Abstract
Detailed offline speciation of gas- and particle-phase organic compounds was conducted using gas/liquid chromatography with traditional and high-resolution mass spectrometers in a hybrid targeted/nontargeted analysis. Observations were focused on an unoccupied home and were compared to two other indoor sites. Observed gas-phase organic compounds span the volatile to semivolatile range, while functionalized organic aerosols extend from intermediate volatility to ultra-low volatility, including a mix of oxygen, nitrogen, and sulfur-containing species. Total gas-phase abundances of hydrocarbon and oxygenated gas-phase complex mixtures were elevated indoors and strongly correlated in the unoccupied home. While gas-phase concentrations of individual compounds generally decreased slightly with greater ventilation, their elevated ratios relative to controlled emissions of tracer species suggest that the dilution of gas-phase concentrations increases off-gassing from surfaces and other indoor reservoirs, with volatility-dependent responses to dynamically changing environmental factors. Indoor–outdoor emissions of gas-phase intermediate-volatility/semivolatile organic hydrocarbons from the unoccupied home averaged 6–11 mg h−1, doubling with ventilation. While the largest single-compound emissions observed were furfural (61–275 mg h−1) and acetic acid, observations spanned a wide range of individual volatile chemical products (e.g., terpenoids, glycol ethers, phthalates, other oxygenates), highlighting the abundance of long-lived reservoirs resulting from prior indoor use or materials, and their gradual transport outdoors.
Keywords: whole-house emission rates, indoor air quality, volatile organic compounds, emissions of volatile chemical products, atmospheric chemistry, surface emissions, aerosols, ventilation, personal care products
Graphical Abstract
INTRODUCTION
Even though humans in developed nations spend approximately 90% of their time indoors, with most of that time (69%) spent in their own residence,1,2 our understanding of indoor air composition, chemistry, and air quality remains relatively understudied compared to outdoors. Airborne reactive gas- and particle-phase organic compounds are often present in a complex mixture that spans a wide range of chemical classes and volatilities, including volatile (VOCs), intermediate-volatility (IVOCs), semivolatile (SVOCs), low volatility (LVOCs), extremely low volatility (ELVOCs), and ultra-low volatility organic compounds (ULVOCs), many of which exist as condensed species in organic aerosol (OA, a key component of particulate matter (PM)).
Elucidating this level of chemical complexity represents an analytical challenge that necessitates advanced methods, including high-resolution mass spectrometry. While speciation across a wide volatility and functionality range has been done for ambient outdoor air,3,4 for specific sources,5,6 to interpret oxidation experiments in chambers,7,8 and for public indoor spaces,9 knowledge gaps remain about the full diversity and spectrum of organic compounds present in the gas and particle phases in residences as well as their emissions to outdoor environments—a major uncertainty related to nontraditional sources, e.g., volatile chemical products (VCPs), in emissions inventories.10−12
Indoor sources, including VCPs,10,11 cooking,13–15 smoking,6,16,17 building materials,18,19 furnishings,20–22 and cleaning,11,23,24 are major contributors to indoor chemical composition and can perturb the chemical and physical conditions of the indoor environment.9,19,25–30 Indoor concentrations are consistently higher than outdoors for a majority of VOCs/IVOCs (i.e., indoor–outdoor ratios; I/O > 1) due to these indoor sources and limited ventilation.25 Ventilation with outdoor air, implemented via both passive (e.g., open windows; infiltration (penetration via gaps in the building envelope)) and mechanical (e.g., HVAC) systems, drives the indoor–outdoor exchange of airborne constituents (i.e., gases and particulate matter (PM)) and often, though not always, temporarily reduces indoor concentrations of gases and particles.31,32 Elevated levels of gas-phase organic compounds (e.g., formaldehyde, acrolein)33,34 and low ventilation rates have been linked to adverse health effects,35,36 though the intrusion of outdoor ozone with greater ventilation can lead to increases in the potentially hazardous ozonolysis products resulting from indoor VOC emissions.37
These indoor sources can also be responsible for a considerable proportion of indoor PM.38 Past measurements of indoor particles focus on general chemical composition (i.e., organic vs inorganic species), analysis of some target species, size distribution, and total particulate mass.6,9,28,31,38–41 Recent research has made it increasingly clear that variations in the elemental composition of the particle phase have significant consequences on physical and chemical properties, including phase state, viscosity, and acidity.4,42–47 These properties also affect how the gas-phase compounds distribute throughout the different phases present in an indoor environment. Equilibrium partitioning between the gas-, particle-, and surface-phase drives the distribution, persistence, and exposure to many organic compounds emitted indoors.29,40,48–52 Gases migrate relatively quickly through an indoor space via advection and turbulent diffusion, while molecular diffusion (near and within surfaces and particles) and multiphase chemical processes occur on longer timescales.49,52–57 Gas–particle and gas–surface partitioning have larger-scale implications on gas-phase concentrations, secondary organic aerosol (SOA) growth, and surface films, hence making them important processes indoors.31,58–61
Here, we present high chemical resolution analyses of indoor gas- and particle-phase organic compounds to study chemical diversity, dynamics, and their transport between phases and to the outdoor environment. Specifically, we (i) speciate a wide volatility and functionality range of organic compounds in both the gas and particle phases via a hybrid targeted/nontargeted approach in a variety of indoor environments; (ii) evaluate the effect of ventilation on their relative concentrations; (iii) examine the magnitude and composition of emissions (i.e., indoor-to-outdoor emissions, source profile speciation) to the outdoor environment across the volatility range of observed compounds; and (iv) assess volatility-dependent gas–particle and gas–surface dynamics with environmental perturbations. An unoccupied home was chosen as the primary study location to focus on baseline conditions while reducing confounding factors from ongoing human-related indoor activities, and the observed chemical composition of organic gases and aerosols was compared to two other distinctly different indoor locations.
MATERIALS/METHODS
Study Design.
The primary study location was a single-family home in suburban St. Louis, MO, from August 4–10, 2018, during the second Air Composition and Reactivity from Outdoor aNd Indoor Mixing campaign (ACRONIM-2). The first campaign, ACRONIM-1, took place in a different St. Louis residence in 2016.31 ACRONIM-2 details, including the site and the other instruments deployed during the study, can be found in the study by Eftekhari et al.62 The ACRONIM-2 home was left unoccupied for a few weeks prior to and during the sampling period and was located approximately 0.5 km from a large highway and within 1.5 km of an interstate freeway. Regional biogenic emissions and occasional influences from local restaurants or biomass burning (e.g., bonfires and smoking) by neighbors were also potential sources.
The approximate volume of the model home was 420 m3, and the air within the house was determined to be well-mixed when the windows were closed.31 The central HVAC system was set to maintain the house at 25 °C, with the fan operating continuously. Adsorbent tube (gas-phase) and poly-(tetrafluoroethylene) (PTFE) filter (particle-phase) samples were concurrently collected in the kitchen (indoors) and on a second-floor deck (outdoors) (Figure S1), using an inlet-free modified 316L passivated stainless steel filter housing (Pall).63 Sample collection parameters, including sample dates/times and volumes, are provided in Table S1.
Three windows were either completely open or closed, which provided ventilation perturbations. Air exchange rates (AER) were measured by releasing and tracking the subsequent decay of CO2 every 4 h (Figure S2, Table S1). In addition, hexafluorobenzene (HFB) and octafluorotoluene (OFT) tracers were continuously released throughout the home to provide a measurement of dilution via ventilation, as previously described.31,62
The offline gas- and particle-phase samples used for comparison in this study were analyzed via the same instrumentation and had been collected by the authors at a movie theater (18–217 people present, 1300 m3) in Mainz, Germany6 and at a commercial workplace in southern Connecticut (CT).64,65 Movie theater samples were collected in a ventilation duct from one of the screening rooms when an audience was present, while the southern CT samples were collected from a variety of indoor locations with occasional foot traffic and minimal occupancy, such as office spaces and hallways. More details can be found in Section S1.
Offline Speciation of Complex Gas-Phase Mixtures via GC-EI-MS and GC-APCI-TOF-MS.
Custom-packed adsorbent tubes containing quartz wool, glass beads, Tenax TA, and Carbopack X were prepared and sampled upon as described in the study by Sheu et al.63 Adsorbent tubes at the St. Louis site were collected for 4 h at a flow rate of 100 SCCM (total volume: 24 L), with the exception of indoor samples collected on August 10, which were collected for 2 h (12 L) instead.
Adsorbent tubes were desorbed via a modified thermal desorption system (Markes TD-100) connected to a gas chromatograph (Agilent 7890B) with a DB-5MS Ultra Inert column (Agilent, 30 m × 320 μm × 0.25 μm). The column outflow was split 1:1 using a two-way splitter board with helium purge gas (Agilent), with one flow path going to a vacuum single quadrupole electron ionization mass spectrometer (Agilent 5977A, GC-EI-MS). The other outflow was ionized via atmospheric pressure chemical ionization (APCI) and detected using high-resolution, time-of-flight mass spectrometry (Agilent 6550 Q-TOF, GC-APCI-TOF-MS). Extensive targeted analysis was conducted on this GC-APCI-TOF-MS spectral data by integrating at specific masses over precalculated retention time ranges (Sections S2 and S3). Due to compound-to-compound variations in response factors (e.g., ionization efficiency) for chemical ionization mass spectrometers and the associated uncertainties with mass calibration, we present mass concentrations where possible and ion counts otherwise.
Offline Speciation of Functionalized Organic Aerosol via LC-ESI-TOF-MS.
Filter sampling, solvent extraction, and analysis followed procedures detailed by Ditto et al.3 Sampling durations for the PTFE filters ranged between 22 and 24 h at a sampling flow rate of 17 SLPM (total volume: 22.4–24.5 m3). Methanol filter extracts were separated via HPLC (Agilent, 1260 Infinity) with a Hypercarb porous graphitic carbon reverse-phase column (Thermo Scientific, 3 μm particle size, 2.1 mm column diameter, 30 mm column length). The column effluent was ionized using electrospray ionization (ESI) and detected in both the positive and negative modes using the same high-resolution Q-TOF as above, where the high mass accuracy (<2 ppm) and mass resolution (M/ΔM > 25 000) of the Q-TOF enable the nontargeted identification of compound formulas in the functionalized OA (additional information in Section S4).
RESULTS AND DISCUSSION
This study presents the detailed speciation of gas- and particle-phase hydrocarbons and functionalized compounds found in indoor air for both the overall complex mixture as well as individual compounds, including variations with ventilation and other environmental perturbations. The results are interpreted both in the context of indoor air composition and indoor-to-outdoor emissions from nontraditional indoor sources (e.g., VCPs), resulting from their transport to outdoors driven by their large indoor/outdoor ratios (i.e., gradients). Until otherwise indicated, the data and discussion pertain to the unoccupied home in St. Louis.
Elevated Gas-Phase Concentrations Indoors and Ventilation Effects in the Unoccupied Home.
Speciation of Complex Hydrocarbon Mixtures.
