Abstract
Cephalad fluid shifts in space have been hypothesized to cause the spaceflight-associated neuro-ocular syndrome (SANS) by increasing the intracranial-ocular translaminal pressure gradient. Lower body negative pressure (LBNP) can be used to shift upper-body blood and other fluids toward the legs during spaceflight. We hypothesized that microgravity would increase jugular vein volume (JVvol), portal vein cross-sectional area (PV), and intracranial venous blood velocity (MCV) and that LBNP application would return these variables toward preflight levels. Data were collected from 14 subjects (11 males) before and during long-duration International Space Station (ISS) spaceflights. Ultrasound measures of JVvol, PV, and MCV were acquired while seated and supine before flight and early during spaceflight at day 45 (FD45) and late at day 150 (FD150) with and without LBNP. JVvol increased from preflight supine and seated postures (46 ± 48% and 646 ± 595% on FD45 and 43 ± 43% and 702 ± 631% on FD150, P < 0.05), MCV increased from preflight supine (44 ± 31% on FD45 and 115 ± 116% on FD150, P < 0.05), and PV increased from preflight supine and seated (51 ± 56% on FD45 and 100 ± 74% on FD150, P < 0.05). Inflight LBNP of −25 mmHg restored JVvol and MCV to preflight supine level and PV to preflight seated level. Elevated JVvol confirms the sustained neck-head blood engorgement inflight, whereas increased PV area supports the fluid shift at the splanchnic level. Also, MCV increased potentially due to reduced lumen diameter. LBNP, returning variables to preflight levels, may be an effective countermeasure.
NEW & NOTEWORTHY Microgravity-induced fluid shifts markedly enlarge jugular and portal veins and increase cerebral vein velocity. These findings demonstrate a marked flow engorgement at neck and splanchnic levels and may suggest compression of the cerebral veins by the brain tissue in space. LBNP (−25 mmHg for 30 min) returns these changes to preflight levels and, thus, reduces the associated flow and tissue disturbances.
Keywords: cerebral vein, jugular vein, LBNP, portal vein, spaceflight
INTRODUCTION
Over 60% of subjects returning from long-duration spaceflights report decreased visual acuity, with some reporting this vision impairment for years after spaceflight (1). It is believed that these ocular structural and vision changes, known as spaceflight-associated neuro-ocular syndrome (SANS), are in part due to increased intracranial and ocular transluminal pressures and ocular edema resulting from cephalad blood and cerebrospinal fluid (CSF) shifts experienced during microgravity exposure (2–4). Noninvasive monitoring of cephalad fluid shifts during spaceflight may provide greater insight into SANS development and assist in the evaluation of countermeasures.
Over the past 20 years, ultrasound assessments of jugular vein volume and portal vein cross-sectional area were frequently used as evidence of cephalad fluid shifts and splanchnic blood pooling. Increased jugular vein volume and portal vein cross-sectional area are documented for short (1 wk) and long (6 mo) spaceflights and with the head-down tilt (HDT) bed rest model of microgravity exposure (5–8). In addition, a 7-day bed rest study noted that enlargement of the jugular vein was accompanied by enlargement of the eye fundus veins and the presence of edema at the fundus level of the eye (7), suggesting a potential link between the observed increase in jugular vein volume and vision changes. When the headward fluid shift was sustained for 30 days of strict HDT bed rest, optic disk edema developed similar to that which occurs during spaceflight (9, 10).
Recent work with a dry immersion model of microgravity exposure has shown increased jugular vein volume, portal vein area, and middle cerebral vein velocity (11, 12) consistent with the hypothesis that dry immersion mimics the fluid shifts observed during spaceflight (13). In clinical practice, patients with increased intracranial pressure have also presented with increased middle cerebral vein velocity (personal observation), suggesting a potential link between these variables. However, in the dry immersion study, middle cerebral vein velocity was only associated with increased intracranial pressure in 50% of the participants investigated, indicating that the change in venous velocity is likely due to other factors associated with cephalad fluid shifts (11, 12). Currently, it is unknown if middle cerebral venous velocity changes with spaceflight and to what degree this measure is affected by lower body negative pressure (LBNP) to counteract cephalad fluid shifts.
Traditionally proposed as a method of inducing orthostatic-like stress in the absence of gravity, LBNP pulls blood toward the lower body and is proposed as a countermeasure against physiological adaptations to microgravity exposure (14). LBNP has been shown to reduce portal vein size (6, 15), jugular vein volume (2, 16), intracranial pressure (2, 16, 17), and optic nerve sheath diameter (18). Therefore, the application of LBNP may be an effective countermeasure in preventing SANS.
The objective of the current study was to determine changes in jugular vein volume, portal vein cross-sectional area, and cerebral venous blood velocity during long-duration spaceflights. It was hypothesized that cephalad fluid shift with microgravity exposure would increase jugular vein volume, portal vein area, and cerebral venous velocity compared with that reported preflight. Moreover, it was hypothesized that the application of LBNP during spaceflight would reduce jugular vein volume, portal vein cross-sectional area, and cerebral venous blood velocity, returning these variables to preflight supine or seated levels.
RESEARCH DESIGN AND METHODS
Subjects
Data were collected from 14 subjects who participated in the study [11 males; age: 47 ± 6 yr; body mass index: 26.4 ± 3 kg/m2 (means ± SD)]. Study protocols and procedures were developed and conducted in accordance with the Declaration of Helsinki and received approval by the National Aeronautics and Space Administration (NASA) and University of California, San Diego (UCSD) research ethics committees. Before participation, each astronaut was informed about the experiment, signed written consent, and was aware of his or her right to withdraw from the study at any time without prejudice. Food and fluid intakes were not strictly controlled during spaceflight, and each subject consumed standard spaceflight diet. Similarly, each subject performed daily physical activity, but this was not standardized across subjects.
