Abstract
The endothelin-B (ETB) receptor is a key regulator of vascular endothelial function in women. We have previously shown that the ETB receptor mediates vasodilation in young women, an effect that is lost after menopause. However, the direct impact of changes in estradiol (E2) on ETB receptor function in women remains unclear. Therefore, the purpose of this study was to test the hypothesis that E2 exposure modulates ETB receptor-mediated dilation in young women. Fifteen young women (24 ± 4 yr, 24 ± 3 kg/m2) completed the study. Endogenous sex hormone production was suppressed with daily administration of a gonadotropin-releasing hormone antagonist (GnRHant; Ganirelix) for 10 days; E2 (0.1 mg/day, Vivelle-Dot patch) was added back on days 4–10. We measured vasodilation in the cutaneous microcirculation (microvascular endothelial function) via local heating (42°C) on day 4 (GnRHant) and day 10 (GnRHant + E2) using laser Doppler flowmetry coupled with intradermal microdialysis during perfusions of lactated Ringer’s (control) and ETB receptor antagonist (BQ-788, 300 nM). During GnRHant, vasodilatory responses to local heating were enhanced with ETB receptor blockade (control: 83 ± 9 vs. BQ-788: 90 ± 5%CVCmax, P = 0.004). E2 administration improved vasodilation in the control site (GnRHant: 83 ± 9 vs. GnRHant + E2: 89 ± 8%CVCmax, P = 0.036). Furthermore, cutaneous vasodilatory responses during ETB receptor blockade were blunted after E2 administration (control: 89 ± 8 vs. BQ-788: 84 ± 8%CVCmax, P = 0.047). These data demonstrate that ovarian hormones, specifically E2, modulate ETB receptor function and contribute to the regulation of microvascular endothelial function in young women.
NEW & NOTEWORTHY The endothelin-B (ETB) receptor mediates vasodilation in young women, an effect lost following menopause. It is unclear whether these alterations are due to aging or changes in estradiol (E2). During endogenous hormone suppression (GnRH antagonist), blockade of ETB receptors enhanced cutaneous microvascular vasodilation. However, during E2 administration, blockade of ETB receptors attenuated vasodilation, indicating that the ETB receptor mediates dilation in the presence of E2. In young women, ETB receptors mediate vasodilation in the presence of E2, an effect that is lost when E2 is suppressed.
Keywords: cutaneous microdialysis, endothelin-B receptor, sex hormones, skin blood flow
INTRODUCTION
The endothelin-1 (ET-1) system is a primary mechanism regulating vascular function, balancing vasoconstriction and vasodilation through binding to ETA and ETB receptors. Although both receptors are located on vascular smooth muscle cells and mediate vasoconstriction (1, 2), ETB receptors are also present on the vascular endothelium and mediate vasodilation (3). The contrasting action of the ETB receptor in regulating vascular function makes it an attractive target for investigating its role in cardiovascular function and disease (4).
Emerging evidence demonstrates that ETB receptors have an important role in maintaining vascular health, particularly in women (5–8). In young adults, ETB receptors contribute to vasodilation in women (8) but contribute to vasoconstriction in men (5). The vasodilatory effect of ETB receptors in women appears to be modulated by fluctuations in endogenous ovarian hormones. We previously demonstrated that ETB receptor blockade decreased cutaneous microvascular vasodilation during the midluteal phase (high estradiol and progesterone) but not the early follicular phase of menstruation (9), demonstrating that ETB receptors are under hormonal control. Subsequently, this ETB receptor-mediated vasodilation is lost in women after menopause (8). Thus, ETB receptor-mediated vasodilation may be a cardioprotective mechanism in young women, yet it is unclear whether this change in ETB receptor function is related to aging or the loss of ovarian hormones, particularly endogenous estradiol.
Accordingly, the aim of the current study was to investigate the impact of 17β-estradiol (E2) on ETB receptor function in women. To accomplish this aim, we perfused ET-1 receptor antagonists via microdialysis and measured cutaneous vasodilation to local heating, an index of microvascular endothelial function (8, 10–12). This protocol was completed during endogenous ovarian hormone suppression and after short-term E2 administration in young, healthy women to isolate the effects of E2 independent of age. We hypothesized that short-term E2 administration would enhance the contribution of ETB receptor-mediated dilation during local heating in young women.
