Abstract
Background
Since July 13, 2021, a third SARS-CoV-2 vaccine BNT162b2 was approved in Israel to immunocompromised and seniors 60 years of age or older. We aimed to evaluate vaccine's reactogenicity.
Methods
A retrospective cohort, using electronic surveys sent to booster vaccine recipients, during July 20–August 10, 2021.
Results
17,820 participated in the survey, with a response rate of 30.2%. 3195 (17.9%) were immunocompromised. Fatigue, myalgia and fever were the most frequent systemic side effects reported (19.6%, 9.2% and 8.1% respectively among immunocompromised; 21.3%, 9.9% and 9.2% respectively among seniors). 67.3% of immunocompromised and 62% of seniors reported experiencing a better or a similar response to the third dose, compared to the second.
Conclusions
Local and systemic reactions after third BNT162b2 vaccine, reported by immunocompromised and seniors, were similar to those observed following previous vaccines and mostly self-resolved. These findings may aid promoting confidence among vaccine providers and recipients.
Keywords: SARS-CoV-2, Booster, Vaccine, Immunocompromised, Side-effects, Elderly
Abbreviations: Immunocompromised individuals, ICI; Maccabi Healthcare Services, MHS
1. Introduction
On July 13, 2021, the Israeli Ministry of Health approved a third SARS-CoV-2 BNT162b2 vaccine dose for individuals who received a second vaccine dose at least 5 months before [1]. Initially, certain immunocompromised individuals (ICI) were eligible to get the third vaccine dose: solid organ transplants, patients with hematologic malignancies and on immunosuppressive treatment. Two weeks later, on July 30, all adults over the age of 60 were offered the third vaccine dose. Data regarding the third vaccine dose's reactogenicity, particularly in these population is scarce.
The aim of this study was to evaluate a third dose of SARS-CoV-2 BNT162b2 vaccine short term safety, as directly reported from two vaccine recipient populations: immunocompromised individuals and seniors 60 years of age or older .
2. Material and methods
2.1. Study design
We conducted an observational cross-sectional study consisting of data collected through an electronic survey.
2.2. Setting and survey administration
A comprehensive COVID-19 vaccine safety monitoring program is routinely conducted since the beginning of the vaccination program by Maccabi Healthcare Services (MHS). MHS is the second largest health maintenance organization in Israel, serving over 2.5 million citizens, representing a quarter of the Israeli population. As part of the safety control program, an online survey was sent from July 20 to August 10, 2021, during the first weeks of the third dose vaccination campaign with SARS-CoV-2 BNT162b2 vaccine. Inclusion criteria for delivering the survey were: a third vaccine administration, ages18–85 and mail or text messages access. Questionnaire were distributed to the respondents by email and text message, seven days after receiving the vaccine. The response was voluntary and without any payment.
2.3. Questionnaire
The survey included questions regarding local and systemic side effects, symptoms duration, medical care required for them and lastly, participants were asked to compare between the overall degree of side effects experienced with those they had following the second vaccine dose. The questionnaires were available only in Hebrew. Each questionnaire was linked to the patient's electronic health record by a unique identifier.
2.4. Statistical examination
We analyzed the two vaccine-targeted populations separately: ICI and seniors aged 60 years and over. Weights were applied in presenting the distribution calculations of the local and systemic side effects, their duration and their overall intensity compared to the second vaccine. Univariate analysis was used to evaluate unweighted local and systemic side effects differences by age groups and sex. Chi square test was used for categorical variables. SPSS (IBM® SPSS® Statistics version 25) was used to conduct all analyses.
2.5. Ethics and data management
The study was approved by the Institutional Review Board of Maccabi Health Services, number 0029–21-MHS.
3. Results
During July 13–August 03, a total of 70,677 individuals received a third dose of BNT162b2 mRNA COVID-19 in MHS. 33,089 (46.8%) were women and 12,407 (15.2%) were ICI (Table 1 ). Among the vaccine recipients, 58,974 (83%) were eligible to receive the survey and the response rate was 30.2%. A total of 17,820 third dose recipients participated in the online survey, with a mean (SD) age of 68.4 (6.5). Of them, 3195 (17.9%) were ICI.
Table 1.