Experimental Protocol
Venous responses to acute fluid shifts and the application of LBNP were assessed ∼90 days before spaceflight. During this session, resting data were collected with subjects in both seated and supine positions. Acute cephalad fluid shifts were achieved by placing the subjects in a 15° head-down tilt (HDT) position, with measurements taken after 40 min in this position. The LBNP (Russian Chibis-M system) was used until now by cosmonauts on the International Space Station (ISS) as a countermeasure against postflight orthostatic intolerance [10-min steps of −25 mmHg and −45 mmHg (19)]. In the present study, the “Chibis” was used to apply only a moderate level of LBNP (−25 mmHg) but for a longer period than until now (45 min) both inflight and in the HDT position preflight. Measurements were taken after 30 min of applied LBNP.
Astronauts spent 210 ± 76 days (means ± SD) on the International Space Station (ISS). Spaceflight data were collected on days 45 (FD45) and 150 (FD150) of the flight. On each of these days, measures were made at rest and after 30 min of −25 mmHg LBNP applied using the Chibis-M system. Postflight measurements supine and seated (jugular vein only) were made 40 days (R + 40) and 180 days (R + 180) after returning to Earth.
Measures
Ultrasound (Vivid Q, GE Healthcare, Chicago, IL) was used for the noninvasive assessment of jugular vein volume (JVvol), portal vein cross-sectional area (PV), and middle cerebral vein velocity (MCV). Left JVvol (cm3) was calculated from a long-axis view and transverse cross-sectional images of the vein between the collar bone and the mandibula (20). The portal vein trunk was visualized by locating the probe at the cross section of the vertical line passing on the right nipple and the xiphoid line, and the diameter was measured for the calculation of PV cross-section area (cm2). Transcranial Doppler ultrasound was used to assess MCV (cm/s) by lowering the Doppler filter and velocity range as previously described (11). The transcranial phased array probe was located on the acoustic window usually used for investigating the middle cerebral artery (right temple, between ear and eye, right above the zygomatic arch). Color Doppler was activated with a low filter for low velocity detection and a wide sample volume. Once the cerebral vein color signal was identified, the pulsed Doppler was activated and the venous velocity spectrum recorded.
Preflight and postflight measurements were made by a trained sonographer, and the subjects performed inflight measurements. Each astronaut received 3 h of ultrasound training preflight to perform all inflight measures. During the spaceflight measurement sessions, subjects received verbal guidance from a trained sonographer on the ground who verified the quality of the captured images and provided instructions on repositioning the ultrasound probe as needed and how to improve the image quality by activating ultrasound equipment functions (gain, depth, frequency, Doppler, and time motion) and the capture of images at the appropriate times.
Statistical Analysis
Statistical analysis was performed using RStudio version 1.1.456 with R software version 3.5.1. The effects of acute fluid shifts, spaceflight, and LBNP were assessed using linear mixed-effects models [packages lme4 (21) version 1.1–18-1 and lmerTest version 3.0–1] with the fixed effect of measurement time point and random effect of subject. Post hoc analyses were conducted using lsmeans (22) version 2.27–62 with Satterthwaite’s approximation for degrees of freedom and Tukey’s adjustments for multiple comparisons. Linear regression was used to test for a relationship between the changes in JVvol and MCV with spaceflight compared with preflight supine and seated positions. Significance was set at P < 0.05, with all results reported as means ± SD.
RESULTS
Some measurements were not available at various time points due to poor ultrasound image quality, the inability to find the middle cerebral vein, or images of the portal vein not being taken at the level of the main trunk. For 14 astronauts, measures of JVvol were successful for 146 of 168 measures (12 time points), PV area was successfully measured at 93 of 140 times (10 time points), and MCV velocity was determined at 79 of the 98 measures (measured at 7 time points). The number of datapoints analyzed at each specific time point is indicated in Table 1.
Table 1.
Measures before, during, and after long-duration spaceflight
| Preflight |
Spaceflight |
Postflight |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supine | Seated | HDT | HDT + LBNP | FD45 | FD45 + LBNP | FD150 | FD150 + LBNP | R + 40 Supine | R + 40 Seated | R + 180 Supine | R + 180 Seated | |
| JVvol, cm3 | 1.73 (0.62) n = 14 |
0.42 (0.22)* n = 14 |
3.42 (1.56)* n = 12 |
1.06 (0.78)† n = 12 |
2.38 (0.79)* n = 14 |
1.55 (0.71)† n = 13 |
2.45 (0.76)* n = 12 |
1.24 (0.59)† n = 11 |
1.85 (0.91) n = 13 |
0.40 (0.31)* n = 10 |
1.63 (0.45) n = 11 |
0.26 (0.11)* n = 10 |
| PV, cm2 | 0.87 (0.23) n = 14 |
0.76 (0.39) n = 8 |
1.06 (0.29) n = 8 |
0.70 (0.16)† n = 7 |
1.03 (0.35) n = 11 |
0.64 (0.19)† n = 8 |
1.25 (0.40)* n = 12 |
0.72 (0.23)† n = 10 |
0.88 (0.26) n 8 |
n/a | 0.75 (0.26) n = 7 |
n/a |
| MCV velocity, cm/s | 9.30 (3.10) n = 14 |
n/a | n/a | 7.33 (1.68) n = 5 |
14.27 (4.00)* n = 9 |
9.28 (2.40)† n = 10 |
17.13 (5.34)* n = 13 |
9.72 (2.25)† n = 12 |
10.06 (3.53) n = 12 |
n/a | 10.16 (3.21) n = 9 |
n/a |
Mean values (SD) and the number of measures (n) are presented for each of the measurement time point. Symbols are as follows: *different from preflight supine (P < 0.05); and †LBNP value different from respective value without LBNP (P < 0.05). FD45, 45th day of spaceflight; FD150, 150th day of spaceflight; HDT, head-down tilt; JVvol, jugular vein volume; LBNP, lower body negative pressure; MCV, middle cerebral vein velocity; n/a, not applicable; PV, portal vein cross-sectional area; R + 40, 40th day after returning to Earth from spaceflight; R + 180, 180th day after returning to Earth from spaceflight.