Of hydrocarbons containing 10 or more carbon atoms, terpenes represented the largest contributing compound type both indoors (48%) and outdoors (45%); a substantial fraction of these were monoterpenes, with some sesquiterpenes observed (Figure 1A,B,A inset). Straight-chain alkanes, cycloalkanes, and aromatics made up most of the remainder, with PAHs comprising a small fraction (3%) of gas-phase hydrocarbon mass. The total hydrocarbon concentrations indoors were on average much higher than their outdoor counterparts by more than one order of magnitude, 140 ± 83 μg m−3 vs 9.1 ± 6.4 μg m−3 (I/O = 16) on average (one-tailed t-test, P = 0.003) (Table S1). The percent contribution of each functional group type (alkanes, cycloalkanes, aromatics, terpenes, and PAHs) was overall quite similar between indoor and outdoor samples (Figure 1A inset). In indoor and outdoor samples, we note relatively higher levels of alkanes indoors and aromatics outdoors (Figure 1A inset).
Figure 1.
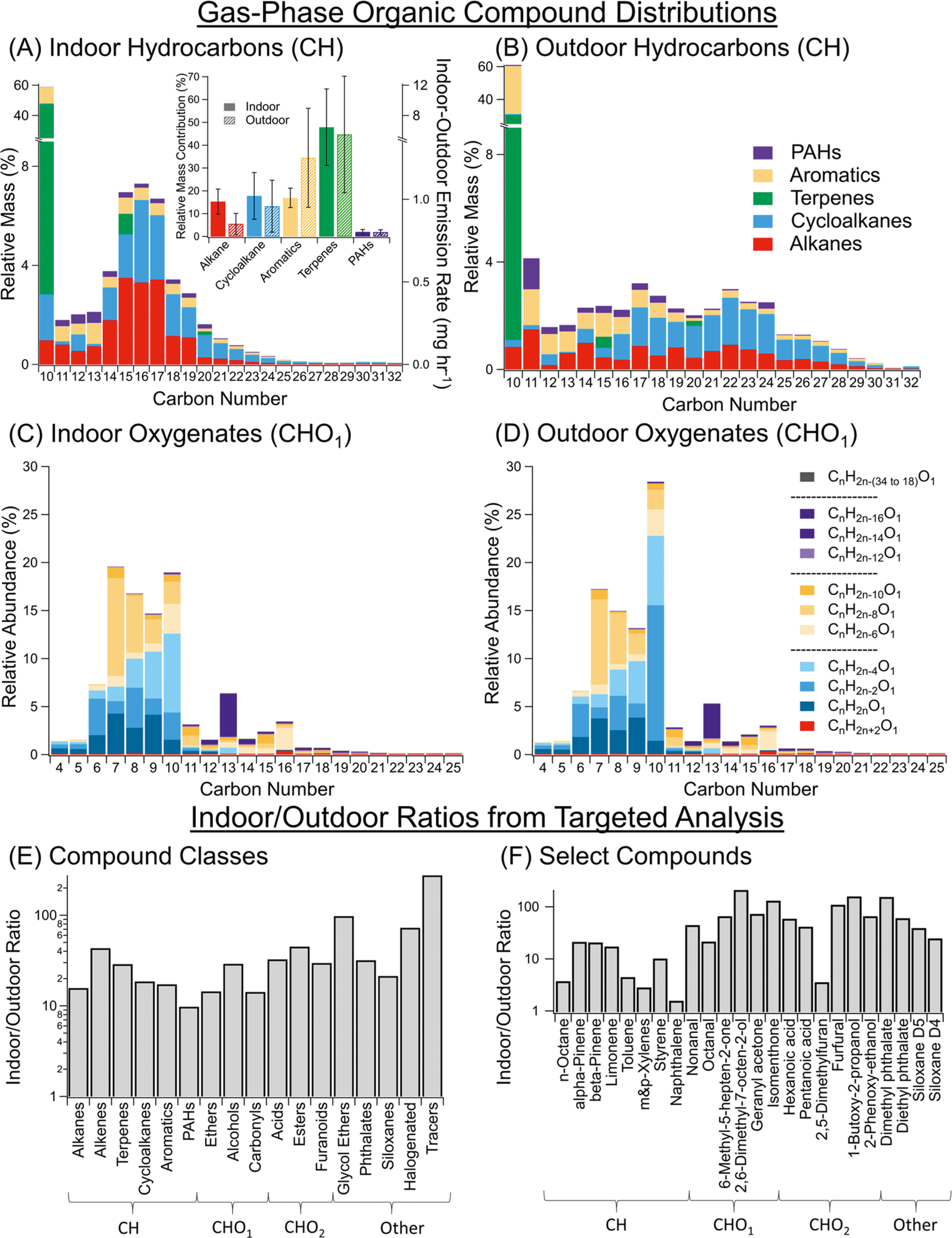
Extensive targeted speciation at the unoccupied home via GC-APCI-TOF-MS for complex mixtures of (A, B) hydrocarbons (CH) and (C, D) oxygen-containing (CHO1) compounds indoors (N = 7) and outdoors (N = 9), with (E) indoor–outdoor ratios (Nindoor = 10, Noutdoor = 11, from GC-EI-MS) presented by the functional group and (F) for selected compounds, shown as a ratio of the geometric means . Hydrocarbon mass concentrations (A, B) were calculated via the response factors in Table S2 using a method similar to prior work with complex and individual standards (Section S3).67 In (A), the CH emission rates for open and closed window conditions are approximated since the exact values (see Figure S3, Table S3) are dependent on outdoor concentrations. See Figure S4 for CHO2 speciation.
In terms of mass concentrations, the gas-phase complex mixture in the intermediate-volatility/semivolatile organic compound (I/SVOC) range peaked around C15–C17 for the indoor environment and was fairly level across C14–C24 for the outdoor samples. This difference may be the result of I/SVOC distributions in emissions from consumer products and building materials and also influenced by I/SVOC partitioning to and from surfaces and other bulk reservoirs. The high surface area to volume ratio of indoor spaces (typically 3 m−1)40,66 may also enhance reversible (and irreversible) partitioning of I/SVOCs (of indoor or outdoor origin) to these reservoirs.
This partitioning to indoor particle- and other condensed-phases will be more pronounced for lower volatility compounds, and as such, hydrocarbon SVOCs (e.g., C19+) comprise a smaller fraction of gas-phase hydrocarbons indoors. In all, the observations of elevated levels of C15–C17 hydrocarbons relative to smaller and larger hydrocarbons emphasize the role of reservoirs of these hydrocarbons from both prior use indoors and active off-gassing.
Speciation of Oxygen-Containing Compounds.
In addition to hydrocarbons, oxygen-containing compounds represent some of the most prevalent compounds present in the indoor environment. By ion abundance, a majority of observed gas-phase compounds observed via GC-APCI (i.e., C4+ compounds) with one oxygen atom had 7–10 carbons (Figure 1C). Monoterpenoids (e.g., camphor (C10H16O), isomenthone (C10H18O)) and skin oil oxidation products were prominent (e.g., 6-methyl-5-hepten-2-one (6-MHO, C8H14O)), whose identifications were confirmed using GC-EI-MS (Figure 1C,D). Most of these aforementioned compounds and the CHO1 compounds detected had volatilities in the VOC-IVOC range and would be expected to be found in the gas phase. However, carrying out an extensive speciation of the complex mixture, as done here, also reveals a noticeable contribution of IVOC CHO1 (C11–C16) to the total ion abundance that would not be identified by GC-EI-MS.
These gas-phase oxygen-containing mixtures were also more abundant indoors for both compounds containing one oxygen (CHO1, (5.4 ± 2.6) × 105 indoors vs. (2.6 ± 2.6) × 104 outdoors, in ion abundance L−1, P = 0.004) and two oxygens (CHO2, (2.8 ± 2.2) × 105 indoors vs (3.0 ± 6.7) × 103 outdoors, in ion abundance L−1, P = 0.008) (Figures 1C,D and S4, Table S1). The more pronounced difference in abundance for compounds with two oxygens can be primarily attributed to the elevated level of furfural, likely from wood off-gassing,19 which was 230 ± 120 ppb indoors compared to 2.4 ± 1.0 ppb outdoors. Because of variations in sensitivity and ionization efficiencies, response factors were not determined for these generally smaller CHO1 and CHO2 compounds; hence, their ion abundances were not converted to mass concentrations. A comparison of their average ion abundances indoors was 2.1 ± 0.6 for CHO1/CH and 1.0 ± 0.3 for CHO2/CH (Figure S6) for the compounds shown in Figure 1.
In addition to the CH, CHO1, and CHO2 compounds in the targeted analysis, other elemental compositions that feature prominently in the nontargeted gas-phase data include CHO3, CHS1, CHO4, and CHO1N1. These seven compound groups account for 93% of the total abundance and 78% of the compounds identified (i.e., by occurrence) in the nontargeted analysis (Figure S5).
Observations of Individual Gas-Phase Compounds and Their Indoor–Outdoor Ratios.
The indoor–outdoor concentration gradients of VOCs–SVOCs signify differences in indoor air composition and sources and are key drivers of indoor-to-outdoor emissions. To enable a compound-specific approach that supplements the complex mixture speciation above, 369 compounds ranging in carbon number from C3 to C25 were identified in this study and their peak areas were quantified. We calculated (i) unmodified indoor–outdoor ratios (I/O), (ii) ratios of indoor abundances between ventilation conditions (Iopen/Iclosed), (iii) ratios of indoor abundances normalized for ventilation dilution via the tracer compounds , and (iv) indoor–outdoor ratios compared between the ventilation conditions (, see Section S2C). In Figure 1E, the presence of specific functional groups was used to classify the compounds. Molecules containing more than one functional group were included in each appropriate category, with the exception of redundant designations, such as glycol ethers and phthalates, which were excluded from ethers/alcohols and esters, respectively. At this site, the functional groups’ I/O ratios ranged from approximately 8 to 100 (Figure 1E). Within these functional groups, we note the significant variation in I/O ratios, perhaps from differences in indoor emission rates. For instance, within the aromatics, C8 aromatics and naphthalenes (I/O ≈ 1–3) had lower indoor emissions at this site (and potentially larger outdoor contributions). By contrast, styrene had a higher I/O (I/O ≈ 10) (Figure 1F), which may be due to emissions from carpets, rugs, and other housing-related materials, in addition to being more reactive outdoors.21 Compounds with higher degrees of functionalization generally tended to have larger I/O ratios, potentially due to their substantial indoor reservoirs/emissions.
Of the hydrocarbons, terpenes and nonterpene alkenes have the highest I/O ratios (Figure 1E). Terpenes have major anthropogenic VCP-related sources, and as such, their indoor concentrations are primarily expected to be driven by indoor emissions. They are highly reactive with both hydroxyl radicals and ozone and are significant SOA precursors.68,69 For example, the timescale for limonene loss to OH oxidation (τ ~ 1–15 h) and terpene loss to ozone oxidation indoors (τ ~ 13 h for limonene, 5.5 for myrcene) can be competitive with ventilation (τ = 1–10 h in this study).70–72
Observed compounds originated from a range of sources at the unoccupied home and included a series of C1–C9 acids and aldehydes, geranyl acetone (e.g., skin oil oxidation product),73 2,5-dimethylfuran (e.g., biomass burning),6 and furfural (wood decomposition).19 It should be noted that previous studies have shown that other smaller organic compounds (C1 and C2, e.g., methane, methanol, ethanol, formaldehyde, and formic acid) comprise a major fraction of total gas-phase organic compound mass.25,66,74,75 For instance, formaldehyde made up a third of the observed gas-phase organic compound mass in a recent unoccupied home study.76 However, for this study, adsorbents were specifically selected to focus on larger compounds.
