Abstract
The quick evaluation of venous thromboembolism is a key point of modern medicine since the delayed diagnosis is associated with a worse prognosis. Venous ultrasound (VU) is a sensitive and rapidly performed test in cases of suspected deep venous thrombosis. Various protocols have been proposed for its execution, such as the study of the whole deep venous circulation of the lower limb or the analysis of the femoral-popliteal area. The aim is to detect a vessel thrombus and the most sensitive element is the non-compressibility with the probe. Initially, the thrombus is hypoechogenic and adherent to the vessel; later, it tends to organize and recanalize. Usually, in the early stages, the risk of embolism is higher. The role of studying the iliac axis and calf veins is still uncertain. VU is not useful for assessing response to anticoagulation therapy and it is unclear whether the persistence of thrombotic abnormalities can guide on a possible prolongation of therapy.
Keywords: Compression ultrasound, Deep venous thrombosis, Venous ultrasound, Venous thromboembolism, Critical care ultrasonography
Core Tip: Venous ultrasound represents an important weapon for emergency setting care. Nevertheless, several different protocols present in the literature could create confusion. In this review our goal is to define a practical and clear guide to support the physician in rapid deep venous thrombosis diagnosis and correct management.
INTRODUCTION
Thrombus formation is a pathological phenomenon caused by an inappropriate hemostatic response; many different factors are involved, often favored in blood stasis points such as venous valves. A major theory delineating the pathogenesis of venous thromboembolism (VTE), often called Virchow's triad, states that VTE occurs as a result of alterations in blood flow, in vascular endothelial injury and in blood constituents. Blood clots could leave these sides to enter the bloodstream, reaching right heart chambers and pulmonary circulation. Rarely, in the presence of patent foramen ovale with right-to-left shunt, there may be systemic embolism. Hence, deep venous thrombosis (DVT) and pulmonary embolism (PE) are two sides of the same coin; about 50% of patients with proximal DVT is affected by an asymptomatic PE, as well as 80% of PEs suffers DVT (often asymptomatic)[1]. DVT may be distal, interesting tibial-peroneal district, or proximal, that affects femoral-popliteal veins; proximal DVT is more frequently related to PE.
DVT is the third most common cardiovascular disease, following heart attack and ischemic stroke[2] (DVT incidence: 150/100000/year, PE incidence: 60-70/ 100000/year). According to Cohen et al[3], based on 6 EU countries data (Italy, Spain, France, Germany, United Kingdom and Sweden), EP-related death rate was 12%[3].
DVT should be suspected in patients presenting with leg swelling, pain or erythema; usually symptoms are unilateral calf-related if isolated distal DVT, whole-leg related if proximal DVT[4]. Many patients are asymptomatic. Although uncommon, it is important to identify patients with phlegmasia cerulea dolens, that ranges from phlegmasia alba dolens to venous gangrene, because it should be considered for more aggressive management[5]. PE has a wide variety of presenting features, ranging from no symptoms to shock or sudden death. The most common presenting symptom is dyspnea, followed by chest pain (classically pleuritic) and cough. Chronic thromboembolic pulmonary hypertension has been estimated to occur in 4.8% (95%CI: 2.3-9.6) of patients who survive a PE[6].
The most used predictive score of DVT is Wells Score[7] (Table 1), more accurate than revised Geneva score in PE suspected patients[8-10]. It predicts an increasing incidence of PE with major probability classes (“low” if ≤ 0 points to “high” ≥ 3 points)[11]. PE incidence ranged from 1%-13% in low probability level (Wells score < 2), 28%-58.3% in medium probability level (Wells score 2-6), and 58.1%-93% in high probability level (Wells score > 6). The sensitivity ranged from 63.8%-79.3%, and the specificity ranged from 48.8%-90.0%.
Table 1.
Wells’ score
|
Features
|
Score, points
|
| Active cancer (in treatment or treated in the last 6 mo or under palliative care) | 1 |
| Paralysis, paresis, or recent plaster immobilization of the lower extremity | 1 |
| Bedridden recently > 3 d or major surgery within 12 wk | 1 |
| Localized tenderness along the deep venous system | 1 |
| Entire leg swollen | 1 |
| Calf swelling > 3 cm compared to the other leg | 1 |
| Pitting edema, confined to symptomatic leg | 1 |
| Collateral (nonvaricose) superficial veins present | 1 |
| Previously documented DVT | 1 |
| Alternative diagnosis to DVT as likely or more likely | -2 |
DVT: Deep venous thrombosis.