Acute Fluid Shifts Preflight
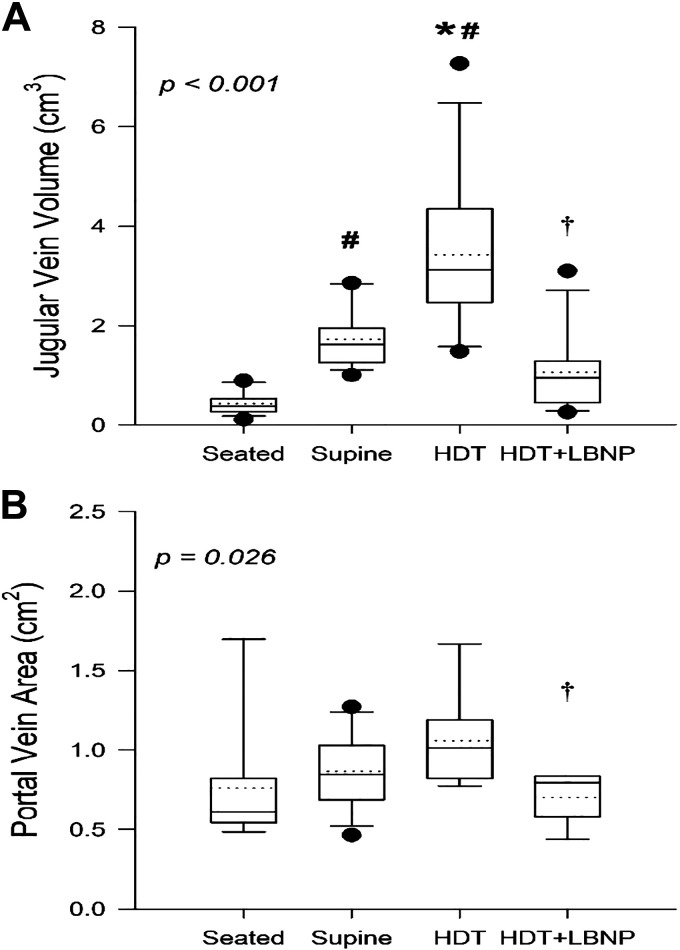
Assessment of preflight response to acute fluid shifts found an effect of body position and LBNP on measures of JVvol (Fig. 1A; P < 0.001) and PV (Fig. 1B; P = 0.026). Compared with seated rest, JVvol increased by 1.31 ± 0.68 cm3 (472 ± 584%; P = 0.001) in the supine position and by 2.99 ± 1.55 cm3 (934 ± 790%; P < 0.001) with HDT. Applying LBNP in the HDT position decreased JVvol to a level that was not different from supine values (P = 0.080). In contrast, PV was not different from seated values, supine (P = 0.656), or with HDT (P = 0.082); however, there was a reduction in PV area with the application of LBNP compared with the HDT value (−0.40 ± 0.22 cm2; −35 ± 12%; P = 0.027).
Figure 1.
Boxes show values of jugular vein volume (A) and portal vein cross-sectional area (B) with preflight seated rest (seated), supine rest (supine), 15° head-down tilt (HDT), and lower body negative pressure (LBNP) applied at −25 mmHg during HDT (HDT + LBNP). Box boundaries represent the 25th and 75th percentiles, with whiskers representing the 10th and 90th percentiles. Median and mean values are shown as solid and dotted lines within the boxes, respectively, with dots indicating outlying points. A linear mixed-effects model was used and found a main effect of measurement time point for jugular vein volume (P < 0.001) and portal vein area (P = 0.026). Tukey’s post hoc analysis results are indicated by the symbols as follows: *different from supine (P < 0.05); #different from seated (P < 0.05); and †HDT + LBNP different from HDT (P < 0.05). The sample number and sex breakdown of each time point are as follows: jugular vein volume in seated, n = 14, 3 females; supine, n = 14, 3 females; HDT, n = 12, 2 females; HDT + LBNP, n = 12, 3 females; portal vein area in seated, n = 8, 2 females; supine, n = 14, 3 females; HDT, n = 8, 3 females; HDT + LBNP, n = 7, 3 females.
Spaceflight and LBNP
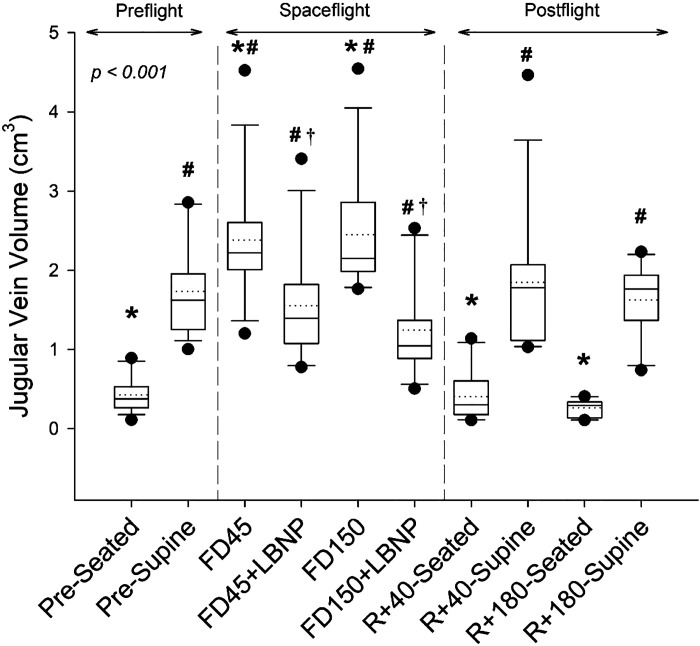
There was a main effect of spaceflight and LBNP on JVvol (Fig. 2, P < 0.001). From preflight supine values, JVvol increased by 0.65 ± 0.70 cm3 (P = 0.007) and 0.64 ± 0.68 cm3 (P = 0.007) on FD45 and FD150, respectively, which corresponded to a respective 46 ± 48% and 43 ± 43% increase (Fig. 5A). Compared with preflight seated rest, there was a much greater increase in JVvol of 1.96 ± 0.81 cm3 (646 ± 595%; P < 0.001) on FD45 and 2.03 ± 0.78 cm3 (702 ± 631%; P < 0.001) on FD150. The application of LBNP on FD45 and FD150 reduced JVvol by −0.87 ± 0.36 cm3 (−37 ± 13%; P < 0.001) and −1.11 ± 0.65 cm3 (−45 ± 17%; P < 0.001; Fig. 5A), respectively, to values that were not different from those measured preflight in the supine position (P = 0.979 and P = 0.145). Postflight JVvol measures were not different from preflight on day R + 40 or R + 180 both in supine or seated position (all P values greater than P = 0.9).
