Abstract
For more than 2 years, health care systems have been floundering in a massive crisis of coronavirus disease 2019 (COVID-19) pandemic. While acute respiratory distress syndrome is the main complication in patients with COVID-19, as the pandemic continues, more data about the nonrespiratory effects of the coronavirus is obtained, including developing Coagulopathy-related manifestations, in the form of venous and arterial thromboembolism. Although arterial thrombosis a rare complication of this disease, it proves to be an effective factor in the mortality and morbidity of COVID-19 patients. The pathophysiology of thrombosis reveals a complex relation between hemostasis and immune system that can be disrupted by COVID-19. Thrombectomy, anticoagulant therapy, and thrombolysis are the main treatments in these patients. In addition, appropriate thromboprophylaxis treatment should be considered in COVID-19 patients. In this article, we have successfully reviewed the arterial thrombotic events in patients reported around the world, including the diagnostic and management method of choice.
Introduction
Emerging in December 2019, Coronavirus Disease 19 (COVID-19) has been declared as a pandemic by WHO; reporting 198,778,175 confirmed cases and 4,235,559 deaths by August 4 2021.1 COVID-19 shows a wide spectrum of clinical presentation and continues to intrigue medical professionals with its variety of symptoms ranging from mostly asymptomatic to fatal conditions following multi-organ involvement mainly involving respiratory system.2 Prior outbursts of coronaviruses, (ie, severe acute respiratory syndrome coronavirus 1 [SARS-CoV-1] and Middle-Eastern respiratory syndrome [MERS]) have shown a significant association with increased risk of thrombosis.3 Likewise, COVID-19 appears to generate a prothrombotic state as evidenced by a surge in global pulmonary embolisms, deep vein thrombosis, cardiac thrombosis, catheter related thrombosis, and arterial thrombosis. Moreover, laboratory tests of COVID-19 patients demonstrate the prothrombic state of patients; including increased level of D-dimer, fibrinogen, factor VIII, and von Willebrand factor.4 Although there has been an extensive focus on deep vein thrombosis and pulmonary embolism as the complications of the COVID-19, there is limited number of studies in fields of peripheral arterial thrombosis. Furthermore, the studies performed on arterial thrombotic events are majorly along with the studies of venous thromboembolism, which decreases the focus on diagnostic and treatment options for this complication. We aim to investigate the literature focusing on arterial thrombotic complications, including the location, method of localization and diagnosis, and management procedure.
Methods
In order to achieve an accelerated qualitative analysis, we performed PubMed search using MeSH (medical subject headings) terms in search strategies mentioned below:
(1 "COVID-19″[Mesh] OR "SARS-CoV-2″[Mesh], (2) "Arteries"[Mesh], "Thrombosis"[Mesh], and "Blood Coagulation"[Mesh], (3) combination and snowballing of (1) and (2). We select the relevant articles to arterial thrombotic events for the purpose of this review. In order to decrease the level of bias, data were extracted independently by FY and EG in a double data extraction method, followed by a thorough reassessment of included articles by AR and IK.
Pathophysiology
COVID-19 may lead to an increased risk of thrombotic events through various pathophysiological means (Fig 1 ):
-
1.
Disseminated intravascular coagulation: Disseminated intravascular coagulation is commonly observed in critically ill patients. Generally, it elicits the initiation of the tissue factor pathway of the coagulation cascade and deposition of platelet-fibrin thrombi in the microvasculature. This ultimately consummates the platelets and the procoagulant factors, resulting in a correlated bleeding diathesis.5
-
2.
Inflammatory cytokines: Excessive cytokine release is assumed to be the cause of the severe illness noted in adolescent patients without prior comorbidities. Higher serum levels of several inflammatory cytokines and chemokines have been related to severe illness and death in several studies.6
-
3.
Macrophage activation syndrome (MAS): MAS may contribute to the aspects of the cytokine storm and hypercoagulopathy observed in COVID-19 patients. MAS occur when activated antigen presenting cells cannot be lysed by CD8+ T cells or natural killer cells. After an initial inflammatory trigger, elevated IL-6 has been shown to diminish natural killer cells cytolytic capacity. Hence, there is a substantial interaction among innate and adaptive immune system that additionally promotes cytokine storm, phagocytosis, and multiorgan dysfunction.7
-
4.
