Keywords: climbing fiber, periodic, prediction, timing, whisker
Abstract
Cerebellar Purkinje neurons help compute absolute subsecond timing, but how their firing is affected during repetitive sensory stimulation with consistent subsecond intervals remains unaddressed. Here, we investigated how simple and complex spikes of Purkinje cells change during regular application of air puffs (3.3 Hz for ∼4 min) to the whisker pad of awake, head-fixed female mice. Complex spike responses fell into two categories: those in which firing rates increased (at ∼50 ms) and then fell [complex spike elevated (CxSE) cells] and those in which firing rates decreased (at ∼70 ms) and then rose [complex spike reduced (CxSR) cells]. Both groups had indistinguishable rates of basal complex (∼1.7 Hz) and simple (∼75 Hz) spikes and initially responded to puffs with a well-timed sensory response, consisting of a short-latency (∼15 ms), transient (4 ms) suppression of simple spikes. CxSE more than CxSR cells, however, also showed a longer-latency increase in simple spike rate, previously shown to reflect motor command signals. With repeated puffs, basal simple spike rates dropped greatly in CxSR but not CxSE cells; complex spike rates remained constant, but their temporal precision rose in CxSR cells and fell in CxSE cells. Also over time, transient simple spike suppression gradually disappeared in CxSE cells, suggesting habituation, but remained stable in CxSR cells, suggesting reliable transmission of sensory stimuli. During stimulus omissions, both categories of cells showed complex spike suppression with different latencies. The data indicate two modes by which Purkinje cells transmit regular repetitive stimuli, distinguishable by their climbing fiber signals.
NEW & NOTEWORTHY Responses of cerebellar Purkinje cells in awake mice form two categories defined by complex spiking during regular trains of brief, somatosensory stimuli. Cells in which complex spike probability first increases or decreases show simple spike suppressions that habituate or persist, respectively. Stimulus omissions alter complex spiking. The results provide evidence for differential suppression of olivary cells during sensory stimulation and omissions and illustrate that climbing fiber innervation defines Purkinje cell responses to repetitive stimuli.
INTRODUCTION
For the correct, well-timed execution of motor and cognitive behaviors, neurons involved in sensorimotor integration must track temporal intervals precisely. Although time is encoded at different scales by several brain regions (1–3), timing of absolute subsecond intervals requires the cerebellum (4, 5). For instance, the cerebellum is necessary for associative learning tasks that involve predicting unconditioned stimuli after conditioned stimuli of 100–1,000 ms (6–10), and cerebellar damage yields motor-independent deficits in timing absolute intervals (11). Whether the cerebellum also contributes to coding relative timing is debated, however. Human functional (f)MRI studies show that such “beat-based” timing activates the basal ganglia rather than the cerebellum (12, 13). Nevertheless, in tasks requiring attention to the regularity of stimulus trains, single-unit electrophysiological recordings in macaques reveal rhythmic firing patterns during periodic stimulation, as well as responses to violations of periodicity, in the cerebellar nuclei as well as the caudate nucleus (14, 15). The finding that cerebellar output neurons encode relative as well as absolute timing raises the question of how Purkinje neurons, which form the sole output of the cerebellar cortex and which project to the cerebellar nuclei, respond to regular stimulus trains and disruptions in periodicity.
Purkinje neurons receive sensory and motor command signals via parallel fibers and climbing fibers, which influence simple and complex spikes. In mice, stimulation of the whiskers evokes well-timed changes in simple spiking with latencies of a few milliseconds and changes in complex spiking after tens of milliseconds; simple and complex spike rates also vary during whisker movements (16–20). As predicted by early theoretical work (21, 22), simple spikes also undergo long-term modulation by complex spikes. Elevating complex spiking in vivo, by electrical or pharmacological activation of the inferior olive, greatly decreases simple spike rates; conversely, reducing complex spikes through silencing of the inferior olive dramatically increases simple spiking (23–26). Well-timed climbing fiber activity also drives plasticity of synaptic inputs to Purkinje cells, altering simple spikes (27–30), and, in awake animals, repeated presentation of complex spike-eliciting stimuli can reduce simple spiking, facilitating behaviors favored by disinhibition of Purkinje cell targets (31–33). Surprisingly, however, it remains a question how persistent, periodic stimulation modulates simple and complex spikes in awake animals in the absence of a learning task.
Here, we investigated Purkinje cell responses in awake, head-fixed mice to prolonged trains of air puffs, applied to the whisker pad at regular subsecond intervals and interrupted by stimulus omissions, in a task-free context. We find that Purkinje cell responses are dominated by either a strong elevation or a strong suppression of complex spike rate and also showed a short-latency, brief simple spike suppression. With repeated stimuli, basal simple spike rates drop below prestimulus levels, and sensory-evoked simple and complex spike patterns also change consistently. The data provide evidence for two response profiles of Purkinje neurons that are distinctly modulated by regular sensory stimulation but similarly sensitive to omissions.
MATERIALS AND METHODS
Mice
All experiments were done in accordance with federal and institutional guidelines and were approved by the Northwestern University Institutional Animal Care and Use Committee (IACUC) (Animal Welfare Assurance Number A3283-01, protocol numbers IS00000242 and IS00014844; PI, I. M. Raman). Pcp2-Cre mice (Jax 004146) and Ai32 mice (Jax 024109) were crossed to produce Pcp2-Ai32 offspring, which expressed channelrhodopsin (ChR2) in Purkinje cells, although optogenetic stimulation was not ultimately used for identification of Purkinje cells. Mice were housed on a 12:12-h day-night cycle, with free access to food and water. Experiments were performed exclusively on female mice since they more readily habituated to the recording setup than did males (see below). The electrophysiological data in the study come from a total of three mice, and three additional mice (C57BL/6; Jackson Laboratories) were used for whisker tracking.
Surgeries
Mice were fully anesthetized with isoflurane (Henry Schein Animal Health, NDC 11695-6776-2). Lidocaine (2%, Henry Schein Animal Health, VINB-0145–8300) was then injected under the scalp, and a metal headplate for head fixation was implanted across the parietal cranial bones (20, 34). Surgical microbind screws (1/16 SL; Fisher) were implanted in frontal and interparietal cranial bones to improve the adherence of the dental cement holding the headplate to the skull. Buprenorphine SR-LAB (1 mg/kg sc; ZooPharm) was administered perioperatively for analgesia. After headplate surgery mice recovered for 3–5 days, after which they were habituated to sitting in a nonconstraining plastic tube on the recording setup for 1–2 h daily for 3 days. During habituation, puffs were randomly administered (∼1/min) to accustom the mouse to the stimulus. Next, under isoflurane anesthesia, mice underwent a craniotomy above cerebellar crus I with the center of craniotomy 7 mm caudal and 3.2 mm lateral from bregma. Both after surgery and between recording sessions, craniotomies were rinsed with povidone iodine (2.5%) and saline (0.9%) and covered with Kwik-Sil (World Precision Instruments) to prevent infection. Buprenorphine SR-LAB (1 mg/kg sc; ZooPharm) was again administered perioperatively for analgesia.
Electrophysiology
Recordings were made from cerebellar Purkinje neurons in awake, head-fixed mice sitting in a plastic tube. Extracellular, loose cell-attached recordings were made with borosilicate patch pipettes (3–5 MΩ) filled with Tyrode solution (mM: 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose, pH 7.35 with NaOH; chemicals from Sigma-Aldrich). Data were acquired with a MultiClamp 700B amplifier in voltage-clamp mode, a Digidata 1550B, and pCLAMP software (Molecular Devices). Recordings were targeted to cerebellar crus I/II at 3–3.5 mm lateral/7–7.2 mm caudal from bregma, 200–2,000 μm below the surface of cortex. The loose cell-attached configuration permitted a high signal-to-noise ratio and good isolation of single units. Purkinje cells were identified by their characteristic simple spike and complex spike waveforms. As many recordings were obtained as possible from each mouse. To prevent the overlap of electrode paths, successive recording sites were separated by 50–100 μm. Mice were euthanized with Euthasol after 1–3 days of recording.
Stimulation Protocol
Air puffs (10 ms, 20 psi) were applied to the whisker pad ipsilateral to the recording site (1–2 cm from face) and controlled by a solenoid valve. Air puffs were triggered by an Arduino Uno programmed with open-source Arduino IDE software. Regular trains of puff stimuli were applied with an interstimulus interval of 300 ms. Each train consisted of 18–22 air puffs, after which 1 puff was omitted, yielding a 600-ms interval between the last puff of one train and the first puff of the next. The irregular occurrence of the omission was chosen to minimize the likelihood that the mice would anticipate the omission. A complete run consisted of 40 trains, for a total of 780 air puffs per cell, with 39 omissions that were flanked by puffs.
In separate experiments without electrophysiological recording, whiskers were tracked in three mice. All whiskers on the left side except C1 and C2 were trimmed for ease of video tracking. As during physiological recording, repetitive puffs were applied to the right whisker pad, where all whiskers remained intact. Whiskers were imaged at 250 frames per second with a Genie Nano M640 NIR camera (Teledyne DALSA Inc.), and the position of the left C1 whisker was tracked off-line with DeepLabCut (35). Each run consisted of 780 puffs with 300-ms intervals, with 39 omissions. In each mouse 3–5 runs were recorded, with a 20-min interval between runs, on 3 consecutive days, for a total of 42 runs (for each of the 3 mice, 13, 14, and 15 runs). Whisker position is reported as whisker angle given as degrees from the midline, with 0° directly behind, 90° directly to the left of, and 180° directly in front of the mouse.
Data Analysis, Exclusion Criteria, and Statistics
Electrophysiological recordings were analyzed with pCLAMP (Molecular Devices), MATLAB (MathWorks), and IGOR Pro (WaveMetrics) software. Recordings were first band-pass filtered between 100 and 5,000 Hz, differentiated, and z scored. Spikes were detected by automated thresholding at a z score of 3–5 [relative to the mean and standard deviation (SD) of the full trace], with threshold inspected in each 1-s block and adjusted manually as necessary to ensure that spikes that decreased in amplitude owing to high-frequency firing were not missed. Next, spikes were classified as simple or complex based on plots of the SD against the mean of trace in a 3-ms window after each spike. Complex spikes clustered as a group with a higher SD and a mean that differed from baseline mean of the full trace, whereas simple spikes clustered with a low SD close to baseline. The record was then visually inspected to verify that simple and complex spikes occurring in rapid succession were correctly classified and to confirm that every complex spike was appropriately identified.