Figure 2.
Boxes show values of jugular vein volume preflight (seated, supine), during spaceflight at rest and with the application of LBNP, and postflight (seated and supine). Box boundaries represent the 25th and 75th percentiles, with whiskers representing the 10th and 90th percentiles. Median and mean values are shown as solid and dotted lines within the boxes, respectively, with dots indicating outlying points. A linear mixed-effects model was used and found a main effect of measurement time point (P < 0.001). Tukey’s post hoc analysis results are indicated by the symbols as follows: *different from supine (P < 0.05); #different from seated (P < 0.05); and †value with LBNP application is significantly different from respective resting value (P < 0.05). The sample number and sex breakdown of each time point are as follows: pre-seated, n = 14, 3 females; pre-supine, n = 14, 3 females; FD45, n = 14, 3 females; FD45 + LBNP, n = 13, 2 females; FD150, n = 12, 2 females; FD150 + LBNP, n = 11, 2 females; R + 40-seated, n = 10, 2 females; R + 40-supine, n = 13, 2 females; R + 180-seated, n = 10, 2 females; R + 180-supine, n = 11, 2 females. FD45, 45th day of spaceflight; FD150, 150th day of spaceflight; LBNP, lower body negative pressure; R + 40, 40th day after returning to Earth from spaceflight; R + 180, 180th day after returning to Earth from spaceflight.
Figure 5.
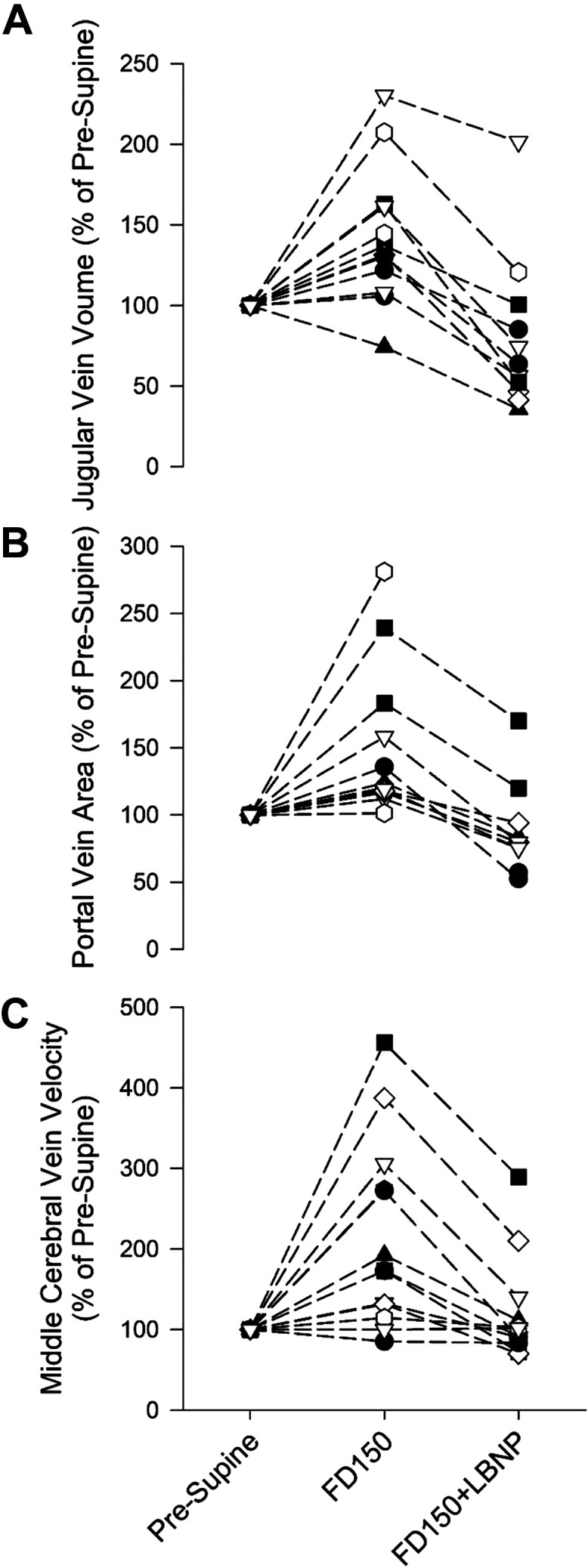
Individual values of jugular vein volume (A; n = 12), portal vein cross-sectional area (B; n = 10), and middle cerebral vein velocity (C; n = 12) preflight (supine) and on FD150 with and without the application of LBNP. Values are presented as a percentage of preflight supine (pre-supine) values. FD150, 150th day of spaceflight; LBNP, lower body negative pressure.