Complement system activation: Complement system activation may also recruit and activate leukocytes, resulting in significant release of the proinflammatory cytokines (ie, IL-1, IL-6, IL-8, and interferon-γ) and promoting microvascular damage. Complement system is strongly activated in sepsis and inhibiting the complement cascade can improve coagulopathy and endothelial dysfunction in animal models with sepsis.8
-
5.
Renin angiotensin system (RAS) overactivation: Angiotensin-converting enzyme 2 (ACE2) is a membrane-bound protein mainly distributed in the lungs, heart, arteries, and veins.9 Angiotensin II(Ang II), the product of ACE2, promotes vasoconstriction, proinflammation, and prothrombotic effects via the Angiotensin II receptor type I (AT1R) and Angiotensin II receptor type IV (AT4R). ACE2 inhibits the RAS activity in 2 ways. First, ACE2 degrades Ang I and Ang II, reducing the substrate for activation of AT1R via the classical RAS. Secondly, Ang II is directly degraded into Angiotensin-(1-7), a vasodilatory peptide with anti-inflammatory effects. COVID-19 uses ACE2 for cellular entry. Through this process, it is assumed that the downregulation of pulmonary of ACE2 is followed by moving the balance towards proinflammatory and prothrombotic effects of Ang II and AT1R.10
-
6.
Hypoxia: Hypoxia-inducible factors can activate platelets and coagulation factors, causing an increased expression of tissue factor and plasminogen activator inhibitor 1, meanwhile inhibiting the endogenous anticoagulant protein leading to worsening of the hypercoagulable state.11
FIG 1.
Mechanisms of thrombosis in Covid-19.
Review of Cases
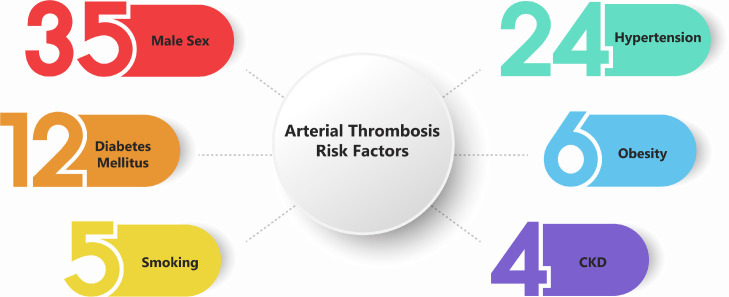
We reviewed 46 patients ranging from 24 to 83 years old with average and median of 61.4 and 61 years old, respectively. Covid 19 infection was confirmed in all patients included in the study by using standard methods that are acceptable worldwide; including polymerase chain reaction, chest X ray, chest computer tomography scan (CT), and serological tests. Details of each case are provided in Table 1 . Male sex, smoking, hypertension, diabetes, chronic kidney disease, and obesity are corelated with preexisting endothelial dysfunction which is a risk factor for thrombosis.12 , 13 We illustrated these risk factors in Figure 2 . We divided these thromboses to 5 groups based on location: 1- head and neck, 2- aorta, 3- abdomen, 4- upper limb, and 5- lower limb. Each group includes several arteries which is illustrated bellow:
TABLE 1.
Review of literature on Covid-19 peripheral artery thrombosis, and what kind of diagnosis modality and management method was chosen.
| Age/Sex | Medical history | Sign and symptoms related to thrombosis | Localization of thrombosis (arteries) | Timing of thrombosis | Diagnosis of thrombosis | Complications | treatments | Outcome and follow up | |
|---|---|---|---|---|---|---|---|---|---|
| Case 118 | 60, M | HTN, DLP, CAD, COPD | Painless vision loss in right eye | Right central retinal | 12 days after covid test + | assessment of intraocular pressure | None | NR | NR |
| Case 219 | 59, M | HTN, hyperuricemia, sickle cell anemia | Painless vision loss in left eye | left central retinal | 14 days after covid symptoms | Optical coherence tomography | None | NR | NR |
| Case 320 | 48, M | Obese, sleep apnea | Painless vision loss in right eye | Right ophthalmic | 38 days after covid test + | Funduscopic examination | DVT | LMWH | Discharged |
| Case 421 | 61, M | HTN, asthma | left hand weakness and numbness | right common carotid | 14 days after covid symptoms | CTA | None | Endarterectomy, UFH, LMWH | Discharged |
| Case 522 | 76, M | HTN, DLP, DM | Right sided loss of strength, aphasia, hemiplegia | Left internal carotid, multiple thrombosis in ascending aorta | 28 days after covid symptoms | CTA | None | LMWH | Discharged |
| Case 623 | 76, F | HTN, DLP, psoriasis | None | Aortic arch, left common carotid | 15 days after covid symptoms | CTA | Cerebral infraction | UFH | Discharged |