Characteristics of third SARS-CoV-2 BNT162b2 -vaccine dose recipients a and survey respondents, MHS national survey, Israel, July 20–August 10, 2021.
| No. of vaccine recipients (%) | No. of Survey respondents (%) | |
|---|---|---|
| Total | 70,677 | 17,820 |
| Age, mean y (SD) | 71.4 (8.1%) | 68.4 (6.5) |
| Age Group | ||
| <60 | 907 (1.3%) | 334 (1.9%) |
| 60–69 | 30,399 (43%) | 10,727 (60.2%) |
| 70–79 | 28,108 (39.8%) | 5735 (32.2%) |
| 80–89 | 9823 (13.9%) | 1024 (5.7%) |
| ≥ 90 | 1440 (2%) | 0 (0%) |
| Sex | ||
| Male | 37,588 (53.2%) | 9813 (55.1%) |
| Female | 33,089 (46.8%) | 8007 (44.9%) |
| Comorbidity | ||
| Immunosuppression | 12,407 (17.5%) | 3195 (17.9%) |
| Solid organ transplant | 806 (1.1%) | 257 (1.4%) |
| Immunosuppressive treatment | 9232 (13.1%) | 2326 (13.1%) |
| Hematologic malignancies | 2278 (3.2%) | 612 (3.4%) |
| Cancer | 17,954 (25.4%) | 4478 (25.1%) |
| Body Mass Index >35 | 5189 (7.4%) | 1165 (6.5%) |
| Diabetes | 19,334 (27.4%) | 4123 (23.1%) |
| Hypertension | 41,281 (58.4%) | 9320 (51.8%) |
| Ethnicity/ sector | ||
| Arabs | 1272 (1.8%) | 146 (0.8%) |
| Ultra- Orthodox Jews | 694 (1.0%) | 118 (0.7%) |
| Non Ulra- Orthodox Jews | 68,711 (97.2%) | 17,556 (98.5) |
| Socioeconomic status†b | ||
| Low | 7171 (10.1%) | 894 (5.0%) |
| Medium | 33,181 (46.9%) | 7578 (42.5%) |
| High | 30,325 (42.9%) | 9348 (52.5%) |
| Smoking status | ||
| Non Smoker | 62,907 (89.0%) | 15,875 (89.1%) |
| Previous Smoker | 999 (1.4%) | 279 (1.6%) |
| Smoker | 6193 (8.8%) | 1552 (8.7%) |
| Unknown | 578 (0.8%) | 114 (0.6%) |
SD- standard deviation: MHS- Maccabi Healthcare Systems.
During July 13–August 03, 2021.
Defined by the Israel Central Bureau of Statistics.
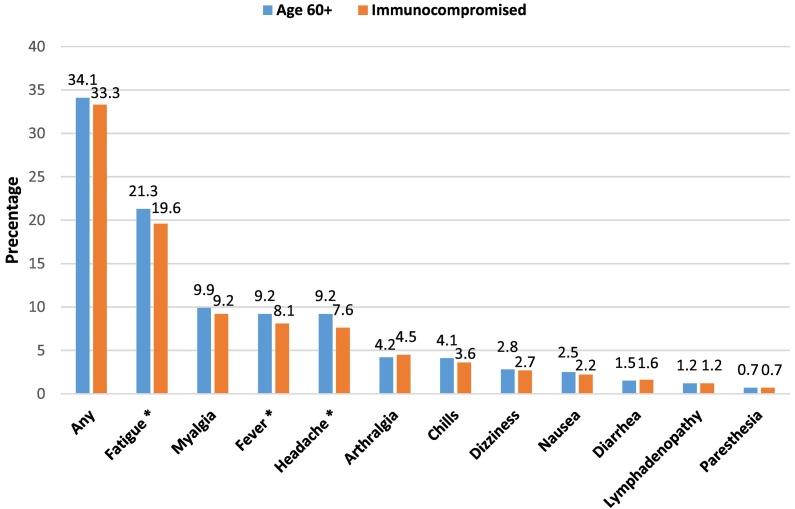
Most immunocompromised (63.3%) and seniors (67.5%) reported injection site pain (Table A.1). The majority of ICI and seniors (66.7% and 65.9%, respectively) did not report any systemic side effect (Fig. 1 ). Fatigue, myalgia, fever and headache were most frequently reported (19.6%, 9.2% and 8.1% respectively among ICI; 21.3%, 9.9% and 9.2% respectively among seniors). 166 (5.2%) ICI and 594 (4.1%) of seniors added supplemental information beyond the questionaries' specific symptom list using free text. No serious side effects were reported.
Fig. 1-.
Local and systemic reaction following the third SARS-CoV-2 BNT162b2 vaccine dose among Immunocompromised individuals a (N = 3195) and Seniors 60 years of age and older (N = 14,625), Maccabi Healthcare Services national survey, Israel, July 20–August 10, 2021. Weights were applied in presenting the distribution calculations of systemic side effects. Chi square test was used, p-values <0.05 were considered statistically significant.
* p < 0.001.
a Solid organ transplants, patients with hematologic malignancies and on immunosuppressive treatment.