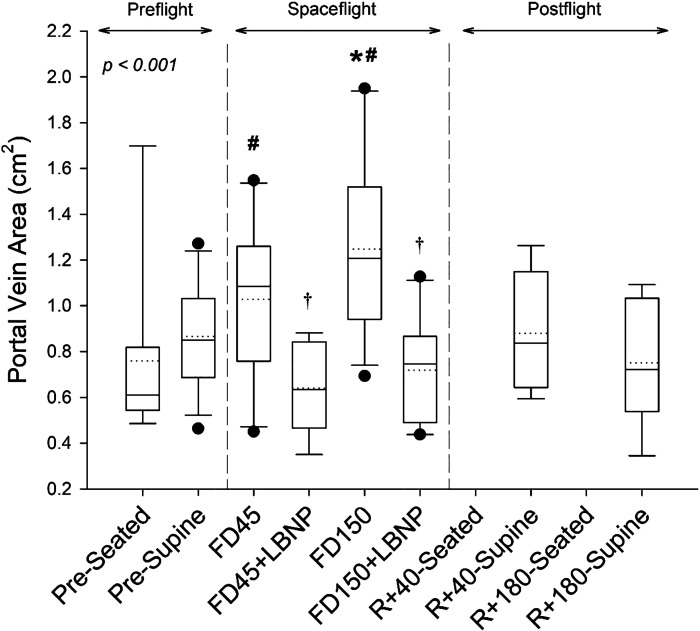
A main effect of spaceflight and LBNP was also found for PV (Fig. 3; P < 0.001). On FD45, PV was not changed from preflight supine (P = 0.603) but was elevated by 0.27 ± 0.54 cm2 (59 ± 85%) compared with preflight seated values (P = 0.041). On FD150, PV was elevated by 0.38 ± 0.38 cm2 (51 ± 56%; P = 0.002; Fig. 5B) with respect to preflight supine values and 0.56 ± 0.50 cm2 (100 ± 74%; P < 0.001) with respect to preflight seated values. LBNP reduced PV on FD45 and FD150, respectively, by −0.45 ± 0.24 cm2 (−40 ± 17%; P = 0.013) and −0.45 ± 25 cm2 (−36 ± 12%; P = 0.001; Fig. 5B) to values that were not different from preflight seated values (P = 1 and P = 0.994, respectively). All postflight measures were not different from preflight (all P values greater than P = 0.9).
Figure 3.
Boxes show values of portal vein area preflight (seated, supine), during spaceflight at rest and with the application of LBNP, and postflight (seated and supine). Box boundaries represent the 25th and 75th percentiles, with whiskers representing the 10th and 90th percentiles. Median and mean values are shown as solid and dotted lines within the boxes, respectively, with dots indicating outlying points. A linear mixed-effects model was used and found a main effect of measurement time point (P < 0.001). Tukey’s post hoc analysis results are indicated by the symbols as follows: *different from supine (P < 0.05); #different from seated (P < 0.05); and †value with LBNP application is significantly different from respective resting value (P < 0.05). The sample number and sex breakdown of each time point are as follows: pre-seated, n = 8, 2 females; pre-supine, n = 14, 3 females; FD45, n = 11, 3 females; FD45 + LBNP, n = 8, 2 females; FD150, n = 12, 2 females; FD150 + LBNP, n = 10, 1 female; R + 40-supine, n = 8, 1 female; R + 180-supine, n = 7, 2 females. FD45, 45th day of spaceflight; FD150, 150th day of spaceflight; LBNP, lower body negative pressure; R + 40, 40th day after returning to Earth from spaceflight; R + 180, 180th day after returning to Earth from spaceflight.
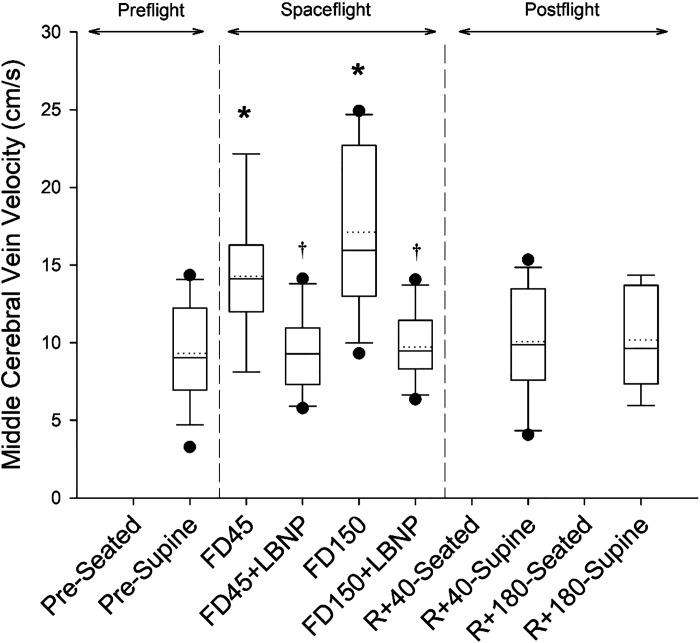
MCV was affected by spaceflight and the application of LBNP (Fig. 4; P < 0.001). MCV increased by 4.03 ± 3.23 cm/s (44 ± 31%; P = 0.016) on FD45 and by 7.89 ± 6.61 cm/s (115 ± 116%; P < 0.001; Fig. 5C) on FD150. There was no relationship between the magnitude of MCV change and JVvol change on FD45 (P = 0.892) or FD150 (P = 0.178). The application of LBNP returned MCV to preflight levels, reducing MCV by −5.34 ± 4.74 cm/s (−33 ± 21%; P = 0.028) on FD45 and by −6.81 ± 5.30 cm/s (−37 ± 22%; P < 0.001; Fig. 5C) on FD150. Postflight values on R + 40 and R + 180 were not different from preflight supine values (P = 0.997 and P = 0.998, respectively).
Figure 4.
Boxes show values of middle cerebral vein velocity preflight (seated, supine), during spaceflight at rest and with the application of LBNP, and postflight (seated and supine). Box boundaries represent the 25th and 75th percentiles, with whiskers representing the 10th and 90th percentiles. Median and mean values are shown as solid and dotted lines within the boxes, respectively, with dots indicating outlying points. A linear mixed-effects model was used and found a main effect of measurement time point (P < 0.001). Tukey’s post hoc analysis results are indicated by the symbols as follows: *different from supine (P < 0.05); #different from seated (P < 0.05); and †value with LBNP application is significantly different from respective resting value (P < 0.05). The sample number and sex breakdown of each time point are as follows: pre-supine, n = 14, 3 females; FD45, n = 9, 2 females; FD45 + LBNP, n = 10, 2 females; FD150, n = 13, 2 females; FD150 + LBNP, n = 12, 2 females; R + 40-supine, n = 12, 2 females; R + 180-supine, n = 9, 1 female. FD45, 45th day of spaceflight; FD150, 150th day of spaceflight; LBNP, lower body negative pressure; R + 40, 40th day after returning to Earth from spaceflight; R + 180, 180th day after returning to Earth from spaceflight.