METHODS
Subjects
This study was approved by the University of Delaware Institutional Review Board, in accordance with the standards outlined in the Declaration of Helsinki and is part of a registered clinical trial (NCT 03236545). Verbal and written consent were obtained voluntarily from all subjects before participation. Fifteen young women [self-reported as Caucasian (n = 11), Black (n = 3), and Hispanic (n = 1)] completed the study. Women underwent a standard physical exam at the University of Delaware Nurse Managed Primary Care Center, including a fasting blood sample to assess plasma glucose, lipid profile, and kidney and liver function. All women were normotensive, nonobese [body mass index (BMI) <30 kg/m2], nonsmokers, and free from any cardiovascular, metabolic, or other chronic diseases. All women reported having regular menstrual cycles (one cycle every ∼28 days). Ten women were using hormonal contraceptives but refrained from usage during the hormone intervention. All women self-reported participating regularly in exercise (average = 4 ± 1 days/wk, minimum = 2 days/wk).
Hormone Intervention
Women underwent a transvaginal ultrasound and blood work by a reproductive endocrinologist (Reproductive Associates of Delaware, Newark, DE) to rule out pregnancy and confirm eligibility to use the study hormones. Women using hormonal contraceptives completed this visit within the last 1–2 days of active pills, whereas women not using hormonal contraceptives completed the visit within the first 3 days of the start of their menstrual cycle. After clearance, women began self-administering the gonadotrophin-releasing hormone antagonist (GnRHant, Ganirelix acetate, 0.25 mg/day in 0.5 mL of normal saline) for 10 days as described previously (13). The GnRHant is given as a daily subcutaneous injection into the lower abdomen. A clinician from Reproductive Associates of Delaware provided initial instructions and training to all women before the start of administration. Ganirelix (Antagon, Organon, Inc., West Orange, NJ) is a synthetic decapeptide with high antagonistic activity against naturally occurring GnRH. Ganirelix induces a rapid and reversible suppression of steroidogenesis, leading to low or undetectable plasma E2 and progesterone (P4) concentrations within 36–48 h of administration (14, 15). To ensure adequate suppression of endogenous reproductive hormone production, women reported to the laboratory after 72 h of GnRHant for the assessment of vascular function. During days 4–10 of the GnRHant administration, E2 (Vivelle-Dot, 0.1 mg/day, transdermal patch on the upper buttocks) was administered in accordance with the package instructions. This dose is the upper limit of what is approved for clinical use and is consistent with Miner et al. (16) who demonstrated changes in endothelial function using this paradigm. Compliance with the hormone intervention was verified at the conclusion of the hormone intervention by a transvaginal ultrasound at Reproductive Associates of Delaware that showed an increase in endometrial thickness and the absence of any follicle growth in conjunction with serum sex steroid measurements.
Experimental Protocol
All women completed two identical experimental protocols to assess vascular function during GnRHant and again following 7 days of GnRHant + E2. The controlled hormone intervention allowed for the isolation of E2-related changes on vascular function, whereas minimizing effects from other endogenous ovarian hormones such as progesterone. Women were instructed to refrain from exercise for 24 h, to avoid caffeine and alcohol for 12 h, and be fasted for at least 4 h before the start of the study visit. All study visits were conducted in a temperature-controlled room (22°C). Participants laid supine for 15 min before measuring resting BP (Dinamap DASH 2000; GE Medical, Chicago, IL) and obtaining a venous blood sample to assess serum concentrations of E2 and P4.
Assessment of Vascular Function
We measured cutaneous microvascular vasodilatory function using laser-Doppler flowmetry coupled with cutaneous microdialysis perfusions of ET-1 receptor antagonists, as both ETA and ETB receptors are present in the cutaneous circulation (5, 10–12, 17). The cutaneous circulation is an ideal model to study microcirculatory function (11) as functional changes that occur in the cutaneous circulation mirror those that occur in other microcirculatory beds such as the heart and kidneys (18–21), and evidence supports that disturbances in microvascular function precede macrovascular dysfunction (22). Thus, this minimally invasive methodological approach allows for the in vivo pharmaco-dissection of various mechanisms involved in regulating microvascular vasodilatory function in humans (11).