In addition to prominent VCPs (e.g., toluene, methylcyclohexane, and limonene), a wide range of oxygenated VCPs with considerable production volumes, use, or estimated potential urban emissions were observed with substantial indoor–outdoor emissions and I/O ratios, driven by their emissions indoors from various sources (Figure 2). Notable VCPs identified in this study (e.g., Table S4) include (i) personal care products (e.g., dihydromyrcenol, D3–D9 siloxanes, homosalate (found in sunscreens), n-hexyl salicylate, 2-ethylhexyl benzoate, diethyltoluamide (DEET, found in bug repellent));14,77,78 (ii) glycols and glycol ethers (e.g., propylene glycol, 2-butoxyethanol, butoxypropanol, butoxyethoxyethanol, phenoxyethanol, C10H22O3 isomers),11,24,79 (iii) alcohols (e.g., butanol, pentanol, hexanol, phenol, benzyl alcohol), (iv) paints/plasticizers (e.g., texanol B [2,2,4-trimethyl-1,3-pentanediol monoisobutyrate], TXIB [2,2,4-trimethyl-1,3-pentanediol diisobutyrate]),80,81 (v) halocarbons (e.g., perchloroethylene [PCE], parachlorotrifluorobenzene [PCBTF]),82 and (vi) phthalates (e.g., dimethyl-, diethyl-, diisobutyl-, dibutyl-, and diisooctyl phthalate).83
Figure 2.
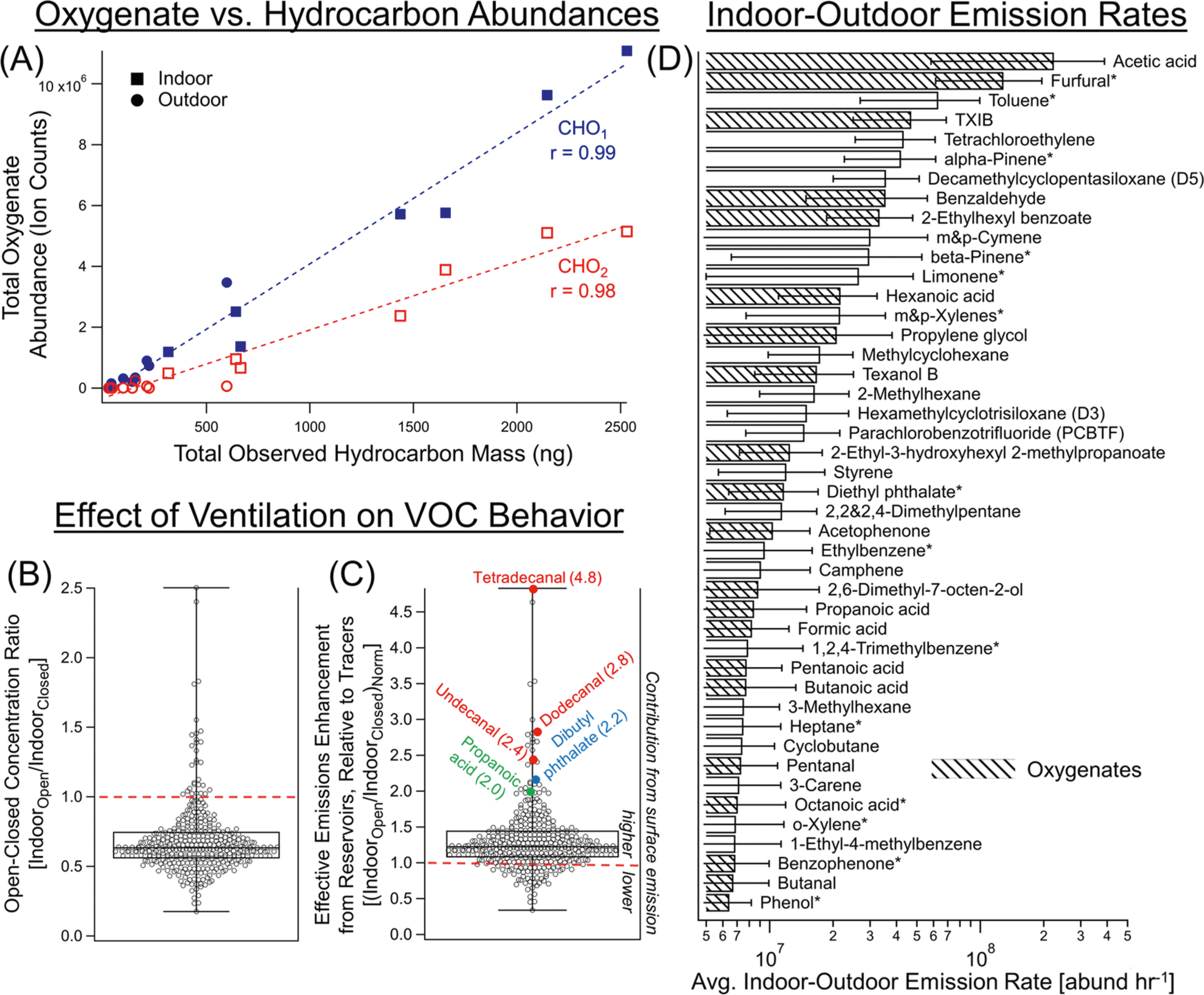
(A) Strongly correlated abundances of CHO vs CH complex mixtures at the unoccupied home (Figure S6A for hydrocarbon abundance), (B, C) changes in individual compound concentrations with ventilation conditions (i.e., windows open vs closed), shown with and without normalization to tracers (measured via EI-MS), and (D) individual compounds with the highest indoor–outdoor emissions (±SD). In the box-and-whisker plots, the box represents the median, 75th, and 25th percentiles, while the whiskers extend to the maximum and minimum values. The effective emission enhancement from surfaces and other indoor reservoirs (into indoor air) are shown in (C) with tracer-normalized abundances (hexafluorobenzene (HFB), octafluorotoluene (OFT)) to account for losses due to ventilation. Emissions in (D) are provided as ion abundances (i.e., peak areas measured via EI-MS) for their most prominent and unique fragment ions (see Table S4 for the complete list and m/z’s used, Table S5 for calibrated mass emissions, and Table S6 for all indoor–outdoor emissions). Compounds calibrated with standards in (D) are denoted with asterisks.
VCPs were also pronounced at the movie theater, primarily because of emissions from the occupants’ personal care products, including fragrance compounds. Unique compounds found at the theater but not at the unoccupied home included carvone, geraniol, linalyl acetate, anethole, pipersonal, galaxolide, hedione, cashmeran, lilial, Vertofix, Tonalid, benzyl salicylate, isoamyl salicylate, α-cetone, and α-hexyl cinnamaldehyde. The greater diversity in personal care products observed in the movie theater compared to the unoccupied residence is likely ascribable to ongoing human-related emissions and potentially occupant demographics in the theater. Together, these two sites demonstrate the importance of active VCP emissions from arriving occupants to the theater, amplified by the recent use of consumer products, as well as the importance of indoor reservoirs of organic compounds as observed in the unoccupied home and their subsequent partitioning and gas-phase transport to the outdoor environment.84–86
Strong Correlations Between Oxygenate and Hydrocarbon Mixtures.
In the unoccupied home, the concentrations of gas-phase complex mixtures varied together. The total abundance of CHO1 and CHO2 was strongly correlated with the total overall mass (and with the total overall abundance) of CH across gas-phase samples, with correlation coefficients of 0.99 and 0.98 for CHO1 and CHO2, respectively (Figure 2A). As further evidence of co-variance between VOCs off-gassing from indoor reservoirs in this unoccupied home, the concentrations of terpenes (α-pinene, β-pinene, and limonene) also strongly correlated during the campaign (Figure S6A). The collection time of the gas-phase samples (2 or 4 h) provided a quasi-steady-state sampling of the indoor concentrations. The strong correlations of CHO1 and CHO2 to CH indicate that the predominant reservoirs of emissions (from surfaces or bulk materials) have a relatively consistent profile over time while the home is unoccupied, with the equilibrium dynamics between the gas-phase and surface/bulk reservoirs likely buffering gas-phase composition.29
Effect of Ventilation on Gas-Phase Organic Compound Concentrations.
To understand the effect of ventilation on gas-phase organic compound concentrations, abundances were compared between the open and closed window conditions. The measured AER in the house was significantly lower (P < 0.0001) when the windows were closed (0.21 ± 0.08 h−1) versus open (0.51 ± 0.22 h−1) (Figure S2). In the GC-APCI-TOF-MS analysis, the average (Iopen/Iclosed) ratios for the CH, CHO1, and CHO2 mixtures were each close to 1, though variance was high due to the small sample size (Nopen = 4, Nclosed = 3) and expected environmental variance (Figure S7A,B, Table S4). An exploration of the effects of window opening/closing was more feasible with individual compound EI-MS data (Nindoor = 10, Noutdoor = 11), where both the absolute ratio (Iopen/Iclosed) and a normalized ratio were calculated (Figure 2B,C). The effects of dilution from ventilation were normalized using the volatile tracers HFB and OFT (Section S2). Once normalized, a ratio at or below 1 suggests that a compound and/or its reservoir is not responsive to changes in ventilation, while a ratio above 1 suggests that opening a window increases the net “emission” rate resulting from chemical transformations or emissions (e.g., repartitioning to the gas-phase from a surface/bulk reservoir) (Section S2). A large proportion of the compounds studied here have ratios higher than 1 (Figure 2C), indicating some emission enhancement from reservoirs occurring in response to ventilation, which bolsters a similar conclusion presented by Fortenberry et al.29,31
Indoor–Outdoor Emission Rates to Inform Emission Inventories.
The transport of air across the building envelope is a key step in the emissions of airborne constituents from indoor environments. Indoor–outdoor emission rates were calculated using data from the concurrent indoor and outdoor samples in a box model under steady-state conditions (Figures 2D and S7, Table S3). Data for individually calibrated (e.g., mg h−1 and μg m−2 h−1 when normalized for the home area) and all compounds (e.g., ion abundance h−1) from EI-MS can be found in Tables S5 and S6 for the unoccupied home (floor area ~167 m2), including monoterpenes (range: 17–110 μg m−2 h−1 for limonene & pinenes) and other compounds used as fragrances (e.g., 13–85 μg m−2 h−1 for nonanal, 4.1–21 μg m−2 h−1 for menthol).
Indoor emissions of VCPs (e.g., TXIB, Texanol, siloxanes, dihydromyrcenol) emitted from a variety of indoor uses and their subsequent indoor–outdoor transport due to ventilation are key contributors of these compounds to outdoor environments.85 For instance, furfural, a well-known by-product of wood decomposition, had the largest quantified emission rate of the identified compounds at an average of 120 mg h−1 (range: 61–275 mg h−1; 370–1650 μg m−2 h−1) from this house primarily constructed with wood. The observation of these large furfural emissions helps in identifying the underlying sources that may drive furfural’s considerable ambient urban concentrations but remain under-represented in emission inventories.85 However, we anticipate that regional differences in construction materials (i.e., wood vs. steel/concrete) and seasonal differences in temperatures will lead to variations in emissions from buildings.
