Abstract
Pulmonary arterial hypertension (PAH) is a fatal cardiopulmonary disease characterized by increased vascular cell proliferation with apoptosis resistance and occlusive remodeling of the small pulmonary arteries. The Notch family of proteins subserves proximal signaling of an evolutionarily conserved pathway that effects cell proliferation, fate determination, and development. In endothelial cells (ECs), Notch receptor 2 (Notch2) was shown to promote endothelial apoptosis. However, a pro- or antiproliferative role for Notch2 in pulmonary endothelial proliferation and ensuing PAH is unknown. We postulated that suppressed Notch2 signaling drives pulmonary endothelial proliferation in the context of PAH. We observed that levels of Notch2 are ablated in lungs from PAH subjects compared with non-PAH controls. Notch2 expression was attenuated in human pulmonary artery endothelial cells (hPAECs) exposed to vasoactive stimuli including hypoxia, TGF-β, ET-1, and IGF-1. Notch2-deficient hPAECs activated Akt, Erk1/2, and antiapoptotic protein Bcl-2 and reduced levels of p21cip and Bax associated with increased EC proliferation and reduced apoptosis. In addition, Notch2 suppression elicited a paradoxical activation of Notch1 and canonical Notch target gene Hes1, Hey1, and Hey2 transcription. Furthermore, reduction in Rb and increased E2F1 binding to the Notch1 promoter appear to explain the Notch1 upregulation. Yet, when Notch1 was decreased in Notch2-suppressed cells, the wound injury response was augmented. In aggregate, our results demonstrate that loss of Notch2 in hPAECs derepresses Notch1 and elicits EC hallmarks of PAH. Augmented EC proliferation upon Notch1 knockdown points to a context-dependent role for Notch1 and 2 in endothelial cell homeostasis.
NEW & NOTEWORTHY This study demonstrates a previously unidentified role for Notch2 in the maintenance of lung vascular endothelial cell quiescence and pulmonary artery hypertension (PAH). A key novel finding is that Notch2 suppression activates Notch1 via Rb-E2F1-mediated signaling and induces proliferation and apoptosis resistance in human pulmonary artery endothelial cells. Notably, PAH patients show reduced levels of endothelial Notch2 in their pulmonary arteries, supporting Notch2 as a fundamental driver of PAH pathogenesis.
Keywords: endothelial cell, Notch, proliferation, pulmonary arterial hypertension, Rb
INTRODUCTION
Pulmonary arterial hypertension (PAH), clinically characterized by extensive vascular remodeling, elevated pulmonary arterial pressure, and reduced right ventricular function, is a degenerative disease which leads to right ventricular failure and death in 38–63% of patients within 5 yr after diagnosis (1, 2). Arterial remodeling associated with PAH is characterized as involving clonal endothelial cell (EC) proliferation, transdifferentiation, and migration concomitant with smooth muscle cell (SMC) hypertrophy and proliferation, which together lead to partial and obliterative occlusion of pulmonary arteries (3–8). Although ECs are known to contribute to the pathogenesis of PAH (8–10), a plethora of studies and, consequently, therapies have been focused on smooth muscle dysfunction, albeit with limited beneficial outcomes.
As is held for ECs in distinct vascular beds, the pulmonary vascular endothelium plays a pivotal role in the maintenance of vascular homeostasis. For instance, decreases in prostacyclin and nitric oxide (NO) production result in exposure of underlying cells to circulating factors that promote proliferation, migration, and resistance to apoptosis (6, 8). Indeed, endothelial damage and monoclonal EC proliferation have been described as fundamental to the hypercellular response of the pulmonary vasculature in PAH. These phenotype changes in ECs appear to trigger SMC proliferation and progressive thickening of the proximal arteries leading to occlusive changes in the PAs and plexiform lesion formation (11–13). Despite extensive studies, there is a dearth of information on cellular signaling in ECs leading to the proliferative phenotype.
The canonical Notch comprises an evolutionarily conserved pathway that plays important roles in cellular and tissue function, including proliferation, apoptosis, differentiation, cell-fate determination, and development (14–18). Upregulation of Notch 1 and 3 receptors and their ligands are implicated in progrowth signaling (18–21). In aortic ECs, Notch receptor 2 (Notch2) promotes endothelial apoptosis (22, 23). However, a pro- or antiproliferative role for Notch2 in pulmonary endothelial proliferation and ensuing PAH is unknown. Therefore, we propose that aberrant Notch2 signaling drives pulmonary endothelial proliferation in the settings of PAH pathogenesis.
Although the degree of hypoxemia varies from mild to moderate in PAH patients, it is well known that PAH patients show increased expression of hypoxia inducible factor-1 (HIF-1)-α and -β subunits, particularly in endothelial cells from obliterative lesions (29). Furthermore, hypoxic stress triggers cellular phenotypic alternations including proliferation and migration of vascular cells that remodel the pulmonary vasculature and ultimately present the characteristic features of PAH. In addition, hypoxia modulates the synthesis and secretion of vasoactive factors and inflammatory cytokines, which are known to contribute to the pathogenesis and histologic changes observed in all forms of PAH. Therefore, in this study, we used a chronic hypoxia model (1% O2) on cultured human pulmonary artery endothelial cells (hPAECs) that recapitulates and approximates the PAH phenotype in vitro.
In this study, we observed a markedly lower Notch2 in the human pulmonary endothelium of PAH versus non-PAH control subjects. Conditions recapitulating the PAH-phenotype in hPAECs in vitro (hypoxia and vasoactive factors) demonstrated a similar suppression of Notch2. Intriguingly, downstream consequences of Notch2 reduction include a previously unidentified promotion of EC proliferation and migration and decreased apoptosis. Here, we present evidence that of the Notch isoforms, Notch2 is essential for the maintenance of lung vascular endothelial quiescence, and its loss leads to ligand-independent activation of endothelial Notch1 via retinoblastoma protein (Rb)-E2F1-mediated signaling leading to the promotion of EC proliferation and resistance to apoptosis.
METHODS
Data Availability
All data generated or analyzed during this study are included within the article.
Human Tissue Samples
All human tissue sample collection and processing were approved by the Institutional Review Board of the University of Pittsburgh. Lung and pulmonary artery tissue samples from PAH and non-PAH controls were obtained from human subjects who provided informed written consent. Tissues were collected either from recipient lungs during transplant surgery or during warm autopsy organ collection. Demographic data of the non-PAH and PAH patient samples are as published in Ghouleh et al. (10). Samples were de-identified and researchers performing the biochemical assays were blinded to patient information (Institutional Review Board No. PRO14010265).
Cells, siRNA Transfection, and Assays
Cell culture, treatments, and siRNA transfection.
Human pulmonary artery endothelial cells (hPAECs) from at least three different lots (Lonza, Walkersville, MD) were grown in EBM-2 media containing EGM-2 bullet kit components (Lonza, Walkersville, MD) at 37°C in a humidified atmosphere of 5% CO2 as described in our previous studies (10, 24). Cells were seeded at 80–90% confluency and synchronized in serum-reduced media (0.2% FBS) for 16 h before further experimentation. Cells between passages 3 and 7 were used in all experiments. Hypoxic treatment (1% O2) in vitro was used to most closely resemble the in vivo disease phenotype as previously described (10, 25, 26). Cells were incubated in either normoxia (21% oxygen) or hypoxia (1% oxygen) for 24 h and subjected to either homogenization in disruption buffer [for immunoblotting and quantitative (q)RT-PCR] or trypsinized for intact cell analysis, such as cell cycle analysis and apoptosis assay. Treatments with transforming growth factor-β (TGFβ; 10 ng/mL), TNFα (10 ng/mL), endothelin-1 (ET-1) (10 ng/mL), IGF-1 (200 ng/mL), or vehicle control were performed for 24 h, and lysates were collected in RIPA buffer thereafter. To gene silence Notch2, Notch1, or HIF-1α, hPAECs were seeded at a density to achieve an overnight 75–80% confluence followed by transfection with Notch2, Notch1, or HIF-1α siRNA or their scrambled control siRNA (Life Technologies) using Lipofectamine 3000 transfection reagent (Life Technologies) according to the manufacturer’s protocol and as documented previously (10). Gene silencing was confirmed after 72 h using immunoblotting (detailed below) and knockdown expressed as percentage of siRNA scrambled controls.
Immunofluorescence.
Formaldehyde-fixed and paraffin-embedded sections of lung tissue were first washed using PBS and blocked in 10% BSA-PBS solution for 30 min at room temperature following permeabilization. To detect specific antigens, sections were stained as described previously (7, 10) using anti-Notch2 antibody (1:50, Cell Signaling, cat. no. 5732) and fluorescent-tagged anti-rabbit secondary antibody and counterstained with DAPI to label the nuclei. Nonspecific rabbit IgG (5 μg/mL) was used instead of primary antibody as a negative control. Tissue sections were encased by glass coverslips using gelvatol mounting media (polyvinylalcohol, glycerol, H2O, sodium azide, and Tris pH 8.5). Confocal images were captured on an Olympus Fluoview 1000 confocal microscope (Olympus America Inc., Bethlehem, PA) and Nikon Analyzer. For each section five to eight images per group were captured. Three independent experiments were performed.
Western blot analysis.
Protein abundance in treated hPAECs was measured as described below. Briefly, treated hPAECs were washed with ice-cold 1× PBS to remove residual media and collected in ice-cold RIPA lysis buffer (Thermo Scientific) containing protease and phosphatase inhibitors (Roche). Samples were homogenized in lysis buffer followed by centrifugation at 4°C for 15 min at 15,000 g, and supernatants were collected. Samples with equal amount of proteins were loaded onto 10 or 15% gel (depending on protein size) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V for 2–2.5 h. Separated proteins were then transferred onto nitrocellulose membranes (Bio-Rad) and blocked using LI-COR TBS blocking buffer for 1 h at room temperature. Membranes were incubated with primary antibodies overnight at 4°C, diluted (as noted): anti-Notch2 (1:1,000, Cell Signaling, cat. no. 5732), anti-Notch1 (1:500, Cell Signaling, cat. no. 3608), anti-cleaved Notch1 (1:1,000, Cell Signaling, cat. no. 4147), anti-Jagged1 (1:500, Santa Cruz, cat. no. 390177), anti-DLL4 (1:1,000, Abcam, cat. no. 7280), anti-p21cip (1:1,000, Cell Signaling, cat. no. 2947), anti-Bax (1:1,000, Cell Signaling, cat. no. 2774), anti-Bcl2 (1:1,000, Abcam, cat. no. 32124), anti-TATA-binding protein (TBP; 1:1,000, Abcam, cat. no. 51841), phospho- and total-p-38 MAPK (1:1,000, Cell Signaling, cat. no. 4631, cat. no. 9228), phospho- and total-SAPK (1:1,000, Cell Signaling, cat. no. 9255, cat. no. 9252), phospho- and total Erk1/2 (1:1,000, Cell Signaling, cat. no. 9101, cat. no. 4695), phospho- and total Akt (1:1,000, Cell Signaling, cat. no. 4060, cat. no. 9272), phospho- and total Rb (1:1,000, Cell Signaling, cat. no. 8516, cat. no. 9309), and anti-β-actin (1:3,000, Santa Cruz, cat. no. 47778). Housekeeping gene β-actin protein was used as a loading control. After overnight incubation, membranes were washed with 1× TBS-Tween and followed by incubation with LI-COR goat anti-rabbit or -mouse secondary antibody at room temperature for 30 min. Proteins were visualized by scanning the fluorescence-labeled membranes using Odyssey Imaging Systems (LI-COR Biosciences). Optical density of protein-of-interest bands were quantified and normalized to β-actin using ImageJ software [National Institutes of Health (NIH)] (7, 10).