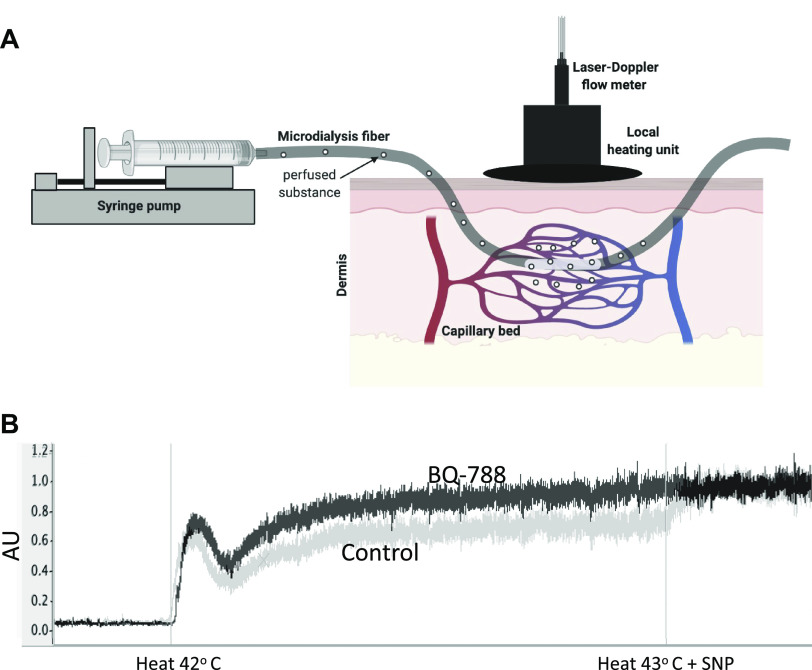
Under sterile conditions, two microdialysis fibers (CMA 31 Linear Microdialysis Probe, Harvard Apparatus, Kista, Sweden) were placed intradermally on the dorsal side of the right forearm, as previously described (8, 9). Briefly, two 23-gauge needles were inserted through the intradermal space, with an entry and exit distance of 2 cm and each site separated by at least 2.5 cm. The microdialysis fibers were next threaded through the lumen of their respective needle (Fig. 1A). Each fiber was secured in place, whereas the needles were individually removed—leaving only the semipermeable portion of the fibers under the dermis. The fibers were connected to a syringe pump (Bee Hive controller and Baby Bee microinfusion pumps, Bioanalytical Systems, West Lafayette, IN) and perfused with a lactated Ringer’s solution (B. Braun Medical, Bethlehem, PA). Perfusions rates were set to 2 μL/min for 60–90 min following fiber insertion to allow cutaneous blood flow to recover from the needle insertion (23). Laser Doppler flow probes (MoorLAB, Temperature Monitor SH02; Moor Instruments, Devon, UK) were placed in local heaters (Moor Instruments) and secured on the surface of the skin directly above the membrane portion of the microdialysis fibers. The laser Doppler probes measure cutaneous red blood cell flux, an index of blood flow, from 1.2 mm2 of skin, whereas the heaters control local skin temperature.
Figure 1.
A: schematic diagram of cutaneous microdialysis fiber placement (image made using BioRender; published with permission), and B: representative tracing of cutaneous vasodilatory responses (arbitrary units, AU) to local heating at the control site (gray line) and ETB receptor blockade (BQ-788; black line) during GnRHant from one woman. ETB, endothelin-B; GnRHant, gonadotropin-releasing hormone antagonist.
Local skin temperature was clamped at 32°C during baseline measures of cutaneous blood flow and for the duration of drug perfusions. Following baseline (5 min), microdialysis fibers were randomly perfused with either lactated Ringer’s or an ETB receptor antagonist BQ-788 (300 nmol/L; Sigma Aldrich, St. Louis, MO) at a rate of 5 μL/min for 45 min (24). In a subset of women (n = 8), a third microdialysis fiber was placed for the perfusion of the ETA receptor antagonist BQ-123 (500 nmol/L; Sigma Aldrich, St. Louis, MO) (24). Local heating was then increased to 42°C (at a rate of 0.1°C/s) to induce vasodilation. The vasodilatory response consists of an initial neutrally mediated peak followed by a prolonged plateau (∼30 min after heating begins) that is predominantly mediated by nitric oxide (12, 17, 25). We used the final 2 min of the plateau phase as our primary indicator of cutaneous vasodilatory responsiveness (26). To elicit maximal vasodilation, local heating was increased to 43°C and sodium nitroprusside (SNP; 28 mmol/L; Marathon Pharmaceuticals, Northbrook, IL) was perfused at a rate of 5 µL/min for ∼10 min (26).
Blood Analysis
Blood samples for the analysis of serum estradiol (s[E2]) and progesterone (s[P4]) concentration were collected in separate tubes without an anticoagulant. A separate blood sample was collected in an EDTA tube for the analysis of plasma ET-1 concentration (p[ET-1]). All tubes were centrifuged, to separate the serum or plasma, which was then pipetted off and frozen at −80°C until the time of analysis. Serum [E2] and [P4] were determined using competitive enzyme-linked immunosorbent assays (ELISA; Alpco, Salem, NH). The range for the s[E2] assay was 0–200 pg/mL with a sensitivity of 1.399 pg/mL. Intra-assay and interassay coefficients of variation for the s[E2] assay were 2.1% and 6.6%, respectively. The range for the s[P4] assay was 0.3–60 ng/mL with a sensitivity of 0.1 ng/mL. Intra-assay and interassay coefficients of variation for the s[P4] assay were 2.4% and 8.8%, respectively. Plasma ET-1 was analyzed using an ELISA (R&D Systems, Minneapolis, MN). Intra-assay and interassay coefficients of variation were <2.5%. All samples were measured at a wavelength of 450 nm on an Infinite F200 Pro microplate reader, and data were analyzed with Magellan IQ software (Tecan Group, Männedorf, CH).