Prior work has examined “whole-house emission rates” for a subset of VOCs and identified acetic acid as a prominently emitted individual compound (e.g., 51.9–818 μg m−2 h−1).87–89 Recent work also observed that whole-house emission rates were sensitive to AER and found that indoor–outdoor emissions (under occupied and closed conditions) were generally consistent for benzene, toluene, and C8 aromatics when normalized for the home area (i.e., μg m−2 h−1, Table S5), though the AER during no-ventilation conditions in that study’s modern home was lower (AER = 0.08 h−1).87 A comparison of our study’s older home to other newly built homes generally span similar ranges when compared across the subset of overlapping compounds measured (e.g., limonene, β-pinene, toluene, m/p-xylenes), with relatively lower emissions of α-pinene and the observed n-alkanes, but greater nonanal emissions in this study’s older home.88,89 Observed monoterpene emissions were ~5 times higher and furfural emission rates from our home with wood-frame construction (167 m2) were multiple orders of magnitude higher compared to Huangfu et al.’s “insulated concrete form” constructed home, which did also contain wood furnishings.87
The chemical distribution of these emissions was similar to the indoor composition since concentrations indoors were much higher than outdoors. Between ventilation conditions, we observed slightly higher indoor–outdoor emissions with opened windows since increased ventilation will transport more of the highly concentrated indoor air to outdoors (Figure 2C). Emissions for the complex hydrocarbon (CH) mixture, including C10+ compounds, averaged 29 mg h−1 (range: 8.2–54 mg h−1) and 13 mg h−1 (3.9–33 mg h−1) in open and closed conditions across the AERs observed in the study, respectively (Table S3), which equates to area-normalized emission rate of 170 and 78 μg m−2 h−1, respectively. Of this, I/SVOC hydrocarbon emissions (e.g., C12+) represent 11 mg h−1 (69 μg m−2 h−1) and 6 mg h−1 (33 μg m−2 h−1) under open and closed window conditions, respectively. A similar calculation for open and closed windows for oxygenated compounds (CHO1 and CHO2 for C4−25) also shows greater indoor–outdoor emissions with increased ventilation. From these observations, we hypothesize that reservoir off-gassing contributes to the indoor–outdoor emissions in response to increased ventilation, which would increase the total indoor–outdoor mass emissions contributing to urban oxygenated VOC emissions.90 In all, this unoccupied home demonstrates the importance of indoor reservoirs, which arise from either prior indoor emissions or indoor materials, and their gradual transport outdoors, even long after use or installation.
Aerosol-Phase Organic Compound Speciation at Indoor Sites.
Aerosol-phase chemical composition varies as a result of indoor activities, gas/particle equilibrium partitioning, and the intrusion of outdoor aerosols with expected variations between sites. Compound formulas and peak areas (in terms of ion abundance) were determined via nontargeted analysis with high-resolution mass spectrometry for particle-phase samples (Section S4). Since hydrocarbons (i.e., CH compounds) do not ionize efficiently in ESI, these results only include functionalized organic aerosols (OA) found in the particle phase.
The functionalized OA volatility distributions from concurrently collected indoor and outdoor aerosol samples (with combined positive- and negative-mode analysis) at the unoccupied home were fairly consistent across the volatility range (Figure S8). Elevated levels of SVOCs and LVOCs in functionalized OA were observed with increased ventilation (i.e., open windows), which may be a result of higher total OA concentrations during these times. The volatility distribution of the functionalized OA for the open window conditions, with a distinctive peak of S/LVOC compounds, aligns well with SOA distributions expected for partitioned oxidized compounds in other studies.91,92
At the unoccupied home, CHO (i.e., CHO ≥ 1, 20%), CHONS (31%), and CHON (40%) compounds are fairly prevalent indoors; with a higher relative abundance of CHO (27%) and CHON (52%) compounds outdoors, where CHON was dominated by more-oxidized CHON compounds (Figure 3A, Table S7). Figure 3A compares the functionalized aerosol speciation from the St. Louis home to two other sampling locations: a German movie theater and a southern Connecticut commercial workplace. A large contribution from nicotine and other off-gassing thirdhand tobacco smoke compounds explains the large fraction of observed CHN abundance in the movie theater.38,63 The data presented here also differs significantly from the large quantity of less-oxidized CHON (i.e., O/N < 3) present in the German outdoor data. The southern CT functionalized OA consists of primarily CHON (both reduced and oxidized nitrogen) and CHO compounds.
Figure 3.
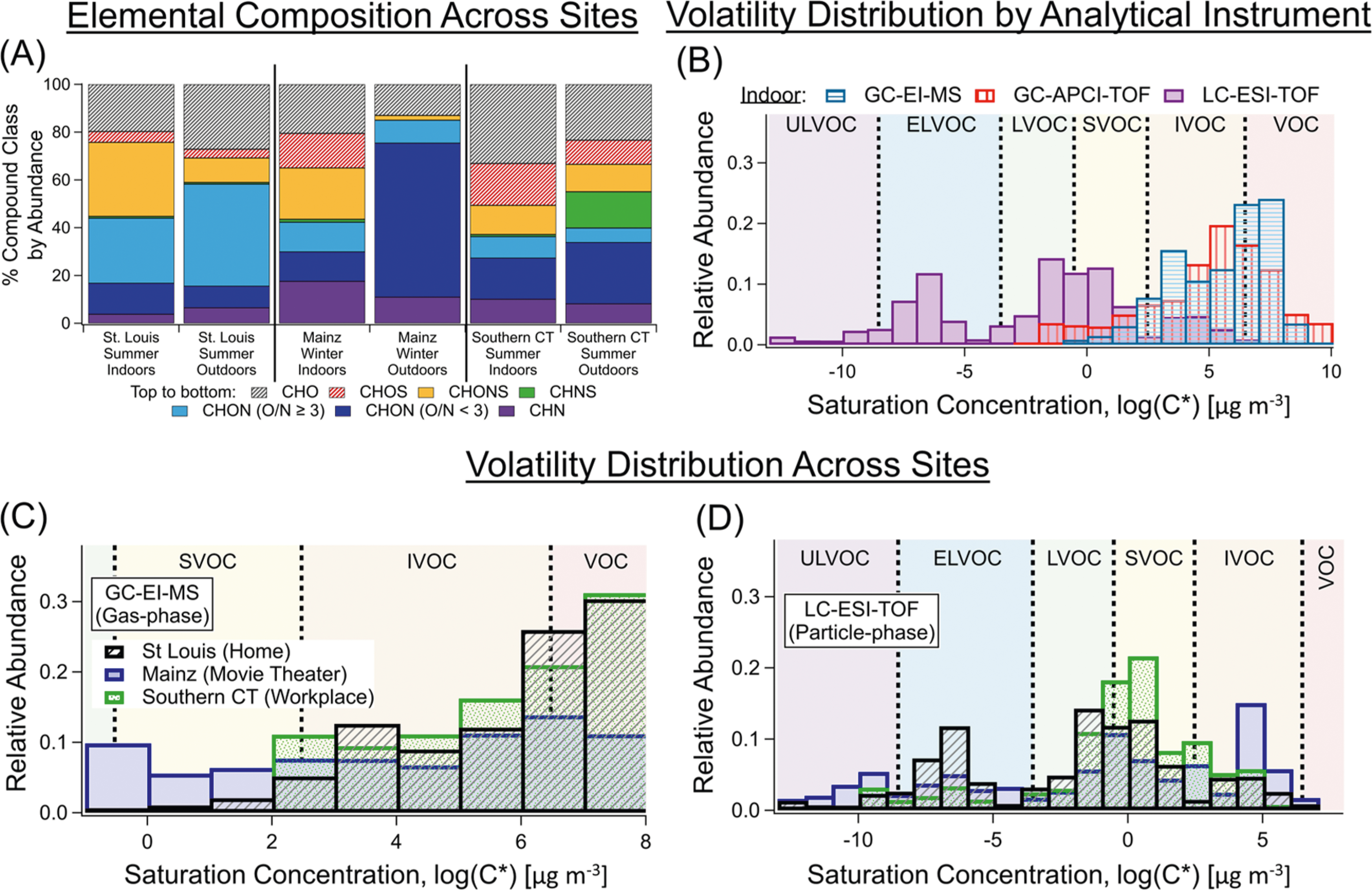
(A) Elemental composition of functionalized OA for indoor and outdoor samples from the unoccupied St. Louis home compared to other sites (NMainz_Indoor = 9, NIndoor_CT = 4, NOutdoor_CT = 5) filters with aggregated positive- and negative-mode ESI data similar to panels (B) and (D) with literature Mainz outdoor data.93 (B) Indoor volatility distributions of the gas- and aerosol-phase observed across offline instruments at the St. Louis home and (C, D) compared, by instrument configuration, to different sites (NMainz_Indoor = 10, NIndoor_CT = 14, NOutdoor_CT = 13 tubes). For more volatility distributions or numerical data, see Figure S8 and Table S7. Effective saturation concentrations (C*) were calculated using the formula-based parameterization from Li et al.94 and divided into different volatility regimes under the Donahue et al. classification (Section S5)95,96 with volatility boundaries (log(C*)) of VOC/IVOC (6.5 μg m−3), IVOC/SVOC (2.5 μg m−3), SVOC/LVOC (−0.5 μg m−3), LVOC/ELVOC (−3.5 μg m−3), and ELVOC/ULVOC (−8.5 μg m−3). While real partitioning behavior indoors generally does not match volatility regime nomenclature,97 these labels can still be used to separate compounds by volatility class. Note: the contributions to the two most volatile VOC bins in (B) and (D) are underestimates given reduced collection efficiencies and lower sensitivity in the high-resolution TOF-MS for smaller hydrocarbons.63,67
Comparison of Measurements across Volatility Space.
The offline collection and analysis of both gas- and particle-phase measurements provide us with chemical data spanning a wide range of volatilities (Figure 3B). The intersection of GC-APCI-TOF-MS and LC-ESI-TOF-MS provides detailed information on gas- and particle-phase I/SVOCs, including both less- and more-functionalized compounds, which have posed analytical challenges in the past. The indoor volatility ranges from this study are also compared to the distributions from the other two sites by the instrument used (Figure 3C,D with more details in Section S5).
For the gas-phase GC-EI-MS distributions, the trend from St. Louis is quite similar to the southern CT data, with both having more VOCs and IVOCs (Figure 3C). The enhanced levels of SVOCs in the Movie Theater data are at least partly attributable to the extensive mixture of personal care products that were identified (e.g., fragrances). The particle-phase distributions also vary somewhat between the three locations (Figure 3D). At the unoccupied home, we observe one main mode near the S/LVOC boundary, as well as some contribution from lower volatility compounds (e.g., ELVOC). The large abundance of particle-phase IVOCs at the movie theater can be explained by high levels of the reduced nitrogen species related to recondensed thirdhand tobacco smoke.6 Meanwhile, all three sites have a peak at the S/LVOC boundary, which might be attributed to typical gas–particle equilibrium partitioning given the overall quasi-steadystate sampling approach in this study. The overall distribution of the particle phase would be expected to be similar to outdoor conditions, albeit with necessary shifts as given by surface interactions and temperature gradients, which distinguish the indoor environment from the outdoor air.9
Dynamic Behavior of Gas-Phase Compounds with Environmental Perturbations.
During a day-long series of samples, we observed variations in VOC and IVOC concentrations at different points during a set of perturbation tests (Figure 4), which suggest varying dynamics between VOCs and IVOCs and could be due to a variety of physical sources and mechanisms.