| Case 724 | 56, NR | NR | Abdominal pain, vomiting | Right middle cerebral, aortic arch, superior mesenteric | Same day as covid test + | CT | None | Thrombectomy, resection of small intestine | NR |
| Case 825 | 69, M | Stroke, ET, HTN | Accidentally found | Aortic arch, descending thoracic aorta | 14 days after covid symptoms | CTA | PE | Medical treatments* | NR |
| Case 926 | 59, M | Schizophrenia, epilepsy, PAD | Mottled skin of lower limb extending to sub umbilical | Midaorta | 9 days after covid symptoms | ultrasound | DVT | Norepinephrine, thrombolysis, UFH | Expired |
| Case 1023 | 69, M | None | Accidentally found | Descending thoracic aorta | 15 days after covid symptoms | CTA | PE | anticoagulant | Discharged |
| Case 1127 | 53, F | None | Dyspnea | Aortic arch | 10 days after covid symptoms | CT | PE | UFH, thrombolysis, argatroban | NR |
| Case 1222 | 78, M | DLP, urothelial carcinoma | None | Aortic arch, descending aorta | 9 days after covid symptoms | CTA | Multiple PE | LMWH | Expired |
| Case 1322 | 64, M | Former smoker, HTN, obstructive sleep apnea, hepatitis B, obese | None | Multiple thrombosis in Descending aorta | 11 days after covid symptoms | CT | None | UFH bridged to LMWH | NR |
| Case 1425 | 58, F | HTN, DM | Accidentally found | Descending aorta | Same day as covid test + | CTA | Non | Medical treatment* | NR |
| Case 1528 | 61, F | DM2, HTN, DLP, GERD, and bipolar disorder | Abdominal pain | Thoracic aorta, abdominal aorta | 14 days after covid symptoms | CTA | RV thrombosis | thrombolysis, LMWH bridged to rivaroxaban | Discharged |
| Case 1629 | 49, M | DM, CAD, obese | Pain and loss of heat in right lower limb | Descending aorta, right femoral | 40 days after covid symptoms | CT | none | Thrombectomy, fasciotomy, right below knee amputation | Discharged |
| Case 1730 | 71, M | None | Left iliac fossa and flank pain | Ascending aorta, left renal | 23 days after covid symptoms | CTA | None | UFH bridged to apixaban, clopidogrel | Discharged |
| Case 1831 | 83, F | HTN, DM2 | Cyanotic and pale lower limbs, distended abdomen | Descending thoracic aorta, abdominal aorta, iliac, superior mesenteric, renal | Same day as covid test + | CT | PE | Comfort measures | NR |
| Case 1932 | 75, M | None | Abdominal pain, vomiting | Descending thoracic aorta, superior mesenteric | 16 days after covid symptoms | CTA | None | thrombolysis, resection of small intestine | NR |
| Case 2033 | 56, M | DM2, HTN, obese | Weakness and hypoesthesia in left lower limb | Aortic arch, aortoiliac, deep femoral, bilateral popliteal | Same day as covid test + | CT, angiography | Recurrent thrombosis | UFH, thrombectomy | Still admitted in ICU |
| Case 2123 | 50, M | None | NR | Aortoiliac | 12 days after covid symptoms | CTA | DVT, cerebellar infarction | Bilateral thrombectomy | Discharged |
| Case 2223 | 67, M | HTN | pain, coldness, and paleness in lower limbs | Aortoiliac | 17 days after covid symptoms | CTA | None | Bilateral thrombectomy, limb amputation | Expired |
| Case 2334 | 73, M | HTN, smoker | Bilateral hip and buttock pain, lower limb paresthesia and paralysis | Aortoiliac, common deep and superficial femoral, bilateral popliteal, tibial | Same day as covid test + | CT | Thrombosis at aortic aneurysm site | thrombolysis, thrombectomy, UFH | expired |
| Case 2432 | 60, M | NR | Weakness, hypoesthesia, and ischemia in lower limbs | Aortoiliac | 14 days after covid symptoms | CTA | None | Thrombectomy | NR |
| Case 2535 | 70, F | HTN, DM2 | cold, pulseless, mottled, and pale left limb | Abdominal aorta, left common iliac, internal iliac, external iliac, popliteal | Same day as covid test + | CTA | Splenic vein, SMV and IMV thrombosis | Thrombectomy, thrombolysis, UFH bridged to warfarin | Discharged |
| Case 2629 | 70, M | HTN, smoker, DLP | Abdominal and lower limbs pain | Abdominal aorta | 29 days after covid symptoms | CT | None | thrombectomy | Expired |
| Case 2736 | 46, M | None | Abdominal pain, diarrhea | right renal branches | 1 day after covid symptoms | CT | None | UFH bridged to LMWH | Discharged |