To quantify timing of simple spikes, peristimulus time histograms (PSTHs) were generated with 2-ms bins. PSTHs were made for each cell and, where noted, then averaged across cells to generate the population response. The number of simple spikes per run per 2-ms bin was multiplied by 500 to convert to a “rate” for ease of comparison, although this analysis best reflects simple spike probability within a 2-ms window. These axes are labeled “2-ms rate.” In all other analyses, firing rates were quantified by calculating the number of spikes per 20-ms bin (except as noted), incremented in 1-ms steps to generate a smoothed “rate trace.”
We obtained recordings from 24 Purkinje cells that were stable over all 40 trains. Visual inspection of the recordings indicated that many cells responded to puffs within 100 ms with reliable changes (either increases or decreases) of complex spikes. All 24 cells were therefore tested for statistically significant (≥2 SDs from the mean) changes in complex spike rate relative to baseline. Of these cells, 22 Purkinje cells showed a biphasic response that consisted of a significant change (increase or decrease) in complex spike rate within 100 ms of the puff that was followed by a rate change of the opposite polarity within 200 ms of the puff (P < 0.06, paired, max or min rate vs. baseline). The remaining two cells lacked this biphasic response in complex spike rate (P > 0.3, paired, max or min rate vs. baseline). We also tested the cells for 1) a transient simple spike suppression after the puff and 2) a modulation in spike parameters with time (detailed in results). The 22 cells with biphasic changes in complex spike rate all exhibited one or both of these additional characteristics, whereas the two additional cells showed neither of these responses and did not display any detectable change in simple spike rate or timing in response to the puffs. These two cells were therefore excluded, and the analyses reported below represent data from the 22 cells with biphasic complex spike responses as well as simple spike modulation.
All further statistical comparisons were made with Student’s two-tailed t test (unpaired, unless noted as paired), and P values are reported. Data are reported as means ± SE unless noted as SD.
RESULTS
Two Categories of Purkinje Cell Responses to Puff Stimulation
To test how Purkinje neurons respond to regular, repetitive sensory stimuli, we made extracellular recordings of crus I/II Purkinje cells of awake, head-fixed female mice trained to sit in a nonconstraining tube (Fig. 1A, left). Trains of air puffs (10 ms, 50 psi) with a 300-ms interpuff interval were applied to the whisker pads of mice. After each train of 18–22 stimuli, a single puff was omitted, producing a 600-ms interval (Fig. 1A, right); 40 such trains constituted a run, for a total of 780 puffs per run. The simple spike and complex spike firing rates of Purkinje cells, each recorded during a full run, were analyzed to assess the responses to repeated stimulation.
Figure 1.
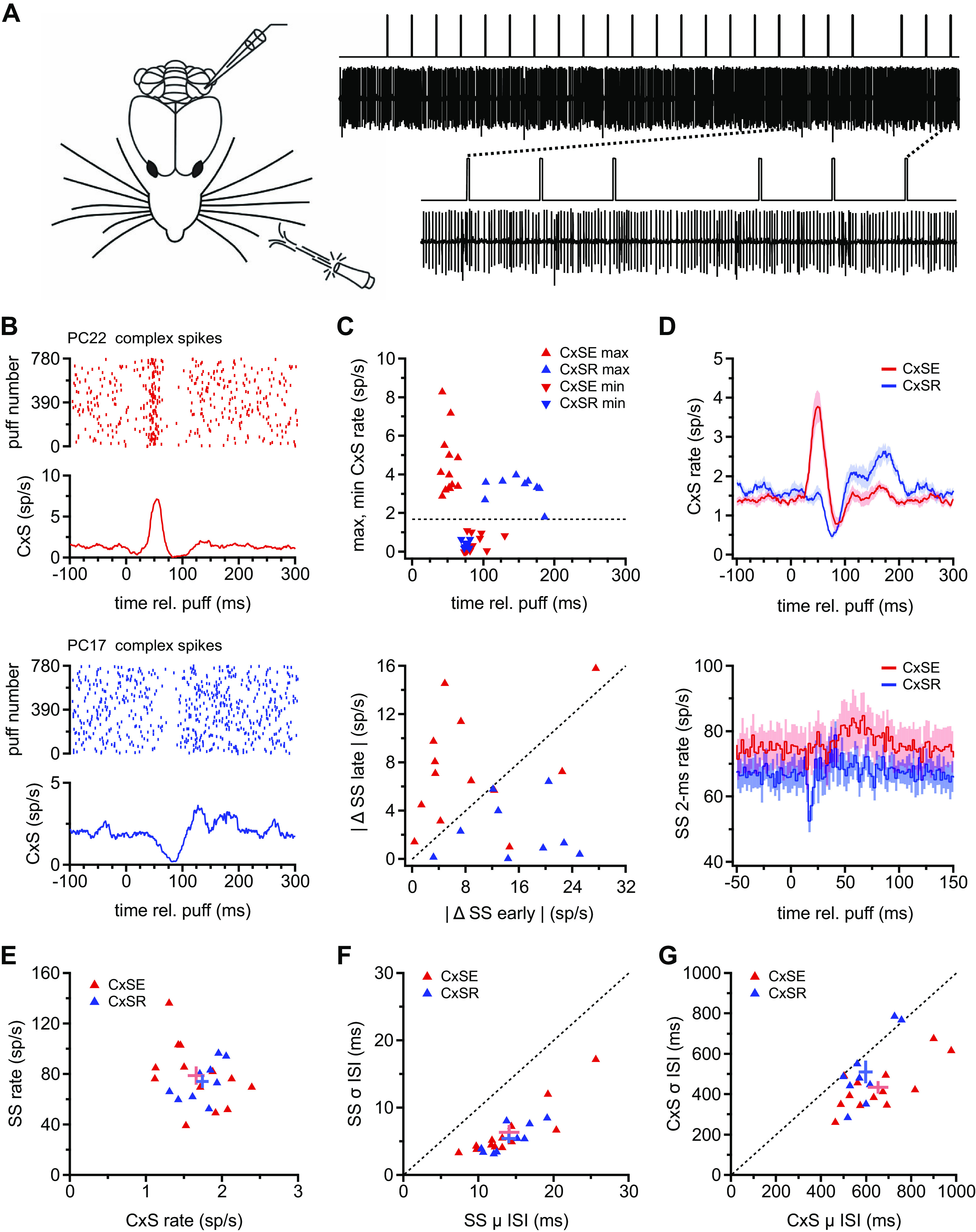
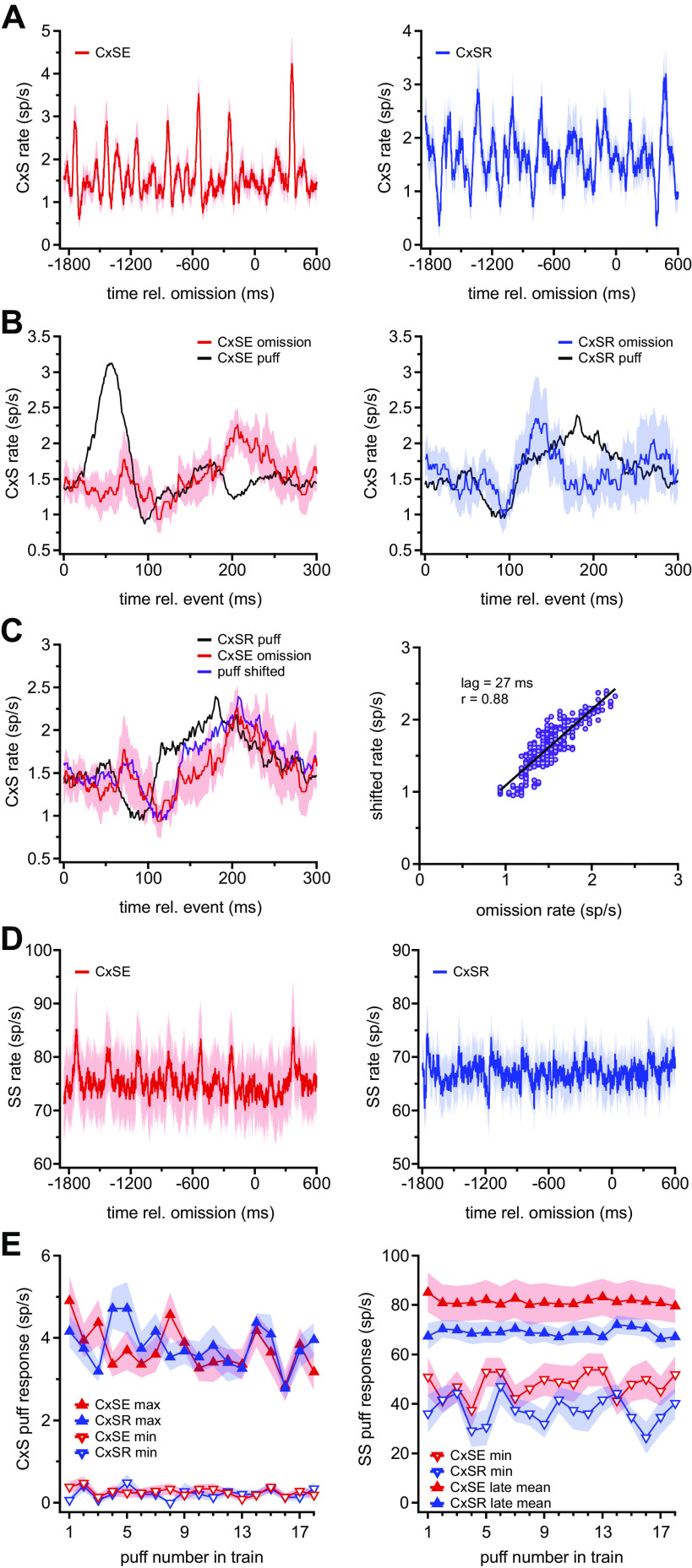
Two types of Purkinje cell responses to repetitive whisker stimulation with air puffs. A, left: schematic of air puff stimulation applied to the ipsilateral whisker pad during Purkinje cell recordings from awake mice. Right: illustration of a sample recording and the stimulus protocol. The stimulus was composed of trains of air puffs with a 300-ms interstimulus interval and an omission every 18–22 stimuli. Dotted lines indicate the region of the record at top that is expanded at bottom. B: sample rasters of complex spikes (CxSs) showing either well-timed spikes (top) or a gap in spikes consistently following air puffs (bottom). C, top: maximum (▴) and minimum (▾) complex spike firing rates from the mean rate traces calculated as in B for each Purkinje cell. Data from cells classed as complex spike elevated (CxSE) and complex spike reduced (CxSR) are red and blue, respectively. Dotted line indicates the mean prestimulus CxS rate across all cells. Bottom: absolute values of the mean change in simple spike (SS) rate between 50 and 74 ms (“late”) vs. the minimum change in SS rate between 14 and 18 ms (“early”) for each Purkinje cell. Data from cells classed as CxSE and CxSR are red and blue, respectively. D, top: traces of mean CxS firing rate for all 780 puffs averaged across all 13 CxSE cells (n = 13, red) and all CxSR cells (n = 9, blue). Bottom: population peristimulus time histograms (2-ms bins) of simple spikes, averaged for all 780 puffs for all CxSE cells (red) and all CxSR cells (blue). E: prestimulus SS rate vs. CxS rate for all cells (symbols). Mean and SE for each group are indicated with the crossed error bars. F: prestimulus SD (σ) on the interspike interval (ISI) vs. mean (μ) SS ISI for all cells. Mean and SE as in E. G: as in F for CxS. rel., Relative to.