For preparation of nuclear extracts, hPAECs seeded onto 100 mm dishes were transfected as described above and processed using the nuclear extract kit (TransAm; Active Motif, Carlsbad, CA). Briefly, cells were washed with ice-cold 1× PBS containing phosphatase inhibitors and scraped gently to collect adherent cells. Cells were lysed using hypotonic buffer and centrifuged at 14,000 g for 1 min to collect a pellet containing the nuclear fraction. Nuclear pellet was resuspended in lysis buffer per the manufacturer’s instructions and isolated nuclear extracts were used for immunoblotting as described above. Key resources are listed in Table 1.
Table 1.
Key resource table
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Notch2 | Cell Signaling Technology | Cat. no. 5732; RRID: AB_10693319 |
| Rabbit monoclonal anti-Notch1 | Cell Signaling Technology | Cat. no. 3608, RRID:AB_2153354 |
| Rabbit monoclonal anti-Notch1, cleaved (Val1744) | Cell Signaling Technology | Cat. no. 4147, RRID:AB_2153348 |
| Rabbit polyclonal anti-Bax | Cell Signaling Technology | Cat. no. 2774, RRID:AB_490806 |
| Rabbit monoclonal anti-p21Waf1/cip1 | Cell Signaling Technology | Cat. no. 2947, RRID:AB_823586 |
| Rabbit polyclonal anti-Bcl2 | Abcam | Cat. no. ab32124, RRID:AB_725644 |
| Rabbit polyclonal anti-DLL4 | Abcam | Cat. no. ab7280, RRID:AB_449562 |
| Mouse monoclonal anti-TATA binding protein (TBP) | Abcam | Cat. no. ab51841, RRID:AB_945758 |
| Rabbit monoclonal anti-phospho-p38 MAPK (Thr180/Tyr182) (12F8) | Cell Signaling Technology | Cat. no. 4631, RRID:AB_331765 |
| Rabbit monoclonal anti-E2F1 | Abcam | Cat. no. ab179445 |
| Mouse monoclonal anti-p38 MAP kinase | Cell Signaling Technology | Cat. no. 9228, RRID:AB_490886 |
| Mouse monoclonal anti-phospho-SAPK/JNK (Thr183/Tyr185) (G9) | Cell Signaling Technology | Cat. no. 9255, RRID:AB_2307321 |
| Rabbit polyclonal anti-SAPK/JNK | Cell Signaling Technology | Cat. no. 9252, RRID:AB_2250373 |
| Rabbit polyclonal anti-p44/42 MAP kinase (phosphorylated Erk1/2) | Cell Signaling Technology | Cat. no. 9101, RRID:AB_331646 |
| Rabbit monoclonal anti-p44/42 MAPK (Erk1/2) (137F5) | Cell Signaling Technology | Cat. no. 4695, RRID:AB_390779 |
| Rabbit monoclonal anti-phospho-Akt (Ser473) | Cell Signaling Technology | Cat. no. 4060, RRID:AB_2315049 |
| Rabbit polyclonal anti-Akt | Cell Signaling Technology | Cat. no. 9272, RRID:AB_329827 |
| Rabbit monoclonal anti-phospho-Rb (Ser807/811) (D20B12 | Cell Signaling Technology | Cat. no. 8516, RRID:AB_11178658 |
| Mouse monoclonal anti-Rb (4H1) | Cell Signaling Technology | Cat. no. 9309, RRID:AB_823629 |
| Mouse monoclonal anti-β-actin | Santa Cruz Biotechnology | Cat. no. sc-47778, RRID:AB_626632 |
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant human TGF-β1 (HEK2-derived) | Peprotech | 100-21; GenPept: P01137 |
| Recombinant human TNF-α | Peprotech | 300-01A; GenPept: P01375 |
| Recombinant human IGF-1 | Millipore | GF138 |
| Endothelin-1 (human, porcine) | R&D Systems | 1160 |
| Critical commercial assays | ||
| Nuclear extraction kit | Active Motif | 40010 |
| ChIP-IT express chromatin immunoprecipitation kit | Active Motif | 53008 |
| Oligonucleotides | ||
| ChIP A-100 | ||
| Forward: 5′-CAC TCA CCA CCC TGT GTT GG-3′ | This article | Not applicable |
| Reverse: 5′-AAG CGC CAG GAG TCC AAA AT-3′ | This article | Not applicable |
| ChIP B-800 | ||
| Forward: 5′-GGC TCC TCC GCT TAT TCA CA-3′ | This article | Not applicable |
| Reverse: 5′-GCC AAA AGT TTG AGC CGG G-3′ | This article | Not applicable |
| Software and algorithms | ||
| ImageJ | Schneider et al. (49) | https://imagej.nih.gov/ij/ |
Quantitative real-time RT-PCR.
Expression of mRNA was quantified as previously described (7, 10). Briefly, total RNA was extracted from human lung tissues and from hPAECs exposed to hypoxia versus normoxia or transfected with Notch2 siRNA or scrambled control using the RNeasy Plus Mini Kit (Qiagen). RNA quality and quantity were assessed by a Nanodrop Spectrophotometer (Thermo Scientific). mRNA abundance was measured by real-time polymerase chain reaction (PCR), wherein total RNA (1–2 µg) was reverse transcribed to cDNA by using Superscript III reverse transcriptase (Life Technologies) following the manufacturer’s protocol. Taqman Fast Advanced Master Mix and Notch1, Notch2, Notch4, Jag1, Jag2, DLL1, DLL4, Hes1, Hey1, Hey2, or 18S primers (Life Technologies) were used for qPCR in a 7900HT Fast Real-Time PCR System (Life Technologies) according to the manufacturer’s protocol for 40 cycles. Relative quantification to corresponding control was obtained using the threshold cycle (Ct) method with 18S as the housekeeping gene and relative expression calculated by 2−ΔΔCt method.
Cell proliferation and in vitro wound scratch assay.
For the CyQuant assay, hPAECs transfected with Notch2 or scrambled siRNA were incubated under normoxic conditions for 72 h and assayed for proliferation by measuring fluorescence at 520 nm using CyQUANT GR dye (Invitrogen, Inc.) per the manufacturer’s protocol. In vitro wound scratch assay was performed as described in Refs. 24, 27. Briefly, hPAECs were seeded in six-well plates in complete media and cultured to confluence. The cells were transfected with Notch2 siRNA or scrambled control, or with Notch1 siRNA (either alone or in combination with Notch2 siRNA) (for Fig. 5E) and serum-starved overnight before initiating the experiment. Confluent cell monolayers were then scraped by dragging a 1,000-µl pipette tip down the center of the well to generate scratch wounds and washed with media to remove cell debris. Cells were incubated at 37°C for 24 h, and bright-field images were captured at 0- and 24-h time points in the same position using a Zeiss Axiovert 40CFL microscope. Two selected fields of images were captured in each sample, and the wound areas are estimated by ImageJ software (NIH).
Figure 5.
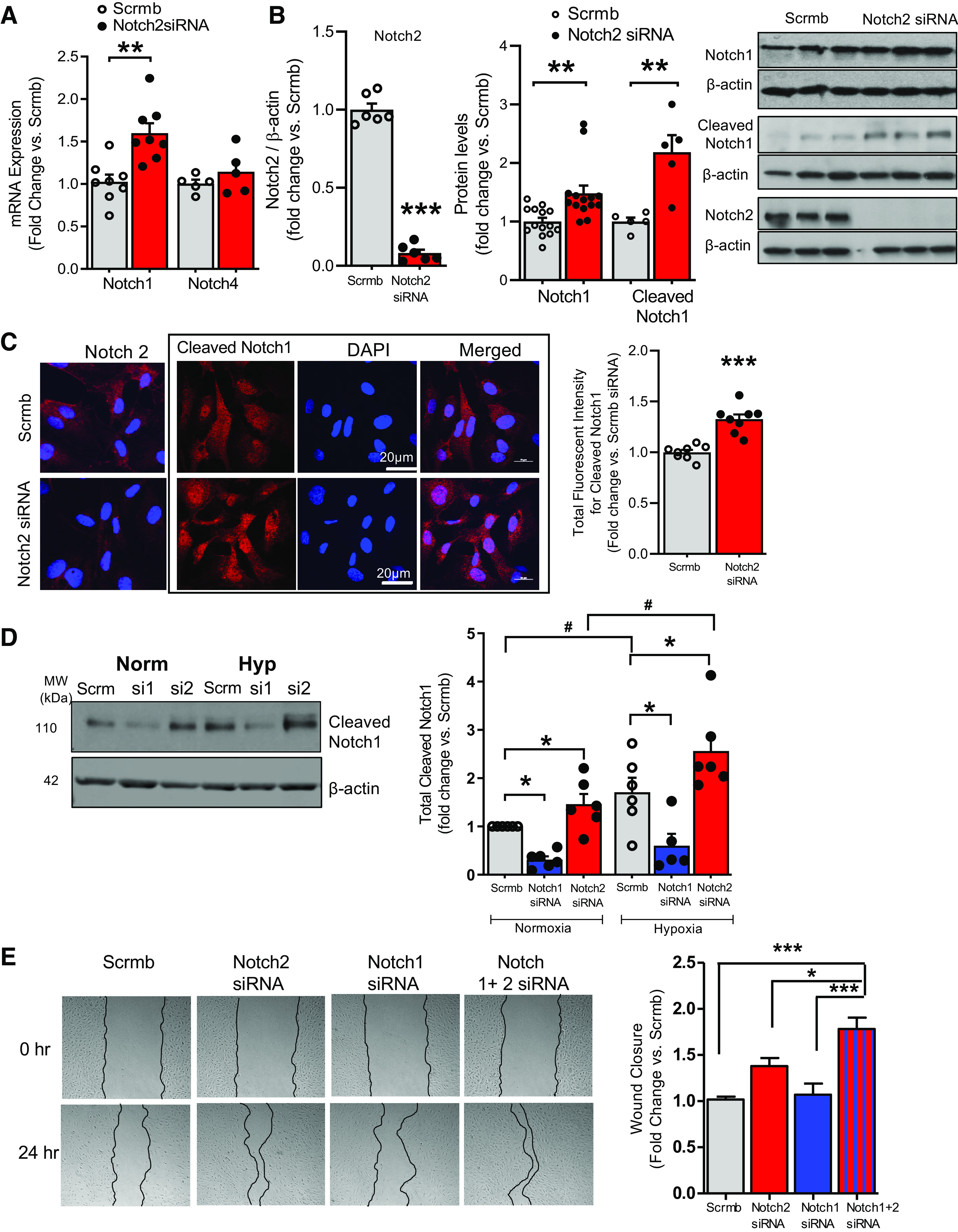
A–E: Notch2 negatively regulates Notch1. Hypoxia augments Notch2 suppression-induced Notch1 levels. A and B: human pulmonary artery endothelial cells (hPAECs) transfected with Notch2 siRNA or scrambled control (Scrmb), synchronized overnight with serum-reduced media, and assayed 72 h posttransfection for gene expression of endothelial Notch1 and Notch4 (n = 8) (A) and immunoblot analysis for Notch2, Notch1, and cleaved Notch1 (B). Representative immunoblot and quantitative bar graphs are provided (n = 3–11). C: immunofluorescence (IF) labelling of hPAECs seeded onto gelatin-coated glass-coverslips and transfected with Notch2 siRNA or scrambled control (Scrmb), 72 h posttransfection. Representative images of cleaved Notch1 (red) with nuclei stained with DAPI (blue). Quantitative bar graphs depicting total fluorescent intensity of cleaved Notch1 protein in Notch2 siRNA-transfected hPAECs vs. scrambled control are provided (n = 5). D: representative immunoblot and quantitative bar graphs of cleaved Notch1 protein in lysates of hPAECs transfected with Notch 1 or 2 siRNA or scrambled control (Scrmb) exposed to hypoxia or normoxia for 24 h. (n = 6). E: representative images of gap-wound injury made on ECs transfected with Notch 1 siRNA and Notch2 siRNA either alone or in combination and scrambled control (Scrmb) as described in Fig. 3. Brightfield images of the wound were captured and percent wound closure in cells was measured as the change in wound size between t = 0 and 24 h vs. the change occurring in Scrmb control-treated cells. Representative brightfield images of the wound at both time points are provided (n = 8). Dots represent individual biological replicates. Bar graphs indicate means ± SE. P values for paired comparisons were calculated using Student’s t test. **P < 0.01, ***P < 0.001. For D, P values were calculated using two-way ANOVA. Post hoc tests applied Bonferroni correction to allow for multiple comparisons. *P < 0.05 vs. scrambled control within group, #P < 0.05 between groups. For E, P values were calculated using one-way ANOVA. Post hoc tests used Bonferroni correction to allow for multiple comparisons. *P < 0.05 vs. Notch2 siRNA, ***P < 0.001 vs. scrambled control or Notch1 siRNA.