| Case 2837 | 82, F | atrial fibrillation for over 4 years, CKD, HTN | abdominal pain and distention | Superior mesenteric | 3 days after covid test + | CT | None | None | Expired during preparation for surgery |
| Case 2938 | 45, M | None | abdominal pain, vomiting | Superior mesenteric | 5 days after covid symptoms | CTA | SMV thrombosis | UFH, thrombectomy, resection of small intestine | NR |
| Case 3039 | 79, F | None | Epigastric pain, diarrhea | Superior mesenteric, jejunal | 8 days after covid symptoms | CT | Portal vein thrombosis | resection of small intestine and right colon, thrombolysis, thrombectomy | Expired |
| Case 3140 | 52, M | NR | Diarrhea, vomiting, abdominal pain | superior mesenteric | 23 days after covid symptoms | CT | None | resection of small intestine | Discharged |
| Case 3241 | 69, M | NR | epigastric pain, constipation, eructation | superior mesenteric, ileocolic branches | NR | CTA | None | resection of small intestine, thrombectomy | Discharged |
| Case 3342 | 61, F | DM, HTN | abdominal pain with distention, vomiting | distal superior mesenteric | 4 days after covid test + | CT | None | UFH, ecosprin, clopidogrel, resection of small intestine | Expired |
| Case 3443 | 44, M | Uncontrolled DM | pain and paresthesia in right upper limb, hypoesthesia in fingers, gangrene of distal arm, forearm, and hand | Right axillary | 13 days after covid symptoms | CTA, ultrasound | Recurrent thrombosis | First time: thrombectomy Second time: steroids, LMWH, antibiotics, right limb amputation above elbow | Discharged |
| Case 3544 | 50, F | HTN, DLP, DM1, CKD, MGUS | Swelling and ischemic signs in the right upper limb | Distal radial | 6 days after covid test + | CTA | None | heparin, iloprost, Amlodipine Forearm amputation | NR |
| Case 3645 | 71, M | DM | Severe pain in right upper limb | right brachiocephalic, subclavian, axillary, brachial, radial, ulnar | More than 15 days after covid symptoms | ultrasound, CTA | None | UFH bridged to LMWH, thrombectomy, endarterectomy | Expired |
| Case 3746 | 68, M | HTN, ESRD | Cold and mottled right upper limb | Right brachial, radial, ulnar | 1 day after covid test + | NR | None | thrombectomy, heparin | Expired |
| Case 3847 | 46, M | HTN, DM | Left sided weakness mainly in lower limb, right upper limb pain, weakness, cyanosis, and coolness, fourth right finger necrosis | Brachial, radial, ulnar | NR | CTA | None | Anticoagulant, limb heating, antibiotics, corticosteroids | Discharged |
| Case 3948 | 31, M | CF, bilateral lung transplantation, chronic lung allograft dysfunction, SVC syndrome | Painful and cold limbs, loss of motricity, and sensitivity on the right side | left internal iliac, Common femoral | 39 days after covid symptoms | CTA | LV thrombosis | thrombectomy, LMWH bridged to vitamin K antagonist, aspirin | Discharged |
| Case 4025 | 82, M | AF, CKD, HTN, PAOD | Ischemia of right lower limb | Right iliac, right femoral, left deep femoral | Right 15 and left 18 days after covid symptoms | CTA, ultrasound | None | Medical treatment*, thrombectomy, amputation | NR |
| Case 4149 | 70, M | lung cancer surgery 4 years ago | ecchymosis of the right lower limb, bluish-purple swelling, and pain to palpation of the lower limb | right femoral, superficial femoral | 23 days after covid symptoms | ultrasound | None | LMWH, lower right extremity amputation | Expired |
| Case 4225 | 59, M | HTN, COPD, smoker, obese, flutter | Ischemia of the right lower limb | Left common femoral | Same day as covid test + | CTA | None | Medical treatments* | NR |
| Case 4325 | 64, M | HTN, PAOD, former smoker | Ischemia of the right lower limb | Right femoropopliteal | Same day as covid test + | CTA, ultrasound | None | Medical treatments*, amputation | NR |
| Case 4450 | 24, M | NR | right lower limb pain, intermittent claudication | right common femoral, profunda femoral, tibial posterior, popliteal | Few days after covid symptoms | ultrasound | None | LMWH, aspirin, thrombectomy | Discharged |