Figure 4.
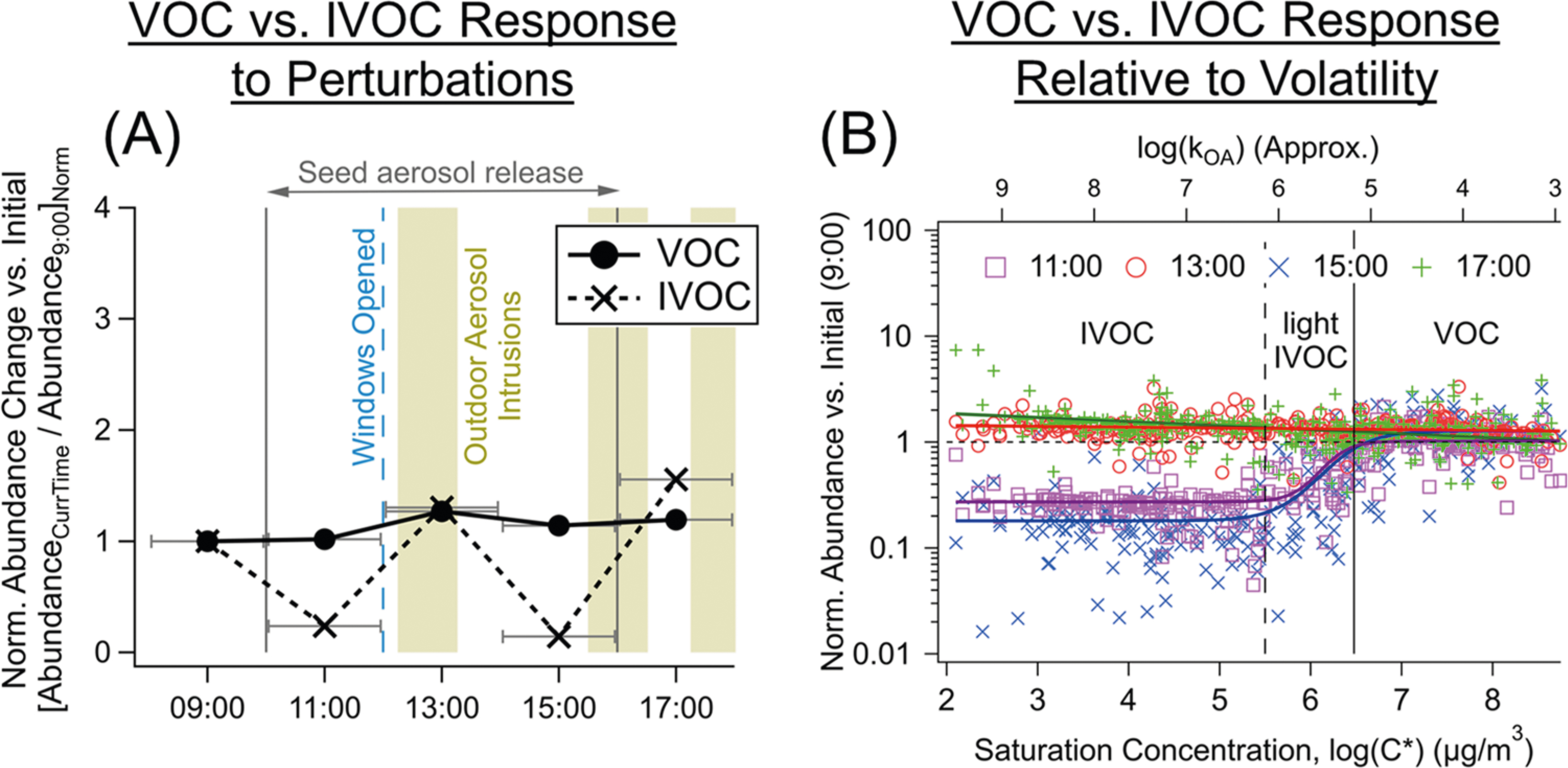
Responses to natural and artificial perturbations over a day-long period: (A) variations in normalized abundances of VOCs and IVOCs measured via GC-EI-MS (grouped consistently with the breakdown in panel (B)), shown with (B) their trends as a function of effective saturation concentration (log(C*)) approximated by GC retention time, and with approximate octanol-air partitioning coefficients (kOA)98 for comparison, which have been used to gauge the persistence of organic compounds indoors.49 Inorganic seed aerosols were released from 10:00–16:00, while the windows were open from 12:00–18:00, with outdoor aerosol intrusion events starting at 12:15, 15:30, and 17:15 (labeled bands on A) identified via SMPS (Figure S9). Samples were collected every 2 h starting at 8:00 (midpoints labeled above), and data were tracer-normalized in (A) by hexafluorobenzene. For additional run-to-run visualizations, see Figure S10.
A series of five 2-hour long gas-phase samples were collected across various perturbations. Windows remained open overnight and were closed prior to the start of the tests (8:00). A dish of Pine-sol was left out at 6:00 for the remainder of the day. After the initial sample (8:00–10:00), ammonium sulfate seed aerosol was released during the following three samples, which led the PM0.75 mass concentration to peak at nearly 12 μg m−3, up from a baseline of 3 μg m−3 (Figure S9F). Using SMPS, we observed an increase in larger particles ranging from 0.1 to 0.5 μm (Aitken mode) in diameter after the introduction of the seed aerosol (Figure S9C). Windows were then opened for the third through fifth samples, accompanied by a decrease in larger aerosols and occasional influxes of ultrafine particles (<100 nm) from outside (Figure S9C,D).
We note that VOCs and IVOCs respond differently over time. After noticing sharp volatility-dependent distinction in behavior at an effective saturation concentration (C*) of C*≈ 106 μg m−3, VOCs and IVOCs were assigned and grouped by their volatility as shown in Figure 4C (derived via GC retention time) (Section S7, Figure S10). For the 11:00 and 15:00 samples, the tracer-normalized abundances of VOCs remained close to 1 relative to the initial (9:00) sample but dropped for IVOCs. For the other two samples at 13:00 and 17:00, the normalized abundance ratios for IVOCs and VOCs were close to 1 (Figure 4A).
In the absence of other perturbations or emissions, we would expect the normalized abundance ratios for VOCs to be 1 for each sampling period, meaning that the concentrations of these primarily gas-phase species should remain fairly constant after accounting for differences in air exchange (via released tracers); the data in Figure 4A support this expected VOC behavior. If IVOCs behaved like VOCs, then their ratios would also be 1, but the abundance ratios for IVOCs were unexpectedly lower than 1 at various times. Consequently, the data in Figure 4B suggest volatility-dependent processes or factors, which have been suggested in prior work,97 but the underlying causes remain uncertain. We acknowledge that this is a limited perturbation experiment and thus present the results without promoting particular conclusions. While this set of five samples reveals some interesting trends, future observations and controlled experiments should consider the following questions and possible contributing factors related to the observed volatility-dependent behaviors (Section S7).
Does the volatility-dependent and unexpected behavior of IVOCs suggest an IVOC-specific sink? A volatility-dependent sink such as gas–particle partitioning likely occurs, but the mass concentration of PM is fairly low in this case (COA < 12 μg m−3) and thus enhanced deposition to PM should not have a substantial effect on overall IVOC concentrations, with some exceptions.6,29,38 Chemical removal as the sink is also unlikely since varying reactivities between compounds would lead to greater variance in normalized abundance ratios, and the ratios are generally consistent throughout the VOC volatility range (Figure 4A).
Are surfaces and/or the diverse range of other bulk indoor reservoirs a possible contributor to volatility-dependent observations, such as an IVOC source/reservoir with a relatively delayed response (to certain perturbations)?29,58,99 Observed gas-phase losses to Teflon chamber walls indicate significant partitioning to/from and mass loading (i.e., Cwall) onto surfaces (ranging 2–24 × 103 μg m−3 for C8–13 alkanes to aldehydes),100 which were several orders of magnitude higher than OA concentrations (COA). The surfaces and more retentive bulk materials frequently found in a home (e.g., carpeting, furnishings, painted drywall) are higher in surface area and less inert than Teflon. As found in the study by Algrim et al., Cwall values for painted tubes were on the order of 108 μg m−3—4–5 orders of magnitude higher than their FEP chamber counterparts. Determining the effective Cwall values of a greater variety of in-home surfaces and for a greater diversity of functionality represents an important area for future work.97 A timescale analysis (Section S6, Figure S11) suggests that while gas–particle partitioning would be rapid, especially for ultrafine particles, equilibrium timescales from bulk reservoirs or surfaces to the gas phase are longer but carry more uncertainty. Gas–surface partitioning timescales are dependent on compound volatility as well as reservoir chemical characteristics, while mass accommodation coefficients and penetration efficiencies of molecules through bulk reservoirs represent major uncertainties in predicting these timescales (Section S6).97
What are the potential effects of HVAC system operation on these observations? Could HVAC cycling in response to changing temperatures during the day (Figure S9) have acted as a volatility-dependent sink or affected the circulation of organic compounds through the residence? Similarly, other dynamic environmental factors (e.g., temperature, RH, solar radiation) during the humid summertime conditions at the unoccupied home (Section S7, Figure S9) may have also contributed to the observed changes, along with the potential role of aerosol perturbations/intrusions, which remains an area for future work.101
In summary, we applied a suite of sampling techniques and analytical instrumentation to speciate the complex mixture of gas- and particle-phase compounds present in an unoccupied indoor environment. An extensive targeted analysis of gas-phase organic compounds revealed greater concentrations indoors, with an overall reduction in mixing ratios when the ventilation was increased by the opening of windows. Enhanced ventilation increased indoor–outdoor emissions of organic compounds. During perturbation tests, we observed volatility-dependent behavior on VOCs-IVOCs that could be the result of environmental factors, which influence the distribution of organic compounds throughout the gas, particle, and surface phases. Our observations and data from this typical home and other indoor sites provide model inputs and points of comparison for future studies. The use of multiple analytical techniques enables greater coverage across the intermediate- to ultra-low volatility ranges and highlights the complex mixture of hydrocarbons and functionalized compounds in the gas and particle phase that should be considered in future studies and models of residences.
Supplementary Material
ACKNOWLEDGMENTS
This work benefits from contributions by Peeyush Khare (Yale University, Paul Scherrer Institute), who added to and refined the APCI Integration code, and Jenna Ditto (Yale University, University of Toronto), who developed the methodologies for filter extraction and the use of LC-ESI-TOF. The authors thank Audrey Dang (Washington University in St. Louis) for assistance with instrumentation at ACRONIM-2, Thomas Klüpfel (Max Planck Institute for Chemistry) and Cinestar Mainz for assistance with the Mainz movie theater sample collection, and Tony Muni for assistance with the southern CT study. Further thanks go to Kai Wang and Thorsten Hoffmann (Johannes Gutenberg-Universtät Mainz) for sharing outdoor aerosol composition data from Mainz, Hugo Destaillats (Lawrence Berkeley National Laboratory) for helpful discussions, and Manabu Shiraiwa (University of California, Irvine) for providing valuable advice on equilibrium timescale calculations. R.S. and D.R.G. acknowledge financial support from NSF and NSF GRFP (CBET-2011362, DGE1122492, and DGE1752134), including support to develop the analytical methods applied here (AGS-1764126). D.R.G. would like to acknowledge support from the Alexander von Humboldt Fellowship for funding his collaboration with MPIC that provided the indoor samples from the theater. B.J.W. recognizes support from NSF (CBET1554061). The authors also thank the Alfred P. Sloan Foundation (G-2018–11133 and G-2015–14134) for funding the home aerosol perturbation study and Connecticut indoor air measurements. B.J.W., A.E., and G.C.M. acknowledge support from the U.S. EPA’s Science to Achieve Results (STAR) program (83575101). D.R.G. appreciates support from the U.S. EPA. This publication was developed under Assistance Agreement RD835871 awarded by the U.S. EPA to Yale University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the EPA. EPA does not endorse any products or commercial services mentioned in this publication.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c01337.
