOI] [PubMed] [Google Scholar]
- 29.Sioofy-Khojine A-B, Lehtonen J, Nurminen N, Laitinen OH, Oikarinen S, Huhtala H, Pakkanen O, Ruokoranta T, Hankaniemi MM, Toppari J, Vähä-Mäkilä M, Ilonen J, Veijola R, Knip M, Hyöty H. Coxsackievirus B1 infections are associated with the initiation of insulin-driven autoimmunity that progresses to type 1 diabetes. Diabetologia 61: 1193–1202, 2018. doi: 10.1007/s00125-018-4561-y. [DOI] [PubMed] [Google Scholar]
- 30.Marroqui L, Lopes M, dos Santos RS, Grieco FA, Roivainen M, Richardson SJ, Morgan NG, Op de Beeck A, Eizirik DL. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic α and β cells. Elife 4: e06990, 2015. doi: 10.7554/eLife.06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Calvo T. Enterovirus infection and type 1 diabetes: unraveling the crime scene. Clin Exp Immunol 195: 15–24, 2019. doi: 10.1111/cei.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanter M, Sork H, Tuomela S, Flodström-Tullberg M. Genetic and environmental interaction in type 1 diabetes: a relationship between genetic risk alleles and molecular traits of enterovirus infection? Curr Diab Rep 19: 82, 2019. doi: 10.1007/s11892-019-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Beeck AO, Eizirik DL. Viral infections in type 1 diabetes mellitus — why the β cells? Nat Rev Endocrinol 12: 263–273, 2016. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vehik K, Lynch KF, Wong MC, Tian X, Ross MC, Gibbs RA, Ajami NJ, Petrosino JF, Rewers M, Toppari J, Ziegler AG, She JX, Lernmark A, Akolkar B, Hagopian WA, Schatz DA, Krischer JP, Hyöty H, Lloyd RE; TEDDY Study Group. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med 25: 1865–1872, 2019. doi: 10.1038/s41591-019-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coomans de Brachène A, Dos Santos RS, Marroqui L, Colli ML, Marselli L, Mirmira RG, Marchetti P, Eizirik DL. IFN-α induces a preferential long-lasting expression of MHC class I in human pancreatic beta cells. Diabetologia 61: 636–640, 2018. doi: 10.1007/s00125-017-4536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marroqui L, Dos Santos RS, Op de Beeck A, Coomans de Brachène A, Marselli L, Marchetti P, Eizirik DL. Interferon-α mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia 60: 656–667, 2017. doi: 10.1007/s00125-016-4201-3. [DOI] [PubMed] [Google Scholar]
- 37.Loi M, Müller A, Steinbach K, Niven J, Barreira da Silva R, Paul P, Ligeon LA, Caruso A, Albrecht RA, Becker AC, Annaheim N, Nowag H, Dengjel J, García-Sastre A, Merkler D, Münz C, Gannagé M. Macroautophagy proteins control MHC class I levels on dendritic cells and shape anti-viral CD8(+) T cell responses. Cell Rep 15: 1076–1087, 2016. doi: 10.1016/j.celrep.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Loi M, Ligeon L-A, Münz C. MHC class I internalization via autophagy proteins. Methods Mol Biol 1880: 455–477, 2019. doi: 10.1007/978-1-4939-8873-0_29. [DOI] [PubMed] [Google Scholar]
- 39.Parekh VV, Pabbisetty SK, Wu L, Sebzda E, Martinez J, Zhang J, Van Kaer L. Autophagy-related protein Vps34 controls the homeostasis and function of antigen cross-presenting CD8α+ dendritic cells. Proc Natl Acad Sci USA 114: E6371–E6380, 2017. doi: 10.1073/pnas.1706504114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jørgensen K, von Herrath M, Pugliese A, Atkinson MA, Morgan NG. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 59: 2448–2458, 2016. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell MA, Redick SD, Blodgett DM, Richardson SJ, Leete P, Krogvold L, Dahl-Jørgensen K, Bottino R, Brissova M, Spaeth JM, Babon JAB, Haliyur R, Powers AC, Yang C, Kent SC, Derr AG, Kucukural A, Garber MG, Morgan NG, Harlan DM. HLA class II antigen processing and presentation pathway components demonstrated by transcriptome and protein analyses of islet β-cells from donors with type 1 diabetes. Diabetes 68: 988–1001, 2019. doi: 10.2337/db18-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed B, Crawford F, Hill RC, Jin N, White J, Krovi SH, Marrack P, Hansen K, Kappler JW. Lysosomal cathepsin creates chimeric epitopes for diabetogenic CD4 T cells via transpeptidation. J Exp Med 218: e20192135, 2021. doi: 10.1084/jem.20192135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vomund AN, Lichti CF, Peterson OJ, Arbelaez AM, Wan X, Unanue ER. Blood leukocytes recapitulate diabetogenic peptide–MHC-II complexes displayed in the pancreatic islets. J Exp Med 218: e20202530, 2021. doi: 10.1084/jem.20202530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carré A, Mallone R. Making insulin and staying out of autoimmune trouble: the beta-cell conundrum. Front Immunol 12: 639682, 2021. doi: 10.3389/fimmu.2021.639682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terman A, Brunk UT. Oxidative stress, accumulation of biological “garbage”, and aging. Antioxid Redox Signal 8: 197–204, 2006. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 46.Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, Gunter JH, de Koning EJ, Walls GV, Gray DW, Johnson PR, Hansen BC, Morris JF, Pipeleers-Marichal M, Cnop I, Clark A. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 53: 321–330, 2010. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4: 202–213, 2003. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 48.Baldwin AC, Naatz A, Bohnsack RN, Bartosiak JT, Oleson BJ, Hansen PA, Dahms NM, Corbett JA. Cation-independent mannose 6-phosphate receptor deficiency enhances β-cell susceptibility to palmitate. Mol Cell Biol 38: e00680-17, 2018. doi: 10.1128/MCB.00680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binger KJ, Neukam M, Tattikota SG, Qadri F, Puchkov D, Willmes DM, Wurmsee S, Geisberger S, Dechend R, Raile K, Kurth T, Nguyen G, Poy MN, Solimena M, Muller DN, Birkenfeld AL. Atp6ap2 deletion causes extensive vacuolation that consumes the insulin content of pancreatic β cells. Proc Natl Acad Sci USA 116: 19983–19988, 2019. doi: 10.1073/pnas.1903678116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assali EA, Shlomo D, Zeng J, Taddeo EP, Trudeau KM, Erion KA, Colby AH, Grinstaff MW, Liesa M, Las G, Shirihai OS. Nanoparticle-mediated lysosomal reacidification restores mitochondrial turnover and function in β cells under lipotoxicity. FASEB J 33: 4154–4165, 2019. doi: 10.1096/fj.201801292R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cabukusta B, Neefjes J. Mechanisms of lysosomal positioning and movement. Traffic 19: 761–769, 2018. doi: 10.1111/tra.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212: 677–692, 2016. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tapper H, Sundler R. Role of lysosomal and cytosolic pH in the regulation of macrophage lysosomal enzyme secretion. Biochem J 272: 407–414, 1990. doi: 10.1042/bj2720407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell 21: 421–430, 2011. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabouille C. Pathways of unconventional protein secretion. Trends Cell Biol 27: 230–240, 2017. doi: 10.1016/j.tcb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy‐based unconventional secretory pathway for extracellular delivery of IL‐1β. EMBO J 30: 4701–4711, 2011. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng HHL, Vong CT, Kwan YW, Lee SM-Y, Hoi MPM. Lysosomal Ca2+ signaling regulates high glucose-mediated interleukin-1β secretion via transcription factor EB in human monocytic cells. Front Immunol 8: 1161, 2017. doi: 10.3389/fimmu.2017.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vomund AN, Zinselmeyer BH, Hughes J, Calderon B, Valderrama C, Ferris ST, Wan X, Kanekura K, Carrero JA, Urano F, Unanue ER. Beta cells transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc Natl Acad Sci USA 112: E5496–E5502, 2015. doi: 10.1073/pnas.1515954112. [DOI] [PMC free article] [PubMed] [Google Scholar]