Around a quarter of people who have had coronavirus disease 2019 (COVID-19) experience symptoms that continue for at least 1 month, but one in ten are still unwell after 12 weeks. This very debilitating condition has been defined by patient groups as “long COVID”, elsewhere called post-COVID, whereas the patients are frequently called COVID-19 long-haulers [1]. Long COVID has a serious impact on patient ability to go back to work or school, to have a social life and may have significant economic consequences for patients, their families and for society. The condition is characterised by long-term sequelae and can involve a range of about 200 different and overlapping symptoms, such as persistent fatigue, chest and muscle pain, headache, shortness of breath, anosmia, muscle weakness, fever, cognitive dysfunction (brain fog), tachycardia, intestinal disorders and skin manifestations. It can affect anyone, but women appear to be twice as likely to develop long COVID as men, but only until around age 60 years, when the risk level becomes similar [2–4].
Short abstract
Long COVID was detected in both adults and children and is characterised by immunological dysregulation. Autoimmune reactions in adult patients and allergic reactions in children appear to be critical factors. https://bit.ly/38RfSyT
Introduction
Around a quarter of people who have had coronavirus disease 2019 (COVID-19) experience symptoms that continue for at least 1 month, but one in ten are still unwell after 12 weeks. This very debilitating condition has been defined by patient groups as “long COVID”, elsewhere called post-COVID, whereas the patients are frequently called COVID-19 long-haulers [1]. Long COVID has a serious impact on patient ability to go back to work or school, to have a social life and may have significant economic consequences for patients, their families and for society. The condition is characterised by long-term sequelae and can involve a range of about 200 different and overlapping symptoms, such as persistent fatigue, chest and muscle pain, headache, shortness of breath, anosmia, muscle weakness, fever, cognitive dysfunction (brain fog), tachycardia, intestinal disorders and skin manifestations. It can affect anyone, but women appear to be twice as likely to develop long COVID as men, but only until around age 60 years, when the risk level becomes similar [2–4]. Long COVID has also been described in paediatric patients [5]. An Italian study reported that at least one symptom persisted 4 months after COVID-19 infection [6] whereas an Australian analysis suggested that only 8% of children had ongoing symptoms 3–6 months after mild SARS-CoV-2 infection [7]. No gender difference was observed in the prevalence of long COVID in this population [5].
Long COVID aetiology
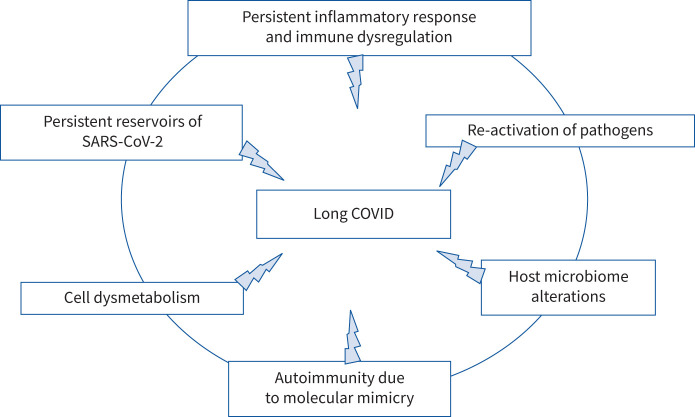
The long COVID syndrome has a similarity to the post-infectious syndromes that followed the outbreaks of chikungunya [8] and Ebola [9]. What are the factors responsible for this syndrome? They could be many and different. Organ damage caused by an excessive inflammatory response activated by the virus, persistent reservoirs of SARS-CoV-2 in certain tissues that could trigger post-infection morbidity, re-activation of pathogens due to immune dysregulation, host microbiome alterations, clotting/coagulation issues and autoimmunity due to molecular mimicry between SARS-CoV-2 and self-proteins have been hypothesised to play a role [10]. Furthermore, it has also been suggested that long COVID symptoms may not be a direct result of the SARS-CoV-2 infection but may be the consequence of COVID-19 inflammation-induced Epstein–Barr virus reactivation [11]. The search for detailed molecular mechanisms underlying long COVID is also underway. Some insights derive from metabolic studies, e.g. an altered tryptophan absorption and metabolism that could underlie the post-infection disease [12], whereas further hypotheses derive from cytopathological studies that suggest that SARS-CoV-2 could be capable of hindering autophagic processes of host cells, thus favouring the “journey” of viral particles inside the cell cytoplasm and their survival. As a consequence, it has been argued that modulators of cellular metabolism or agents bolstering autophagy could represent a therapeutic strategy against post-COVID sequelae, helping to eliminate viral particles or fragments potentially immunogenic and active [13]. However, to date, the plethora of different long COVID symptoms and disturbances seems to indicate that different concurrent mechanisms could be involved (figure 1) and that different therapeutic approaches should be settled in order to cure these patients.
