Abstract
Background
Meningitis retention syndrome (MRS) is a rare condition that presents with acute urinary retention as a complication of aseptic meningitis. Cases of MRS due to varicella zoster virus (VZV) infection without a rash are rare. We report the case of a patient who had no signs of meningitis or VZV infection, including a rash.
Case presentation
A 58-year-old man presented with dysesthesia of the lower limbs and acute urinary retention. He had fever but no rash and no signs of meningitis. He was diagnosed to have VZV infection based on the detection of VZV DNA in the cerebrospinal fluid. He responded satisfactorily to a course of intravenous acyclovir and experienced no sequelae during a 2-year follow-up period.
Conclusion
MRS due to aseptic meningitis of viral origin should be considered in the differential diagnosis of acute urinary retention even in the absence of specific signs and symptoms of meningitis or a suggestive rash.
Keywords: Meningitis retention syndrome, Varicella zoster virus, Zoster sine herpete, Acute urinary retention, Lower limb dysesthesia
Background
The usual causes of acute urinary retention in adults include prostatic hyperplasia, peripheral nerve diseases involving the sacral spinal cord, such as diabetic neuropathy and Guillain-Barré syndrome, and diseases of the lumbar spinal canal, such as lumbar spondylosis and lumbar disc herniation. However, acute urinary retention has also been reported in association with cerebral demyelinating diseases such as acute disseminated encephalomyelitis and multiple sclerosis, aseptic meningitis, and herpes in the sacral region [1]. Meningitis retention syndrome (MRS) is a rare condition associated with aseptic meningitis presenting with acute urinary retention [2]. Enteroviruses, flaviviruses, arboviruses, and herpes simplex viruses are common viral causes of MRS, whereas varicella zoster virus (VZV) is rarely associated with MRS [4, 5].
A typical skin rash is not observed in about 40% of patients with meningoencephalitis caused by VZV [6]. However, besides fever, most patients with aseptic meningitis, including VZV meningitis, report symptoms such as headache, nausea and vomiting, signs of meningeal irritation, disturbance of consciousness, and brain dysfunction (cognitive dysfunction, behavioral changes, neurological symptoms, and convulsions) [7–9].
Here, we report about a patient with MRS associated with aseptic meningitis due to VZV who had no typical symptoms of meningitis or VZV infection.
Case presentation
A 58-year-old man with no particular past medical history had fever of 38 °C 11 days before admission. Dysesthesia of both the lower limbs and acute urinary retention were observed in the same day. Although the fever improved after a few days, 7 days before admission, the patient developed urinary retention 7 days before admission, which required intervention by a urologist. As urinary drainage and oral administration of silodosin, urapidil, baclofen, and distigmine did not improve the patient’s condition, however, a spinal cord lesion was suspected and he was referred to our institution.
On systemic review during a medical examination at our institution, bilateral lower limb dysesthesia, dysuria, and constipation were present, whereas fever, headache, nausea, vomiting, and rash were absent.
He was fully conscious, and with pulse rate, temperature, blood pressure, and respiratory rate of 117 beats/min, 36.5 °C, 153/110 mmHg, and 15 breaths/min, respectively. A physical examination did not reveal any rash, or any other obvious abnormal findings while neurological examination was negative for Jolt accentuation, no nuchal rigidity, and Kerning's and Brudzinski's signs. However, the Babinski reflexes wore positive bilaterally, and he had right patellar tendon hyperreflexia and positive Romberg and Mann tests. In addition, he had urinary retention and constipation, and decreased anal sphincter reflex, a reduction in vibratory sensation in both lower limbs and dysesthesia in parts of the lower limbs as illustrated in Fig. 1. There were no other abnormalities neurologic examination. The Laboratory test results on presentation are shown in Table 1. The abnormalities included relative neutrophilia, and elevated levels of D-dimer, procalcitonin, lactic dehydrogenase, blood urea nitrogen and creatinine.
Fig. 1.
Areas showing dysesthesia (red diagonal)
Table 1.