Sample collection and data processing details; speciation comparisons across various experimental conditions (i.e., indoor vs outdoor, by ventilation condition); more volatility distribution detail for the aerosol-phase and across analytical methods; size distribution, temperature, and relative humidity data; a list of experimental trials; and various indoor/outdoor ratios and indoor-to-outdoor emission rates (in mg h−1, μg m−2 h−1, and ion abundance h−1) for an extensive list of 369 individual compounds (PDF)
Contributor Information
Roger Sheu, Department of Chemical and Environmental Engineering, Yale University, New Haven, Connecticut 06511, United States; Present Address: Department of Environmental Health and Engineering, Johns Hopkins University, 3400 N Charles St., Baltimore, Maryland 21218, United States.
Claire F. Fortenberry, Department of Energy, Environmental, & Chemical Engineering and Center for Aerosol Science and Engineering, Washington University in St. Louis, St. Louis, Missouri 63130, United States; Present Address: Universities Space Research Association and NASA Glenn Research Center, 21000 Brookpark Rd, Cleveland, Ohio 44135, United States.
Michael J. Walker, Department of Energy, Environmental, & Chemical Engineering and Center for Aerosol Science and Engineering, Washington University in St. Louis, St. Louis, Missouri 63130, United States.
Azin Eftekhari, Department of Environmental Sciences and Engineering, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27515, United States; Present Address: College of Medicine, University of South Alabama, 307 N University Blvd., Mobile, Alabama 36688, United States..
Christof Stönner, Department of Atmospheric Chemistry, Max Planck Institute for Chemistry, Mainz 55128, Germany.
Alexa Bakker, Department of Chemical and Environmental Engineering, Yale University, New Haven, Connecticut 06511, United States; Present Address: McGill University, 845 Sherbrooke St W, Montreal, Quebec H3A 0G4, Canada..
Jordan Peccia, Department of Chemical and Environmental Engineering, Yale University, New Haven, Connecticut 06511, United States.
Jonathan Williams, Department of Atmospheric Chemistry, Max Planck Institute for Chemistry, Mainz 55128, Germany.
Glenn C. Morrison, Department of Environmental Sciences and Engineering, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27515, United States.
Brent J. Williams, Department of Energy, Environmental, & Chemical Engineering and Center for Aerosol Science and Engineering, Washington University in St. Louis, St. Louis, Missouri 63130, United States
Drew R. Gentner, Department of Chemical and Environmental Engineering, Yale University, New Haven, Connecticut 06511, United States.
REFERENCES
- (1).The Use of Time. Daily Activities of Urban and Suburban Populations in Twelve Countries; Szalai A, Ed.; Mouton: The Hague, Paris, 1972. [Google Scholar]
- (2).Klepeis NE; Nelson WC; Ott WR; Robinson JP; Tsang AM; Switzer P; Behar JV; Hern SC; Engelmann WH The National Human Activity Pattern Survey (NHAPS): A Resource for Assessing Exposure to Environmental Pollutants. J. Exposure Anal. Environ. Epidemiol. 2001, 11, 231–252. [DOI] [PubMed] [Google Scholar]
- (3).Ditto JC; Barnes EB; Khare P; Takeuchi M; Joo T; Bui AAT; Lee-Taylor J; Eris G; Chen Y; Aumont B; Jimenez JL; Ng NL; Griffin RJ; Gentner DR An Omnipresent Diversity and Variability in the Chemical Composition of Atmospheric Functionalized Organic Aerosol. Commun. Chem. 2018, 1, 1–13. [Google Scholar]
- (4).Ditto JC; Joo T; Slade JH; Shepson PB; Ng NL; Gentner DR Nontargeted Tandem Mass Spectrometry Analysis Reveals Diversity and Variability in Aerosol Functional Groups across Multiple Sites, Seasons, and Times of Day. Environ. Sci. Technol. Lett. 2020, 7, 60–69. [Google Scholar]
- (5).Gentner D; Isaacman G Elucidating Secondary Organic Aerosol from Diesel and Gasoline Vehicles through Detailed Characterization of Organic Carbon Emissions. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 18318–18323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sheu R; Stönner C; Ditto JC; Klüpfel T; Williams J; Gentner DR Human Transport of Thirdhand Tobacco Smoke: A Prominent Source of Hazardous Air Pollutants into Indoor Nonsmoking Environments. Sci. Adv. 2020, 6, No. eaay4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Isaacman-Vanwertz G; Massoli P; O’Brien R; Lim C; Franklin JP; Moss JA; Hunter JF; Nowak JB; Canagaratna MR; Misztal PK; Arata C; Roscioli JR; Herndon ST; Onasch TB; Lambe AT; Jayne JT; Su L; Knopf DA; Goldstein AH; Worsnop DR; Kroll JH Chemical Evolution of Atmospheric Organic Carbon over Multiple Generations of Oxidation. Nat. Chem. 2018, 10, 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hunter JF; Day DA; Palm BB; Yatavelli RLN; Chan AWH; Kaser L; Cappellin L; Hayes PL; Cross ES; Carrasquillo AJ; Campuzano-Jost P; Stark H; Zhao Y; Hohaus T; Smith JN; Hansel A; Karl T; Goldstein AH; Guenther A; Worsnop DR; Thornton JA; Heald CL; Jimenez JL; Kroll JH Comprehensive Characterization of Atmospheric Organic Carbon at a Forested Site. Nat. Geosci. 2017, 10, 748–753. [Google Scholar]
- (9).Price DJ; Day DA; Pagonis D; Stark H; Algrim LB; Handschy AV; Liu S; Krechmer JE; Miller SL; Hunter JF; De Gouw JA; Ziemann PJ; Jimenez JL Budgets of Organic Carbon Composition and Oxidation in Indoor Air. Environ. Sci. Technol. 2019, 53, 13053–13063. [DOI] [PubMed] [Google Scholar]
- (10).Khare P; Gentner DR Considering the Future of Anthropogenic Gas-Phase Organic Compound Emissions and the Increasing Influence of Non-Combustion Sources on Urban Air Quality. Atmos. Chem. Phys. 2018, 18, 5391–5413. [Google Scholar]
- (11).McDonald BC; de Gouw JA; Gilman JB; Jathar SH; Akherati A; Cappa CD; Jimenez JL; Lee-Taylor J; Hayes PL; McKeen SA; Cui YY; Kim S-W; Gentner DR; Isaacman-VanWertz G; Goldstein AH; Harley RA; Frost GJ; Roberts JM; Ryerson TB; Trainer M Volatile Chemical Products Emerging as Largest Petrochemical Source of Urban Organic Emissions. Science 2018, 359, 760–764. [DOI] [PubMed] [Google Scholar]
- (12).Seltzer KM; Pennington E; Rao V; Murphy BN; Strum M; Isaacs KK; Pye HOT Reactive Organic Carbon Emissions from Volatile Chemical Products. Atmos. Chem. Phys. 2021, 21, 5079–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mohr C; DeCarlo PF; Heringa MF; Chirico R; Slowik JG; Richter R; Reche C; Alastuey A; Querol X; Seco R; Peñuelas J; Jimenez JL; Crippa M; Zimmermann R; Baltensperger U; Prév̂ot AS Identification and Quantification of Organic Aerosol from Cooking and Other Sources in Barcelona Using Aerosol Mass Spectrometer Data. Atmos. Chem. Phys. 2012, 12, 1649–1665. [Google Scholar]
- (14).Kristensen K; Lunderberg DM; Liu Y; Misztal PK; Tian Y; Arata C; Nazaroff WW; Goldstein AH Sources and Dynamics of Semivolatile Organic Compounds in a Single-family Residence in Northern California. Indoor Air 2019, 29, No. ina.12561. [DOI] [PubMed] [Google Scholar]
- (15).Klein F; Baltensperger U; Prévôt ASH; El Haddad I Quantification of the Impact of Cooking Processes on Indoor Concentrations of Volatile Organic Species and Primary and Secondary Organic Aerosols. Indoor Air 2019, 29, 926–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Nazaroff WW; Singer BC Inhalation of Hazardous Air Pollutants from Environmental Tobacco Smoke in US Residences. J. Exposure Anal. Environ. Epidemiol. 2004, 14, S71–S77. [DOI] [PubMed] [Google Scholar]
- (17).Singer BC; Hodgson AT; Nazaroff WW Gas-Phase Organics in Environmental Tobacco Smoke: 2. Exposure-Relevant Emission Factors and Indirect Exposures from Habitual Smoking. Atmos. Environ. 2003, 37, 5551–5561. [Google Scholar]
- (18).Poppendieck DG; Hubbard HF; Weschler CJ; Corsi RL Formation and Emissions of Carbonyls during and Following Gas-Phase Ozonation of Indoor Materials. Atmos. Environ. 2007, 41, 7614–7626. [Google Scholar]
- (19).Liu Y; Misztal PK; Xiong J; Tian Y; Arata C; Weber RJ; Nazaroff WW; Goldstein AH Characterizing Sources and Emissions of Volatile Organic Compounds in a Northern California Residence Using Space- and Time-Resolved Measurements. Indoor Air 2019, 29, 630–644. [DOI] [PubMed] [Google Scholar]
- (20).Salthammer T Emission of Volatile Organic Compounds from Furniture Coatings. Indoor Air 1997, 7, 189–197. [Google Scholar]
- (21).Weschler CJ; Hodgson AT; Wooley JD Indoor Chemistry: Ozone, Volatile Organic Compounds, and Carpets. Environ. Sci. Technol. 1992, 26, 2371–2377. [Google Scholar]
- (22).Hodgson AT; Wooley JD; Daisey JM Emissions of Volatile Organic Compounds from New Carpets Measured in a Large-Scale Environmental Chamber. Air Waste 1993, 43, 316–324. [DOI] [PubMed] [Google Scholar]
- (23).Destaillats H; Lunden MM; Singer BC; Coleman BK; Hodgson AT; Weschler CJ; Nazaroff WW Indoor Secondary Pollutants from Household Product Emissions in the Presence of Ozone: A Bench-Scale Chamber Study. Environ. Sci. Technol. 2006, 40, 4421–4428. [DOI] [PubMed] [Google Scholar]
- (24).Singer BC; Destaillats H; Hodgson AT; Nazaroff WW Cleaning Products and Air Fresheners: Emissions and Resulting Concentrations of Glycol Ethers and Terpenoids. Indoor Air 2006, 16, 179–191. [DOI] [PubMed] [Google Scholar]