FIGURE 1.
Possible concurrent mechanisms leading to long COVID.
Long COVID in children
Concerning the paediatric population, clinical data on long COVID-19 are limited. The most common complications were muscle and/or joint pain, headache, chest pain or a feeling of chest tightness, palpitations, respiratory problems and sleep disturbances [5, 6]. In their study, Osmanov et al. [5], thanks to a standardised follow-up data collection protocol developed by the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) [14], observed that older age and allergic diseases were associated with higher risk of persistent symptoms at follow-up. Osmanov et al. [5] suggested that, at least in children, immunological mechanisms may be responsible for an increased risk of long-term consequences of infection. In particular, COVID-19 sequelae could be linked with the mast cell activation syndrome and the T-helper type 2 (Th2)-biased immunological response in children with allergic diseases [5]. Importantly, these findings are in line with a recent case report describing an adolescent with persisting symptoms with first documentation of ongoing immune dysfunction and lung perfusion defects after mild COVID-19 [15].
Gender disparity: a “black hole” for long COVID
As mentioned above, long COVID and its symptom reporting is more frequent by women than men [2]. This is both a gender and sex disparity that is being discussed and underscored from public health policy makers in different terms. One deals with the fact that women frequently pay more attention to their body and to its alterations. This often leads to a more rapid diagnostic and therapeutic intervention. Unfortunately, there is also “a school of thought” sustaining that the long COVID gender skew could simply represent an artefact: it could just be conceived by “hysterical, middle-aged women”. This sexist approach could certainly represent a strong bias from both an ethical and a clinical point of view and it could also impact the healthcare and working rights for female patients with long COVID.
Actually, females have both innate and acquired immunological responses stronger than males and both genes and hormones are involved in this sex difference [16, 17]. These sex-based immunological differences contribute to variations in the incidence of autoimmune diseases, higher in females than in males and in susceptibility to malignancies and infectious diseases, more frequent in males than in females, and probably represent the major cause of female prevalence of long COVID in adults. Finally, as happens with Lyme disease, in which the pathogen remains hidden and generates higher levels of inflammatory cytokines in women than men [18], it can be hypothesised that fragments of SARS-CoV-2 could remain hidden in reservoirs such as the kidneys or brain, igniting some chronic inflammation-associated cascade giving rise to symptoms such as pain or brain fog experienced by patients with long COVID. Importantly, as mentioned above, no significant difference was reported to date between the youngest male and female patients [5]. This could reinforce the hypothesis that sex hormones and their immunomodulating activity could play a role in adult long COVID patients [19, 20].
Autoimmunity and long COVID
It has been observed that, compared with uninfected controls and as for Epstein–Barr virus, cytomegalovirus and HIV, SARS-CoV-2 infection is associated with the generation of a wide range of autoantibodies that can attack tissues of infected subjects [21]. Some infected individuals were found to have autoantibodies against proteins involved in several immunological activities, including interferon responses, leukocyte trafficking and lymphocyte function/activation [22]. Other autoantibodies are tissue-specific, including autoantibodies specific to blood vessels, heart and brain. The propensity of some patients to develop over 15 separate types of autoantibodies and above 10 distinct autoimmune diseases has been observed [23]. The main mechanisms that may contribute to the development of autoimmunity in COVID-19 are the following: 1) hyper-activation of the immune system, 2) the induction of excessive neutrophil extracellular trap formation, and 3) SARS-CoV-2 cross-reaction with self-components of the host. In fact, SARS-CoV-2 has been shown to cross-react with gut, kidney, lung, heart and brain antigens, and SARS-CoV-2 proteins can share homology with some self-protein epitopes, leading to molecular mimicry paths [23]. Furthermore, under conditions of inflammation, other organisms of the microbiome/virome communities, which could vary widely between different patients, may also contribute to autoantibody production and cause the great variety of autoantibody reactivity. This complex scenario could explain the significant percentage of clinical variations detected in patients with long COVID.
Conclusions
Long COVID is characterised by specific long-lasting inflammatory/immunological dysregulation and cannot be considered as a unique pathology but a huge series of different morbidity states. Hence, a better understanding of the heterogeneity of this pathology, assessing the appearance of autoantibodies in the serum of adult patients, in particular women, and evaluating the Th2 immune response and plasma IgE levels in children, could be important goals to begin to identify personalised and specific treatments for patients with long COVID.