Comparing between age groups and sex (Table A1), younger ages reported systemic reactions more commonly than older ages (37.8% of 60–69 years versus 26.5% of 80 years or over among the ICI group, p < 0.001; 39.4% of 60–69 years versus 25.9% of 80 years or over among the seniors group, p < 0.001). In addition, females reported more systemic side effects as compared to males (45.3% versus 27.1% respectively among the ICI group, p < 0.001 and 47.9% versus 26.4% among the seniors group, p < 0.001). This pattern is found consistently regarding each side effect in both of the study groups. 94.4% of respondents who reported a side effect did not needed any medical care for their symptoms. About a half of respondents that had systemic symptoms (52.2% of ICI and 55.1% of seniors) reported them to resolve within 24 h, and only 4.8% and 4.5% (respectively) reported them to last more than six days.
In a subjective comparison of side effects with those following the second vaccine dose, 67.3% of ICI and 62% of seniors reported to experience a similar or a better reaction after receiving the third dose. Females experienced a worse reaction than males in both groups (Fig. A.1).
4. Discussion
This first of its kind COVID-19 booster vaccine's short term safety survey was conducted on two populations who were eligible for a third SARS-CoV-2 BNT162b2 vaccine dose – ICI and seniors aged 60 years and over. Results indicate that overall short term side effects were similar to those observed following the previous two vaccine doses administrated [[2], [3]] and were mostly self-resolved. Duration of most symptoms lasted 24 h or less and the majority of respondents reacted better than or similar to the second vaccine dose.
Females and younger age groups report more frequent side effects after the third dose administration and they tend to rank these side effect more severely than after the second dose. Differences in reactogenicity among vaccine recipients were also found following the first and second SARS-CoV-2 BNT162b2 vaccine administration [4]. Although sex difference in reactogenicity was not reported in the SARS-CoV-2 BNT162b2 vaccine trial, real world data demonstrated this difference after the two vaccine doses [3]. Age differences were reported in the phase 3 clinical trials of this vaccine, as well as in the real world data [2,3]. Comprehension of these age and sex disparities by healthcare workers can contribute in personalized counseling for vaccine recipients.
Elderly and immunosuppressed individuals are most vulnerable to COVID-19 disease [5] and more likely to have breakthrough infection [6], two reasons for the recommendation of an additional vaccine dose. To date, small researches evaluated short term safety of a third mRNA vaccine dose [7,8], where patients presented only mild short term side effects. The limited information available limits the publics' trust and acceptance of an additional dose recommendation. This voluntary survey sheds light on the common side effects following the booster vaccine as openly reported by the patients themselves. This valuable information can contribute to vaccine acceptance and relieve fear of potential short-term side effects.
Our study has some limitations. First, this survey was voluntary and required internet or telephone access, therefore, information might not be representative or generalizable. Second, although survey responses were weighted to approximate representativeness of MHS' vaccine recipients, findings might not be representative to all vaccine recipients nationally. Third, self-reports are subject to recall biases. Forth, as with all public surveys, this was a snapshot taken at a given point in time and cannot reflect long-term side effects.
5. Conclusions
In this real world community survey study, local and systemic reactions after third vaccination with BNT162b2 vaccine, reported by ICI and seniors, were similar to those observed following administration of the previous vaccines and mostly self-resolved. This data gives reassurance for healthcare providers and patients, adds further weight to vaccination in the benefit versus risk equation and is of substantial importance to decrease vaccine hesitancy and to elevate vaccine confidence.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgments
Acknowledgement
We would like to thank Rada Kovatch for conducting the survey.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2021.108860.
Appendix A. Supplementary data
Supplementary material
References
- 1.Ministry of Health Israel, COVID-19 vaccination. https://govextra.gov.il/ministry-of-health/covid19-vaccine/en-covid-19-vaccine-3rd-dose/ (accessed 31 August 2021)
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G. Pérez, Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., Hu C., Selvachandran S., Antonelli M., Murray B., Canas L.S., Molteni E., Graham M.S., Modat M., Joshi A.D., Mangino M., Hammers A., Goodman A.L., Chan A.T., Wolf J., Steves C.J., Valdes A.M., Ourselin S., Spector T.D. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatakrishnan A.J., Kumar-M P., Silvert E., Garcia-Rivera E., Szenk M., Suratekar R., Lenehan P., Lindemer E., O’Horo J.C., Williams A.W., Badley A.D., Virk A., Swift M.D., Gores G.J., Soundararajan V. Female-male differences in COVID vaccine adverse events have precedence in seasonal flu shots: a potential link to sex-associated baseline gene expression patterns. MedRxiv. 2021 doi: 10.1101/2021.04.01.21254798. [DOI] [Google Scholar]
- 5.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosh-Nissimov T., Orenbuch-Harroch E., Chowers M., Elbaz M., Nesher L., Stein M., Maor Y., Cohen R., Hussein K., Weinberger M., Zimhony O., Chazan B., Najjar R., Zayyad H., Rahav G., Wiener-Well Y. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werbel W.A., Boyarsky B.J., Ou M.T., Massie A.B., Tobian A.A.R., Garonzik-Wang J.M., Segev D.L. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann. Intern. Med. 2021 doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material