- (25).Brown SK; Sim MR; Abramson MJ; Gray CN Concentrations of Volatile Organic Compounds in Indoor Air–a Review. Indoor Air 1994, 4, 123–134. [Google Scholar]
- (26).Logue JM; McKone TE; Sherman MH; Singer BC Hazard Assessment of Chemical Air Contaminants Measured in Residences. Indoor Air 2011, 21, 92–109. [DOI] [PubMed] [Google Scholar]
- (27).Jia C; Batterman S; Godwin C VOCs in Industrial, Urban and Suburban Neighborhoods, Part 1: Indoor and Outdoor Concentrations, Variation, and Risk Drivers. Atmos. Environ. 2008, 42, 2083–2100. [Google Scholar]
- (28).Weisel CP; Zhang J; Turpin BJ; Morandi MT; Colome S; Stock TH; Spektor DM; Korn L; Winer A; Alimokhtari S; Kwon J; Mohan K; Harrington R; Giovanetti R; Cui W; Afshar M; Maberti S; Shendell D Relationship of Indoor, Outdoor and Personal Air (RIOPA) Study: Study Design, Methods and Quality Assurance/Control Results. J. Exposure Anal. Environ. Epidemiol. 2005, 15, 123–137. [DOI] [PubMed] [Google Scholar]
- (29).Wang C; Collins DB; Arata C; Goldstein AH; Mattila JM; Farmer DK; Ampollini L; DeCarlo PF; Novoselac A; Vance ME; Nazaroff WW; Abbatt JPD Surface Reservoirs Dominate Dynamic Gas-Surface Partitioning of Many Indoor Air Constituents. Sci. Adv. 2020, 6, No. eaay8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Singer BC; Coleman BK; Destaillats H; Hodgson AT; Lunden MM; Weschler CJ; Nazaroff W W Indoor Secondary Pollutants from Cleaning Product and Air Freshener Use in the Presence of Ozone. Atmos. Environ. 2006, 40 (35), 6696–6710. [DOI] [PubMed] [Google Scholar]
- (31).Fortenberry C; Walker M; Dang A; Loka A; Date G; Cysneiros de Carvalho K; Morrison G; Williams B Analysis of Indoor Particles and Gases and Their Evolution with Natural Ventilation. Indoor Air 2019, 29, 761–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hodgson AT; Faulkner D; Sullivan DP; DiBartolomeo DL; Russell ML; Fisk WJ Effect of Outside Air Ventilation Rate on Volatile Organic Compound Concentrations in a Call Center. Atmos. Environ. 2003, 37, 5517–5527. [Google Scholar]
- (33).Brinke JT; Selvin S; Hodgson AT; Fisk WJ; Mendell MJ; Koshland CP; Daisey JM Development of New Volatile Organic Compound (VOC) Exposure Metrics and Their Relationship to “Sick Building Syndrome” Symptoms. Indoor Air 1998, 8, 140–152. [Google Scholar]
- (34).Allen JG; MacNaughton P; Satish U; Santanam S; Vallarino J; Spengler JD Associations of Cognitive Function Scores with Carbon Dioxide, Ventilation, and Volatile Organic Compound Exposures in Office Workers: A Controlled Exposure Study of Green and Conventional Office Environments. Environ. Health Perspect. 2016, 124, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sundell J; Levin H; Nazaroff WW; Cain WS; Fisk WJ; Grimsrud DT; Gyntelberg F; Li Y; Persily AK; Pickering AC; Samet JM; Spengler JD; Taylor ST; Weschler CJ Ventilation Rates and Health: Multidisciplinary Review of the Scientific Literature. Indoor Air 2011, 21, 191–204. [DOI] [PubMed] [Google Scholar]
- (36).Carrer P; Wargocki P; Fanetti A; Bischof W; De Oliveira Fernandes E; Hartmann T; Kephalopoulos S; Palkonen S; Seppänen O What Does the Scientific Literature Tell Us about the Ventilation–Health Relationship in Public and Residential Buildings? Build. Environ. 2015, 94, 273–286. [Google Scholar]
- (37).Salvador CM; Bekö G; Weschler CJ; Morrison G; Le Breton M; Hallquist M; Ekberg L; Langer S Indoor Ozone/Human Chemistry and Ventilation Strategies. Indoor Air 2019, 29, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).DeCarlo PF; Avery AM; Waring MS Thirdhand Smoke Uptake to Aerosol Particles in the Indoor Environment. Sci. Adv. 2018, 4, No. eaap8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Avery AM; Waring MS; DeCarlo PF Seasonal Variation in Aerosol Composition and Concentration upon Transport from the Outdoor to Indoor Environment. Environ. Sci.: Processes Impacts 2019, 21, 528–547. [DOI] [PubMed] [Google Scholar]
- (40).Abbatt JPDD; Wang C The Atmospheric Chemistry of Indoor Environments. Environ. Sci.: Processes Impacts 2020, 22, 25–48. [DOI] [PubMed] [Google Scholar]
- (41).Weschler CJ; Fong KL Characterization of Organic Species Associated with Indoor Aerosol Particles. Environ. Int. 1986, 12, 93–97. [Google Scholar]
- (42).Koop T; Bookhold J; Shiraiwa M; Pöschl U Glass Transition and Phase State of Organic Compounds: Dependency on Molecular Properties and Implications for Secondary Organic Aerosols in the Atmosphere. Phys. Chem. Chem. Phys. 2011, 13, 19238. [DOI] [PubMed] [Google Scholar]
- (43).Ye P; Zhao Y; Chuang WK; Robinson AL; Donahue NM Secondary Organic Aerosol Production from Pinanediol, a Semi-Volatile Surrogate for First-Generation Oxidation Products of Monoterpenes. Atmos. Chem. Phys. 2018, 18, 6171–6186. [Google Scholar]
- (44).Ditto JC; Joo T; Khare P; Sheu R; Takeuchi M; Chen Y; Xu W; Bui AATT; Sun Y; Ng NL; Gentner DR Effects of Molecular-Level Compositional Variability in Organic Aerosol on Phase State and Thermodynamic Mixing Behavior. Environ. Sci. Technol. 2019, 53, 13009–13018. [DOI] [PubMed] [Google Scholar]
- (45).Ye J; Gordon CA; Chan AWH Enhancement in Secondary Organic Aerosol Formation in the Presence of Preexisting Organic Particle. Environ. Sci. Technol. 2016, 50, 3572–3579. [DOI] [PubMed] [Google Scholar]
- (46).Jang M; Kamens RM Characterization of Secondary Aerosol from the Photooxidation of Toluene in the Presence of NO x and 1-Propene. Environ. Sci. Technol. 2001, 35, 3626–3639. [DOI] [PubMed] [Google Scholar]
- (47).Weber RJ; Guo H; Russell AG; Nenes A High Aerosol Acidity despite Declining Atmospheric Sulfate Concentrations over the Past 15 Years. Nat. Geosci. 2016, 9, 282–285. [Google Scholar]
- (48).Morrison G Interfacial Chemistry in Indoor Environments. Environ. Sci. Technol. 2008, 42, 3495–3499. [DOI] [PubMed] [Google Scholar]
- (49).Weschler CJ; Nazaroff WW Semivolatile Organic Compounds in Indoor Environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar]
- (50).Weschler CJ; Nazaroff WW SVOC Partitioning between the Gas Phase and Settled Dust Indoors. Atmos. Environ. 2010, 44, 3609–3620. [Google Scholar]
- (51).Pelletier M; Bonvallot N; Ramalho O; Mandin C; Wei W; Raffy G; Mercier F; Blanchard O; Le Bot B; Glorennec P Indoor Residential Exposure to Semivolatile Organic Compounds in France. Environ. Int. 2017, 109, 81–88. [DOI] [PubMed] [Google Scholar]
- (52).Eichler CMA; Cao J; Isaacman-VanWertz G; Little JC Modeling the Formation and Growth of Organic Films on Indoor Surfaces. Indoor Air 2019, 29, 17–29. [DOI] [PubMed] [Google Scholar]
- (53).Weschler CJ; Nazaroff WW Growth of Organic Films on Indoor Surfaces. Indoor Air 2017, 27, 1101–1112. [DOI] [PubMed] [Google Scholar]
- (54).Shiraiwa M; Seinfeld JH Equilibration Timescale of Atmospheric Secondary Organic Aerosol Partitioning. Geophys. Res. Lett. 2012, 39, 1–6. [Google Scholar]
- (55).Mai H; Shiraiwa M; Flagan RC; Seinfeld JH Under What Conditions Can Equilibrium Gas−Particle Partitioning Be Expected to Hold in the Atmosphere? Environ. Sci. Technol. 2015, 49, 11485–11491. [DOI] [PubMed] [Google Scholar]
- (56).Li Y; Shiraiwa M Timescales of Secondary Organic Aerosols to Reach Equilibrium at Various Temperatures and Relative Humidities. Atmos. Chem. Phys. 2019, 19, 5959–5971. [Google Scholar]
- (57).Liu C; Shi S; Weschler C; Zhao B; Zhang Y Analysis of the Dynamic Interaction Between SVOCs and Airborne Particles. Aerosol Sci. Technol. 2013, 47, 125–136. [Google Scholar]
- (58).Pagonis D; Price DJ; Algrim LB; Day DA; Handschy AV; Stark H; Miller SL; De Gouw J; Jimenez JL; Ziemann PJ Time-Resolved Measurements of Indoor Chemical Emissions, Deposition, and Reactions in a University Art Museum. Environ. Sci. Technol. 2019, 53, 4794–4802. [DOI] [PubMed] [Google Scholar]
- (59).Jimenez JL; Canagaratna MR; Donahue NM; Prevot ASH; Zhang Q; Kroll JH; DeCarlo PF; Allan JD; Coe H; Ng NL; Aiken AC; Docherty KS; Ulbrich IM; Grieshop AP; Robinson AL; Duplissy J; Smith JD; Wilson KR; Lanz VA; Hueglin C; Sun YL; Tian J; Laaksonen A; Raatikainen T; Rautiainen J; Vaattovaara P; Ehn M; Kulmala M; Tomlinson JM; Collins DR; Cubison MJ; Dunlea EJ; Huffman JA; Onasch TB; Alfarra MR; Williams PI; Bower K; Kondo Y; Schneider J; Drewnick F; Borrmann S; Weimer S; Demerjian K; Salcedo D; Cottrell L; Griffin R; Takami A; Miyoshi T; Hatakeyama S; Shimono A; Sun JY; Zhang YM; Dzepina K; Kimmel JR; Sueper D; Jayne JT; Herndon SC; Trimborn AM; Williams LR; Wood EC; Middlebrook AM; Kolb CE; Baltensperger U; Worsnop DR Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. [DOI] [PubMed] [Google Scholar]
- (60).Hodzic A; Jimenez JL; Madronich S; Canagaratna MR; Decarlo PF; Kleinman L; Fast J Modeling Organic Aerosols in a Megacity: Potential Contribution of Semi-Volatile and Intermediate Volatility Primary Organic Compounds to Secondary Organic Aerosol Formation. Atmos. Chem. Phys. 2010, 10, 5491–5514. [Google Scholar]
- (61).Donahue N; Robinson A; Pandis S Atmospheric Organic Particulate Matter: From Smoke to Secondary Organic Aerosol. Atmos. Environ. 2009, 43, 94–106. [Google Scholar]