Shareable PDF
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Support statement: This work was supported by Ministero della Salute (grant: COVID-2020-12371817). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Yelin D, Wirtheim E, Vetter P, et al. . Long-term consequences of COVID-19: research needs. Lancet Infect Dis 2020; 20: 1115–1117. doi: 10.1016/S1473-3099(20)30701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. . Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 2021; 18: 2621. doi: 10.3390/ijerph18052621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivan M, Rayner C, Delaney B. Fresh evidence of the scale and scope of long COVID. BMJ 2021; 373: n853. doi: 10.1136/bmj.n853 [DOI] [PubMed] [Google Scholar]
- 5.Osmanov IM, Spiridonova E, Bobkova P, et al. . Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J 2022; 59: 2101341. doi: 10.1183/13993003.01341-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonsenso D, Munblit D, De Rose C, et al. . Preliminary evidence on long COVID in children. Acta Paediatr 2021; 110: 2208–2211. doi: 10.1111/apa.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Say D, Crawford N, McNab S, et al. . Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health 2021; 5: 22–23. doi: 10.1016/S2352-4642(21)00124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillot X, Ribera A, Gasque P. Chikungunya-induced arthritis in Reunion Island: a long-term observational follow-up study showing frequently persistent joint symptoms, some cases of persistent chikungunya immunoglobulin M positivity, and no anticyclic citrullinated peptide seroconversion after 13 years. J Infect Dis 2020; 222: 1740–1744. doi: 10.1093/infdis/jiaa261 [DOI] [PubMed] [Google Scholar]
- 9.Clark DV, Kibuuka H, Millard M, et al. . Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 2015; 15: 905–912. doi: 10.1016/S1473-3099(15)70152-0 [DOI] [PubMed] [Google Scholar]
- 10.Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021; 12: 698169. doi: 10.3389/fmicb.2021.698169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold JE, Okyay RA, Licht WE, et al. . Investigation of long COVID prevalence and its relationship to epstein-barr virus reactivation. Pathogens 2021; 10: 763. doi: 10.3390/pathogens10060763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eroğlu İ, Eroğlu BÇ, Güven GS. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 2021; 90: 111308. doi: 10.1016/j.nut.2021.111308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gassen NC, Papies J, Bajaj T, et al. . SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat Commun 2021; 12: 3818. doi: 10.1038/s41467-021-24007-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ISARIC Global COVID-19 Paediatric Follow-Up Working Group. ISARIC Global COVID-19 paediatric follow-up. 2021. https://isaric.org/research/covid-19-clinical-research-resources/paediatric-follow-up
- 15.Buonsenso D, Di Giuda D, Sigfrid L, et al. . Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc Health 2021; 5: 677–680. doi: 10.1016/S2352-4642(21)00196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis ML, Maselli A, Pagano MT, et al. . Immune response and autoimmune diseases: a matter of sex. Ital J Gender Specific Med 2019; 5: 11–20. [Google Scholar]
- 17.Klein S, Flanagan K. Sex differences in immune responses. Nat Rev Immunol 2016; 16: 626–638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 18.Jarefors S, Bennet L, You E, et al. . Lyme borreliosis reinfection: might it be explained by a gender difference in immune response? Immunology 2006; 118: 224–232. doi: 10.1111/j.1365-2567.2006.02360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadakedath S, Kandi V, Mohapatra RK, et al. . Immunological aspects and gender bias during respiratory viral infections including novel coronavirus disease-19 (COVID-19): a scoping review. J Med Virol 2021; 93: 5295–5309. doi: 10.1002/jmv.27081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortona E, Buonsenso D, Carfi A, et al. . Long COVID: an estrogen-associated autoimmune disease? Cell Death Discov 2021; 7: 77. doi: 10.1038/s41420-021-00464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khamsi R. Rogue antibodies could be driving severe COVID-19. Nature 2021; 590: 29–31. doi: 10.1038/d41586-021-00149-1 [DOI] [PubMed] [Google Scholar]
- 22.Wang EY, Mao T, Klein J, et al. . Diverse functional autoantibodies in patients with COVID-19. Nature 2021; 595: 283–288. doi: 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- 23.Dotan A, Muller S, Kanduc D, et al. . The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 2021; 20: 102792. doi: 10.1016/j.autrev.2021.102792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02245-2021.Shareable (226.2KB, pdf)