Initial inspection of the data suggested that the most salient difference across cells was that the puff-evoked complex spike probability appeared to increase in some Purkinje cells and decrease in others. These changes were evident both in the raster plots from all 780 stimuli and in the mean puff-associated complex spike rates (per 20-ms sliding bin; see materials and methods) calculated across all stimuli (Fig. 1B). Individual cells differed, however, in the magnitude of the firing rate change as well as the degree to which the rates oscillated after the initial increase or decrease. To evaluate the extent to which these different puff-associated activity patterns were consistent across subsets of Purkinje cells, we examined the time and polarity of the apparent change in complex spiking. A mean rate trace was computed for complex spikes from each cell, and the maximum and minimum rate were plotted against its time of occurrence. Of 24 Purkinje cells recorded (see materials and methods), 22 showed significant changes in complex spike rate within 75 ms of the stimulus, followed by a switch in the polarity of the response within 200 ms. In 13 neurons, the complex spike rate rose to a maximum of 4.5 ± 0.5 spikes (sp)/s with a latency of 51 ± 2.1 ms after the puff and then fell to a minimum rate of 0.4 ± 0.1 sp/s with a latency of 88.0 ± 4.4 ms (Fig. 1C, top, red symbols). Because the elevation preceded the reduction of firing rate, these neurons are referred to as “complex spike elevated” (CxSE) Purkinje cells. In contrast, in the other nine Purkinje cells, a minimum rate of 0.4 ± 0.1 sp/s occurred 75.4 ± 1.2 ms after the puff, followed by a maximal rate of 3.3 ± 0.2 sp/s at 148.8 ± 10.6 ms (Fig. 1C, top, blue symbols). Because the minimum complex spike rate preceded the maximum, neurons with this response profile are referred to as “complex spike reduced” (CxSR) Purkinje cells. Averaging the puff-evoked changes in complex spike rate in each response category illustrates the within-class similarity and between-class difference in response profiles of CxSE and CxSR cells (Fig. 1D, top).
The existence of two patterns of complex spike responses raised the question of whether the puff-evoked simple spike responses also fell into two (or more) distinct categories. To examine the temporal pattern of puff-evoked simple spikes, PSTHs with 2-ms bins were plotted over all 780 stimuli for each cell. As previously reported (20), many Purkinje cells showed a sensory-evoked, short-latency (14–18 ms), brief (4–6 ms) suppression of simple spiking and/or a longer-latency, more temporally dispersed increase in simple spike rate ∼50–75 ms after the puff, previously correlated with the motor command associated with whisker protraction. The magnitude of the early spike suppression and late rate increase varied across individual Purkinje cells. To test whether these attributes might offer another mode of categorizing Purkinje responses, we plotted the maximum change in simple spike rate between 50 and 74 ms against the maximal simple spike suppression between 14 and 18 ms for each cell (Fig. 1C, bottom). The scatterplot revealed that suppressions were sometimes evident without rate changes and vice versa. Color-coding the cells according to the previous classification based on complex spike responses illustrated that most CxSE cells (10/13) fell above the half-unity line, indicative of large, late responses at least half as large as the preceding suppression in these cells, whereas all CxSR cells (9/9) fell below it, showing that the simple spike suppression in these cells was always more than double the subsequent elevation in rate. Thus, although alternate classification schemes might be informative, the scatterplot indicates that separating the cells on the basis of complex spikes can predict key elements of simple spike responses with reasonable (86%) accuracy.
Indeed, averaging the simple spike response based on the CxSE and CxSR classification (Fig. 1D, bottom) demonstrated that although the short-latency rate decrease deviated significantly from the 100-ms prepuff baseline rate in both groups (CxSE and CxSR: minimum Δ rates: −6.3 ± 2.5 sp/s, P = 0.03 and −14.5 ± 2.6 sp/s, P = 0.0005, paired), the extent of suppression was indeed more prominent in CxSR than CxSE cells (P = 0.036, unpaired). Likewise, the simple spike rate increase between 50 and 75 ms was greater with respect to baseline in CxSE cells versus CxSR cells (maximum Δ rates 50–74 ms: 6.6 ± 1.5 sp/s vs. 1.9 ± 0.9 sp/s, P = 0.03). Thus, Purkinje cells fall into two categories that differ in the pattern of their complex spike responses as well as the pattern of their simple spike responses to tactile sensory input.
To test whether CxSE and CxSR Purkinje cells might differ in stimulus-independent respects, we compared their simple spike rates and their complex spike rates before the application of any puffs. The prestimulus firing rates (before application of any puffs) were indistinguishable between groups, however (Fig. 1E; CxSE vs. CxSR: simple spikes, 78.9 ± 7.1 sp/s vs. 74.2 ± 5.2 sp/s, P = 0.6; complex spikes, 1.7 ± 0.1 sp/s vs. 1.7 ± 0.1 sp/s, P = 0.6). The regularity of simple spike firing, measured from the mean and SD of the interspike interval (ISI) distributions, was also comparable in the two categories of cells (Fig. 1F; CxSE vs. CxSR: mean, 14.1 ± 1.4 ms vs. 14.0 ± 1.0 ms, P = 0.99; SD, 6.4 ± 1.1 ms vs. 5.4 ± 0.7 ms, P = 0.5). Consequently, the coefficients of variation were similar (0.43 ± 0.03 vs. 0.38 ± 0.03, P = 0.2). The mean and SD of ISIs for complex spikes were statistically indistinguishable in the two groups (Fig. 1G; CxSE vs. CxSR: mean, 652.9 ± 44.8 ms vs. 596.9 ± 30.1 ms, P = 0.3; SD, 434.4 ± 31.7 ms vs. 510.8 ± 56.6 ms, P = 0.3), but the relative mean and SD values differed on a per-cell basis, giving a lower coefficient of variation in CxSE cells (CxSE vs. CxSR: 0.67 ± 0.04 vs. 0.84 ± 0.06; P = 0.02). Taken together, although CxSR and CxSE Purkinje cells differ in their responses to sensory input, they largely share common basal firing properties.
Modulation of Purkinje Cell Responses by Repetitive Sensory Stimulation
Observations over the course of the recordings suggested that aspects of the responses to the puffs were not stationary. Although complex spike rates appeared generally stable, simple spike firing rates noticeably decreased in some cells (Fig. 2A). To quantify these data, we averaged the 18–22 puff-evoked responses for each train and then tested for changes in firing rates across all 40 trains. We first examined the mean complex spike rate over the full ∼6-s duration of each train. This analysis, which does not consider the time of occurrence of complex spikes, indicated that overall rates remained stable over the course of the 40 trains for either CxSE or CxSR cells (Fig. 2B; mean rates in first 5 trains vs. last 5 trains: CxSE, 1.6 ± 0.0 sp/s vs. 1.6 ± 0.1 sp/s, P = 0.8; CxSR: 1.7 ± 0.1 sp/s vs. 1.8 ± 0.0 sp/s, P = 0.3, paired). These rates are also similar to the firing rate during the prestimulus baseline (1.7 sp/s in both cell groups), emphasizing that the effect of puffs is to reorganize spike timing rather than to elicit or suppress complex spikes.
Figure 2.