Data and Statistical Analyses
Data were collected at 1,000 Hz using PowerLab (ADInstruments, Bella Vista, NSW, Australia) and LabChart 8.0 (ADInstruments). Data segments were analyzed during the local heating plateau (42°C; 2 min) and maximal dilation with SNP + local heating (43°C; 2 min). Cutaneous vascular conductance (CVC) was calculated as skin blood flow/mean arterial pressure (MAP) and is expressed as a percent of maximal dilation (%CVCmax) to account for any site-to-site variations in blood flow.
Vasodilatory responses to local heating in the ET-1 receptor-blocked sites were analyzed using a two-way repeated-measure analysis of variance (ANOVA), with factors of hormone profile (2 levels: GnRHant and GnRHant + E2) and skin blood flow site [2 levels: control (lactated Ringer’s) and BQ-788, or BQ-123 in a subset of participants]. The effect of time on MAP was analyzed using a two-way repeated-measure ANOVA, with factors of hormone profile (2 levels: GnRHant and GnRHant + E2), and time (3 levels: baseline, heat to 42 degrees, and heat + SNP). Significant interactions were further investigated with the least significant differences. Two-tailed paired t tests were used to compare serum concentrations of E2 and P4, along with body mass and MAP between visits (i.e., GnRHant and GnRHant + E2). Significance was set at α of <0.05, and results are expressed as means ± SD. All data analyses were performed using SPSS Statistics 25 (SPSS, Chicago, IL).
RESULTS
Baseline subject characteristics are presented in Table 1 and a representative tracing is of the cutaneous vasodilatory response is illustrated in Fig. 1B. All women were nonobese, normotensive, and had blood values within normal clinical limits. As expected, serum [E2] was higher during GnRHant + E2 (P = 0.004 vs. GnRHant; Table 2). Serum [P4], p[ET-1], body mass, and resting blood pressure did not change during the hormone intervention (all P ≥ 0.191; Table 2).
Table 1.
Demographic and screening characteristics
| Demographic Information | |
| Subjects, n | 15 |
| Age, yr | 24 ± 4 |
| Height, cm | 167 ± 8 |
| Mass, kg | 68 ± 10 |
| BMI, kg/m2 | 24 ± 3 |
| Hemodynamic measurements | |
| Systolic BP, mmHg | 108 ± 9 |
| Diastolic BP, mmHg | 72 ± 6 |
| MAP, mmHg | 84 ± 6 |
| Blood chemistry | |
| Total cholesterol, mg/dL | 185 ± 37 |
| High-density lipoprotein, mg/dL | 70 ± 15 |
| Low-density lipoprotein, mg/dL | 98 ± 30 |
| Triglycerides, mg/dL | 81 ± 23 |
| Hemoglobin, mg/dL | 13.4 ± 1.0 |
| Hematocrit, % | 40.3 ± 3.0 |
| Fasting plasma glucose, mg/L | 84 ± 5 |
Values are means ± SD; n = number of subjects. BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure.
Table 2.
Subject characteristics and serum sex steroids during experimental visits
| GnRHant | GnRHant + E2 | P Value | |
|---|---|---|---|
| Demographic and hemodynamic measurements | |||
| Mass, kg | 68 ± 10 | 68 ± 10 | 0.412 |
| Systolic BP, mmHg | 109 ± 9 | 107 ± 8 | 0.332 |
| Diastolic BP, mmHg | 67 ± 6 | 67 ± 6 | 0.504 |
| MAP, mmHg | 81 ± 7 | 80 ± 7 | 0.399 |
| Hormones, pg/mL | |||
| Estradiol, serum | 68 ± 63 | 83 ± 65 | 0.004 |
| Progesterone, serum | 0.75 ± 0.84 | 0.62 ± 0.90 | 0.191 |
| Endothelin-1, plasma | 1.30 ± 0.58 | 1.22 ± 0.30 | 0.577 |
Values are means ± SD; n = 15 subjects. BP, blood pressure; E2, estradiol; GnRHant, gonadotropin-releasing hormone antagonist; MAP, mean arterial pressure. P values are from paired t test comparisons.