Laboratory data on admission
| Parameter | Recorded value | Standard value |
|---|---|---|
| White blood cell count | 7920/µL | 4500–7500/µL |
| Neutrophils | 81.4% | 42–74% |
| Lymphocytes | 13.5% | 18–50% |
| Monocytes | 4.7% | 1–10% |
| Hemoglobin | 16.8 g/dL | 11.3–15.2 g/dL |
| Platelet count | 335 × 103/µL | 130–350 × 103/µL |
| Prothrombin time/international normalized ratio | 1.02 | 0.80–1.20 |
| Activated partial thromboplastin time | 27.9 s | 26.9–38.1 s |
| D-dimer | 2.1 μg/mL | < 1.0 μg/mL |
| C-reactive protein | 0.03 mg/L | ≤ 0.60 mg/dL |
| Procalcitonin | 0.97 ng/mL | ≤ 0.05 ng/mL |
| Total protein | 8.0 g/dL | 6.9–8.4 g/dL |
| Albumin | 4.4 g/dL | 3.9–5.1 g/dL |
| Total bilirubin | 1.2 mg/dL | 0.2–1.2 mg/dL |
| Aspartate aminotransferase | 26 U/L | 11–30 U/L |
| Alanine aminotransferase | 37 U/L | 4–30 U/L |
| Lactase dehydrogenase | 190 U/L | 109–216 U/L |
| Creatine kinase | 44 U/L | 40–150 U/L |
| Blood urea nitrogen | 35.8 mg/dL | 8–20 mg/dL |
| Creatinine | 1.50 mg/dL | 0.63–1.03 mg/dL |
| Sodium | 138 mEq/L | 136–148 mEq/L |
| Potassium | 4.4 mEq/L | 3.6–5.0 mEq/L |
| Chloride | 100 mEq/L | 98–108 mEq/L |
| Glucose | 117 mg/dL | 70–109 mg/dL |
| Hemoglobin A1c | 5.8% | 5.6–5.9% |
No abnormalities were noted in urinalysis. Abdominal computed tomography (CT) revealed urinary and fecal retentions, but there was no space-occupying lesion in the pelvis nor a lumbar spinal canal stenosis. No abnormalities were observed in the spine on magnetic resonance imaging (MRI), but cranial contrast-enhanced MRI showed meningeal thickening (Fig. 2).
Fig. 2.

Head contrast-enhanced MRI showing meningeal thickening and contrast enhancement (red circle)
Because the cranial MRI indicated meningitis and MRS was suspected to be the cause of his acute urinary retention, his cerebrospinal fluid was examined for confirmation of the diagnosis. The results were as follows: cell count of 232/μL (mononuclear cells 227/μL, polymorphonuclear cells 5/μL), protein level of 331 mg/dL, sugar level of 69 mg/dL (47.6% of the simultaneous blood glucose level of 145 mg/dL), and adenosine deaminase concentration of 14.5 U/L. Furthermore, the CSF was positive for VZV DNA, but negative for cryptococcal Ag, and general bacterial, and mycobacterium cultures. The titers of various viruses in the serum are shown in Table 2. The elevated VZV IgM level indicated acute phase infection, while the high VZV IgG level indicated a reactivation of VZV. Elevated Epstein-Barr virus viral capsid antigen IgG and Epstein-Barr virus nuclear antigen IgG indicated past Epstein-Barr virus infection, while the negative Epstein-Barr virus IgM ruled-out acute Epstein-Barr virus infection.
Table 2.