Cell cycle assay.
For cell cycle assay, propidium-iodide staining followed by flow cytometry assay was conducted as described below. Briefly, hPAECs seeded into six-well tissue culture dishes were transfected with Notch2 siRNA or scrambled siRNA, and serum deprived (0.2% FBS) for 16h. Thereafter, serum-deprived media were replaced with complete media (2% FBS), and cells were incubated for 24 h under normoxia. Cells were trypsinized, fixed in ice-cold 70% ethanol/PBS, and treated with ribonuclease A (0.2 mg/mL), and DNA content was stained with propidium iodide (50 μg/mL) at room temperature for 45 min. Cell cycle analysis was performed using a BD LSR Fortessa flow cytometer and analyzed using FlowJo software (version 10). A minimum of 10,000 events was recorded for each sample. The data were tabulated as the sum of cells in S + G2/M phases and normalized as fold change from scrambled controls.
Apoptosis assay.
HPAECs cultured in six-well plates containing EBM-2 media were transfected with Notch2 siRNA or scrambled control and incubated under normoxic conditions for 72 h. Cells were trypsinized and washed twice with 1× PBS. The harvested cells were resuspended in 100 µl of annexin V buffer, incubated with FITC-conjugated annexin V and propidium iodide for 15 min in the dark at room temperature following the manufacturer’s (BD Biosciences, Germany) instructions. Subsequently, 400 µl of annexin V buffer were added to each of the samples before analysis by fluorescence-activated cell sorting methods using BD LSR Fortessa flow cytometer. Flow cytometry data was analyzed using FlowJo Software (version 10).
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assays were performed on hPAECs grown in 15-cm dishes and transfected with Notch2 siRNA or scrambled control. Cells were processed 72 h posttransfection using the ChIP-IT Express kit (Active Motif) with the following modifications. Cells were fixed, lysed, and DNA collected per the manufacturer’s instructions. Isolated chromatin was sheared using the Bioruptor sonicator (Diagenode). Cross-linked and sonicated chromatin (∼10 μg) from Notch2 siRNA- or scrambled control-transfected hPAECs were subjected to immunoprecipitation with 1–3 μg E2F1 antibody (Abcam ChIP grade ab179445) or nonspecific IgG (Vector Laboratories) and an overnight incubation at 4°C with Protein G Magnetic Beads. Thereafter, the antibody-chromatin complexes were eluted, and cross-linking between protein and chromatin was then reversed and DNA was recovered following the manufacturer’s instructions. The amount of chromatin bound to E2F1 was then assayed for Notch1 promoter binding by using primers flanking specified regions on the human Notch1 promoter. Specific primers were designed to bind to two different sites (∼100 and 800 bp before the transcription start site (TSS) on the Notch1 promoter sequence. Primer sequences for amplifying specific regions on Notch1 promoter were as follows: ChIP A-100 forward: 5′-CAC TCA CCA CCC TGT GTT GG-3′; ChIP A-100 reverse: 5′-AAG CGC CAG GAG TCC AAA AT-3′; ChIP B-800 forward: 5′-GGC TCC TCC GCT TAT TCA CA-3′; and ChIP B-800 reverse: 5′-GCC AAA AGT TTG AGC CGG G-3′. PCR cycling conditions were as follows: Initial denaturation step at 96°C for 60 s and then 40 cycles [96°C for 30 s, 55°C for 60 s, and 72°C for 30 s] followed by a final hold at 4°C. The PCR products were analyzed on a 1.5% agarose gel with a 1-kB DNA ladder and imaged on a ChemiDoc System (Bio-Rad).
Statistical Analysis
Experimental data are represented as bar graphs with means ± SE. Unpaired Student’s t test was used to analyze the statistical differences of greater than two groups. One-way and two-way ANOVA followed by Bonferroni’s correction was used for the comparison of greater than two groups. Differences with P < 0.05 were considered statistically significant. All statistical analyses were performed using Graphpad Prism 5 software (Graphpad Software Inc., La Jolla, CA). Statistical details can be found within respective figure legends. Statistical tests used and power determinations were made in consultation with a professional statistician acknowledged herein.
RESULTS
Notch2 is Suppressed in Human PAH and in Response to Hypoxia
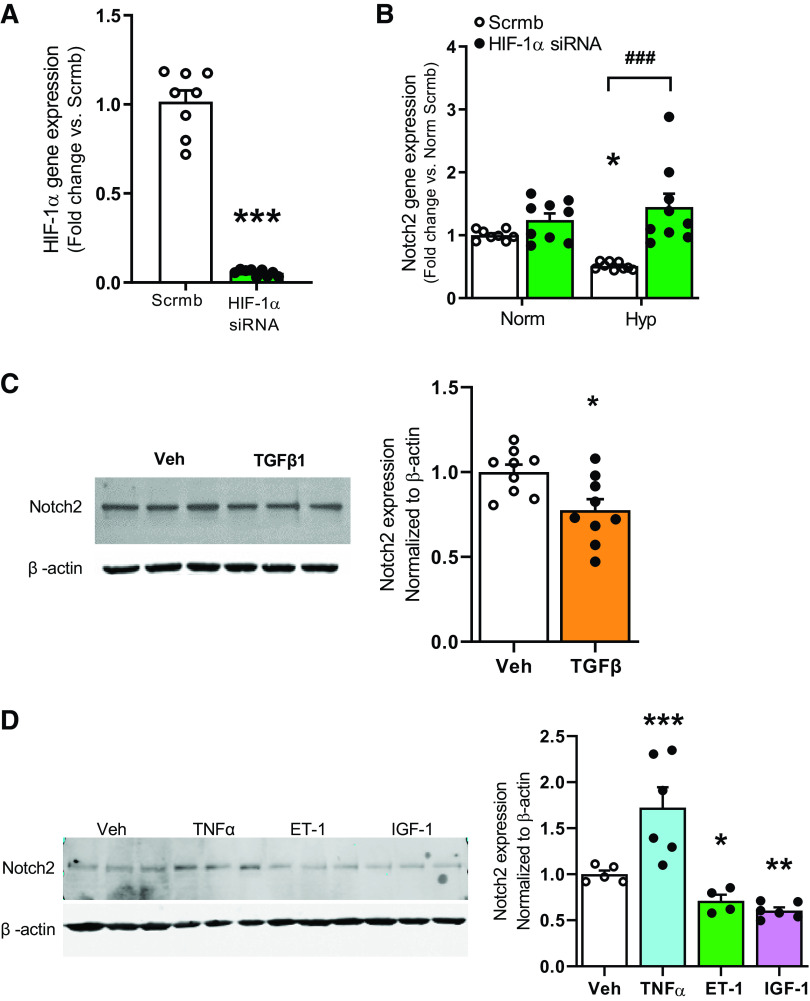
To interrogate whether Notch2 might play a role in human PAH, we localized and quantified Notch2 in lung tissue sections of human PAH versus non-PAH donor controls. Immunofluorescence (IF) of Notch2 revealed its presence in the vessel’s intimal layer in non-PAH control lung sections (Fig. 1A). In contrast, cross sections of PAH samples showed sharply lower levels of Notch2. Furthermore, quantitative RT-PCR (qRT-PCR) of arterial homogenates showed significantly lower levels of Notch2 mRNA in PAH versus non-PAH controls (∼70% less vs. control, Fig. 1B). To explore a role for endothelial Notch2 in vitro, we employed human pulmonary artery endothelial cells (hPAECs, Lonza) to examine whether Notch2 expression would be similarly affected in cell culture upon hypoxic exposure: conditions that recapitulate cellular changes occurring in PAH [hypoxia (Hyp), 1% vs. normoxia (Norm), 21% O2] (7, 10, 25, 26). Quantitative RT-PCR and immunoblot analyses displayed decreased amounts of Notch2 both at mRNA (Fig. 1C) and protein levels (Fig. 1D) upon hypoxic exposure of hPAECs. Moreover, IF staining of hypoxic hPAECs displayed reduced cellular Notch2 compared with normoxic controls (Fig. 1E). Furthermore, it is well established that lung sections of PAH patients show increased levels of hypoxia-inducible factor (HIF) (28, 29). To investigate whether hypoxia-mediated suppression of Notch2 could be abrogated by knockdown of HIF-1α, we transfected hPAECs with HIF-1α siRNA. Gene expression analysis confirmed >90% reduction in HIF-1α transcript levels in HIF-1α siRNA-transfected ECs versus scrambled control (Fig. 2A) and showed that hypoxia-mediated suppression of Notch2 is rescued upon blockade of HIF-1α in hPAECs (Fig. 2B).
Figure 1.