| Case 4525 | 71, M | HTN, DVT, obese, homozygous factor V Leiden mutation | Ischemia of the right lower limb | Right popliteal | 4 days after covid symptoms | Ultrasound | DVT | Medical treatments* | NR |
| Case 4651 | 40, M | NR | Left lower limb pain | Right popliteal | NR | Ultrasound | None | NR | NR |
AF, atrial fibrillation; CAD, coronary artery disease; CF, cystic fibrosis; CKD, chronic kidney disease; COPD; chronic obstructive pulmonary disease; CT, computer tomographic; CTA, computer tomographic angiogram; DVT, deep venous thrombosis; DLP, dyslipidemia; DM, diabetes mellitus; ESRD, end stage renal disease; ET, essential thrombocytopenia; F, female; GERD, gastroesophageal reflux disease; HTN, hypertension; IMV, inferior mesenteric vein; LMWH, low molecular weight heparin; LV, left ventricle; M, male, MGUS, monoclonal gammopathy of undetermined significance; NR, not reported; PAD, peripheral arterial disease; PAOD, peripheral arterial occlusive disease; PE, pulmonary embolism; RV, right ventricle; SVC, superior vena cava; SMV, superior mesenteric vein; UFH, unfractionated heparin.
Medical treatments: exact treatment was not mentioned in the original literature.
FIG 2.
Risk factors of arterial thrombosis in Covid-19. The numbers represent the count of each risk factor associated with 46 cases.
Head and Neck
We reviewed 7 cases of thrombosis in central retinal artery, ophthalmic artery, internal carotid artery, common carotid, and middle cerebral artery. CT-angiography (CTA) was used as the diagnostic method for 3 patients, CT for 1 patient, and other methods in 3 patients. For treatment, low molecular weight heparin (LMWH) was given in 3 patients, unfractionated heparin (UFH) in 2 patients, and thrombectomy and endarterectomy were performed each in 1 patient.
Thoracic Aorta
We observed 16 cases of arterial thrombosis localized in the thoracic Aorta as a result of covid-19 infection; Of which ascending aorta (n = 2), aortic arch (n = 6), and descending aorta (n = 8) were specifically mentioned as the region of involvement. Two studies did not mention the exact region of the thrombosis. CTA was the method of choice for diagnosis in 9 patients, CT in 6, angiography in 1, and ultrasound in 1 patient. For treatment purposes UFH was given in 6 patients, LMWH in 4, thrombolysis in 4, and thrombectomy in 3. Other treatments that were not commonly used include norepinephrine, clopidogrel, direct oral anticoagulants, and argatroban. Two case reports did not accomplish to address the exact treatment and management method.
Abdomen
We reviewed 20 cases of abdominal thrombosis occurring in abdominal aorta, superior mesenteric, jejunal, ileocolic branch arteries, and renal arteries. The most common diagnostic method included CT (n = 10) and CTA (n = 9), followed by angiography (n = 1) and ultrasound (n = 1). Common treatment choices consisted of thrombectomy (n = 10), resection (n = 7), UFH administration (n = 7), thrombolysis (n = 5), clopidogrel (n = 2), and LMWH administration (n = 2). Less frequent treatments were apixaban, warfarin, vit K antagonist, aspirin, and ecosprin.
Upper Limb
We reviewed 5 patients of the upper limb thrombosis in brachiocephalic, subclavian, axillary, brachial, radial, ulnar as a result of covid-19 infection. The diagnostic method was CTA alone (n = 2) or CTA and Doppler-ultrasound (n = 2). UFH (n = 3) and LMWH (n = 2) were administered to the patients for treatment. Thrombectomy was performed in 3 patients. Amputation was carried out in 2 patients.
Lower Limb
We reviewed 16 cases of lower limb thrombosis in common iliac, internal, and external iliac, deep, and superficial femoral, popliteal, tibial, and posterior tibial as a result of covid-19 infection. Diagnostic methods included CTA (n = 8), ultrasound (n = 6), CT (n = 4), and angiography (n = 1). For management, thrombectomy (n = 9) and amputation (n = 6) were performed. The remaining patients underwent medical treatment with LMWH (n = 3), UFH (n = 2), thrombolysis (n = 1), and aspirin (n = 1).