Titers of various viruses in the serum
| Parameter | Recorded value | standard value |
|---|---|---|
| Human immunodeficiency virus antigen antibody | (−) | (−) |
| Hepatitis B surface antigen | (−) | (−) |
| Hepatitis C virus antibody | (−) | (−) |
| Rapid plasma reagent | (−) | (−) |
| Treponema pallidum latex agglutination | (−) | (−) |
| EB-VCA IgM | 0.0 EIA value | < 0.4 EIA value |
| EB-VCA IgG | 7.4 EIA value | < 0.4 EIA value |
| EBNA IgG | 4.7 EIA value | < 0.4 EIA value |
| HSV IgM | 0.15 EIA value | < 0.7 EIA value |
| HSV IgG | < 2.0 EIA value | < 2.0 EIA value |
| VZV IgM | 8.24 EIA value | < 0.8 EIA value |
| VZV IgG | 128.0 EIA value | < 2.0 EIA value |
| Mumps virus IgM | 0.04 EIA value | < 0.7 EIA value |
| Mumps virus IgG | 2.6 EIA value | < 2.0 EIA value |
EB-VCA Epstein-Barr virus viral capsid antigen, EBNA EBV Epstein-Barr virus nuclear antigen, HSV herpes simplex virus, VZV varicella zoster virus, EIA enzyme immunometric assay
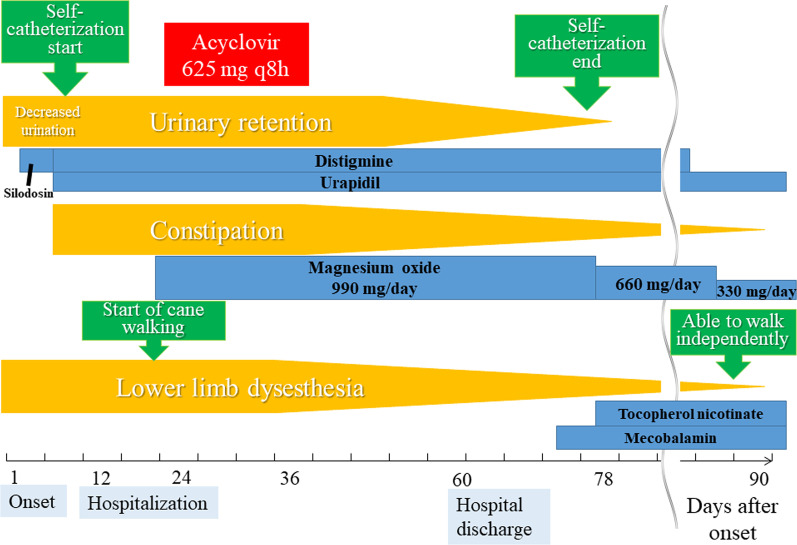
The final diagnosis was MRS owing to aseptic meningitis due to VZV. After the diagnosis, he was given intravenous acyclovir at a dose of 625 mg every 8 h for 14 days. The subsequent course is shown in Fig. 3. The symptoms gradually improved, and management with oral medications (distigmine, urapidil, magnesium oxide) became feasible.
Fig. 3.
Clinical course of symptoms
The medications for dysuria and dyschezia have been discontinued, and 2 years have passed since the onset of MRS. No sequelae have been observed, and the patient is being followed up.
Discussion and conclusions
Except for fever, no findings indicative of meningitis were observed on presentation, and diagnosing the condition as VZV meningitis without a rash was difficult.
MRS is a rare condition that presents with complications of aseptic meningitis and dysuria [2]. Furthermore, MRS due to VZV is rare [2, 4, 5, 7, 10–12]. To the best of our knowledge, only one previous case of VZV meningitis without a rash that led to MRS has been reported [5]. The mechanism of MRS is presumed to include spinal shock owing to meningeal irritation, inflammation of upper motor neurons of the spinal cord, direct viral entry, and onset of acute disseminated encephalomyelitis after viral infection [13].
No rash was not observed before and after the onset. A previous study reported that skin findings were not observed in 40% of patients with meningoencephalitis caused by VZV [6]. Thus, the possibility of VZV should be considered in the differential diagnosis of aseptic meningitis, despite the absence of a rash. If urinary retention without an identifiable cause is present, MRS due to aseptic meningitis should be considered in the differential diagnosis, and cerebrospinal fluid should be examined, despite a lack of signs of meningitis.
In conclusion, meningitis due to VZV without a rash is possible, as is meningitis without specific signs and symptoms. Therefore, despite the absence of symptoms or signs suggestive of meningitis, MRS owing to aseptic meningitis, including VZV, should be considered in the differential diagnosis of acute urinary retention.
Acknowledgements
None.