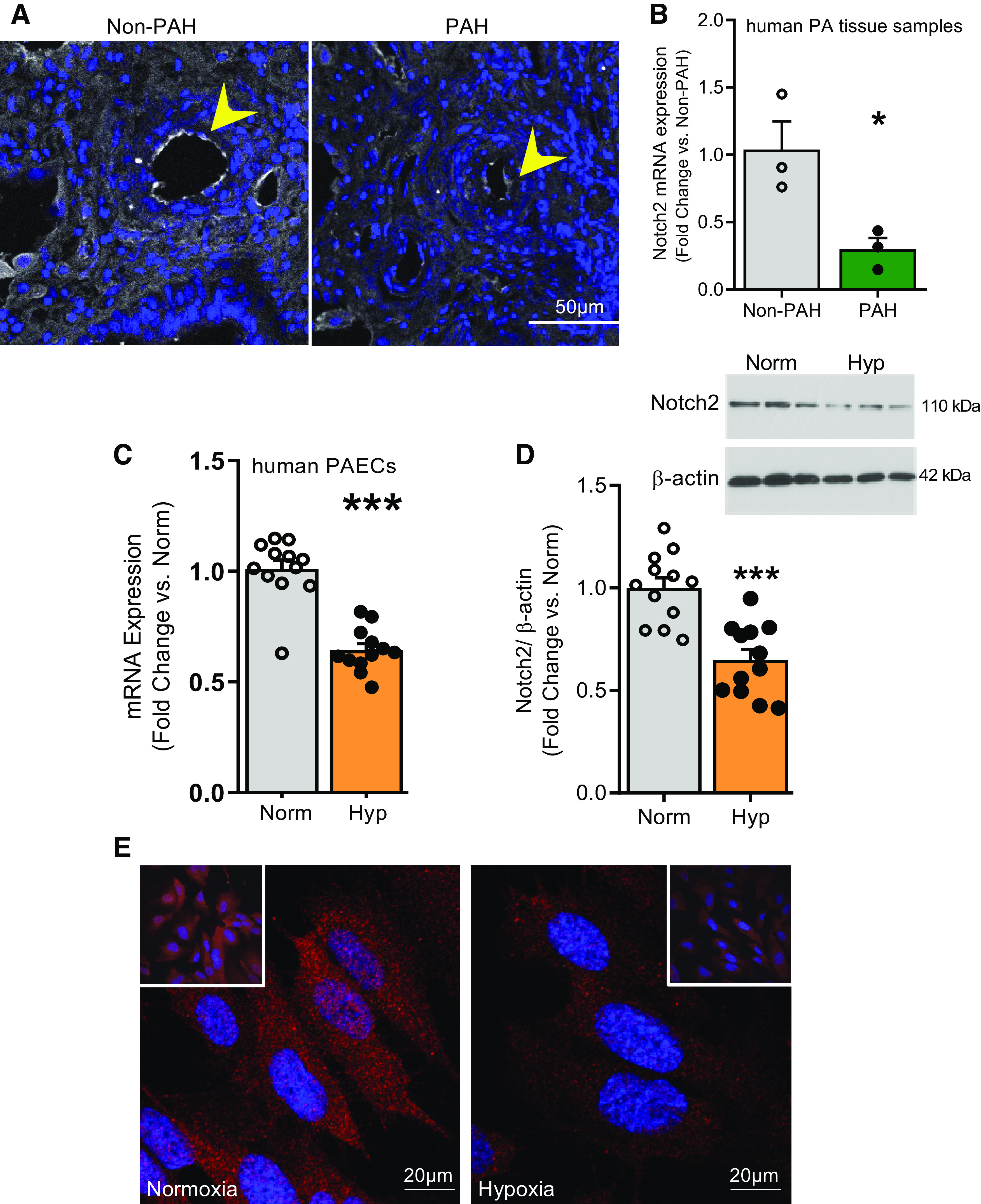
A–E: expression of Notch2 is reduced in the pulmonary vasculature in pulmonary arterial (PA) hypertension (PAH) and in human pulmonary artery endothelial cells (hPAECs) exposed to hypoxia vs. normoxia. A and B: Notch2 expression is reduced in lung arteries of subjects with PAH. A: Notch2 localization and abundance in human PAH and non-PAH lung samples by immunofluorescence (IF). Expression of Notch2 (white) and nuclei (DAPI, blue) are shown (n = 3). B: quantification of transcript levels of Notch2 in pulmonary artery tissue homogenates of human non-PAH and PAH samples (n = 3). C–E: Notch2 expression in commercially obtained hPAECs (Lonza) exposed to hypoxia to mimic cell conditions of pulmonary hypertension in vitro. C: mRNA expression of Notch2 upon 24 h hypoxia (Hyp) vs. normoxia (Norm) quantified by quantitative RT-PCR (n = 12). D: representative immunoblot and cumulative quantification (bar graph) of Notch2 in hPAECs in response to 24 h hypoxia (Hyp) vs. normoxia (Norm) (n = 12). E: IF labelling of Notch2 (red) in hPAECs upon 24 h hypoxia vs. normoxia. Nuclei stained with DAPI are shown in blue. Insets allow visualization of greater cell numbers (lower magnification). Dots represent individual data points of biological replicates. Bar graphs indicate means ± SE. P values for paired comparisons were calculated using Student’s t test. *P <0.05, ***P <0.001.
Figure 2.
A and B: hypoxia inducible factor-1α (HIF-1α) gene silencing rescues hypoxia-mediated suppression of Notch2 in human pulmonary artery endothelial cells (hPAECs). A and B: hPAECs were transfected with HIF-1α siRNA or scrambled control (Scrmb). Transfected cells were exposed to hypoxia (Hyp) vs. normoxia (Norm), and samples for quantitative RT-PCR analysis were collected 24 h postexposure. Gene expression analyses for HIF-1α and Notch2 performed in hPAECs are shown (n = 9). C and D: vasoactive peptide endothelin-1 (ET-1), growth factors, and cytokines differentially regulate Notch2 expression in hPAECs. hPAECs were serum-starved overnight followed by stimulation with TGFβ1 (10 ng/mL), TNFα (10 ng/mL), ET-1 (10 ng/mL), and IGF-1 (200 ng/mL) for 24 h. Cells were lysed in RIPA buffer containing protease inhibitors and subjected to Western blot. Representative immunoblots and quantitative bar graphs depicting Notch2 in hPAECs (n = 6–9) are shown. P values for paired comparisons were calculated using Student’s t test. *P < 0.05, **P < 0.01, ***P <0.001. For B, values were determined using ANOVA. Post hoc tests applied the Bonferroni correction to allow for multiple comparisons. *P < 0.05 hypoxia vs. normoxia scrambled control, ###P < 0.001 HIF-1α siRNA vs. scrambled control hypoxia.
Vasoactive Factors, Proinflammatory Cytokines, and Growth Factors Released by Injured ECs Suppress Notch2 Pathway
As hypoxia is known to modulate synthesis and secretion of vasoactive factors and inflammatory cytokines (30), we proceeded to explore whether TGFβ1 (10 ng/mL), TNFα (10 ng/mL), ET-1 (10 ng/mL), and IGF-1 (200 ng/mL) for 24 h could attenuate Notch2 in serum-deprived hPAECs. Immunoblotting demonstrated a reduced abundance of Notch2 with TGFβ1, ET-1, and IGF-1 (∼30–40% reduction compared with vehicle control), while treatment with TNFα resulted in 1.7-fold increase in Notch2 (Fig. 2, C and D). These data illustrate that proinflammatory cytokines and growth factors likely contribute toward the downregulation in Notch2 in pulmonary ECs.
Loss of Notch2 Induces Proliferation and Migration of Pulmonary ECs, Akt and Erk1/2 Activation, and Cell Cycle Progression
Replicating Notch2 suppression of PAH patient pulmonary arteries, control hPAECs were transfected with Notch2 siRNA or scrambled control (Scrmb) and examined for alterations in proliferation and migration of pulmonary ECs: hallmarks of PAH. Notch2 gene silencing resulted in a significantly increased EC proliferation (Fig. 3A). Wound-scratch assays as described in Refs. 24, 27 were performed on Notch2 siRNA-transfected hPAECs. Quantitative analysis of the wound area showed Notch2 suppression potentiating the proliferative/migratory ability of hPAECs compared with controls (Fig. 3B). Furthermore, we used flow cytometry on hPAECs transfected with nontargeting scrambled control or Notch2 siRNA to explore a potential role of Notch2 in cell cycle progression. Propidium iodide staining of hPAECs demonstrated significantly increased numbers of cells in S and G2/M phases of the cell cycle in Notch2 siRNA-transfected hPAECs compared with controls (Fig. 3C). Activation of Akt and Erk1/2 (change in p-Akt to total Akt ratio; p-42/44 to total 42/44 MAPK ratio, respectively) pathways is known to promote proliferation, migration, and cell survival (31–34). Thus, we evaluated levels of activated Akt and Erk1/2 in hPAECs transfected with Notch2 siRNA versus Scrmb control. Immunoblot of hPAEC lysates confirmed knockdown of Notch2 and a significantly increased phospho-Akt and phospho-ERK 1/2 MAP kinase (Fig. 3D); no changes in total Akt or Erk 1/2 expression were observed. Notch2 attenuation also resulted in reduced expression of cyclin-dependent kinase inhibitor p21cip (Fig. 3D) compared with scrambled control.
Figure 3.