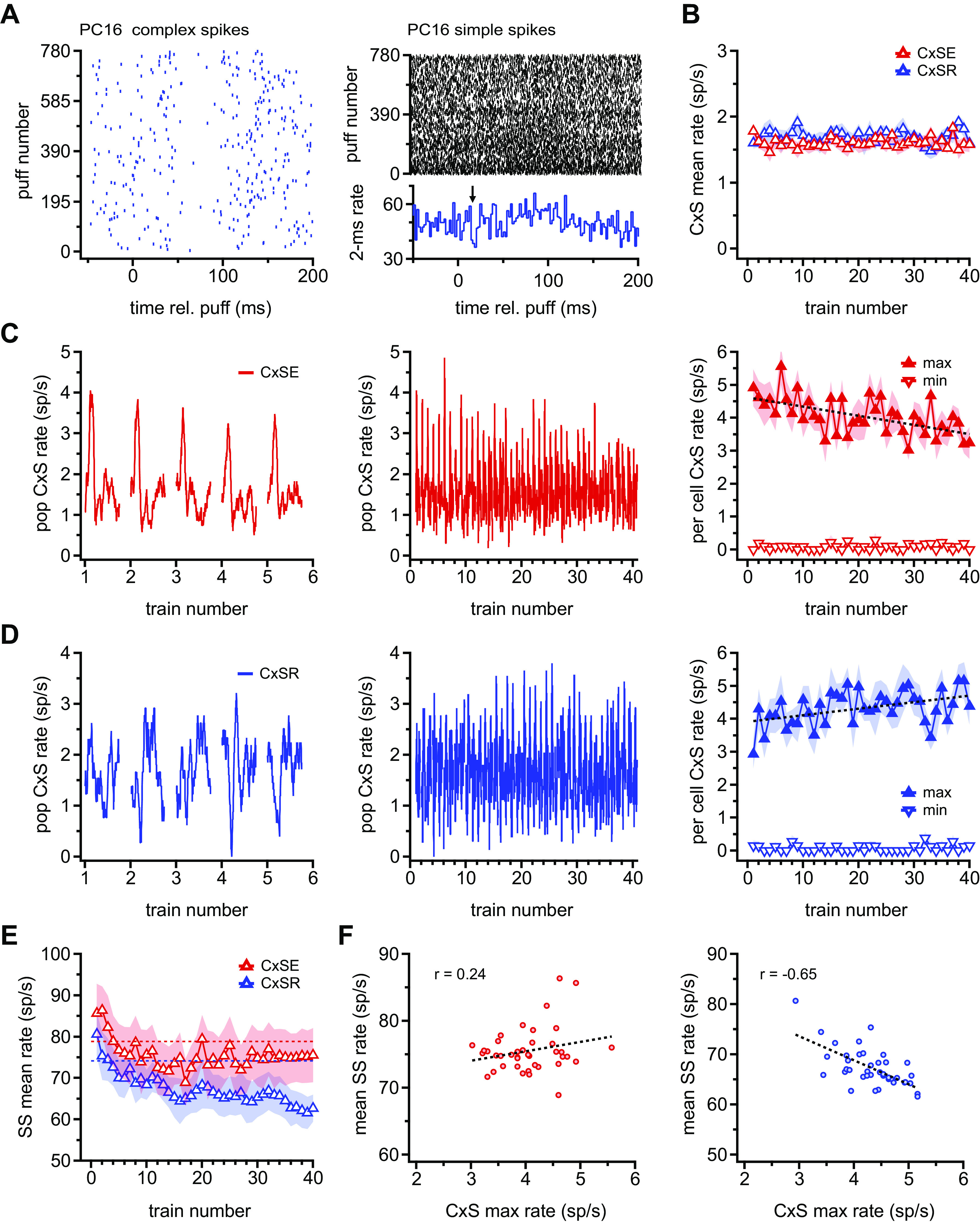
Modulation of Purkinje cell firing with repetitive sensory stimulation. A: sample rasters of complex spikes (CxSs) (left) and simple spikes (SSs) (right) over all 780 puffs for a single complex spike reduced (CxSR) cell. Only the first 200 ms after a puff is shown for clarity. Note the decrease in SS rate across puffs. The 2-ms peristimulus time histogram below the SS raster illustrates the short-latency transient suppression (arrow) and delayed rate increase of SSs of this cell. B: population mean for the CxS rate during each train. Barely visible shading indicates SE. CxSE, complex spike elevated. C, left: population rate traces for CxSs in CxSE cell for trains 1–5. Each 300-ms rate trace is the mean response to the ∼20 puffs in a single train, such that train 1 was calculated from the mean response of each cell to puffs 1 to ∼20, train 2 is the response to puffs ∼21 to ∼40, etc., averaged across all 13 CxSE cells. Center: as at left, for all 40 trains. Right: mean (symbols) and SE (shading) of maximum and minimum CxS rate responses for individual cells, rather than the population mean, over all 40 trains. The window was constrained to 0–75 ms after a puff for the maximum rate and 50–150 ms for the minimum rate. D: as in C, for all 9 CxSR cells. The window was constrained to 50–150 ms for the minimum rate and 100–200 ms after a puff for the maximum rate. E: as in B for SSs. Horizontal dotted lines indicate mean prestimulus firing rates averaged for CxSE (red) and CxSR (blue) cells. F: mean SS firing rate vs. mean CxS for all 40 trains for CxSE (left) and CxSR (right) Purkinje cells. Lines indicate linear fits to the data, and correlation coefficients are indicated. rel., Relative to.
Therefore, we next examined the instantaneous rate traces as in Fig. 1, to take into account the temporal clustering of complex spikes through the postpuff interval. For each cell, a mean rate trace (with 40-ms sliding bins) was calculated for each of the 40 trains. The traces were then averaged across CxSE or CxSR cells to obtain 40 population traces, one for each train, which were plotted sequentially from the first to the fortieth (Fig. 2, C and D, left for an expansion of trains 1–5 and center for trains 1–40). The analysis demonstrated that the maximal complex spike rate evoked by the puff changed across trains in both categories of Purkinje cell responses, but in opposite directions. The population plot, however, slightly underestimated the changes on a per-cell basis, since the peak complex spike rate did not occur at exactly the same time in each cell. To quantify the change more accurately, therefore, we measured the maximum and minimum complex spike rate for each train, for each cell, within the time windows in which these extremes occurred in the full-run (780 puff) averages. In CxSE cells, the per-cell maximal complex spike rate, measured between 0 and 75 ms after puff, dropped from 4.5 ± 0.2 sp/s in the first 5 trains to 3.6 ± 0.2 sp/s in the last 5 trains (Fig. 2C, right; P = 0.0003, paired, slope = −0.03 sp/s/train), whereas the minimum firing rate, measured between 50 and 150 ms, remained stable (in first 5 trains vs. last 5 trains: 0.1 ± 0.0 sp/s vs. 0.1 ± 0.0 sp/s, P = 0.95, paired). In contrast, in CxSR cells, the per-cell maximal complex spike rate, measured between 100 and 200 ms, increased from 3.8 ± 0.2 sp/s in the first 5 trains to 4.6 ± 0.2 sp/s in the last 5 trains (Fig. 2D, right; P = 0.008, paired, slope = +0.02 sp/s/train); the minimum complex spike rate, measured between 50 and 150 ms, was stable (in first 5 trains vs. last 5 trains: first 5 trains: 0.1 ± 0.0 sp/s vs. 0.1 ± 0.1 sp/s, P = 0.7, paired). Because the total number of complex spikes remained approximately constant across trains for both CxSE and CxSR cells, the changes in instantaneous rate indicated that the relative timing of the spikes was altered. Thus, the temporal precision of complex spikes decreased with repetitive stimulation in CxSE cells and increased in CxSR cells.
Next, we tested whether prolonged repetitive stimulation also modulated simple spikes. We again began by averaging the simple spike rate over the full ∼6 s of each train and examining how mean rates changed over 40 trains. In CxSE cells, the mean simple spike rate initially increased above the prestimulus baseline and then decreased over the next 10 trains (Fig. 2E; first train vs. final train: 85.7 ± 5.8 sp/s vs. 75.5 ± 6.8 sp/s, P = 0.04, paired) to a new steady state only slightly below baseline (mean of last 3 trains: 75.4 ± 6.5 sp/s, P = 0.086 vs. baseline, paired). CxSR cells also increased their mean simple spike firing rate during the first train, but the rate then fell through the 40 trains, without reaching a clear steady state (first train vs. final train: 80.6 ± 5.8 sp/s vs. 62.7 ± 4.4 sp/s, P = 0.04, paired; mean of last 3 trains: 62.1 ± 3.8 sp/s, P = 0.066 vs. baseline, paired). Thus, repetitive stimulation led to a gradual change in simple spike rate, accounting for the different prepuff firing rates (Fig. 1D, bottom) when the responses to all puffs are averaged. The data also raise the possibility that in CxSR cells the simple spike rate may provide an approximate readout of the number of stimuli presented.
Since well-timed complex spike activity can induce plasticity of synaptic inputs to Purkinje cells and thereby change simple spike activity, we next tested for any relationship between the gross changes in simple spike rate observed across trains and the alterations of complex spike timing. In CxSE cells, the mean simple spike firing rate in each train did not significantly correlate with the maximum complex spike rate, reflecting the different time courses of the changes in the two responses (Fig. 2F; r = 0.24). In contrast, in CxSR cells, in which both complex and simple spike response patterns changed gradually, a negative correlation was significant (r = −0.65). Thus, as complex spikes occurring 100–200 ms after the puff became more precisely timed, the overall firing rate of simple spikes decreased in CxSR Purkinje cells.
To examine whether short-latency, puff-evoked simple spiking also changed over the course of repeated stimulation, we divided the stimuli into quartiles, each consisting of 195 consecutive puffs, and plotted the mean puff-evoked simple spike PSTHs. Remarkably, in CxSE cells, the early suppression of simple spikes completely disappeared (Fig. 3A, top). The gradual loss of suppression was quantified by plotting the difference between the bin of minimum value and the mean prepuff bin to obtain the change in simple spike rate (Fig. 3B); by the end of the run, the change in spikes per bin at the time of the initial short-latency suppression decreased significantly and was indistinguishable from baseline (first 10 trains vs. last 10 trains: −15.0 ± 3.3 sp/s vs. 2.3 ± 3.6 sp/s, P = 0.0003, paired). In contrast, in CxSR cells the early suppression of simple spikes remained stable (Fig. 3, A, bottom, and C; first 10 trains vs. last 10 trains: −15.8 ± 3.5 sp/s vs. −13.3 ± 3.6 sp/s, P = 0.6, paired). The decrement in CxSE cells but not CxSR cells accounts for the different magnitudes of synchronous suppression when responses were averaged across all 780 stimuli (Fig. 1D, bottom). Thus, Purkinje cells differ in whether or not their simple spikes encode sensory stimuli in a history-dependent or history-independent manner, and how they respond correlates with their complex spike responses to the puff.
Figure 3.