Abbreviations
- CT
Computed tomography
- Ig
Immunoglobulin
- MRI
Magnetic resonance imaging
- MRS
Meningitis retention syndrome
- VZV
Varicella zoster virus
Authors' contributions
TK managed the case, and prepared and revised the manuscript. KG and AK assisted with the preparation and revision of the manuscript. All co-authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All co-authors take full responsibility for the integrity of the study and the final manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Ethics approval and consent to participate does not apply, because this is a case report. The ethics committee of Hyogo Prefectural Tamba Medical Center exempted protocols from ethics because it not a research study. Consent to participate does not apply.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choong S, Emberton M. Acute urinary retention. BJU Int. 2000;85:186–201. doi: 10.1046/j.1464-410x.2000.00409.x. [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara R, Kishi M, Tsuyusaki Y, Tateno A, Tateno F, Uchiyama T, et al. "Meningitis-retention syndrome": a review. Neurourol Urodyn. 2013;32:19–23. doi: 10.1002/nau.22279. [DOI] [PubMed] [Google Scholar]
- 3.Gregušová A, Klézl P, Mašková V, Smíšková D, Pícha D, Malikova H. Acute urinary retention in aseptic meningitis: meningitis-retention syndrome. Neuro Endocrinol Lett. 2019;40:166–168. [PubMed] [Google Scholar]
- 4.Erol B, Avci A, Eken C, Ozgok Y. Urinary retention, erectile dysfunction and meningitis due to sacral herpes zoster: a case report and review of the literature. Urol Int. 2009;82:238–241. doi: 10.1159/000200807. [DOI] [PubMed] [Google Scholar]
- 5.Abe M, Araoka H, Kimura M, Yoneyama A. Varicella zoster virus meningoencephalitis presenting with Elsberg syndrome without a rash in an immunocompetent patient. Intern Med. 2015;54:2065–2067. doi: 10.2169/internalmedicine.54.4341. [DOI] [PubMed] [Google Scholar]
- 6.Koskiniemi M, Piiparinen H, Rantalaiho T, Eränkö P, Färkkilä M, Raiha K, et al. Acute central nervous system complications in varicella zoster virus infections. J Clin Virol. 2002;25:293–301. doi: 10.1016/S1386-6532(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 7.Takeshima S, Shiga Y, Himeno T, Tachiyama K, Kamimura T, Kono R, et al. Clinical, epidemiological and etiological studies of adult aseptic meningitis: report of 11 cases with varicella zoster virus meningitis. Rinsho Shinkeigaku. 2017;57:492–498. doi: 10.5692/clinicalneurol.cn-001054. [DOI] [PubMed] [Google Scholar]
- 8.Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175–81. doi: 10.1001/jama.282.2.175. [DOI] [PubMed] [Google Scholar]
- 9.Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, et al. Viral meningoencephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol. 2010;17:999–e57. doi: 10.1111/j.1468-1331.2010.02970.x. [DOI] [PubMed] [Google Scholar]
- 10.Dinh A, Salomon J, Schoindre Y, Mathez D, Denys P, Durand MC, et al. Acute urinary retention due to viral coinfections (HIV, HBV, VZV) J Int Assoc Phys AIDS Care (Chic) 2010;9:20–2. doi: 10.1177/1545109709356475. [DOI] [PubMed] [Google Scholar]
- 11.Saito H, Ebashi M, Kushimoto M, Ikeda J, Egashira F, Yamaguchi S, et al. Elsberg syndrome related to varicella zoster virus infection with painless skin lesions in an elderly woman with poorly controlled type 2 diabetes mellitus. Ther Clin Risk Manag. 2018;14:1951–4. doi: 10.2147/TCRM.S178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TW, Whang JC, Lee SH, Choi JI, Park SM, Lee JB. Acute urinary retention due to aseptic meningitis: meningitis-retention syndrome. Int Neurourol J. 2010;14:122–4. doi: 10.5213/inj.2010.14.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishna A, Devulapally P, Ghobrial I. Meningitis retention syndrome. J Community Hosp Intern Med Perspect. 2012 doi: 10.3402/jchimp.v2i1.15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.