Vascular Function
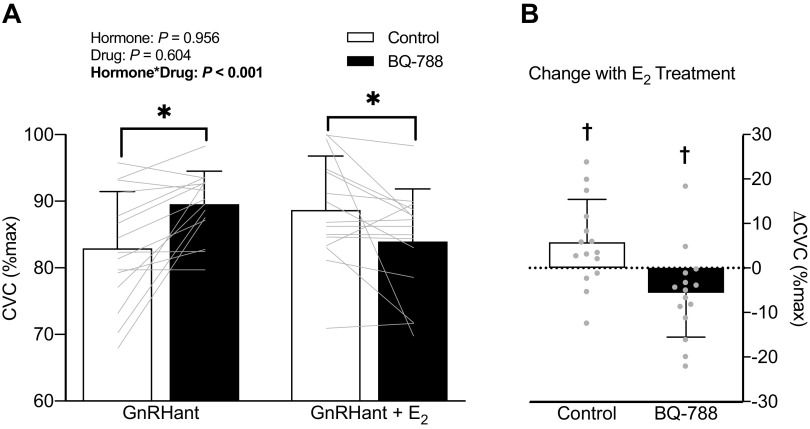
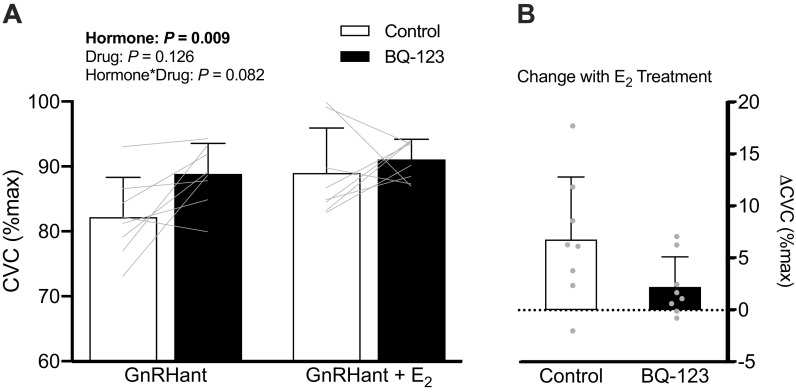
There was a significant hormone-by-drug interaction (P < 0.001; Fig. 2) for the effect of E2 on ETB receptor function. During hormone suppression with GnRHant, ETB receptor blockade increased cutaneous vasodilation to local heating compared with the control site (Δ6.6 ± 7.5%CVCmax, P < 0.004, 95% CI [2.5, 10.8]). However, during E2 administration, ETB receptor blockade attenuated cutaneous vasodilation to local heating compared with the control site (Δ−4.8 ± 8.5%CVCmax, P = 0.047, 95% CI [−9.5, −0.1]). E2 administration improved cutaneous vasodilation in control sites (Δ5.8 ± 9.7%CVCmax, P = 0.036, 95% CI [0.5, 11.2]) compared with GnRHant. In a subset of women (n = 8), E2 had no significant effect on the vasodilatory response during ETA receptor blockade (GnRHant: 89 ± 5% vs. GnRHant + E2: 91 ± 3%; interaction: P = 0.082, Fig. 3). MAP decreased slightly throughout the protocol (P < 0.01) with no difference between GnRHant and GnRHant + E2 hormone profiles (P > 0.43). Maximal vasodilatory capacity was similar across sites and between hormone conditions (skin blood flow site: P = 0.159; hormone: P = 0.295; skin blood flow site × hormone: P = 0.308).
Figure 2.
A: cutaneous vasodilatory responses expressed as a percent of maximal cutaneous vascular conductance (%CVCmax) during local heating and microdialysis perfusion of lactated Ringer’s (control) and ETB blockade (BQ-788) during hormone suppression without (GnRHant) and with estradiol administration (GnRHant + E2) and B: the change in %CVCmax due to E2 treatment within control and ETB blocked sites in 15 women. Data are presented as means (SD) with individual values. *P ≤ 0.047 vs. control (within hormone phase). †P ≤ 0.047 vs. GnRHant (E2 treatment effect). E2, estradiol; ETB, endothelin-B; GnRHant, gonadotropin-releasing hormone antagonist.
Figure 3.
A: cutaneous vasodilatory responses expressed as a percent of maximal cutaneous vascular conductance (%CVCmax) during local heating and microdialysis perfusion of lactated Ringer’s (control) and ETA blockade (BQ-123) during hormone suppression without (GnRHant) and with estradiol administration (GnRHant + E2) and B: the change in %CVCmax due to E2 treatment within control and ETA blocked sites in a subset of women (n = 8). Data are presented as means (SD) with individual values. E2, estradiol; ETA, endothelin-A; GnRHant, gonadotropin-releasing hormone antagonist.