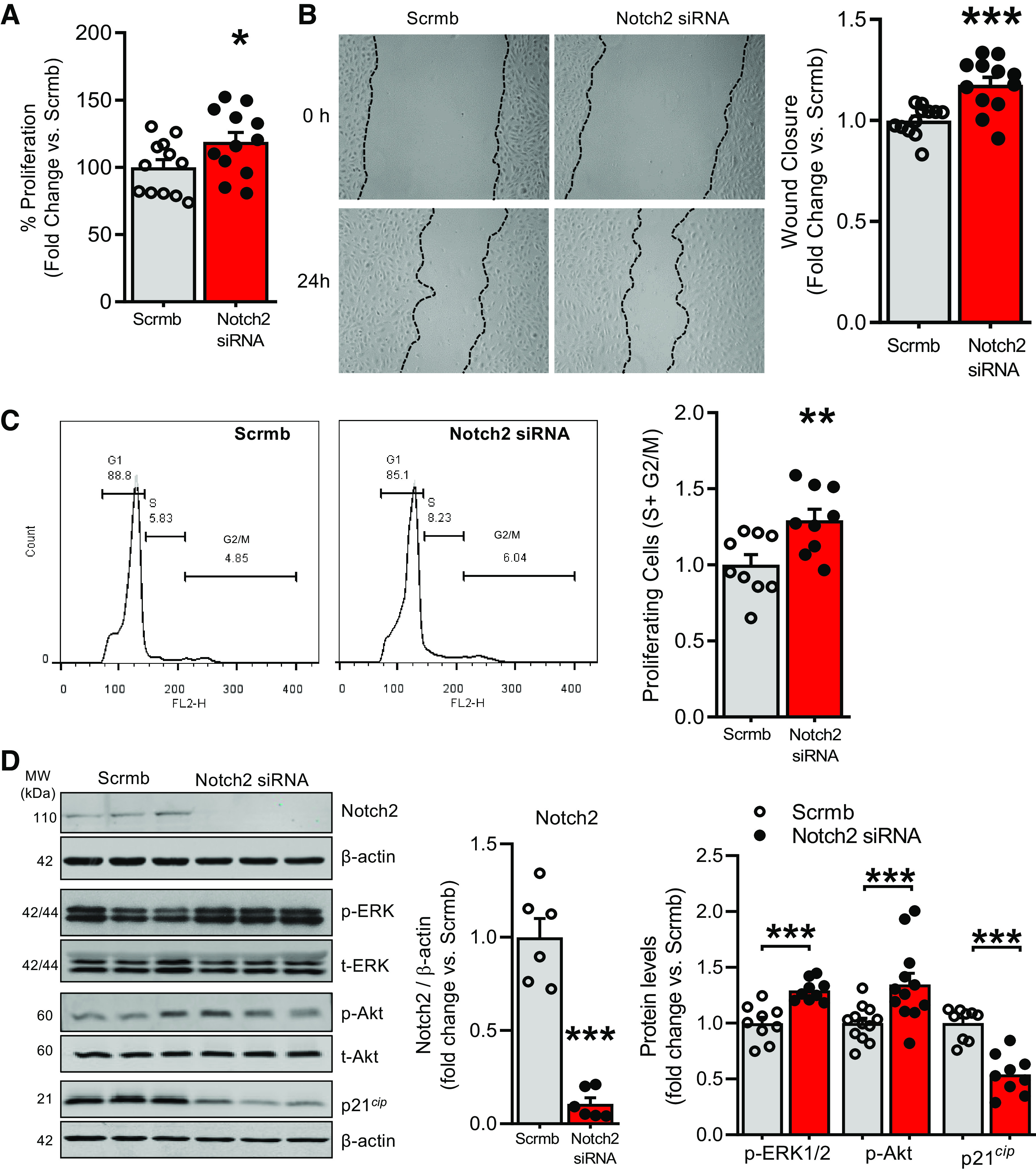
A–D: Notch2 silencing in control pulmonary artery endothelial cells (PAECs) increases EC proliferation and migration, activates phosphorylation of Erk1/2 and Akt, and reduces cyclin-dependent kinase inhibitor p21cip levels. A–C: human (h)PAECs were transfected with Notch2 siRNA or scrambled control (Scrmb) and assays performed 72 h posttransfection. A: transfected hPAECs were seeded and synchronized overnight, and proliferation was measured in a plate-based fluorescence assay using CyQuant (n = 12). B: gap-wound injury was made swiping a sterile 1,000-µl pipette tip across a lawn of transfected hPAECs. Brightfield images of the wound were captured and percent wound closure in cells treated with Notch2 siRNA was measured as the change in wound size between t = 0 and 24 h vs. the change occurring in Scrmb control-treated cells (n = 12). C: analysis of cell cycle distribution of transfected hPAECs harvested and stained with propidium iodide (PI) was performed by flow cytometry. Representative flow cytometry histograms show G1, S, and G2 + M phases of cell cycle distribution in Notch2 gene silenced vs. scrambled control hPAECs. Bar graphs depict proliferating cells quantified as total number of cells in S + G2/M phases of cell cycle as a function of control (n = 9). D: representative immunoblot and quantitative bar graphs of expression levels of Notch2, phospho-Erk1/2 vs. total Erk1/2 (p-42/44 MAPK vs. total 42/44 MAPK), phospho-Akt ser473 vs. total Akt, and cell cycle protein cyclin-dependent kinase inhibitor 1 (p21cip) in lysates from Notch2 siRNA-transfected vs. scrambled control hPAECs (n = 6–9). Dots represent individual biological replicates. Bar graphs indicate means ± SE. P values for paired comparisons were calculated using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Notch2 Silencing Inhibits Endothelial Cell Apoptosis
Inhibition of EC apoptosis is thought to be an essential factor underlying EC survival (8). Therefore, we investigated whether Notch2 downregulation could be promoting proliferation via evasion of apoptosis and increased cell survival. Notch2 siRNA- or scrambled siRNA-transfected ECs were stained with FITC-annexin V and propidium iodide (PI) followed by flow cytometry to assess apoptosis. Cells staining for annexin V with or without PI [represented in the top and bottom right quadrants of the scatter plot (Q2 and Q3; Fig. 4A)] are indicative of apoptotic cells. The percentage of apoptotic ECs was significantly lower in Notch2 siRNA-treated versus control (10.76 ± 1.17 vs. 16.18 ± 1.4 for Scrmb), consistent with Notch2 silencing favoring EC evasion of apoptosis and increased survival (Fig. 4A). Several members of Bcl-2 family of proteins, including Bcl-2 and Bax (35) as well as stress-activated MAPK kinases, such as SAPK and p38 MAP kinases, are involved in positive and negative regulation of apoptotic cell death in response to myriad cellular injuries (36). We assessed whether reductions in Notch2 altered levels of Bcl-2, Bax, SAPK, and p38 MAPK proteins. Immunoblots of Notch2 siRNA-treated cell lysates showed higher Bcl-2 (antiapoptotic protein) and lower proapoptotic protein, Bax, compared with controls (Fig. 4B) as well as significantly lower phospho-SAPK and phospho-p38 MAPK compared with controls (Fig. 4C) with no significant changes in the total amount of SAPK or p38 MAPK.
Figure 4.
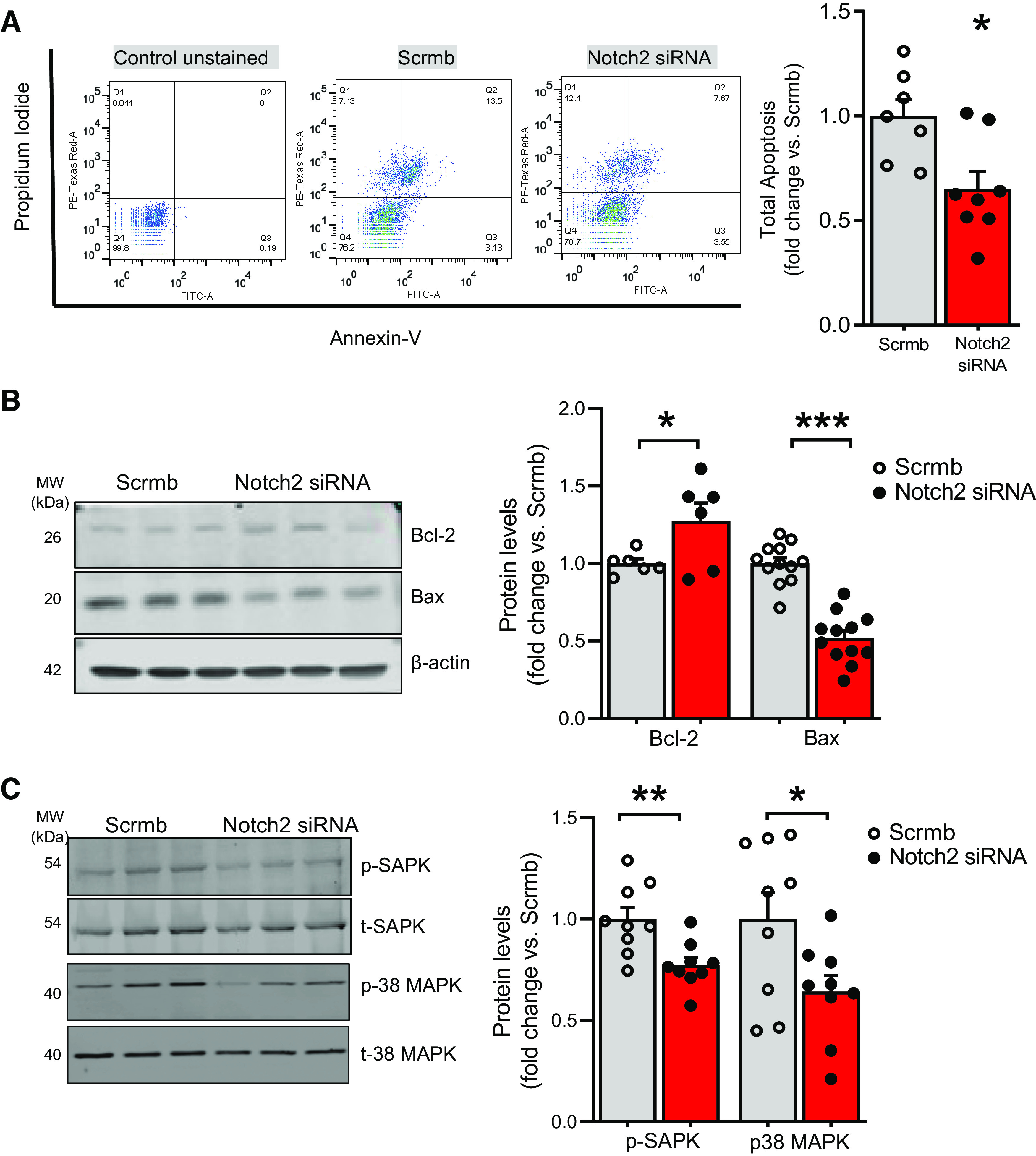
A–C: Notch2 gene silencing in control human pulmonary artery endothelial cells (hPAECs) inhibits endothelial cell apoptosis. hPAECs were transfected with Notch2 siRNA or scrambled control (Scrmb), synchronized overnight, and analyzed 72 h posttransfection. A: annexin V-propidium iodide apoptosis assay was performed to study cell apoptosis. Living cells are double negative for annexin V and propidium iodide (Q4), early apoptotic cells are annexin V positive and propidium iodide negative (Q3), late apoptotic cells are double positive for annexin V and propidium iodide (Q2), and necrotic/dead cells are propidium iodide-positive only (Q1). Values in Q2 and Q3 represent all apoptotic cells. Reduced numbers in Q2 and Q3 are representative of apoptosis resistance. Representative scatterplot and quantitative bar graph of total apoptosis is presented as fold change (n = 7–8). B and C: representative immunoblots and quantitative bar graphs of Bcl-2 and Bax (B) and phospho-SAPK and p38 MAPK (C) levels in Notch2 siRNA-transfected vs. scrambled control hPAEC lysates (n = 9). Dots represent individual biological replicates. Bar graphs indicate means ± SE. P values for paired comparisons were calculated using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Notch2-Directed Gene Silencing Increases Notch1 Levels and Activation
Next, we examined whether gene silencing of endothelial Notch2 alters levels of other Notch receptors present in the pulmonary ECs. Quantitative RT-PCR demonstrated Notch2 gene silencing in hPAECs under normoxia significantly increases transcript levels of Notch1 while Notch4 levels remained unchanged (Fig. 5A). The Notch1 increase was corroborated at the protein level (Fig. 5B). As Notch protein undergoes posttranslational processing including cleavage to an active signaling molecule, increased expression of Notch1 leading to elevated levels of activated (cleaved) Notch1 upon Notch2 silencing was interrogated. Immunoblots showed that in addition to total Notch1 levels, Notch2 siRNA increased levels of cleaved Notch1 (Fig. 5B). IF staining of hPAECs showed widespread increases in cleaved Notch1 in Notch2-suppressed cells (Fig. 5C, box images quantified in graph to the right), corroborating findings shown in Fig. 5B. IF staining confirmed knockdown of Notch2 in Notch2-gene silenced ECs compared with controls (Fig. 5C, 1st column). We next questioned whether hypoxic exposure of Notch2-deficient ECs would further augment Notch2 silencing-mediated activation of Notch1. Immunoblot analysis demonstrated an elevated cleaved Notch1 level in cells exposed to hypoxia versus normoxia (gray hypoxic versus gray normoxic; Fig. 5D) (18). Moreover, an increase in cleaved Notch1 in Notch2 siRNA-treated versus scrambled control ECs (red Notch2 siRNA vs. gray scrambled bars in normoxia) is further enhanced when Notch2 siRNA-transfected ECs are exposed to hypoxia (red vs. red Notch2 siRNA bars, hypoxia vs. normoxia) (Fig. 5D). To investigate whether silencing of Notch1 would mitigate Notch2 deficiency-mediated effects on EC phenotype, we transfected ECs with either Notch1 or Notch2 or in combination and scrambled control siRNAs and examined EC wound closure. Quantitative analysis of the wound area showed that while Notch2 gene silencing potentiated the migratory ability of hPAECs compared with controls, contrary to our assumption, additional knockdown of Notch1 further increased EC wound closure (Fig. 5E).