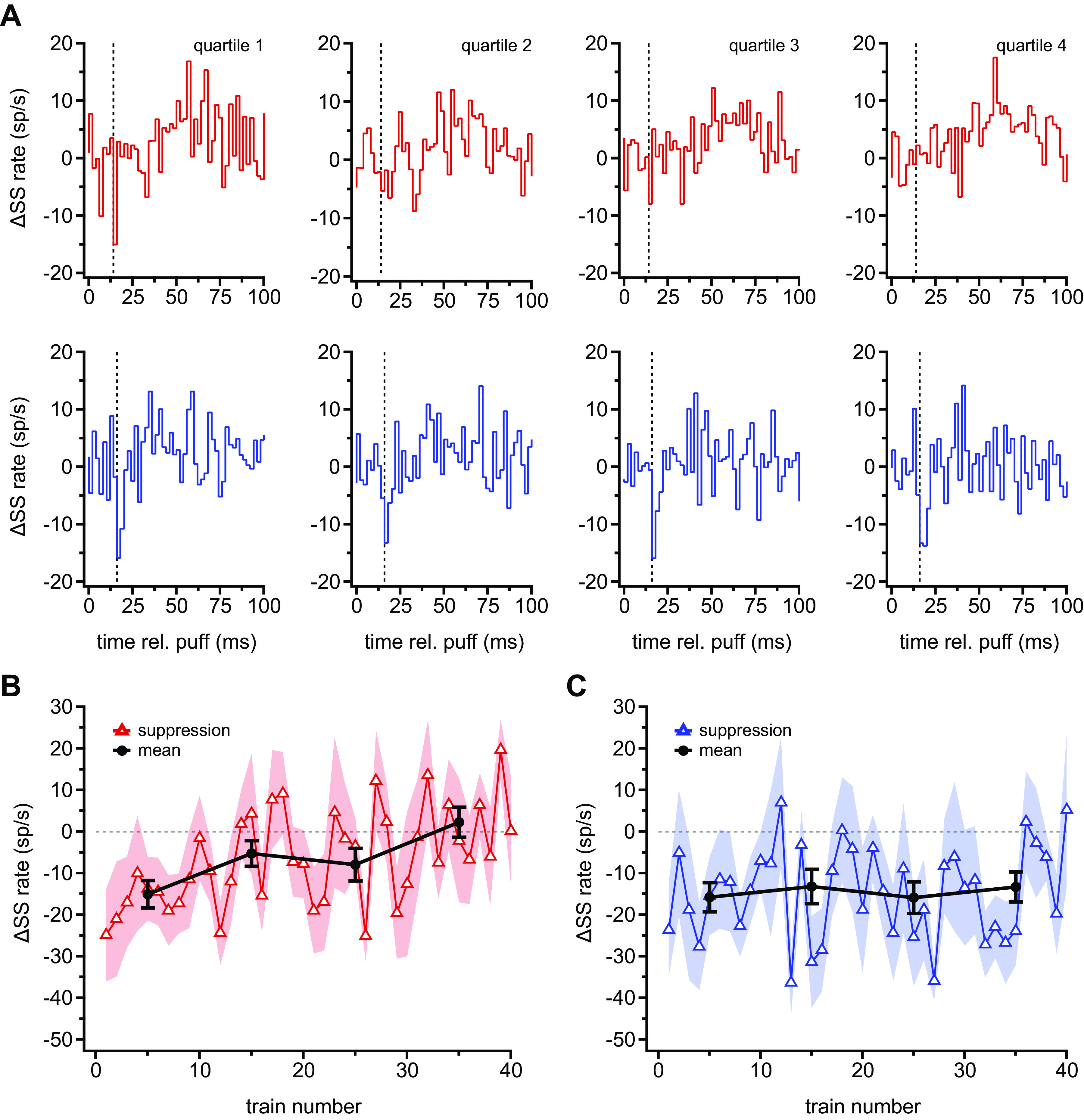
Gradual loss of well-timed simple spike (SS) suppressions in complex spike elevated (CxSE) but not complex spike reduced (CxSR) cells. A: population peristimulus time histograms of SSs (2-ms bins) for successive quartiles (series of 195 puffs) for all 13 CxSE (top) and all 9 CxSR (bottom) cells. Vertical dashed line indicates 14 ms for CxSE cells and 16 ms for CxSR cells, the usual latency to the puff-evoked SS suppression. B: maximal suppression in SS firing rate (measured in the 14–16 ms bin) vs. train number in CxSE cells (△, mean; shading, SE), with the average of each 10-train segment superimposed (black, mean ± SE). C: as in B for CxSR cells, showing maximal suppression (measured in the 16–18 ms bin).
Purkinje Cell Simple and Complex Spikes during Stimulus Omissions
Since elements of the responses of both CxSE and CxSR cells changed consistently over the course of stimulation, and since Purkinje cells are implicated in tasks that require tracking of absolute time intervals, we next investigated whether Purkinje cells showed evidence of responses to the omitted puff. The complex spike rate traces (40-ms sliding bins) for each of the 40 trains were averaged and plotted to illustrate the responses to six stimuli preceding and one stimulus following the omission. In CxSE cells (Fig. 4A, left), the baseline rate for 50 ms before the omission was 1.6 ± 0.2 sp/s. Overlaying the omission response and the mean of three previous puff responses illustrated the absence of the early elevation evoked by the physical stimulus (Fig. 4B, left). Instead, the complex spike firing rate after the omission first fell to 0.9 ± 0.2 sp/s at 111 ms (P = 0.055, paired) and then increased to 2.3 ± 0.2 sp/s, at a latency of 206 ms after omission (P = 0.004, paired; Fig. 4B, left). The suppression phase of the omission response overlapped temporally with the complex spike reduction in the puff-evoked response. The emergence of a late elevation in complex spiking is reminiscent of the CxSR cell response to puffs.
Figure 4.
Complex (CxS) and simple (SS) spikes during stimulus omissions. A: mean CxS rate for all 13 complex spike elevated (CxSE) cells (left) and all 9 complex spike reduced (CxSR) cells (right), for 6 puffs before and 1 puff after each omission, averaged over 39 omissions. Omission occurs at time = 0 ms. B: CxSE (left) and CxSR (right) CxS rate after omission (mean trace, red or blue; SE, shading), mean rate after the stimulus, averaged for the 3 puffs before the omission (black). C, left: as in B, but with omission response of CxSE cells (red), the puff response for CxSR cells (black), and the time-shifted puff response of CxSR cells that gave the highest cross-correlation (purple). Right: solid symbols, correlation of omission response and the time-shifted puff response from the plot at left. Black line, linear fit, with correlation coefficient and lag as indicated. D: as in A for SSs. Note that with the 20-ms sliding bins used to compute rate, the puff-evoked well-timed 2-ms suppressions of SSs are filtered out, and only the later slow rate increases are visible. E: maximal and minimal CxS (left) and SS (right) firing rates in response to repetitive stimuli over the time course of each train, averaged over all 40 trains for all CxSE cells (red) or all CxSR cells (blue). Puff 1 indicates the first stimulus in each train. rel., Relative to.
In CxSR cells (Fig. 4A, right), the baseline rate preceding the omission was likewise 1.6 ± 0.2 sp/s. After the omission, the complex spike rate first dropped to 1.0 ± 0.3 sp/s at a latency of 92 ms (P = 0.04, paired) and then increased to 2.4 ± 0.6 sp/s at a latency of 131 ms (P = 0.3, paired; Fig. 4B, right). As in the case of CxSE cells, superimposing the traces indicated that the response to omissions and to the three preceding puffs both shared an early reduction of complex spiking. The reasonably good overlap during the suppression phase diverges, however, in the elevation phase, which is more broadly timed in the response to puffs. Since CxSE cells also showed a significant suppression followed by elevation of complex spikes after the omission, we tested whether the omission response of CxSE cells might be better correlated with the stimulus-evoked response of CxSR cells, whose biphasic complex spike response follows the same sequence, than it was with the stimulus-evoked response of CxSE cells. Superimposing these records indeed showed a similarity with a delay (Fig. 4C, left), and cross-correlation revealed a remarkably high correlation at a lag of 27 ms (r = 0.88; Fig. 4C, right). Thus, omitted stimuli evoke a delayed pattern of complex spiking in CxSE cells, which closely resembles that evoked by physical stimuli in CxSR cells.
Next, we examined simple spiking during the omission period. Because the limited number of omissions (n = 39) made the PSTHs with 2-ms bins extremely noisy, we tested for the presence of the well-timed suppression of simple spikes associated with real sensory stimulation by examining the z score of the response in the 14–16 ms bin (i.e., when puffs reliably evoked suppressions), calculated relative to the SD of the responses for the 50 bins 100 ms before the omission. For the CxSE cells the z score was +0.11, and for the CxSR cells the z score was +1.49, indicating that no transient suppression response was present in either cell type in the absence of a puff; this result is not surprising especially for CxSE cells, in which the brief synchronous suppression even to physical stimuli disappears over the time that an omission response might be acquired. The results suggest that the simple spike patterns do not predict an omitted sensory stimulus and instead exclusively report true physical stimuli.
To look for less well-timed omission responses, we analyzed simple spike rates with 20-ms sliding bins rather than spike times. In CxSE cells, from a baseline of 74.6 ± 6.0 sp/s, the simple spike rate rose slightly, to 77.1 ± 6.3 sp/s, 140 ms after the omitted stimulus (P = 0.08) and then dropped to a minimum of 70.5 ± 6.4 sp/s at 101 ms (P = 0.02; Fig. 4D, left). In CxSR cells, the baseline simple spike rate was 66.1 ± 4.0 sp/s. After the omission, the maximum rate was 70.7 ± 4.1 sp/s at a latency of 28 ms (P = 0.03; Fig. 4D, right) and the minimum was 62.8 ± 4.8 sp/s at 164 ms (P = 0.02; Fig. 4D, right). Since simple spike rate can correlate with whisker position (19, 20), these small changes may be indicative of movements in the omission interval.
On the rate plots (Fig. 4A), the responses to the first puff after the omission appeared distinct from responses late in the train, particularly for complex spikes. We therefore tested for short-term changes in the magnitude of the response to each puff in a train by averaging the complex or simple spike responses across all 40 trains and measuring the maximum and minimum rates for each of the sequential 18 puffs (the minimal number of puffs in a train; see materials and methods). The maximal firing rates evoked by the first puff after an omission were slightly enlarged in CxSE but not CxSR cells (Fig. 4E); linear fits to the maximal and minimal spike rates over the course of the trains indicated a significant correlation only in the decrease in maximal complex spike rate for CxSE cells (slope = −0.05 spikes/s/puff, r = −0.5, P = 0.03). Together, the data support the idea that the responses of CxSE cells are sensitive to the longer interval, or to the omission itself.