DISCUSSION
The current study investigated jugular vein volume, portal vein cross-sectional area, and cerebral venous velocity as indicators of cephalad fluid shifts during prolonged spaceflight. In addition, this study tested the ability of prolonged, moderate levels (−25 mmHg) of LBNP to reverse the headward fluid shift and return these variables to resting, preflight levels. Consistent with the hypotheses, JVvol, PV, and MCV were all elevated during spaceflight. Also consistent with the hypotheses, LBNP application lowered all three variables back to at least preflight supine levels, suggesting that LBNP is an effective countermeasure against physiological adaptations to sustained cephalad fluid shifts with spaceflight.
Acute Fluid Shifts Preflight
Before spaceflight, response to acute cephalad fluid shifts induced by HDT was assessed with and without the application of LBNP. As expected, HDT data in the present study resulted in increased JVvol consistent with exposure to acute dry immersion (11, 12) and acute HDT −6° (16) and HDT −15° (23). Also, consistent with previous work (16), LBNP reduced JVvol from HDT values back to supine levels. In contrast to results from previous HDT and acute dry immersion (11, 12), PV was not increased with HDT potentially due to individual variability and small sample size from missing measures. However, the effect of LBNP was noted, as PV was significantly reduced with LBNP compared with HDT values as previously observed during HDT (6).
Venous Responses to Spaceflight
With spaceflight, JVvol was increased on both FD45 and FD150. This is consistent with the observations previously reported with spaceflight (5, 8, 20), HDT (5, 6, 23), and dry immersion (12). This increase in JVvol, which was sustained throughout the flight, and reduced venous blood flow velocity potentially increased risk of thrombus formation (8). Stagnation of fluid may also impact organs of the head and neck as indicated by eye fundus edema (7), optic disk edema (1), and increased thyroid volume (11). In addition, increased JVvol may be indicative of and may serve as a surrogate example of the overall fluid shift toward the head (cerebral spinal fluid and lymph) in the absence of gravity and potential increase in intracranial pressure. At present, we do not have information inflight on how the brain tissue could be affected by the headward fluid shift, but postflight MRI shows residual modification of the brain vessel lumen and tissue and CSF volume and placement (24, 25).
Spaceflight increased MCV, which is consistent with results from dry immersion studies (11, 12). The observed increase in MCV may result from a narrowing of venous vessels, as cerebral arterial blood velocity and carotid artery blood velocity are not different from preflight values after 1 wk to 6 mo in space (5, 26). An investigation of the astronaut brain structure following long-duration spaceflight also noted a narrowing of cerebral spinal fluid spaces and intracerebral veins (24), which suggests the possibility of a similar narrowing of cerebral venous structures in the present study. As the skull is a ridged structure with a set volume, the narrowing of cerebral spinal fluid spaces and cerebral veins (as observed postflight) could act as a buffer against increasing intracranial pressure by allowing for a volume expansion in other areas without a corresponding increase in intracranial pressure, thus resulting in increased MCV with limited or no increase in intracranial pressure. Subsequently, increased MCV may be an indicator of conditions that could result in structural remodeling as has been observed with postflight MRI assessments (24).
Contrary to dry immersion (11), the magnitude of MCV increase was not related to the magnitude of jugular vein volume increase with spaceflight. This may be due to the timing of measurements, as values were assessed after only the first 2 h of dry immersion and spaceflight assessments after 45 or 150 days in the microgravity environment with sustained cephalad fluid shifts. Alternatively, the lack of a relationship after prolonged exposure to fluid shifts could indicate different adaptations for the venous structures within the confines of a rigid skull and those of the neck. Regardless, the observed cerebral vein velocity increase, measured for the first time during spaceflight, suggests the existence of a potentially unsuitable brain venous network compression during spaceflight. Unfortunately, an unsuitable modification of the surrounding brain tissue volume and content cannot be investigated at present.
In addition to increased JVvol and MCV, PV also increased with spaceflight. This is consistent with previous investigations with spaceflight (20), head-down tilt (6, 7), and dry immersion (11). Although the increase in JVvol indicates an engorgement of fluid in the head and neck, increased PV suggests fluid pooling within the splanchnic region with a potential influence on organ function and metabolism.
LBNP Effects during Spaceflight
LBNP is frequently used to induce fluid shifts similar to what is experienced when transitioning from supine to standing in a gravitational environment (LBNP of −45 mmHg). Therefore, during spaceflight, LBNP can be used to apply an orthostatic-like stress in the absence of gravity. As such, LBNP has been previously used during spaceflight to determine changes in orthostatic tolerance and to assist subjects readapting to Earth’s gravity (14, 19). In the current study, consistent with the study hypotheses, LBNP (−25 mmHg) applied on both FD45 and FD150 counteracted the cephalad fluid shift and reduced JVvol, PV, and MCV to preflight supine levels.
LBNP induces a visible venous blood shift from the jugular vein and a reduction of the stroke volume, which lowers middle cerebral arterial flow mean velocity as previously reported in 6-mo inflight LBNP studies (−5% at −25 mmHg and −10% at −45 mmHg) (19, 26). LBNP also likely induced a nonvisible displacement of cerebral spinal fluid from the head to the lumbar area (spinal cul-de-sac), as the bottom of the lumbar column is submitted to the drop in pressure inside the LBNP chamber (Chibis suit) (9). Thus, the drop in intracranial vein velocity during LBNP might be related to the blood and CSF shift away from the head (resulting in less compression of the cerebral veins) but also to the drop in middle cerebral arterial flow.
Although LBNP during spaceflight returned JVvol to preflight supine levels, it was unable to return this variable to preflight seated levels in contrast to the assessment of LBNP with acute fluid shift preflight. This could be related to increased tissue edema experienced with long-duration spaceflights. Superficial tissue edema has been observed with spaceflight and simulated microgravity (9, 11, 27). Although LBNP promotes the translocation of fluid toward the legs, the duration and the intensity used in the current study were only sufficient to restore all variables to preflight supine level and PV to preflight seated level. The effect of LBNP on each of these variables does suggest a potential benefit of LBNP if used more frequently throughout the spaceflight or for longer duration or at higher magnitudes.