DISCUSSION
The current investigation isolated the effects of E2 on ET-1 receptor function in young women. The primary novel findings are 1) during hormone suppression, blockade of ETB receptors enhanced cutaneous vasodilation; 2) during E2 administration, ETB receptor blockade attenuated cutaneous vasodilation, indicating a restoration of ETB receptor-mediated dilation; and 3) E2 administration improved the cutaneous vasodilatory response to local heating. Taken together, ETB receptors mediate vasodilation in the presence of E2, an effect that is lost when E2 is suppressed.
Greater ETB receptor-mediated vasodilation in the presence of E2 may be explained by a change in ETB receptor function on either the endothelium or smooth muscle cells. We previously reported functional changes in ETB receptor-mediated dilation throughout the menstrual cycle (9), such that heightened E2 in the midluteal phase of young premenopausal women resulted in greater ETB receptor-mediated vasodilation. The current data suggest that this was likely driven by the changes in E2. Conversely, in animal models, the ETB receptor agonist sarafotoxin elicited greater constriction of the afferent arteriole in ovariectomized (OVX) rats compared with sham (27) demonstrating greater ETB-mediated vasoconstriction when E2 is absent. Therefore, ETB receptors may mediate vasoconstriction when E2 is low, and mediate vasodilation when E2 is present or elevated. Evidence from animal models also supports E2-mediated control of the ETB receptor expression. Compared with ovariectomized (OVX) rats, aortic ETB receptor expression was higher in intact females and OVX + E2 rats, suggesting that E2 upregulates ETB receptors. Similar findings of E2-induced changes in ETB receptor expression have also been demonstrated in the kidney (28) and heart. Although we were not able to discern between the contribution of endothelial cells from smooth muscle ETB receptors in young women, we recently demonstrated that ETB receptor expression on endothelial cells is lower in postmenopausal women (29). This reduction in expression of ETB receptors is likely driven, in part, by a loss of endogenous ovarian hormones. Taken together, fluctuations in sex hormones regulate ETB receptor expression and function and are important for vascular control in women.
Postmenopausal women are at greater risk for developing CVD and have decreased vascular function compared with young premenopausal women (30, 31). This increased risk may be, in part, linked to a dysregulation of the ET-1 system. We previously measured cutaneous vasodilatory responses to local heating in premenopausal and postmenopausal women during microdialysis perfusions of ETB receptor antagonists (8). Premenopausal women had a reduction in vasodilation during blockade of ETB receptors, demonstrating that ETB receptors mediate dilation in young women. Conversely, postmenopausal women experienced an increase in vasodilation and restoration of vasodilatory capacity when ETB receptors were blocked. Interestingly, the current study reports a similar response profile during suppression of endogenous ovarian hormones in young women as we had previously noted in postmenopausal women. Thus, either aging or menopause-associated declines in E2 resulted in a shift in ETB receptor function between premenopausal and postmenopausal women. Based on the results of the current study, we speculate the latter explanation; that menopause-associated declines in endogenous E2 modulate ETB receptor function.
The E2-associated effects on the ET-1 system appear to be specific to the ETB receptor, as we did not observe changes in cutaneous microvascular responses with ETA receptor blockade. This was somewhat surprising since our previous findings demonstrated that ETA receptor blockade reduced vasodilation during the midluteal phase of the menstrual cycle (high E2 and P4), but not during early follicular phase (9). This may be due, in part, to different concentrations of E2 observed during the hormone intervention versus a natural menstrual cycle (change of 18% vs. 63% increase in s[E2], respectively). Other evidence supports that ETA receptor expression is influenced by progesterone (32), and may require larger shifts in hormone concentrations to see functional changes (33). In older women, the ETA receptor appears to be secondary to vascular control mechanisms mediated by the ETB receptor (7). Thus, the ETA receptor may be less sensitive to hormonal changes; however, additional work is needed to understand the impact of the ETA receptor on aging and sex differences in vascular function.
The changes in microvascular vasodilatory function with E2 administration may also be impacted by changes in ET-1 bioavailability. Previous research supports that E2 administration attenuates the production and release of ET-1 (34–36) and reduces the vasoconstrictor effects of ET-1 (37, 38). Furthermore, plasma ET-1 concentrations are modulated by E2 in young women throughout the menstrual cycle (39) and reduced after E2 therapy in male-to-female transgender adults (40, 41). However, we did not observe a significant reduction in plasma ET-1 in the current study with short-term E2 administration. Because ET-1 is secreted abluminally, plasma levels may not best reflect production of ET-1. Further investigation into the interactions among sex hormones, aging, and ET-1 is warranted.