Concomitant Venous Thrombosis and Pulmonary Embolism
Overall, there were 4 patients with pulmonary embolism and 8 patients with venous thrombotic events classified in 4 patients with deep vein thrombosis, 3 with inferior/superior mesenteric vein and 1 with portal vein thrombosis. there were also 2 patients with cardiac thrombosis with thrombi located in the ventricles.
Discussion and Conclusions
Thrombotic events are frequently seen in COVID-19 and contribute to poorer outcome.7 As for the other reviews have frequently discussed pulmonary embolism, vein thrombosis and cardiovascular thrombotic events, the purpose of this review is to fill the gap in the literature about arterial thrombosis along with future perspectives regarding diagnostic and therapeutic methods. Here we summarized 46 cases of arterial thrombosis categorized into 5 groups based on location of event.
Cheruiyot et al14 reported the majority of the COVID-19 patients with arterial thrombosis have been male patients, and the median age has been 50 years old. However, due to the fact that venous and arterial involvement has not been separated in this literature, the data reported for arterial involvement have been missing the age-sex information. The other possible pitfall in this field is that the prevalence of COVID-19 infection is more in male population and the median age of infection is 50 years old.3 Hence, it should be further investigated whether the male sex and age is indeed a risk factor for arterial thrombosis, or the predominance of arterial thrombosis in male patients in certain age group is merely a result of age-sex prevalence of COVID-19 infected patients.
Comparing the venous thromboembolism, arterial thrombosis is an uncommon event. Di Minno et al15 reported the incidence of venous thromboembolism 24.3-39.2% in a meta-analysis, whereas the incidence of arterial thrombosis is 4.4%.14 Higher D-dimer value is mentioned as a risk factor of both venous and arterial thromboembolic events.15 , 16 Both asymptomatic and symptomatic venous thrombosis show high levels of D-dimer. Contrarily, there are limitations in studying d-Dimer level in asymptomatic arterial thrombosis, as a result of lower prevalence rate of arterial thrombosis in COVID-19 patients.
Most frequently used diagnostic methods were CTA, CT, and ultrasound. CTA was the most used diagnostic method with 24 times in all discussed categories, followed by CT (n = 13), and ultrasound (n = 9). The therapeutic method of choice was thrombectomy with total of 18 times. In the next place UFH with 13 and LMWH with 12 times were used. Additionally, amputation or resection were performed 14 times and thrombolysis therapy 7 times.
Future Therapeutic Targets and Areas of Research
Elevated levels of D-dimer were seen at least in 31 patients. Previously collected data clearly suggests that an elevated D-dimer, and presence of coagulopathy, indicates a poorer prognosis in COVID-19 patients.2 , 17 Heightened clinical vigilances and observation of D-dimer levels is needed in hospitalized covid-19 patients, although the decision-making process should be case-based for every individual. Thrombosis is a major complication in COVID-19 patients with optimal thromboprophylaxis being unknown. At least 14 patients were given anticoagulant as prophylaxis which was proven inadequate and highlights the necessity of novel or additional therapeutic approaches.
Footnotes
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization website 2021 [Available from: https://covid19.who.int/.