Notch1 Upregulation in Notch2-Deficient ECs Increases Downstream Effectors Hes and Hey and Is Independent of Changes in Canonical Notch Ligands
We examined whether there is an increased nuclear translocation of Notch intracellular domain and transcriptional activation of Notch target genes in Notch2 gene-silenced ECs. Immunoblot of nuclear fractions isolated from Notch2 siRNA- and scrambled control-transfected hPAECs displayed a near fourfold increased cleaved Notch1 in Notch2 siRNA ECs (Fig. 6A), indicative of enhanced translocation to the nucleus. Hes1, Hey1, and Hey2 are among the key canonical Notch effector gene products (15), that are known to transduce signals to multiple pathways key to cell survival. We examined Hes1, Hey1, and Hey2 by qRT-PCR and found that Notch2 silencing under normoxic conditions increased transcript levels of Hes1, Hey1, and Hey2 (Fig. 6B). We interrogated whether Notch2 silencing-mediated cleavage of Notch1 is ligand-dependent. Examining the expression of key canonical ligands of the Notch pathway, DLL1, DLL4, Jagged1 (Jag1), and Jagged2 (Jag2) by qRT-PCR and DLL4 and Jag1 by Western blot analysis, no change was observed upon gene silencing of Notch2 (Fig. 6, C and D, respectively).
Figure 6.
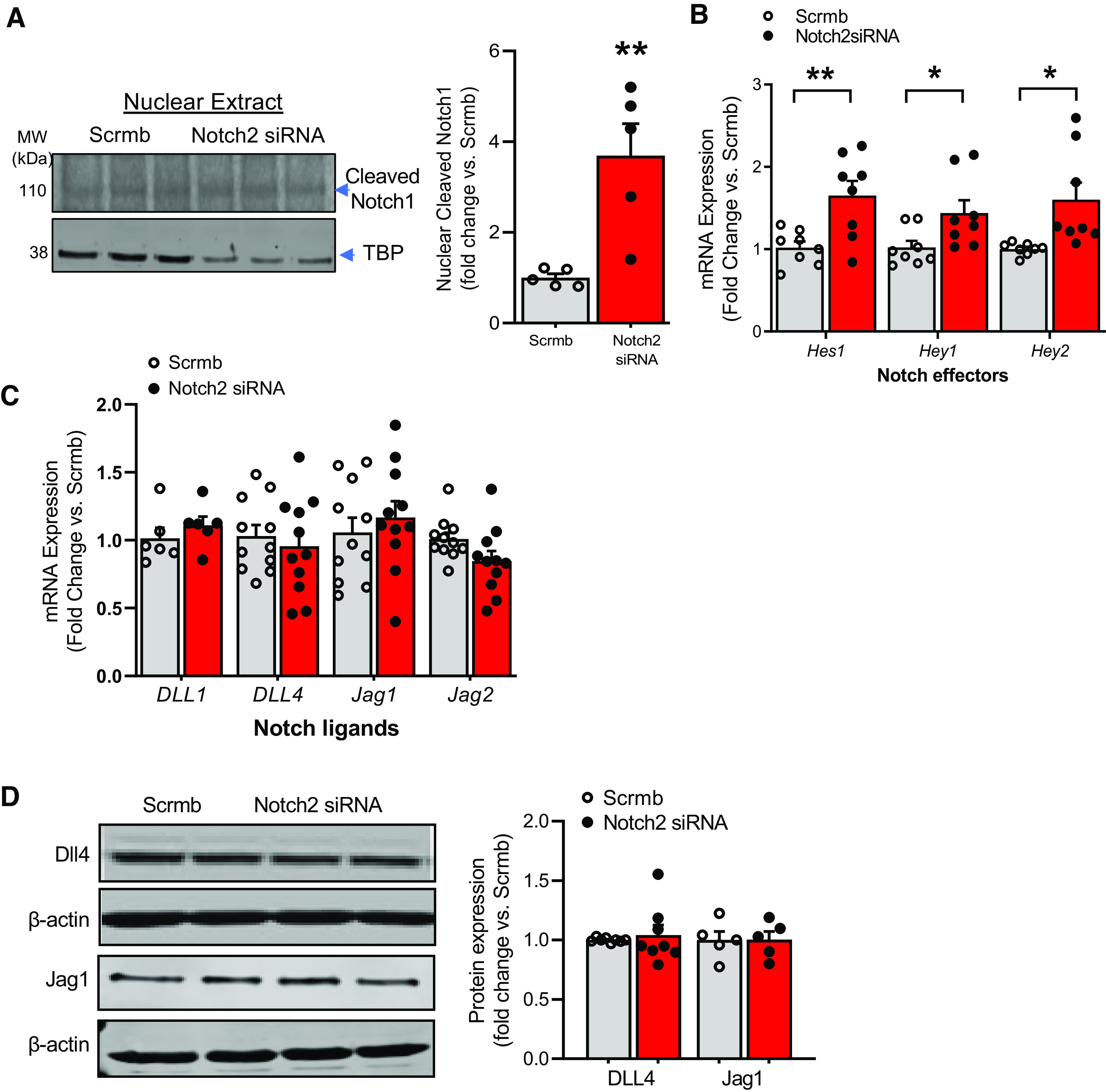
A–D: Notch2 induces nuclear Notch1 and canonical Notch effectors, independent of changes in Notch ligands in human pulmonary artery endothelial cells (hPAECs). hPAECs transfected with Notch2 siRNA or scrambled control (Scrmb), synchronized overnight with serum-reduced media, and assayed 72 h posttransfection for quantitative (q)RT-PCR or Western blot analysis. A: representative immunoblot and quantitative bar graphs of nuclear cleaved Notch1 in nuclear lysates of Notch2 siRNA-treated cells vs. scrambled control 72 h posttransfection (n = 3). TBP, TATA-binding protein. B: gene expression analysis for Hes1, Hey1, and Hey2 was performed in transfected hPAECs 72 h posttransfection by qRT-PCR (n = 8). C: gene expression analysis for Notch ligands Dll1, DLL4, Jag1, and Jag2 was performed in transfected hPAECs 72 h posttransfection (n = 6–11). D: representative immunoblot and quantitative bar graphs depicting level of endothelial DLL4 and Jag1 in hPAECs transfected with Notch2 siRNA (siRNA) or scrambled control (Scrmb) are provided (n = 5–8). Dots represent individual biological replicates. Bar graphs indicate means ± SE. P values for paired comparisons were calculated using Student’s t test. *P < 0.05; **P < 0.01.
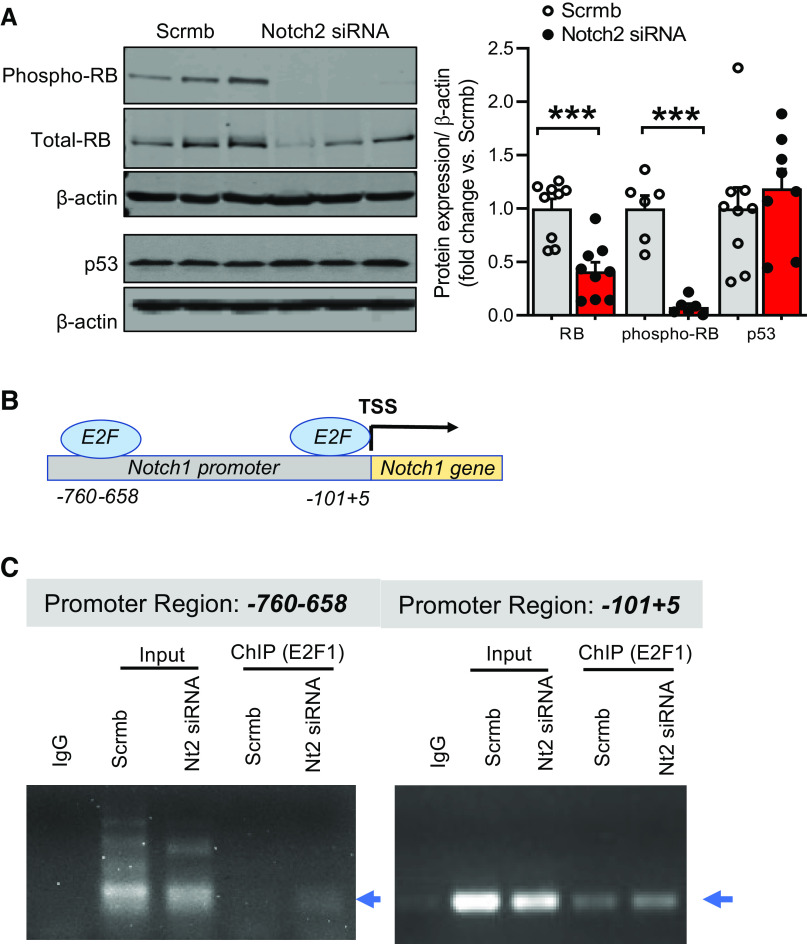
Notch2 Deficiency Decreases Retinoblastoma Protein and Modulates E2F1 Transcriptional Activity to Promote Notch1 Levels
To gain mechanistic insight into how Notch2 deficiency promotes Notch1 expression and mediates EC proliferation, the expression of tumor suppressor genes Rb and p53 in pulmonary ECs was tested. Immunoblot of Notch2 siRNA-treated cell lysates showed a significant reduction in Rb protein (both at the phosphorylated and total level) and no change in p53 expression compared with controls (Fig. 7A). These findings led us to posit that the loss of Rb upon Notch2 deficiency frees E2F1 from Rb-E2F1-bound inhibitory complex (37) and fosters E2F1 binding to Notch1 promoter, thus increasing Notch1 levels in Notch2-deficient ECs. As such, the decrease in Rb levels in Notch2-deficient ECs prompted us to investigate the effect of a loss in Notch2 on E2F1 binding to the Notch1 promoter. Using TRANSFAC and Notch1 promoter bioinformatic analysis, we identified several sequence-specific E2F1-binding domains in the Notch1 promoter and focused our analysis on two consensus regions within ∼1,000 bp upstream of the Notch1 transcriptional start site (TSS) (Fig. 7B). Chromatin immunoprecipitation (ChIP) assays were performed to identify the functional E2F1 binding sites in the Notch1 promoter. ChIP assays using primers flanking two E2F1 binding regions on the Notch1 promoter (that specifically amplified DNA sequence immunoprecipitated by E2F1-specific antibody) revealed that compared with controls, Notch2 gene silencing increases E2F1 binding to DNA-binding elements of the Notch1 promoter (1.5-fold vs. scrambled control for the binding site ∼100 bp from TSS; Fig. 7C). These data are consistent with Notch1 as an E2F1-directed target gene in Notch2-deficient ECs. Taken together, the immunoblot analysis displaying a decrease in Rb protein (Fig. 7A) and in vitro-ChIP assay (Fig. 7C) demonstrating increased E2F1 binding on the Notch1 promoter upon knockdown of Notch2 are consistent with the Rb-E2F1 nexus being involved in feed-forward reciprocal upregulation of Notch1 in Notch2-deficient ECs.