Limitations
We recognize other mechanistic pathways that are involved in the regulation of vascular function such as prostaglandins, angiotensin II, and norepinephrine (42), and are also modulated by E2. Furthermore, there is evidence that oxidative stress is upregulated during GnRH antagonism and across the menopausal transition (43), which may interfere with nitric oxide bioavailability and therefore reduce cutaneous microvascular vasomotor function. These pathways all interact with the endothelin system and its receptors (44). It is possible that E2 administration impacted pathways either upstream or downstream for ET-1 and ETB receptors, which we were not able to discern. Additional work is needed to understand the interactions among these pathways and the effects on vascular function in women throughout their lifespan. Second, we were not able to determine whether the functional changes observed were specific to either the endothelium or vascular smooth muscle since ETB receptors are located in both areas. Finally, our investigation purposefully studied young women to examine the influence of E2 on ETB receptors independent of aging or menopause. It is unclear whether the same conclusions would be reached in postmenopausal women due to an aging vasculature and impaired endothelial function.
Conclusions
In conclusion, we demonstrate that in young women ETB receptors mediate vasodilation in the presence of E2, an effect that is lost when E2 is suppressed. These data extend our previous findings from the menstrual cycle (9) to demonstrate that changes in E2 are a strong regulator of ETB receptor function. Furthermore, these data also extend our previous findings showing this ETB receptor-mediated vasodilation in young women is lost after menopause (8), which may be related, in part, to the declining E2 that occurs after menopause. Thus, these findings are an important contribution to our understanding of how ovarian hormones, particularly E2, regulate vascular function in women, and further support the importance of the ETB receptor as a primary mechanism for vascular health in women.
GRANTS
This work was supported by American Heart Association Award 16SDG30700015 (to M. M. Wenner) and National Institutes of Health Grants U54-GM104941 (to M. M. Wenner; PI: Binder-Macleod), P20 GM113125 (to D. G. Edwards), and HL R01 146558 (M. M. Wenner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.H., D.G.E., R.F.F., and M.M.W. conceived and designed research; L.N.S., K.M.H., A.V.K., S.J.M., L.M.W., J.C.H., and M.M.W. performed experiments; L.N.S. and M.M.W. analyzed data; L.N.S., K.M.H., A.V.K., S.J.M., L.M.W., D.G.E., R.F.F., and M.M.W. interpreted results of experiments; L.N.S. and M.M.W. prepared figures; L.N.S. and M.M.W. drafted manuscript; L.N.S., K.M.H., A.V.K., S.J.M., L.M.W., J.C.H., D.G.E., R.F.F., and M.M.W. edited and revised manuscript; L.N.S., K.M.H., A.V.K., S.J.M., L.M.W., J.C.H., D.G.E., R.F.F., and M.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mark Modelski, Beth Sutton, Rhonda Wright, Wendy Nichols, Carolyn Haines, the Nurse Managed Primary Care Center, and participants for time.
REFERENCES
- 1.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348: 730–732, 1990. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 2.Lin HY, Kaji EH, Winkel GK, Ives HE, Lodish HF. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc Natl Acad Sci USA 88: 3185–3189, 1991. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Fukuroda T, Fukami T, Ozaki S, Nagase T. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA 91: 4892–4896, 1994. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohar EY, Pollock DM. Sex-specific contributions of endothelin to hypertension. Curr Hypertens Rep 20: 58, 2018. doi: 10.1007/s11906-018-0856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KelloggDL, Jr., Liu Y, Pergola PE. Selected contribution: gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol (1985) 91: 2407–2411, 2001. doi: 10.1152/jappl.2001.91.5.2407. [DOI] [PubMed] [Google Scholar]
- 6.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin Sci (Lond) 131: 2777–2789, 2017. doi: 10.1042/CS20171292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 298: R261–R265, 2010. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenner MM, Sebzda KN, Kuczmarski AV, Pohlig RT, Edwards DG. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 313: R51–R57, 2017. doi: 10.1152/ajpregu.00410.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebzda KN, Kuczmarski AV, Pohlig RT, Lennon SL, Edwards DG, Wenner MM. Ovarian hormones modulate endothelin-1 receptor responses in young women. Microcirculation 25: e12490, 2018. doi: 10.1111/micc.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 11.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 12.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 13.Wenner MM, Haddadin AS, Taylor HS, Stachenfeld NS. Mechanisms contributing to low orthostatic tolerance in women: the influence of oestradiol. J Physiol 591: 2345–2355, 2013. doi: 10.1113/jphysiol.2012.247882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberye JJ, Mannaerts BM, Huisman JA, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril 72: 1006–1012, 1999. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 15.Oberye JJ, Mannaerts BM, Kleijn HJ, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril 72: 1001–1005, 1999. doi: 10.1016/s0015-0282(99)00413-6. [DOI] [PubMed] [Google Scholar]