The use of LBNP as a countermeasure during head-down bed rest has been found to maintain the cardiac volume and peripheral vascular resistance, with LBNP applied several times a day for 23 min at −25 mmHg (28). The repeated use of prolonged (1 h), moderate-level (−25 mmHg) LBNP has not previously been investigated. Therefore, it is unknown if, or for how long, the observed venous changes persist after LBNP is removed or if there is a training effect that may help to prevent SANS. Work with thigh cuffs (Russian bracelets), which are worn for 12 h and also produce a fluid shift toward the legs, has demonstrated that cardiovascular effects dissipate within the 12 h when the thigh cuffs were not in use (29). Therefore, it can be assumed that the effects of LBNP would also disappear within a few hours after the LBNP had been stopped; however, this has yet to be confirmed. Currently, an LBNP system is being developed that could be worn by subjects during daily activities (30) and may be used to determine the effectiveness of LBNP as a countermeasure against physiological adaptations to fluid shifts during spaceflight.
Limitations
Although this study reported on data from 14 subjects, data were missing from several measurement time points. This was in part due to the technical nature of the measurements being conducted. Subjects were given 3 h of training preflight, which was sufficient for some measures but not others. As a result, the measurement success rate was much higher on FD150, as subjects were more practiced at acquiring the ultrasound measurements.
The current study used JVvol as an indication of cephalad fluid shifts and a potential lack of venous outflow. However, the jugular vein only represents one pathway for blood to leave the cerebral circulation. Currently, it is unclear how other veins of the head and neck are affected by spaceflight, which may contribute to changes in cerebral blood flow, intracranial pressure, tissue edema, and the development of SANS.
Conclusions
The increase in JVvol and PV confirmed a sustained cephalad fluid shift resulting in blood accumulation at the head and splanchnic regions, respectively. The observed increase in MCV suggests that this fluid shift impacts cerebral venous circulation and possibly the surrounding structures (CSF network and brain tissue). The application of −25 mmHg LBNP restored these variables at least back to preflight supine levels. Therefore, LBNP application during spaceflight may be an effective countermeasure against SANS and other physiological consequences of sustained cephalad fluid shifts.
GRANTS
This study was supported by NASA Grant Nos. NNX13AJ12G “Fluid Distribution before, during and after Prolonged Space Flight,” NSSC19K0301 “Venous Congestion and Directed Countermeasures,” 80NSSC19K0409 “Self-Generated LBNP for Deep-Space Missions,” and NNJ11ZSA002NA “Distribution of Body Fluids during Long Duration Space Flight and Subsequent Effects on Intraocular Pressure and Vision Disturbance” (to M.B.S.); Doug/Dulchavsky FS NASA Grant No. NNX13AK30G; and CNES (French space agency) Grant No. DAR: 480000510 (to P.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.A., B.R.M., D.J.E., S.S.L., A.E.S., S.M.C.L., and S.A.D., M.B.S., and A.R.H., conceived and designed research; A.E.S. and D.S.M. performed experiments; K.A.Z. and D.S.M. analyzed data; P.A., K.A.Z., D.J.E., S.S.L., and A.R.H. interpreted results of experiments; P.A. prepared figures; P.A. drafted manuscript; K.A.Z., B.R.M., D.J.E., S.S.L., A.E.S., D.S.M., S.M.C.L., S.A.D., M.B.S., and A.R.H. edited and revised manuscript; P.A. and A.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our research collaborators and technical assistants at NASA JSC and KBR, Houston, Texas. We also thank the ISS crew members who volunteered for these studies.
REFERENCES
- 1.Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R, Polk JD. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118: 2058–2069, 2011. doi: 10.1016/j.ophtha.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Macias BR, Liu JHK, Grande-Gutierrez N, Hargens AR. Intraocular and intracranial pressures during head-down tilt with lower body negative pressure. Aerosp Med Hum Perform 86: 3–7, 2015. doi: 10.3357/AMHP.4044.2015. [DOI] [PubMed] [Google Scholar]
- 3.Zhang LF, Hargens AR. Spaceflight-induced intracranial hypertension and visual impairment: Pathophysiology and countermeasures. Physiol Rev 98: 59–87, 2018. doi: 10.1152/physrev.00017.2016. [DOI] [PubMed] [Google Scholar]
- 4.Wiener TC. Space obstructive syndrome: Intracranial hypertension, intraocular pressure, and papilledema in space. Aviat Space Environ Med 83: 64–66, 2012. doi: 10.3357/asem.3083.2012. [DOI] [PubMed] [Google Scholar]
- 5.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol 86: 157–168, 2001. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- 6.Arbeille P, Kerbeci P, Mattar L, Shoemaker JKK, Hughson RLL. WISE-2005: tibial and gastrocnemius vein and calf tissue response to LBNP after a 60-day bed rest with and without countermeasures. J Appl Physiol (1985) 104: 938–943, 2008. doi: 10.1152/japplphysiol.01021.2007. [DOI] [PubMed] [Google Scholar]
- 7.Besnard S, Roumy J, Tobal N, Herault S, Porcher M, Boulay J, Arbeille P. Venous stagnation induced by 7 days in HDT, in the cerebral, opthalmic, renal and splanchnic territories. J Gravit Physiol 9: P75–P76, 2002. [PubMed] [Google Scholar]