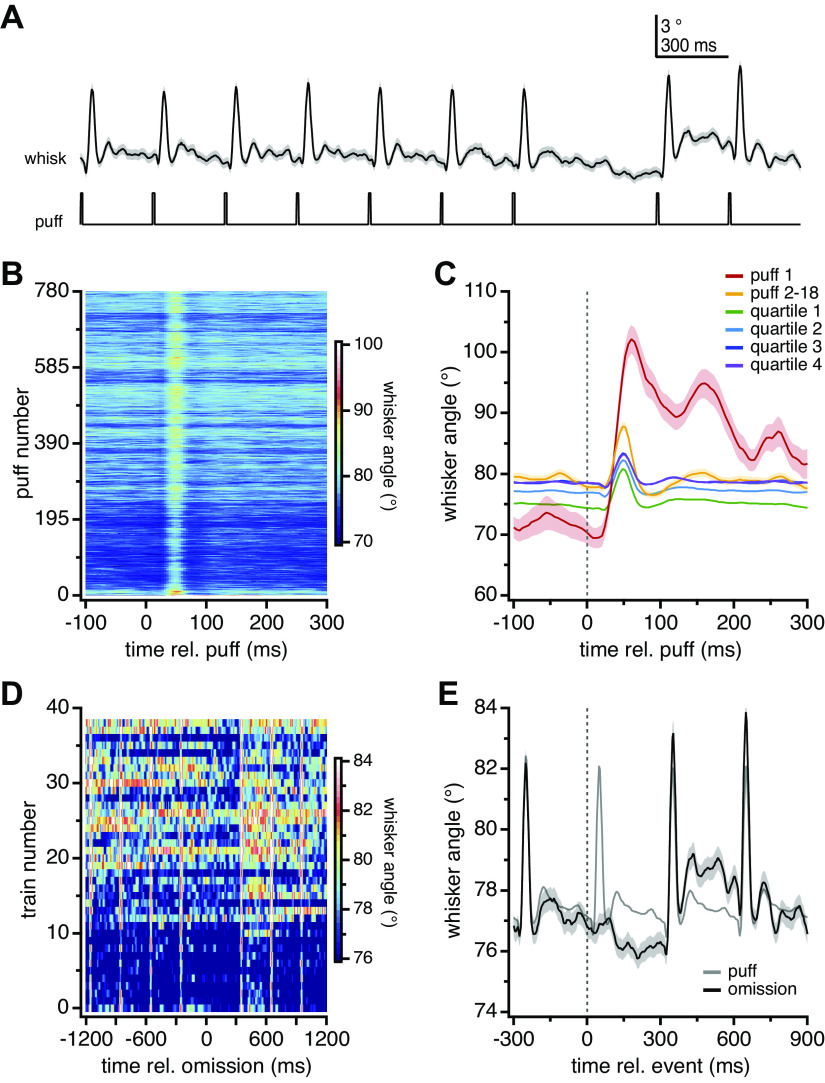
Puffs applied to the whisker pad generally elicited whisker protractions, as described previously (20), although these were not tracked during the present recordings. Therefore, to examine the movements associated with the observed patterns of Purkinje cell activity, whiskers were tracked in three mice exposed to the same stimuli that had been applied during electrophysiological recording, namely 40 trains with 39 omissions (3–5 runs/mouse/day for 3 days, for a total of 42 runs; see materials and methods). Averaged responses of all trains preceded by an omission (n = 39 trains/run for 42 runs) illustrated that whisker protractions reliably followed each puff, and a small retraction occurred during the omission interval (Fig. 5A). As shown in the heat maps of Fig. 5B, the magnitude of the whisking response changed over the course of the 780 puffs per run, however. The first puff of each run elicited the largest movement (30.3 ± 2.3°), which decreased about threefold (8.6 ± 0.7°) through the first train. With repeated stimulation, the magnitude of the puff-evoked protractions became progressively smaller, falling to 4.6 ± 0.2° in the last quartile, whereas the whisker position between stimuli became first more protracted, then more retracted, and then more protracted again. Superimposing the averaged whisking responses to 1) the first puff only, 2) all but the first puff in the first train, and 3) all 195 puffs in each of the four quartiles (Fig. 5C) shows that the protraction magnitude dropped after the first puff and through the first train but stabilized through the last three quartiles. The latency remained similar, however, with the peak deflection occurring at 48 ms in each quartile. This period of maximal protraction correlates with the period of the maximal increase in simple spike rate in CxSE cells, consistent with previous work (20).
Figure 5.
Modulation of whisker movements by repetitive sensory stimulation. A: mean whisker position ± SE (black) and stimulus protocol for 3 s encompassing the 7 puffs before and 2 puffs after the omission (average of 39 omissions per run × 42 runs in 3 mice). B: heat map of whisker position for each puff in the run for all 42 runs in 3 mice. C: mean whisker angle ± SE for all first puffs (n = 42 runs), all puffs in the first train excluding the first puff (n = 17 puffs × 42 runs), and all puffs in each quartile (n = 195 puffs × 42 runs). D: heat map of the mean whisker angle for 4 puffs before and 3 puffs after each omission (42 runs in 3 mice). Note that scale is different from B. E: magnified plot of mean whisker angle (± SE) during the omission (same trace as in A), with the whisker angle during the puff (averaged over all 780 puffs, in all 42 runs in all mice) superimposed for comparison (gray). rel., Relative to.
During the omission, the most salient movement was a retraction of 1.1–1.3° between 140 and 300 ms, evident in both the heat map (Fig. 5D) and the overlaid responses to puffs and omissions (Fig. 5E); the postomission puff also evokes a movement with a more protracted late component (Fig. 5E). Whether the retraction is an active response to the omission per se or simply a reaction to a longer interval between puffs cannot be determined; nevertheless, it loosely correlates in time with the omission-related suppression of complex spikes that occurs first in CxSR cells and then CxSE cells, as well as with the small changes in simple spiking in both cell groups.
DISCUSSION
These results indicate that Purkinje cells in crus I/II of mouse cerebellar cortex produce two distinct patterns of responses to trains of tactile stimulation of the ipsilateral whisker pad. After the stimulus, some cells (CxSE) generate a short-latency, well-timed elevation of complex spike probability followed by a suppression, whereas others (CxSR) show a well-timed reduction followed by a broadly timed elevation of complex spike probability. CxSE and CxSR Purkinje cells are similar in several respects, including their basal simple and complex spike rates; the presence of a sensory-evoked, short-latency suppression of simple spikes that is synchronous across the population; a drop in complex spike probability ∼70 ms after a puff; and complex spike suppression after omissions that interrupt regular stimulus trains. Cells with each response profile differ, however, in the degree to which puff stimuli increase simple spike rates, as well as in the modulation of simple and complex spiking by repeated stimulation. With prolonged trains of puffs, the complex spikes of CxSE cells become more temporally dispersed, whereas the short-latency suppression of simple spikes decreases, suggesting adaptation to the stimulus itself. In contrast, in CxSR cells, complex spikes become more well timed as the overall simple spike rate decreases. Meanwhile, the sensory-evoked, transient suppression of simple spikes remains, indicating a consistent response to the stimulus. Together, the data provide evidence that the pattern of complex spiking displayed by Purkinje cells in response to sensory input is characteristic of a broader response profile that can predict the cells’ responses to repeated stimulation and disruptions of rhythmicity.
Categorization of Purkinje Cells by Complex Spike Responses
Just over half the Purkinje cells tested were CxSE cells, which responded to puff stimuli with an increased complex spike probability ∼50 ms after a puff, producing an a 2.6-fold elevation of firing rate measured in 20-ms windows. Conversely, CxSR cells responded with a 4.2-fold reduction of complex spike rate 70 ms after a puff. In both groups, however, complex spike rates averaged over the full 300-ms interpuff interval remained constant, indicating that both elevated and reduced responses involved retiming rather than adding or subtracting complex spikes. The responses of CxSE cells in particular are suggestive of stimulus-evoked complex spike synchrony (36). Since the degree of such synchrony (over tens of milliseconds) can alter spiking in the cerebellar nuclei (37), these changing spike patterns may modulate cerebellar output in real time.
The distinct response profiles raise the question of whether these are actually two classes of cells or stimulus-dependent responses of a common cell type. The latter seems more likely, since Purkinje cells have complex spike receptive fields that depend on stimulus characteristics (38–41), including distinct fields associated with individual whiskers (16, 42). Indeed, the air puff covered only 30–40% of the pad area, so different whiskers received distinct sensory input. Also, both sets of Purkinje cells had overlapping distributions of basal complex and simple spike rates, suggesting no systematic differences in zebrin expression (43–45). Finally, the timing of complex spike suppression was similar in CxSE and CxSR cells, as expected for a common mechanism. It is possible that these Purkinje cells have climbing fiber inputs that are themselves tuned to the direction of whisker deflection. Given that whiskers protracted after puffs and slightly retracted during omission intervals, this idea seems particularly plausible for CxSE cells, whose shortest-latency complex spike responses changed polarity in the two conditions. Whether cells classified as CxSR cells might generate short-latency increases in complex spike probability with stronger retraction-inducing stimuli has yet to be studied. Regardless of whether individual Purkinje cells can switch category, classifying cells by complex spike responses is useful for inferring how simple and complex spikes are modulated by repetitive stimulation.
Complex Spike Suppression and Implications for Cerebello-Olivary Circuitry
Sensory-evoked increases in complex spiking have been reported frequently (16, 46–49), but complex spike suppression by sensory input has been observed less commonly. Complex spike rates decrease in mice upon omission of expected unconditioned stimuli in delay eyelid conditioning tasks (33) and in monkeys upon receiving smaller-than-expected rewards in eye movement tasks (50). In specific cerebellar microzones in mice, complex spiking can also decrease with normal rewards in visuomotor integration tasks (51). Here, in all Purkinje cells, task-independent sensory input reduced complex spiking with about a 70-ms latency, regardless of whether an elevation of complex spiking preceded this drop. The reduction necessarily results from a period of decreased climbing fiber activity.