- 2.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. Journal of Thrombosis and Haemostasis. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller M.C., Meijers J.C., Vroom M.B., Juffermans N.P. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Critical Care. 2014;18(1):R30. doi: 10.1186/cc13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Li R., Wang J., Jiang Q., Gao C., Yang J., et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infectious Diseases. 2020;20(1) doi: 10.1186/s12879-020-05242-w. 4:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am J Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupu F., Keshari R.S., Lambris J.D., Coggeshall K.M. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133(Suppl 1(0 1)):S28–S31. doi: 10.1016/j.thromres.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., Van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 12.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shashar M., Francis J., Chitalia V. Thrombosis in the uremic milieu–emerging role of "thrombolome". Seminars in dialysis. 2015;28(2):198–205. doi: 10.1111/sdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheruiyot I., Kipkorir V., Ngure B., Misiani M., Munguti J., Ogeng'o J. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Annals of vascular surgery. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Minno A., Ambrosino P., Calcaterra I., Di Minno M.N.D., editors. Seminars in thrombosis and hemostasis. Thieme Medical Publishers; 2020. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. Journal of Thrombosis and Haemostasis. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya S., Diamond M., Anwar S., Glaser A., Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867. doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montesel A., Bucolo C., Mouvet V., Moret E., Eandi C.M. Case Report: Central Retinal Artery Occlusion in a COVID-19 Patient. Frontiers in pharmacology. 2020;11 doi: 10.3389/fphar.2020.588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumitrascu O.M., Volod O., Bose S., Wang Y., Biousse V., Lyden P.D. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. Journal of Stroke and Cerebrovascular Diseases. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer-Perez S., Alfayate-García J., Vicente-Jiménez S., Ruiz-Muñoz M., Dhimes-Tejada F.P., Gutiérrez-Baz M., et al. Symptomatic Common Carotid Free-Floating Thrombus in a COVID-19 Patient, Case Report and Literature Review. Annals of Vascular Surgery. 2021;73:122–128. doi: 10.1016/j.avsg.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Carranza M., Salazar D.-E., Troya J., Alcázar R., Peña C., Aragón E., et al. Aortic thrombus in patients with severe COVID-19: review of three cases. Journal of Thrombosis and Thrombolysis. 2021;51(1):237–242. doi: 10.1007/s11239-020-02219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Arbelaez D., Ibarra-Sanchez G., Garcia-Gutierrez A., Comanges-Yeboles A., Ansuategui-Vicente M., Gonzalez-Fajardo J.A. COVID-19-Related Aortic Thrombosis: A Report of Four Cases. Annals of Vascular Surgery. 2020;67:10–13. doi: 10.1016/j.avsg.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azouz E., Yang S., Monnier-Cholley L., Arrivé L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Medicine. 2020;46(7):1464–1465. doi: 10.1007/s00134-020-06079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashi M., Jacquin A., Dakhil B., Zaimi R., Mahé E., Tella E., et al. Severe arterial thrombosis associated with Covid-19 infection. Thrombosis research. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minalyan A., Thelmo F.L., Chan V., Tzarnas S., Ahmed F. Severe acute respiratory syndrome coronavirus 2-induced acute aortic occlusion: a case report. Journal of Medical Case Reports. 2021;15(1):2–6. doi: 10.1186/s13256-021-02692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandotra P., Supariwala A., Selim S., Garra G., Gruberg L. Aortic Arch Thrombus and Pulmonary Embolism in a COVID-19 Patient. The Journal of Emergency Medicine. 2021;60(2):223–225. doi: 10.1016/j.jemermed.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afari H., Tefera L., Rosovsky R.P. Case of right ventricular and aortic thrombi in a patient with severe COVID-19. BMJ Case Reports. 2021;14(4) doi: 10.1136/bcr-2020-240745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borulu F., Erkut B. Severe Aortic Thrombosis in the Early Period after COVID-19: Two Cases. Ann Vasc Surg. 2021;73:114–118. doi: 10.1016/j.avsg.2021.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee A., Ghosh R., Furment M.M. Case Report: COVID-19 Associated Renal Infarction and Ascending Aortic Thrombosis. The American Journal of Tropical Medicine and Hygiene. 2020;103(5):1989–1992. doi: 10.4269/ajtmh.20-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katchanov J., Kalisch J., Herzing W., Knorr F., Havla M., Klink T., et al. Extensive Aortic Thrombosis in a Patient With COVID-19. Annals of Emergency Medicine. 2020;76(3):373–374. doi: 10.1016/j.annemergmed.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vulliamy P., Jacob S., Davenport R.A. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. British Journal of Haematology. 