- 8.Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón-Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz-Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw open 2: e1915011, 2019. doi: 10.1001/jamanetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurie SS, Lee SMC, Macias BR, Patel N, Stern C, Young M, Stenger MB. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol 138: 165–172, 2020. doi: 10.1001/jamaophthalmol.2019.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurie SS, Macias BR, Dunn JT, Young M, Stern C, Lee SMC, Stenger MB. Optic disc edema after 30 days of strict head-down tilt bed rest. Ophthalmology 126: 467–468, 2019. doi: 10.1016/j.ophtha.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Arbeille P, Avan P, Treffel L, Zuj K, Normand H, Denise P. Jugular and portal vein volume, middle cerebral vein velocity, and intracranial pressure in dry immersion. Aerosp Med Hum Perform 88: 457–462, 2017. doi: 10.3357/AMHP.4762.2017. [DOI] [PubMed] [Google Scholar]
- 12.Arbeille P, Greaves D, Guillon L, Besnard S. Thigh cuff effects on venous flow redistribution during 4 days in dry immersion. Aerosp Med Hum Perform 91: 697–702, 2020. doi: 10.3357/AMHP.5524.2020. [DOI] [PubMed] [Google Scholar]
- 13.Navasiolava NM, Custaud MA, Tomilovskaya ES, Larina IM, Mano T, Gauquelin-Koch G, Gharib C, Kozlovskaya IB. Long-term dry immersion: Review and prospects. Eur J Appl Physiol 111: 1235–1260, 2011. doi: 10.1007/s00421-010-1750-x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell MR, Charles JB. Historical review of lower body negative pressure research in space medicine. Aerosp Med Hum Perform 86: 633–640, 2015. doi: 10.3357/AMHP.4246.2015. [DOI] [PubMed] [Google Scholar]
- 15.Edgell H, Grinberg A, Gagné N, Beavers KR, Hughson RL. Cardiovascular responses to lower body negative pressure before and after 4 h of head-down bed rest and seated control in men and women. J Appl Physiol (1985) 113: 1604–1612, 2012. doi: 10.1152/japplphysiol.00670.2012. [DOI] [PubMed] [Google Scholar]
- 16.Watkins W, Hargens AR, Seidl S, Clary EM, Macias BR. Lower-body negative pressure decreases noninvasively measured intracranial pressure and internal jugular vein cross-sectional area during head-down tilt. J Appl Physiol (1985) 123: 260–266, 2017. doi: 10.1152/japplphysiol.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen LG, Lawley JS, Lilja-Cyron A, Petersen JCG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Juhler M, Levine BD. Lower body negative pressure to safely reduce intracranial pressure. J Physiol 597: 237–248, 2019. doi: 10.1113/JP276557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall-Goebel K, Terlević R, Gerlach DA, Kuehn S, Mulder E, Rittweger J. Lower body negative pressure reduces optic nerve sheath diameter during head-down tilt. J Appl Physiol (1985) 123: 1139–1144, 2017. doi: 10.1152/japplphysiol.00256.2017. [DOI] [PubMed] [Google Scholar]
- 19.Herault S, Fomina G, Alferova I, Kotovskaya A, Poliakov V, Arbeille P. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets). Eur J Appl Physiol 81: 384–390, 2000. doi: 10.1007/s004210050058. [DOI] [PubMed] [Google Scholar]
- 20.Arbeille P, Provost R, Zuj K, Vincent N. Measurements of jugular, portal, femoral, and calf vein cross-sectional area for the assessment of venous blood redistribution with long duration spaceflight (Vessel Imaging Experiment). Eur J Appl Physiol 115: 2099–2106, 2015. doi: 10.1007/s00421-015-3189-6. [DOI] [PubMed] [Google Scholar]
- 21.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 22.Lenth RV. Least-Squares Means: The R Package lsmeans. J Stat Softw 69: 1–33, 2016. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- 23.Marshall-Goebel K, Mulder E, Bershad E, Laing C, Eklund A, Malm J, Stern C, Rittweger J. Intracranial and intraocular pressure during various degrees of head-down tilt. Aerosp Med Hum Perform 88: 10–16, 2017. doi: 10.3357/AMHP.4653.2017. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DR, Albrecht MH, Collins HR, Asemani D, Chatterjee AR, Spampinato MV, Zhu X, Chimowitz MI, Antonucci MU. Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 377: 1746–1753, 2017. doi: 10.1056/NEJMoa1705129. [DOI] [PubMed] [Google Scholar]
- 25.Kramer LA, Hasan KM, Stenger MB, Sargsyan A, Laurie SS, Otto C, Ploutz-Snyder RJ, Marshall-Goebel K, Riascos RF, Macias BR. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology 295: 640–648, 2020. doi: 10.1148/radiol.2020191413. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki KI, Levine BD, Zhang R, Zuckerman JH, Pawelczyk JA, Diedrich A, Ertl AC, Cox JF, Cooke WH, Giller CA, Ray CA, Lane LD, Buckey JC, Baisch FJ, Eckberg DL, Robertson D, Biaggioni I, Blomqvist CG. Human cerebral autoregulation before, during and after spaceflight. J Physiol 579: 799–810, 2007. doi: 10.1113/jphysiol.2006.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirsch KA, Baartz F-J, Gunga H-C, Röcker L. Fluid shifts into and out of superficial tissues under microgravity and terrestrial conditions. Clin Investig 71: 687–689, 1993. doi: 10.1007/BF00209721. [DOI] [PubMed] [Google Scholar]
- 28.Arbeille P, Gauquelin G, Pottier JM, Pourcelot L, Güell A, Gharib C. Results of a 4-week head-down tilt with and without LBNP countermeasure: II. Cardiac and peripheral hemodynamics–comparison with a 25-day spaceflight. Aviat Space Environ Med 63: 9–13, 1992. [PubMed] [Google Scholar]
- 29.Arbeille P, Herault S, Fomina G, Roumy J, Alferova I, Gharib C. Influences of thigh cuffs on the cardiovascular system during 7-day head-down bed rest. J Appl Physiol (1985) 87: 2168–2176, 1999. doi: 10.1152/jappl.1999.87.6.2168. [DOI] [PubMed] [Google Scholar]
- 30.Petersen LG, Hargens A, Bird EM, Ashari N, Saalfeld J, Petersen JCG. Mobile lower body negative pressure suit as an integrative countermeasure for spacelight. Aerosp Med Hum Perform 90: 993–999, 2019. doi: 10.3357/AMHP.5408.2019. [DOI] [PubMed] [Google Scholar]