- (62).Eftekhari A; Fortenberry CF; Williams BJ; Walker MJ; Dang A; Pfaff A; Ercal N; Morrison GC Continuous Measurement of Reactive Oxygen Species inside and Outside of a Residential House. Indoor Air 2020, 31, 1199–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Sheu R; Marcotte A; Khare P; Charan S; Ditto JC; Gentner DR Advances in Offline Approaches for Chemically Speciated Measurements of Trace Gas-Phase Organic Compounds via Adsorbent Tubes in an Integrated Sampling-to-Analysis System. J. Chromatogr. A 2018, 1575, 80–90. [DOI] [PubMed] [Google Scholar]
- (64).Bakker A; Siegel JA; Mendell MJ; Peccia J Building and Environmental Factors That Influence Bacterial and Fungal Loading on Air Conditioning Cooling Coils. Indoor Air 2018, 28, 689–696. [DOI] [PubMed] [Google Scholar]
- (65).Bakker A; Siegel JA; Mendell MJ; Prussin AJ; Marr LC; Peccia J Bacterial and Fungal Ecology on Air Conditioning Cooling Coils Is Influenced by Climate and Building Factors. Indoor Air 2020, 30, 326–334. [DOI] [PubMed] [Google Scholar]
- (66).Salthammer T; Mentese S; Marutzky R Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Khare P; Marcotte A; Sheu R; Walsh AN; Ditto JC; Gentner DR Advances in Offline Approaches for Trace Measurements of Complex Organic Compound Mixtures via Soft Ionization and High-Resolution Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1598, 163–174. [DOI] [PubMed] [Google Scholar]
- (68).Kanakidou M; Seinfeld JH; Pandis SN; Barnes I; Dentener FJ; Facchini MC; Van Dingenen R; Ervens B; Nenes A; Nielsen CJ; Swietlicki E; Putaud JP; Balkanski Y; Fuzzi S; Horth J; Moortgat GK; Winterhalter R; Myhre CEL; Tsigaridis K; Vignati E; Stephanou EG; Wilson J Organic Aerosol and Global Climate Modelling: A Review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar]
- (69).Gao S; Keywood M; Ng NL; Surratt J; Varutbangkul V; Bahreini R; Flagan RC; Seinfeld JH Low-Molecular-Weight and Oligomeric Components in Secondary Organic Aerosol from the Ozonolysis of Cycloalkenes and α-Pinene. J. Phys. Chem. A 2004, 108, 10147–10164. [Google Scholar]
- (70).Bouvier-Brown NC; Goldstein AH; Gilman JB; Kuster WC; De Gouw JA In-Situ Ambient Quantification of Monoterpenes, Sesquiterpenes and Related Oxygenated Compounds during BEARPEX 2007: Implications for Gas- And Particle-Phase Chemistry. Atmos. Chem. Phys. 2009, 9, 5505–5518. [Google Scholar]
- (71).Gomez Alvarez E; Amedro D; Afif C; Gligorovski S; Schoemaecker C; Fittschen C; Doussin J-F; Wortham H Unexpectedly High Indoor Hydroxyl Radical Concentrations Associated with Nitrous Acid. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 13294–13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Atkinson R; Arey J Gas-Phase Tropospheric Chemistry of Biogenic Volatile Organic Compounds: A Review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar]
- (73).Wisthaler A; Weschler CJ Reactions of Ozone with Human Skin Lipids : Sources of Carbonyls, Dicarbonyls, and Hydroxycarbonyls in Indoor Air. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 6568–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Pitts JN; Biermann HW; Tuazon EC; Green M; Long WD; Winer AM Time-Resolved Identification and Measurement of Indoor Air Pollutants by Spectroscopic Techniques: Gaseous Nitrous Acid, Methanol, Formaldehyde and Formic Acid. JAPCA 1989, 39, 1344–1347. [DOI] [PubMed] [Google Scholar]
- (75).Salthammer T Keynote : Indoor Air 2014 Very Volatile Organic Compounds : An Understudied Class of Indoor Air Pollutants. Indoor Air 2014, 26, 25–38. [DOI] [PubMed] [Google Scholar]
- (76).Singer BC; Delp WW; Black DR; Destaillats H; Walker IS Reducing In-Home Exposure to Air Pollution; Berkeley, CA; 2016. [Google Scholar]
- (77).Nazaroff WW; Weschler CJ Cleaning Products and Air Fresheners: Exposure to Primary and Secondary Air Pollutants. Atmos. Environ. 2004, 38, 2841–2865. [Google Scholar]
- (78).Tang X; Misztal PK; Nazaroff WW; Goldstein AH Siloxanes Are the Most Abundant Volatile Organic Compound Emitted from Engineering Students in a Classroom. Environ. Sci. Technol. Lett. 2015, 2, 303–307. [Google Scholar]
- (79).Choi H; Schmidbauer N; Spengler J; Bornehag C-G Sources of Propylene Glycol and Glycol Ethers in Air at Home. Int. J. Environ. Res. Public Health 2010, 7, 4213–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Kim JL; Elfman L; Mi Y; Wieslander G; Smedje G; Norbäck D Indoor Molds, Bacteria, Microbial Volatile Organic Compounds and Plasticizers in Schools? Associations with Asthma and Respiratory Symptoms in Pupils. Indoor Air 2007, 17, 153–163. [DOI] [PubMed] [Google Scholar]
- (81).Weschler CJ; Nazaroff WW Dermal Uptake of Organic Vapors Commonly Found in Indoor Air. Environ. Sci. Technol. 2014, 48, 1230–1237. [DOI] [PubMed] [Google Scholar]
- (82).Shah RU; Coggon MM; Gkatzelis GI; McDonald BC; Tasoglou A; Huber H; Gilman J; Warneke C; Robinson AL; Presto AA Urban Oxidation Flow Reactor Measurements Reveal Significant Secondary Organic Aerosol Contributions from Volatile Emissions of Emerging Importance. Environ. Sci. Technol. 2020, 54, 714–725. [DOI] [PubMed] [Google Scholar]
- (83).Rudel RA; Camann DE; Spengler JD; Korn LR; Brody JG Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [DOI] [PubMed] [Google Scholar]
- (84).Gkatzelis GI; Coggon MM; Mcdonald BC; Peischl J; Gilman JB; Aikin KC; Robinson MA; Canonaco F; Prevot ASH; Trainer M; Warneke C Observations Confirm That Volatile Chemical Products Are a Major Source of Petrochemical Emissions in U.S. Cities. Environ. Sci. Technol. 2021, 55, 4343. [DOI] [PubMed] [Google Scholar]
- (85).Gkatzelis GI; Coggon MM; McDonald BC; Peischl J; Aikin KC; Gilman JB; Trainer M; Warneke C Identifying Volatile Chemical Product Tracer Compounds in U.S. Cities. Environ. Sci. Technol. 2021, 55, 188–199. [DOI] [PubMed] [Google Scholar]
- (86).Stockwell CE; Coggon MM; Gkatzelis GI; Ortega J; McDonald BC; Peischl J; Aikin K; Gilman JB; Trainer M; Warneke C Volatile Organic Compound Emissions from Solventand Water-Borne Coatings – Compositional Differences and Tracer Compound Identifications. Atmos. Chem. Phys. 2021, 21, 6005–6022. [Google Scholar]
- (87).Huangfu Y; Lima NM; O’Keeffe PT; Kirk WM; Lamb BK; Walden VP; Jobson BT Whole-House Emission Rates and Loss Coefficients of Formaldehyde and Other Volatile Organic Compounds as a Function of the Air Change Rate. Environ. Sci. Technol. 2020, 54, 2143–2151. [DOI] [PubMed] [Google Scholar]
- (88).Hodgson AT; Rudd AF; Beal D; Chandra S Volatile Organic Compound Concentrations and Emission Rates in New Manufactured and Site-Built Houses. Indoor Air 2000, 10, 178–192. [DOI] [PubMed] [Google Scholar]
- (89).Offermann FJ; Hodgson AT In Emission Rates of Volatile Organic Compounds in New Homes, 12th International Conference on Indoor Air Quality and Climate, 2011; pp 177–182. [Google Scholar]
- (90).Karl T; Striednig M; Graus M; Hammerle A; Wohlfahrt G Urban Flux Measurements Reveal a Large Pool of Oxygenated Volatile Organic Compound Emissions. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Donahue NM; Robinson AL; Stanier CO; Pandis SN Coupled Partitioning, Dilution, and Chemical Aging of Semivolatile Organics. Environ. Sci. Technol. 2006, 40, 2635–2643. [DOI] [PubMed] [Google Scholar]
- (92).Robinson AL; Donahue NM; Shrivastava MK; Weitkamp EA; Sage AM; Grieshop AP; Lane TE; Pierce JR; Pandis SN Rethinking Organic Aerosols: Semivolatile Emissions and Photochemical Aging. Science 2007, 315, 1259–1262. [DOI] [PubMed] [Google Scholar]
- (93).Wang K; Zhang Y; Huang RJ; Cao J; Hoffmann T UHPLC-Orbitrap Mass Spectrometric Characterization of Organic Aerosol from a Central European City (Mainz, Germany) and a Chinese Megacity (Beijing). Atmos. Environ. 2018, 189, 22–29. [Google Scholar]
- (94).Li Y; Pöschl U; Shiraiwa M Molecular Corridors and Parameterizations of Volatility in the Chemical Evolution of Organic Aerosols. Atmos. Chem. Phys. 2016, 16, 3327–3344. [Google Scholar]
- (95).Donahue NM; Kroll JH; Pandis SN; Robinson AL A Two-Dimensional Volatility Basis Set-Part 2: Diagnostics of Organic-Aerosol Evolution. Atmos. Chem. Phys. 2012, 12, 615–634. [Google Scholar]
- (96).Schervish M; Donahue NM Peroxy Radical Chemistry and the Volatility Basis Set. Atmos. Chem. Phys. 2020, 20, 1183–1199. [Google Scholar]
- (97).Algrim LB; Pagonis D; de Gouw JA; Jimenez JL; Ziemann PJ Measurements and Modeling of Absorptive Partitioning of Volatile Organic Compounds to Painted Surfaces. Indoor Air 2020, 30, 745–756. [DOI] [PubMed] [Google Scholar]
- (98).Xiao H; Wania F Is Vapor Pressure or the Octanol-Air Partition Coefficient a Better Descriptor of the Partitioning between Gas Phase and Organic Matter? Atmos. Environ. 2003, 37, 2867–2878. [Google Scholar]
- (99).Pagonis D; Krechmer JE; De Gouw J; Jimenez JL; Ziemann PJ Effects of Gas-Wall Partitioning in Teflon Tubing and Instrumentation on Time-Resolved Measurements of Gas-Phase Organic Compounds. Atmos. Meas. Tech. 2017, 10, 4687–4696. [Google Scholar]
- (100).Matsunaga A; Ziemann PJ Gas-Wall Partitioning of Organic Compounds in a Teflon Film Chamber and Potential Effects on Reaction Product and Aerosol Yield Measurements. Aerosol Sci. Technol. 2010, 44, 881–892. [Google Scholar]
- (101).Duncan SM; Tomaz S; Morrison G; Webb M; Atkin J; Surratt JD; Turpin BJ Dynamics of Residential Water-Soluble Organic Gases: Insights into Sources and Sinks. Environ. Sci. Technol. 2019, 53, 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