2020;189(6):1053–1054. doi: 10.1111/bjh.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naudin I., Long A., Michel C., Devigne B., Millon A., Della-Schiava N. Acute aortoiliac occlusion in a patient with novel coronavirus disease-2019. Journal of Vascular Surgery. 2021;73(1):18–21. doi: 10.1016/j.jvs.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel P., Yu Y., Zia S., Padberg F., Curi M., Huang J. Systemic Thrombolysis as Initial Treatment of COVID-19 Associated Acute Aortoiliac and Lower Extremity Arterial Thrombosis. Annals of Vascular Surgery. 2021;70:297–301. doi: 10.1016/j.avsg.2020.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merz L.E., Sinnenberg L., Gerhard-Herman M.D., Vaduganathan M. Extensive Arterial Thrombosis in Covid-19. The American journal of cardiology. 2020;134:148–149. doi: 10.1016/j.amjcard.2020.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varner K.B., Cox E.J. COVID-19 as the cause of thrombosis: recognising COVID-19 infection in apparently asymptomatic patients. BMJ Case Reports. 2021;14(1) doi: 10.1136/bcr-2020-241027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Superior Mesenteric Artery Thrombosis in a Patient with COVID-19: A Unique Presentation. Journal of the College of Physicians and Surgeons Pakistan. 2020;30(2):112–114. doi: 10.29271/jcpsp.2020.supp2.112. [DOI] [PubMed] [Google Scholar]
- 38.Amaravathi U., Balamurugan N., Muthu Pillai V., Ayyan S.M. Superior Mesenteric Arterial and Venous Thrombosis in COVID-19. The Journal of emergency medicine. 2021;60(5):e103–e1e7. doi: 10.1016/j.jemermed.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivier de Barry A.M., Caroline Diffre, Martin Seror, Mostafa El Hajjam, Robert-Yves Carlier. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. 2020;2-6. [DOI] [PMC free article] [PubMed]
- 40.Lia A.B., Carlotta P., Sara P., Simone F., Antonio C. Arterial Mesenteric Thrombosis as a Complication of SARS-CoV-2 Infection. European Journal of Case Reports in Internal Medicine. 2020;7(5) doi: 10.12890/2020_001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell J.M., Rakheja D., Gopal P. SARS-CoV-2-related Hypercoagulable State Leading to Ischemic Enteritis Secondary to Superior Mesenteric Artery Thrombosis. Clinical Gastroenterology and Hepatology. 2020:1. doi: 10.1016/j.cgh.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karna S.T., Panda R., Maurya A.P., Kumari S. Superior Mesenteric Artery Thrombosis in COVID-19 Pneumonia: an Underestimated Diagnosis—First Case Report in Asia. Indian Journal of Surgery. 2020;82(6):1235–1237. doi: 10.1007/s12262-020-02638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramachandran R., Vasudevan Pillai A., Raja S., Sailesh S. Axillary artery thrombosis resulting in upper limb amputation as a COVID-19 sequela. BMJ Case Reports. 2021;14(1) doi: 10.1136/bcr-2020-240981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makhoul K., Shukha Y., Hanna L.A., Nitecki S., Leiderman M., Hayek T., et al. A case of rapidly progressive upper limb ischemic necrosis in a patient with COVID-19. International Journal of Infectious Diseases. 2021;106:401–404. doi: 10.1016/j.ijid.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur P., Qaqa F., Ramahi A., Shamoon Y., Singhal M., Shamoon F., et al. Acute upper limb ischemia in a patient with COVID-19. Hematology/oncology and stem cell therapy. 2020:S1658-3876(20)30096-0. [DOI] [PMC free article] [PubMed]
- 46.Dugue D., Hsu K.T., Wagner I.J., Jones C.M. Implications of COVID-19-associated Coagulopathy on Reconstructive Surgery: A Case of Ongoing Tissue Necrosis. Plast Reconstr Surg Glob Open. 2020;8(12):e3366. doi: 10.1097/GOX.0000000000003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Almeida Lima A.N., Santos Leite Pessoa M., Franco Costa Lima C., Picasso De Araújo Coimbra P., Bezerra Holanda J.L. Images in Vascular Medicine: Acute peripheral artery occlusion and ischemic stroke in a patient with COVID-19. Vascular Medicine. 2020;25(5):482–483. doi: 10.1177/1358863X20945020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renaud-Picard B., Gallais F., Ohana M., Zeyons F., Kretz B., Andre J., et al. Bilateral Acute Cardioembolic Limb Ischemia After Coronavirus Disease 2019 Pneumonia in a Lung Transplant Recipient: A Case Report. Transplant Proc. 2020;52(9):2715–2718. doi: 10.1016/j.transproceed.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Chen P., Mutar M., Hung M., Shao Z., Han Y., et al. Ischemic Necrosis of Lower Extremity in COVID-19: A Case Report. Journal of Atherosclerosis and Thrombosis. 2021;28(1):90–95. doi: 10.5551/jat.57950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veyre F., Poulain-Veyre C., Esparcieux A., Monsarrat N., Aouifi A., Lapeze J., et al. Femoral Arterial Thrombosis in a Young Adult after Nonsevere COVID-19. Annals of Vascular Surgery. 2020;69:85–88. doi: 10.1016/j.avsg.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aslan S., Bekci T., Cakir I.M. Isolated posterior tibial artery thrombosis after non-severe SARS-CoV-2 infection. Revista da Sociedade Brasileira de Medicina Tropical. 2021;54 doi: 10.1590/0037-8682-0205-2021. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]