- 16.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.KelloggDL, Jr., Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal SC, Allen J, Murray A, Purcell IF. Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res 84: 55–59, 2012. doi: 10.1016/j.mvr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 20.I Jzerman R, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33: 536–542, 2003. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 21.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 22.Gates PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp Physiol 94: 311–316, 2009. doi: 10.1113/expphysiol.2008.043349. [DOI] [PubMed] [Google Scholar]
- 23.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol 296: H51–H56, 2009. doi: 10.1152/ajpheart.00919.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenner MM, Taylor HS, Stachenfeld NS. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol 589: 4671–4679, 2011. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gohar EY, Cook AK, Pollock DM, Inscho EW. Afferent arteriole responsiveness to endothelin receptor activation: does sex matter? Biol Sex Differ 10: 1, 2019. doi: 10.1186/s13293-018-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gohar EY, Yusuf C, Pollock DM. Ovarian hormones modulate endothelin A and B receptor expression. Life Sci 159: 148–152, 2016. doi: 10.1016/j.lfs.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczmarski AV, Shoemaker LN, Hobson JC, Edwards DG, Wenner MM. Altered endothelial ETB receptor expression in postmenopausal women. Am J Physiol Heart Circ Physiol 319: H242–H247, 2020. doi: 10.1152/ajpheart.00342.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 31.Moreau KL, Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med 2014: 204390, 2014. doi: 10.1155/2014/204390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Knutsen GR, Brown MD, Ruest LB. Control of endothelin-a receptor expression by progesterone is enhanced by synergy with Gata2. Mol Endocrinol 27: 892–908, 2013. doi: 10.1210/me.2012-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen SH, Nielsen LB, Mortensen A, Nilas L, Ottesen B. Progestins oppose the effects of estradiol on the endothelin-1 receptor type B in coronary arteries from ovariectomized hyperlipidemic rabbits. Menopause 15: 503–510, 2008. doi: 10.1097/gme.0b013e318156f803. [DOI] [PubMed] [Google Scholar]
- 34.Bilsel AS, Moini H, Tetik E, Aksungar F, Kaynak B, Ozer A. 17Beta-estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc Res 46: 579–584, 2000. doi: 10.1016/s0008-6363(00)00046-8. [DOI] [PubMed] [Google Scholar]
- 35.David FL, Carvalho MH, Cobra AL, Nigro D, Fortes ZB, Reboucas NA, Tostes RC. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension 38: 692–696, 2001. doi: 10.1161/01.hyp.38.3.692. [DOI] [PubMed] [Google Scholar]
- 36.Webb CM, Ghatei MA, McNeill JG, Collins P. 17beta-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation 102: 1617–1622, 2000. doi: 10.1161/01.CIR.102.14.1617. [DOI] [PubMed] [Google Scholar]
- 37.Lamping KG, Nuno DW. Effects of 17 beta-estradiol on coronary microvascular responses to endothelin-1. Am J Physiol Heart Circ Physiol 271: H1117–H1124, 1996. doi: 10.1152/ajpheart.1996.271.3.H1117. [DOI] [PubMed] [Google Scholar]
- 38.Sudhir K, Ko E, Zellner C, Wong HE, Hutchison SJ, Chou TM, Chatterjee K. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation 96: 3626–3632, 1997. doi: 10.1161/01.CIR.96.10.3626. [DOI] [PubMed] [Google Scholar]
- 39.Polderman KH, Stehouwer CD, van Kamp GJ, Schalkwijk CG, Gooren LJ. Modulation of plasma endothelin levels by the menstrual cycle. Metabolism 49: 648–650, 2000. doi: 10.1016/S0026-0495(00)80042-6. [DOI] [PubMed] [Google Scholar]
- 40.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med 118: 429–432, 1993. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 41.van Kesteren PJ, Kooistra T, Lansink M, van Kamp GJ, Asscheman H, Gooren LJ, Emeis JJ, Vischer UM, Stehouwer CD. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost 79: 1029–1033, 1998. doi: 10.1055/s-0037-1615115. [DOI] [PubMed] [Google Scholar]
- 42.Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Effects of estrogen on the vascular system. Braz J Med Biol Res 36: 1143–1158, 2003. doi: 10.1590/s0100-879x2003000900002. [DOI] [PubMed] [Google Scholar]
- 43.Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 42: 1699–1714, 2020. doi: 10.1007/s11357-020-00236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubanyi GM, Botelho LH. Endothelins. FASEB J 5: 2713–2720, 1991. doi: 10.1096/fasebj.5.12.1916094. [DOI] [PubMed] [Google Scholar]