Figure 7.
A–C: Notch2 gene silencing abrogates Rb protein expression and modulates E2F1 binding to the Notch1 promoter. A: representative immunoblot and quantitative bar graphs for tumor suppressors Rb (total and phospho) and p53 performed in human pulmonary artery endothelial cells (hPAECs) transfected with Notch2 siRNA or scrambled control (Scrmb) (n = 6–9). B: schematic diagram of the human Notch1 promoter illustrating the E2F binding sites in the regulatory region; upstream regions were numbered in relation to the transcription start site (TSS). C: for chromatin immunoprecipitation (ChIP) assay, hPAECs transfected with Notch2 (Nt2) siRNA or scrambled control (Scrmb) were fixed with formaldehyde and cross-linked chromatin was subjected to immunoprecipitation with E2F1 antibody. The amount of chromatin bound to E2F1 was assayed by PCR using primers flanking specific regions on Notch1 promoter (arrowheads). Controls include PCR reactions performed with no DNA, input DNA and DNA immunoprecipitated by normal species-matched IgG antibody. Dots represent individual biological replicates. Bar graphs indicate means ± SE. P values for paired comparisons were calculated using Student’s t test. ***P < 0.001.
DISCUSSION
This study provides the first evidence to our knowledge that Notch2 is central to foundational homeostatic processes in the pulmonary endothelium. Specifically, the salient novel and mechanistic findings of the current study are 1) EC Notch2 is suppressed in response to hypoxia (a model that recapitulates PAH), proinflammatory cytokines, and growth factors in vitro; 2) gene silencing of Notch2 shifts the homeostatic balance in favor of cell cycle progression, a proproliferative, promigratory phenotype; 3) Notch2 knockdown perturbs apoptosis signaling and attenuates EC apoptosis; 4) Notch2 knockdown upregulates activated Notch1; and 5) these effects are mediated via liberation of transcription factor E2F1 from Rb-E2F1 complex allowing for E2F1-Notch1 promoter-driven increases in Notch1.
Our data are derived primarily in vitro using commercially available human pulmonary artery endothelial cells. However, the significance of these data is underscored by the clinically relevant findings reported here that Notch2 is markedly lower in human lung endothelium and total lung lysates of human PAH versus non-PAH subjects. Mutations leading to changes in Notch2 function have been linked to various cancers (38) and certain inherited cardiovascular defects, including Alagille syndrome (39). Herein, we emphasize the functional relevance of loss of function for Notch2 in pulmonary endothelium and by extension PAH. Notch2 polymorphisms in PAH cohorts have to our knowledge not been reported to date. In aggregate, our findings suggest that Notch2 is at the nexus of a homeostatic pathway repressing endothelial cell division and migration while promoting apoptosis, and conversely inform the field of a previously unidentified role for Notch2 loss-of-function and Notch1 activation in promoting these phenotypes and PAH.
PAH pathogenesis is a multifactorial process involving the primacy of exacerbated endothelial cell proliferation and, in turn, smooth muscle hyperplasia. This study was focused on the potential role for endothelial Notch2 suppression in vitro as a component of PAH pathogenesis. Immunohistochemical studies on pulmonary artery cross sections from human PAH patients display increased expression of HIF-1 subunits, particularly in ECs from plexiform lesions (29). This increase in HIF-1 levels is partially modeled by exposing cells or animals to hypoxia and is, therefore, a well-established model for recapitulating PAH both in vitro and in vivo. Furthermore, since hypoxia is known to regulate synthesis and secretion of vasoactive factors and inflammatory cytokines (30), we proceeded to explore whether such factors could alter Notch2 expression. Growth factors such as IGF-1 (40), vasoactive molecule (ET-1) (41), and cytokines TNFα and TGFβ1 (17, 42) are also heightened in complex lesions in PAH and implicated in abnormal proliferation. Indeed, we show that HIF-1α is responsible for the suppression of Notch2 (Fig. 2B) and that TGFβ, ET-1, and IGF-1 (regulated by hypoxia) were all capable of suppressing Notch2 (Fig. 2, C and D). Other than the factors described above, it is tempting to speculate that hypoxia-mediated suppression of Notch2 is also mediated by miRNA-214. miRNA-214 expression is upregulated by hypoxia as we have previously published for vascular SMCs (7) (as well as ECs, unpublished data). Furthermore, bioinformatic analysis for potential targets for miR-214 shows binding sites on Notch2 3′-untranslated region. We therefore reason that hypoxia-induced miR-214 could explain, in part, the downregulation of Notch2 in ECs. It is also well established that ECs not only function as major generators of vasoactive mediators, such as NO, prostacyclin, and ET-1 that alter SMC function but also maintain homeostasis. Therefore, an understanding of cellular signaling mechanisms that promote abnormal proliferation is of major importance.
The current study demonstrates that Notch2 expression is markedly attenuated in lung arterial intima in PAH patients and upon exposure of hPAECs to hypoxia or growth factors such as TGFβ1 and IGF-1 (Figs. 1 and 2). While dysfunction in Notch2 has been reported for various cancers and certain congenital cardiovascular defects, this is the first study to our knowledge in which this link has been made with PAH. Such loss of endothelial Notch2 is in contrast with other endothelial members of Notch receptor family including Notch1 (18) and Notch4 and SMC-specific Notch3 (19–21), which are shown to be upregulated in PAH. Admittedly, antagonistic effects of Notch2 and Notch1 have been reported in various cancers (43, 44). Consistent with previous studies (17, 23), we found that TNFα, which is released by perturbed ECs, induces Notch2 protein expression (Fig. 2D). It is therefore plausible that the magnitude, specific mediator, and strength and duration of stimulation triggers differential Notch signaling (45). It is also logical from an evolutionary standpoint that individual Notch receptor homologs confer distinct biological functions, and educe divergent signaling pathways (43, 46).
Aberrant EC proliferation, migration, and resistance to apoptosis are among pivotal determinants driving pathogenesis of PAH (3–6). In this study, Notch2 suppression led to an increase in proliferative capacity, migration (wound closure), and cell cycle progression of ECs. Moreover, we found that loss of Notch2 activated Akt and Erk1/2 pathways (Fig. 3) consistent with their roles in enhanced proliferation, migration and cell survival (31–33). The p21cip downregulation we observed is also consistent with an unabated progression of the cell cycle and proliferation. Additionally, we found that Notch2 knockdown decreases the number of cells expressing indicators of apoptosis; and increased Bcl-2 and decreased Bax fall in line as drivers of the phenotype (Fig. 4). Furthermore, we observed a decrease in the levels of p-SAPK and p38 MAPK, instigators of apoptosis (36). Taken together, our findings suggest crossover signaling causing resistance to apoptosis, on the one hand, and proliferation/migration on the other in Notch2-suppressed ECs, all leading to enhanced cell survival boosting the latter.
One of the most unique findings of our study is that Notch2-deficient ECs display a transcriptional and translational upregulation of Notch1 as well as activation (cleavage) of Notch1 via mechanisms independent of changes in cognate ligands (Figs. 5 and 6). Similar compensatory response is reported in breast cancer cells (47) where opposing expression of Notch1 and Notch2 proteins are demonstrated during tumor development. Thus it is not illogical that Notch2 suppression would upregulate Notch1 in ECs, although this is the first study to our knowledge in which this linkage has been made in ECs or PAH. Furthermore, hypoxia-induced Notch1 activation was robustly augmented by Notch2 suppression (Fig. 5D). Either Notch2 suppression or attendant Notch 1 upregulation and activation leads to an increase in Hes1, Hey1, and Hey2. Indeed, the latter is likely to be true as enhanced levels of activated Notch1 resulted in increased nuclear abundance which is consistent with the cleaved Notch1 acting as a transcription factor (Fig. 6A) in the upregulation of Hes1, Hey1, and Hey2 (Fig. 6B). Furthermore, Hes1, Hey1, and Hey2 are known to reduce expression of checkpoint inhibitor p21cip (and p27kip) causing disinhibition of the cell cycle and hence proliferation (48). This would explain the propagated cycle of accelerated growth of ECs in our hands.
Based on these observations and the prior literature, we postulated that Notch1 inhibition in Notch2-deficient cells would diminish wound closure, yet it had the opposite effect. Thus it appears that the primacy of endothelial Notch2 suppression sets up a condition in which subsequent endothelial Notch1 inhibition feedforward enhances the effect of Notch2. Thinking of this a little more broadly, the effect of increased Notch1 on a Notch2-suppressed background is likely to be cell type and milieu specific. That is, we observed that Notch1 suppression in ECs causes an autocrine impact to elevate EC proliferation and migration (wound closure). Clearly, the functional roles of Notch1 and 2 and their interplay deserve closer scrutiny and their cross talk in vivo is predictably more complex.
Finally, our findings of increased E2F1 binding to the human Notch1 promoter upon Notch2 gene silencing may be explained in some part by reduced levels of Rb in Notch2-deficient ECs (Fig. 7). Mechanistically, we proffer that loss of Rb results in liberation of E2F1, Notch1 promotion, and ubiquitous proliferation via regulation of cell cycle and proliferation genes (37). Both Rb and E2F family of proteins function to regulate multiple cell processes, including cell proliferation, migration, apoptosis, and differentiation (37). Thus our findings provide a rational and clear link between Notch2 and Notch1. It is important to point out, however, that other potential effectors under the control of E2F1 could also modulate cell proliferation and survival signals in Notch2-deficient ECs.
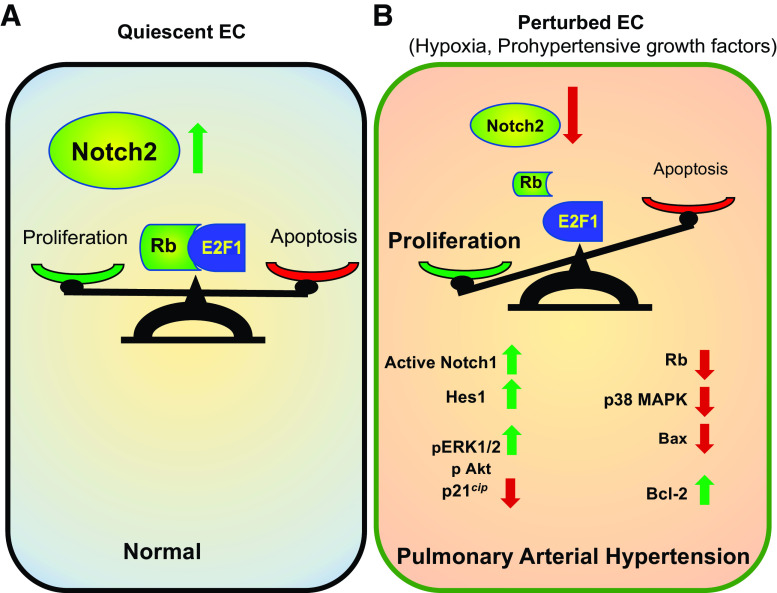
Overall, our results suggest that expression of Notch2 in the endothelium protects against the development of PAH by maintaining a checkpoint balance in the rates of EC proliferation and apoptosis. In the normal endothelium, the findings suggest that Notch2 preserves the quiescence and integrity of endothelium by quelling cell cycle progression. On the other hand, in response to hypoxia, and/or attendant prohypertensive growth factors and cytokines, the ensuing loss of Notch2 appears to govern a complex, orchestrated balance between EC proliferation and antiapoptosis mediated by a variety of effectors including those elicited by a disruption of the Rb-E2F1 complex and promotion of Notch1. All told, these endothelial signaling events are expected to lead to vascular remodeling and the development of PAH (Fig. 8).