Such suppression of the inferior olive may arise from active inhibition by cerebellar nucleo-olivary cells (52) or by the long afterhyperpolarizations of coupled inferior olivary cells (53). Regarding the former possibility, nucleo-olivary cells release GABA asynchronously, yielding inhibitory postsynaptic currents (IPSCs) in inferior olivary cells that slowly activate over tens of milliseconds (54–56), raising the question of how puffs might increase spiking by nucleo-olivary cells. One possibility is that Purkinje-mediated disinhibition might raise nucleo-olivary cell firing sufficiently to suppress climbing fiber activity. The only puff-evoked drop in Purkinje cell firing is the brief gap in simple spike probability <20 ms after a puff, however, which seems too early and too transient to evoke a nucleo-olivary response strong enough to inhibit the inferior olive ∼50–60 ms later, especially since nucleo-olivary cells do not produce well-timed action potentials upon disinhibition (57). A second idea is that puff-activated mossy fibers directly excite nucleo-olivary cells. The sensory input indeed activates neurons of the trigeminal nuclei, which form mossy fibers (58), but the excitation again appears too short latency (∼12 ms) and transient (20) to evoke effective inhibition of the inferior olive. A third possibility is that nucleo-olivary excitation may be evoked by puff-activated inferior olivary cells. Climbing fiber collaterals form synaptic contacts onto nucleo-olivary cells (59), and such collaterals effectively excite large premotor neurons of the cerebellar nuclei in mature mice (60). If climbing fibers responsible for early complex spikes (50 ms after a puff) in CxSE cells also excite nucleo-olivary cells, then nucleo-olivary feedback might broadly inhibit the inferior olive with a latency of tens of milliseconds. The resulting drop in climbing fiber activity could produce the delayed suppression of complex spikes in both CxSE and CxSR Purkinje cells (70 ms after a puff). This idea of a direct negative feedback loop between the cerebellar nuclei and the inferior olive appears compatible with the present data.
An alternative, perhaps simpler, scenario is that the suppression of climbing fibers arises within the inferior olive itself. Inferior olivary action potentials are followed by afterhyperpolarizations of a few hundred milliseconds in brain slices and anesthetized mice (53, 61). Given the electrical coupling of inferior olivary cells (53, 62), it is possible that firing by a subset of neurons suppresses the activity of a larger group of neurons, producing short-latency complex spikes in CxSE neurons followed by a longer-latency reduction in complex spikes in both CxSE and CxSR cells.
Simple Spike Responses during Repetitive Stimulation
Several studies have demonstrated that elevating complex spiking can reduce simple spiking. Early work in decerebrate cats showed that harmaline injection into the inferior olive, which increases firing by climbing fibers (24), can slow or silence simple spikes in Purkinje cells (23). Likewise, in barbiturate-anesthetized cats, direct stimulation of climbing fibers at 2.5–10 Hz for ∼10 s greatly reduces simple spike rates for many seconds (63, 64). Conversely, Purkinje cell simple spike rates in anesthetized rats increase severalfold with destruction of the inferior olive but can be restored back down to baseline by direct stimulation of climbing fibers (25). The most plausible bases for these changes are through long-term potentiation (LTP) and climbing fiber-dependent long-term depression (LTD) of parallel fibers, possibly along with climbing fiber-dependent regulation of inhibitory neurons (29, 65, 66).
The present experiments illustrate a similar phenomenon in awake mice with natural sensory stimuli. Applying puffs at 300-ms intervals (3.3 Hz) reliably evoked short-latency complex spikes in CxSE cells and longer- latency complex spikes in CxSR cells. Interestingly, the complex spikes retimed rather than increased in quantity, yet long-term changes in simple spike rates were evident. Simple spike rates first rose by ∼10 sp/s in both groups of Purkinje cells but then fell to a greater extent in CxSR cells than CxSE cells. Notably, complex spike timing became less precise on a per-cell basis in CxSE cells and more precise in CxSR cells; only in the latter category were complex and simple spike rates strongly negatively correlated. These results suggest that the clustering of complex spikes 200 ms after (or 100 ms before) a puff may be most efficacious at weakening the net effect of parallel fiber excitation of crus I/II Purkinje cells, at least under these stimulus conditions.
In addition to modulating the average simple spike rate, repetitive stimulation affected simple spike responses to the puff itself. Studies in anesthetized (16, 17) and awake (20, 67) mice have shown that Purkinje cells respond to single brief puffs to the whisker pad with short-latency, precisely timed, transient inhibition of firing. In awake mice, this suppression of simple spikes synchronously across the Purkinje cell population correlates with a transient elevation of cerebellar output that augments whisker movements (20). Here, we find that the sensory response is differentially labile in the two Purkinje cell categories. In CxSE cells, puff-evoked synchronous inhibition is evident for the first ∼200 puffs but gradually decreases, becoming undetectable in the last ∼200 puffs, consistent with reports that firing rates of molecular layer interneurons can decrease after bursts of air puffs (68, 69). In CxSR cells, however, synchronous inhibition remains robust and stable throughout the 780 puffs. Thus, whereas CxSE cells habituate, CxSR cells reliably report sensory stimuli for as long as they persist.
Simple spikes of Purkinje cells, however, can also carry whisking-related motor signals. Simple spike rates increase during spontaneous whisking (19) and upon direct stimulation of motor cortex (18). With puff-evoked whisking, elevations of firing rate over 100–200 ms are seen in both anesthetized (16) and awake (20) mice. The rate increase depends on activity in whisk-generating brain stem nuclei, suggesting that it represents the whisking motor command (20). Here, we find a similarly broadly timed puff-evoked increase in simple spike rate, with latency and duration that correlate with maximal whisker protraction, in CxSE but not CxSR cells. The results raise the possibility that the motor command-related simple spike response of a Purkinje cell may vary according to its climbing fiber receptive field.
Omission Response
Studies of macaques demonstrate that Purkinje-target neurons in the dentate nuclei respond strongly to omissions of regular visual stimuli applied at subsecond intervals, particularly in tasks requiring attention to periodicity over months of training (14, 15). Here, despite a limited signal-to-noise ratio owing to the small number of omission stimuli, we find that mouse Purkinje neurons demonstrate modest but significant alterations of complex spike firing during stimulus omissions within a few hundred stimuli. The omission responses of both CxSE and CxSR cells consisted of a suppression followed by an elevation of complex spike probability. This decrease is consistent with the drops in complex spike rate associated with prediction error (33, 50, 70). Notably, however, in CxSR cells the omission-associated response was timed much like the stimulus-associated response. In CxSE cells the decrease was delayed by ∼30 ms, but the subsequent increase closely matched the time course of the stimulus-evoked response in CxSR cells. Whether the small omission responses of simple spikes are reliable or biologically meaningful is harder to assess, but it is possible that they reflect small, delayed whisker retractions following omissions.
Together, the results raise the possibility that the omitted stimulus may be detected by the inferior olive, but by cells distinct from those responsive to puffs. Omission-sensitive cells might suppress the activity of other climbing fibers, through gap junctions or nucleo-olivary feedback loops, which would affect the Purkinje cells from which we recorded, leading to complex spike suppression. This scenario, which is consistent with studies of harmaline effects in the fastigial nuclei (24) and responses of the dentate nuclei to periodic stimuli (14), suggests that Purkinje cells may participate in encoding regular stimuli with subsecond intervals and detecting violations of periodicity.
GRANTS
This work was supported by NIH R37-NS39395 (I.M.R.) and R35-NS116854 (I.M.R.), a Northwestern University Weinberg College of Arts and Sciences Summer Undergraduate Research Grant (G.W.Z.), and American Heart Association Fellowship 829841 to M.H.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.W.Z., S.T.B., and I.M.R. conceived and designed research; G.W.Z., S.T.B., and M.H. performed experiments; G.W.Z., S.T.B., and M.H. analyzed data; G.W.Z., S.T.B., and I.M.R. interpreted results of experiments; G.W.Z., S.T.B., and M.H. prepared figures; G.W.Z. and S.T.B. drafted manuscript; G.W.Z., S.T.B., and I.M.R. edited and revised manuscript; G.W.Z., S.T.B., M.H., and I.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the late Dr. Jerry Simpson for early discussions of the classical literature and to Dr. Eric Lang for helpful comments on the manuscript, especially regarding possible effects of inferior olivary coupling. We also thank Dr. Mauricio Medina for surgical advice and training that greatly improved recording stability and quality.
Present addresses: G. W. Zempolich: The Neuroscience Institute, New York University Grossman School of Medicine, New York, NY 10016; S. T. Brown: Wolfson Institute for Biomedical Research, University College London, Gower St., London WC1E 6BT, UK.