Figure 8.
A and B: proposed protective function of Notch2 signaling axis in pulmonary arterial hypertension. A: in the normal endothelium, abundance of Notch2 maintains quiescence via balancing the rate of endothelial cell proliferation and apoptosis, and thus supports preservation of pulmonary artery endothelium integrity. B: perturbed endothelia, however, express decreased levels of Notch2 resulting in increased active Notch1 and increased rates of proliferation and apoptosis-resistant pulmonary artery endothelial cells (EC), leading to endothelial dysfunction and subsequent vascular remodeling associated with pulmonary arterial hypertension.
This study reveals a previously unidentified role for Notch2 in EC quiescence and pulmonary artery homeostasis. The clinical significance of these findings is underscored by the observation that Notch2 is markedly reduced in human PAH specimens. Our results show that this suppression drives activated Notch1 leading to a shift toward a proproliferative, apoptosis-resistant EC phenotype. It has been suggested that an imbalance between apoptotic and survival pathways channels endothelial cells toward a more synthetic pro-proliferative and promigratory phenotype in human PAH. Altogether, the above-mentioned evidence suggests that a therapeutic Notch2 activation or Notch1 inhibition preserving EC homeostasis is worthy of evaluation. Additional in vivo work utilizing mice harboring endothelial-specific deletion of Notch2 is needed to identify specific molecular mechanisms at play in PAH pathogenesis and to study the relative contribution of other Notch receptors in causing or driving PAH progression in a Notch2-deficient background. Moreover, a broader and more in-depth understanding of the mechanisms by which individual Notch pathways relay with other Notch isoforms is likely to shed new light on the control of cellular apoptosis and survival in ECs and multiple other cell types.
GRANTS
This work was funded by National Institutes of Health (NIH) Grants R01-HL-142248, R01-HL-079207, R01-HL-112914, and T32-GM-008424 and American Heart Association (AHA) Grant 18TPA34170069 (to P.J.P.); NIH Grant R01-HL-113178 (to E.G); NIH Grant R01-HL-142638 (to M.M.R.); and NIH Grants R01-HL-133864 and R01-HL-128304 and AHA Established Investigator Grant 19 EIA34770095 (to A.C.S.); and Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania and Vitalant (to P.J.P., A.C.S., and E.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and P.J.P. conceived and designed research; S.S., Y.L., D.d.J., J.S., and M.M.R. performed experiments; S.S., Y.L., D.d.J., E.G., E.C.-P., and P.J.P. analyzed data; S.S., Y.L., E.C.-P., A.C.S., and P.J.P. interpreted results of experiments; S.S. and E.C.-P. prepared figures; S.S. drafted manuscript; S.S., Y.L., E.C.-P., and P.J.P. edited and revised manuscript; S.S., Y.L., D.d.J., J.S., M.M.R., E.G., E.C.-P., A.C.S., and P.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge imaging facilities provided by the Center for Biologic Imaging (University of Pittsburgh). We thank Dr. S. M. Nouraie for expert assistance with statistical analyses and power determinations.
REFERENCES
- 1.Hopper RK, Feinstein JA, Manning MA, Benitz W, Hudgins L. Neonatal pulmonary arterial hypertension and Noonan syndrome: two fatal cases with a specific RAF1 mutation. Am J Med Genet A 167A: 882–885, 2015. doi: 10.1002/ajmg.a.37024. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 188: 365–369, 2013. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Morrell NW, Adnot S, Archer SL, Dupuis J, Lloyd Jones P, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–31, 2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131: 1006–1018, 2015. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo S, Meijles DN, Al Ghouleh I, Tandon M, Cifuentes-Pagano E, Sembrat J, Rojas M, Goncharova E, Pagano PJ. MEF2C-MYOCD and Leiomodin1 auppression by miRNA-214 promotes smooth muscle cell phenotype switching in pulmonary arterial hypertension. PLoS One 11: e0153780, 2016. doi: 10.1371/journal.pone.0153780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 10: 95, 2009. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humbert M, Montani D, Perros F, Dorfmüller P, Adnot S, Eddahibi S. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul Pharmacol 49: 113–118, 2008. doi: 10.1016/j.vph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Ghouleh IA, Sahoo S, Meijles DN, Amaral JH, de Jesus DS, Sembrat J, Rojas M, Goncharov DA, Goncharova EA, Pagano PJ. Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin Sci (Lond) 131: 2019–2035, 2017. doi: 10.1042/CS20160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 12.Jonigk D, Merk M, Hussein K, Maegel L, Theophile K, Muth M, Bockmeyer CL, Mengel M, Gottlieb J, Welte T, Haverich A, Golpon H, Kreipe H, Laenger F. Obliterative airway remodeling: molecular evidence for shared pathways in transplanted and native lungs. Am J Pathol 178: 599–608, 2011. doi: 10.1016/j.ajpath.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43: 25S–32S, 2004. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689, 2006. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 15.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18: 901–911, 2004. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JK, Lee TW, Do EK, Moon HJ, Kim JH. Role of Notch1 in the arterial specification and angiogenic potential of mouse embryonic stem cell-derived endothelial cells. Stem Cell Res Ther 9: 197, 2018. doi: 10.1186/s13287-018-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B, Inman GJ, Bradley JR, Rana AA, Upton PD, Morrell NW. TNFalpha drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun 8: 14079, 2017. doi: 10.1038/ncomms14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabral S, Tian X, Kojonazarov B, Savai R, Ghofrani HA, Weissmann N, Florio M, Sun J, Jonigk D, Maegel L, Grimminger F, Seeger W, Savai Pullamsetti S, Schermuly RT. Notch1 signalling regulates endothelial proliferation and apoptosis in pulmonary arterial hypertension. Eur Respir J 48: 1137–1149, 2016. doi: 10.1183/13993003.00773-2015. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwate PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 15: 1289–1297, 2009. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu YR, Mao L, Piantadosi CA, Gunn MD. CCR2 deficiency, dysregulation of Notch signaling, and spontaneous pulmonary arterial hypertension. Am J Respir Cell Mol Biol 48: 647–654, 2013. doi: 10.1165/rcmb.2012-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Xie X, Zhu Y, Liu L, Feng W, Pan Y, Zhai C, Ke R, Li S, Song Y, Fan Y, Fan F, Wang X, Li F, Li M. Inhibition of Notch3 prevents monocrotaline-induced pulmonary arterial hypertension. Exp Lung Res 41: 435–443, 2015. doi: 10.3109/01902148.2015.1060545 [DOI] [PubMed] [Google Scholar]
- 22.Quillard T, Devalliere J, Chatelais M, Coulon F, Séveno C, Romagnoli M, Barillé Nion S, Charreau B. Notch2 signaling sensitizes endothelial cells to apoptosis by negatively regulating the key protective molecule survivin. PLoS One 4: e8244, 2009. doi: 10.1371/journal.pone.0008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quillard T, Devalliere J, Coupel S, Charreau B. Inflammation dysregulates Notch signaling in endothelial cells: implication of Notch2 and Notch4 to endothelial dysfunction. Biochem Pharmacol 80: 2032–2041, 2010. doi: 10.1016/j.bcp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G, Pagano PJ. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem 288: 36437–36450, 2013. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gammella E, Leuenberger C, Gassmann M, Ostergaard L. Evidence of synergistic/additive effects of sildenafil and erythropoietin in enhancing survival and migration of hypoxic endothelial cells. Am J Physiol Lung Cell Mol Physiol 304: L230–L239, 2013. doi: 10.1152/ajplung.00112.2012. [DOI] [PubMed] [Google Scholar]
- 26.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol 298: L600–L606, 2010. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2: 329–333, 2007. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 28.Lei W, He Y, Shui X, Li G, Yan G, Zhang Y, Huang S, Chen C, Ding Y. Expression and analyses of the HIF-1 pathway in the lungs of humans with pulmonary arterial hypertension. Mol Med Rep 14: 4383–4390, 2016. doi: 10.3892/mmr.2016.5752. [DOI] [PubMed] [Google Scholar]
- 29.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 30.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 31.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell 169: 381–405, 2017. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene 26: 3113–3121, 2007. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 33.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res 86: 892–896, 2000. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 34.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med 195: 425–437, 2017. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hata AN, Engelman JA, Faber AC. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 5: 475–487, 2015. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23: 2838–2849, 2004. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 37.Sachdeva UM, O’Brien JM. Understanding pRb: toward the necessary development of targeted treatments for retinoblastoma. J Clin Invest 122: 425–434, 2012. doi: 10.1172/JCI57114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang NJ, Sanborn Z, Arnett KL, Bayston LJ, Liao W, Proby CM, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci U S A 108: 17761–17766, 2011. doi: 10.1073/pnas.1114669108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn 237: 1144–1152, 2008. doi: 10.1002/dvdy.21502. [DOI] [PubMed] [Google Scholar]
- 40.Sun M, Ramchandran R, Chen J, Yang Q, Raj JU. Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice. Am J Respir Cell Mol Biol 55: 779–791, 2016. doi: 10.1165/rcmb.2015-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 42.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol 49: 1135–1145, 2013. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res 64: 7787–7793, 2004. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 44.Kohlhof H, Hampel F, Hoffmann R, Burtscher H, Weidle UH, Hölzel M, Eick D, Zimber-Strobl U, Strobl LJ. Notch1, Notch2, and Epstein-Barr virus-encoded nuclear antigen 2 signaling differentially affects proliferation and survival of Epstein-Barr virus-infected B cells. Blood 113: 5506–5515, 2009. doi: 10.1182/blood-2008-11-190090. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, Bocchetta M. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res 67: 7954–7959, 2007. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- 46.Graziani I, Eliasz S, De Marco MA, Chen Y, Pass HI, De May RM, Strack PR, Miele L, Bocchetta M. Opposite effects of Notch-1 and Notch-2 on mesothelioma cell survival under hypoxia are exerted through the Akt pathway. Cancer Res 68: 9678–9685, 2008. doi: 10.1158/0008-5472.CAN-08-0969. [DOI] [PubMed] [Google Scholar]
- 47.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med 14: 779–786, 2004. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- 48.Rostama B, Turner JE, Seavey GT, Norton CR, Gridley T, Vary CP, Liaw L. DLL4/Notch1 and BMP9 interdependent signaling induces human endothelial cell quiescence via P27KIP1 and thrombospondin-1. Arterioscler Thromb Vasc Biol 35: 2626–2637, 2015. doi: 10.1161/ATVBAHA.115.306541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included within the article.