REFERENCES
- 1.Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol 14: 225–232, 2004. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Issa JB, Tocker G, Hasselmo ME, Heys JG, Dombeck DA. Navigating through time: a spatial navigation perspective on how the brain may encode time. Annu Rev Neurosci 43: 73–93, 2020. doi: 10.1146/annurev-neuro-101419-011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz G, Harris R, Shrom D, Berry MJ 2nd.. Detection and prediction of periodic patterns by the retina. Nat Neurosci 10: 552–554, 2007. doi: 10.1038/nn1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci 27: 307–340, 2004. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 5.Breska A, Ivry RB. Taxonomies of timing: where does the cerebellum fit in? Curr Opin Behav Sci 8: 282–288, 2016. doi: 10.1016/j.cobeha.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science 223: 296–299, 1984. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 7.Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci 19: 10940–10947, 1999. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning dependent timing of conditioned eyelid responses. J Neurosci 13: 1708–1718, 1993. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiney SA, Wohl MP, Chettih SN, Ruffolo LI, Medina JF. Cerebellar dependent expression of motor learning during eyeblink conditioning in head-fixed mice. J Neurosci 34: 14845–14853, 2014. doi: 10.1523/JNEUROSCI.2820-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jirenhed DA, Hesslow G. Are Purkinje cell pauses drivers of classically conditioned blink responses? Cerebellum 15: 526–534, 2016. doi: 10.1007/s12311-015-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 300: 1437–1439, 2003. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 12.Teki S, Grube M, Griffiths TD. A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Front Integr Neurosci 5: 90, 2012. doi: 10.3389/fnint.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teki S, Grube M, Kumar S, Griffiths TD. Distinct neural substrates of duration-based and beat-based auditory timing. J Neurosci 31: 3805–3812, 2011. doi: 10.1523/JNEUROSCI.5561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmae S, Uematsu A, Tanaka M. Temporally specific sensory signals for the detection of stimulus omission in the primate deep cerebellar nuclei. J Neurosci 33: 15432–15441, 2013. doi: 10.1523/JNEUROSCI.1698-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kameda M, Ohmae S, Tanaka M. Entrained neuronal activity to periodic visual stimuli in the primate striatum compared with the cerebellum. eLife 8: e48702, 2019. doi: 10.7554/eLife.48702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosman LW, Koekkoek SK, Shapiro J, Rijken BF, Zandstra F, van der Ende B, Owens CB, Potters JW, de Gruijl JR, Ruigrok TJ, De Zeeuw CI. Encoding of whisker input by cerebellar Purkinje cells. J Physiol 588: 3757–3783, 2010. doi: 10.1113/jphysiol.2010.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu CP, Bing YH, Liu QR, Qiu DL. Synaptic responses evoked by tactile stimuli in Purkinje cells in mouse cerebellar cortex Crus II in vivo. PLoS One 6: e22752, 2011. doi: 10.1371/journal.pone.0022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proville RD, Spolidoro M, Guyon N, Dugué GP, Selimi F, Isope P, Popa D, Léna C. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat Neurosci 17: 1233–1239, 2014. doi: 10.1038/nn.3773. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Augustine GJ, Chadderton P. The cerebellum linearly encodes whisker position during voluntary movement. eLife 5: e10509, 2016. doi: 10.7554/eLife.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown ST, Raman IM. Sensorimotor integration and amplification of reflexive whisking by well-timed spiking in the cerebellar corticonuclear circuit. Neuron 99: 564–575, 2018. doi: 10.1016/j.neuron.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marr D. A theory of cerebellar cortex. J Physiol 202: 437–470, 1969. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albus JS. A theory of cerebellar function. Math Biosci 10: 25–61, 1971. doi: 10.1016/0025-5564(71)90051-4. [DOI] [Google Scholar]
- 23.de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res 53: 81–95, 1973. doi: 10.1016/0006-8993(73)90768-3. [DOI] [PubMed] [Google Scholar]
- 24.Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res 18: 69–87, 1973. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- 25.Colin F, Manil J, Desclin JC. The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res 187: 3–27, 1980. doi: 10.1016/0006-8993(80)90491-6. [DOI] [PubMed] [Google Scholar]
- 26.Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci 24: 4510–4517, 2004[Erratum inJ Neurosci24: following 5456, 2004]. doi: 10.1523/JNEUROSCI.4530-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33: 253–258, 1982. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 28.Konnerth A, Dreessen J, Augustine GJ. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc Natl Acad Sci USA 89: 7051–7055, 1992. doi: 10.1073/pnas.89.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittmann W, Häusser M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J Neurosci 27: 5559–5570, 2007. doi: 10.1523/JNEUROSCI.5117-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M, Yamaguchi K, Nagao S, Yamazaki T. Long-term depression as a model of cerebellar plasticity. Prog Brain Res 210: 1–30, 2014. doi: 10.1016/B978-0-444-63356-9.00001-7. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res 128: 309–328, 1977. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 32.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci 27: 2493–2502, 2007. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohmae S, Medina JF. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci 18: 1798–1803, 2015. doi: 10.1038/nn.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56: 43–57, 2007. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21: 1281–1289, 2018. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- 36.Tang T, Suh CY, Blenkinsop TA, Lang EJ. Synchrony is key: complex spike inhibition of the deep cerebellar nuclei. Cerebellum 15: 10–13, 2016. doi: 10.1007/s12311-015-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang T, Blenkinsop TA, Lang EJ. Complex spike synchrony dependent modulation of rat deep cerebellar nuclear activity. eLife 8: e40101, 2019. doi: 10.7554/eLife.40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kano MS, Kano M, Maekawa K. Receptive field organization of climbing fiber afferents responding to optokinetic stimulation in the cerebellar nodulus and flocculus of the pigmented rabbit. Exp Brain Res 82: 499–512, 1990. doi: 10.1007/BF00228792. [DOI] [PubMed] [Google Scholar]
- 39.Brown IE, Bower JM. Congruence of mossy fiber and climbing fiber tactile projections in the lateral hemispheres of the rat cerebellum. J Comp Neurol 429: 59–70, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 40.Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of action by the Purkinje cells of the cerebellum. Nature 526: 439–442, 2015. doi: 10.1038/nature15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jörntell H, Bengtsson F. Climbing fiber receptive fields—organizational and functional aspects and relationship to limb coordination. Cerebellum 14: 360–363, 2015. doi: 10.1007/s12311-015-0647-y. [DOI] [PubMed] [Google Scholar]
- 42.Axelrad H, Crepel F. Représentation sélective des vibrisses mystaciales au niveau des cellules de Purkinje du cervelet par la voie des fibres grimpantes chez le rat [Selective representation of vibrissa at the level of cerebellar Purkinje cells by way of climbing fibers in rats]. C R Acad Hebd Seances Acad Sci D 284: 1321–1324, 1977. doi: 10.1016/s0370-4475(77)80013-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, Schonewille M. Cerebellar modules operate at different frequencies. eLife 3: e02536, 2014. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao J, Cerminara NL, Kotsurovskyy Y, Aoki H, Burroughs A, Wise AK, Luo Y, Marshall SP, Sugihara I, Apps R, Lang EJ. Systematic regional variations in Purkinje cell spiking patterns. PLoS One 9: e105633, 2014. doi: 10.1371/journal.pone.0105633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerminara N, Lang E, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci 16: 79–93, 2015. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown IE, Bower JM. The influence of somatosensory cortex on climbing fiber responses in the lateral hemispheres of the rat cerebellum after peripheral tactile stimulation. J Neurosci 22: 6819–6829, 2002. doi: 10.1523/JNEUROSCI.22-15-06819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, Häusser M. Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. J Neurosci. 29: 8005–8015, 2009. doi: 10.1523/JNEUROSCI.4919-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Najafi F, Giovannucci A, Wang SS, Medina JF. Sensory-driven enhancement of calcium signals in individual Purkinje cell dendrites of awake mice. Cell Rep 6: 792–798, 2014. doi: 10.1016/j.celrep.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harmon TC, Magaram U, McLean DL, Raman IM. Distinct responses of Purkinje neurons and roles of simple spikes during associative motor learning in larval zebrafish. eLife 6: e22537, 2017. doi: 10.7554/eLife.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larry N, Yarkoni M, Lixenberg A, Joshua M. Cerebellar climbing fibers encode expected reward size. eLife 8: e46870, 2019. doi: 10.7554/eLife.46870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kostadinov D, Beau M, Blanco-Pozo M, Häusser M. Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 22: 950–962, 2019. doi: 10.1038/s41593-019-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol (Berl) 184: 225–243, 1991. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- 53.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol 315: 549–567, 1981. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron 62: 555–565, 2009. doi: 10.1016/j.neuron.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turecek J, Regehr WG. Neuronal regulation of fast synaptotagmin isoforms controls the relative contributions of synchronous and asynchronous release. Neuron 101: 938–940.e4, 2019. doi: 10.1016/j.neuron.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turecek J, Regehr WG. Cerebellar and vestibular nuclear synapses in the inferior olive have distinct release kinetics and neurotransmitters. eLife 9: e61672, 2020. doi: 10.7554/eLife.61672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Najac M, Raman IM. Integration of Purkinje cell inhibition by cerebellar nucleo-olivary neurons. J Neurosci 35: 544–549, 2015. doi: 10.1523/JNEUROSCI.3583-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yatim N, Billig I, Compoint C, Buisseret P, Buisseret-Delmas C. Trigeminocerebellar and trigemino-olivary projections in rats. Neurosci Res 25: 267–283, 1996. doi: 10.1016/0168-0102(96)01061-9. [DOI] [PubMed] [Google Scholar]
- 59.De Zeeuw CI, Van Alphen AM, Hawkins RK, Ruigrok TJ. Climbing fibre collaterals contact neurons in the cerebellar nuclei that provide a GABAergic feedback to the inferior olive. Neuroscience 80: 981–986, 1997. doi: 10.1016/s0306-4522(97)00249-2. [DOI] [PubMed] [Google Scholar]
- 60.Najac M, Raman IM. Synaptic excitation by climbing fibre collaterals in the cerebellar nuclei of juvenile and adult mice. J Physiol 595: 6703–6718, 2017. doi: 10.1113/JP274598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khosrovani S, Van Der Giessen RS, De Zeeuw CI, De Jeu MT. In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proc Natl Acad Sci USA 104: 15911–15916, 2007. doi: 10.1073/pnas.0702727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci 26: 1739–1748, 2006. doi: 10.1523/JNEUROSCI.3677-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rawson JA, Tilokskulchai K. Repetitive firing of cerebellar Purkinje cells in response to impulse in climbing fibre afferents. Neurosci Lett 25: 131–135, 1981. doi: 10.1016/0304-3940(81)90320-7. [DOI] [PubMed] [Google Scholar]
- 64.Andersson G, Hesslow G. Activity of Purkinje cells and interpositus neurones during and after periods of high frequency climbing fibre activation in the cat. Exp Brain Res 67: 533–542, 1987. doi: 10.1007/BF00247286. [DOI] [PubMed] [Google Scholar]
- 65.Coddington LT, Rudolph S, Van de Lune P, Overstreet-Wadiche L, Wadiche JI. Spillover-mediated feedforward inhibition functionally segregates interneuron activity. Neuron 78: 1050–1062, 2013. doi: 10.1016/j.neuron.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nietz AK, Vaden JH, Coddington LT, Overstreet-Wadiche L, Wadiche JI. Non-synaptic signaling from cerebellar climbing fibers modulates Golgi cell activity. eLife 6: e29215, 2017. doi: 10.7554/eLife.29215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romano V, De Propris L, Bosman LW, Warnaar P, ten Brinke MM, Lindeman S, Ju C, Velauthapillai A, Spanke JK, Middendorp Guerra E, Hoogland TM, Negrello M, D’Angelo E, De Zeeuw CI. Potentiation of cerebellar Purkinje cells facilitates whisker reflex adaptation through increased simple spike activity. eLife 7: e38852, 2018. doi: 10.7554/eLife.38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramakrishnan KB, Voges K, De Propris L, De Zeeuw CI, D’Angelo E. Tactile stimulation evokes long-lasting potentiation of Purkinje cell discharge in vivo. Front Cell Neurosci 10: 36, 2016. doi: 10.3389/fncel.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bing YH, Wu MC, Chu CP, Qiu DL. Facial stimulation induces long-term depression at cerebellar molecular layer interneuron-Purkinje cell synapses in vivo in mice. Front Cell Neurosci 9: 214, 2015. doi: 10.3389/fncel.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hull C. Prediction signals in the cerebellum: beyond supervised motor learning. eLife 9: e54073, 2020. doi: 10.7554/eLife.54073. [DOI] [PMC free article] [PubMed] [Google Scholar]