Quick diagnosis of DVT/PE represents a fundamental weapon for modern medicine. The early detection of DVT is crucial to reduce the risk of thromboembolism in the critical patient, thus reducing the related morbidity and mortality. A delayed diagnosis of PE has poor outcomes, ranges from shock to hospital death[12]: Therefore, the 30 d mortality rate exceeds 3% in patients with DVT who are not anticoagulated, and this mortality risk increases 10-fold in patients who develop PE.
The most diffused vascular diagnostic tools are: (1) Phlebography: Gold standard, not widely used for its invasiveness; (2) Computed tomography (CT) angiogram: Utilized for PE and proximal districts (pelvic, iliac, caval); (3) Magnetic resonance imaging angiogram: Utility comparable to CT angiogram; and (4) Echocolordoppler: The most employed instrumental methodic for quick diagnosis and screening of pathology (limited just in the most peripheral venous tracts).
Therefore, we realized a review of clinical studies comparing outcomes of patients with a history of DVT subjected to different managements. We achieved this by doing formal searches of the electronic database MEDLINE (source PubMed) and the Cochrane Controlled Clinical Trials Register Database. About 40 studies were selected from 1989 to 2017, by a combination of medical subject headings including the following terms: Compression ultrasound (CUS), DVT, venous ultrasound (VU) and VTE. References from reviews and selected articles were also examined for potentially relevant citations. Our analysis was restricted to the trials that focused on the comparison between different existing diagnostic protocols, with a special focus on emergency department experiences.
VU
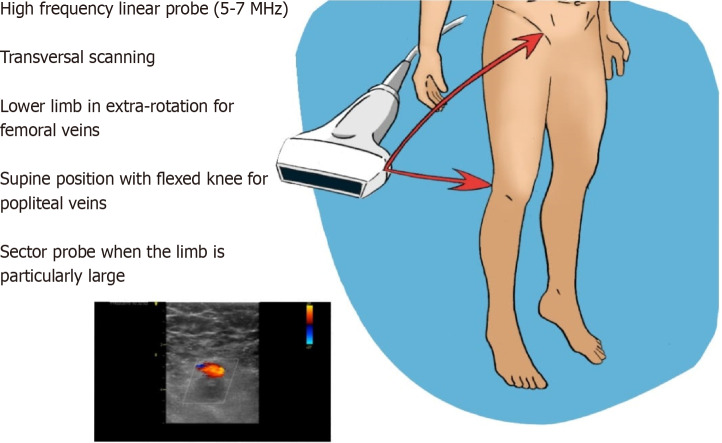
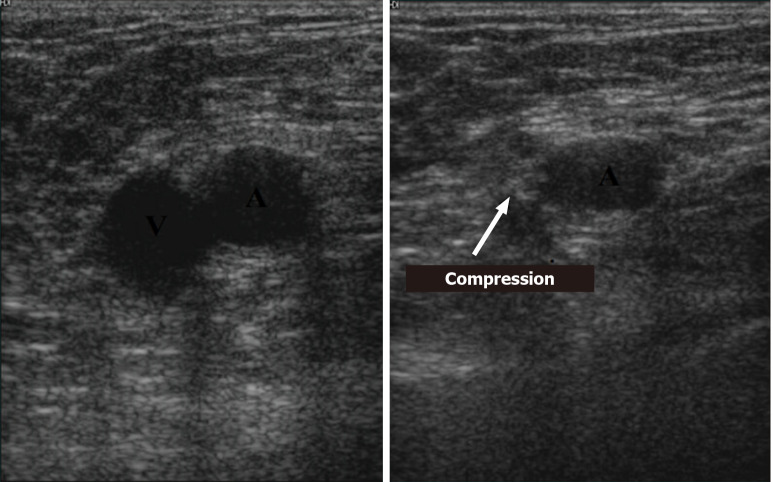
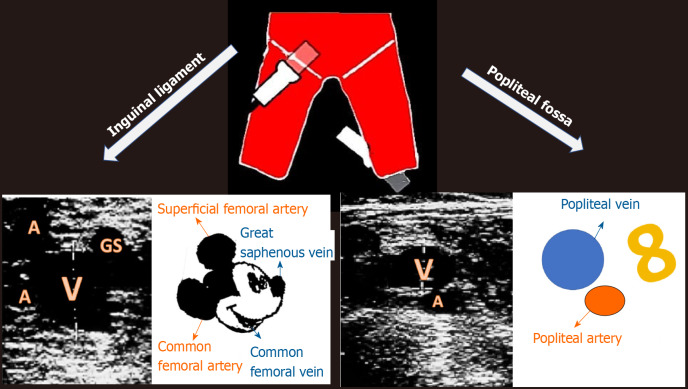
VU is the commonest methodic used for DVT assessment. Many different protocols have been proposed, thus incrementing the confusing about its management. The study of deep veins is performed with high-frequency linear probes (5-7.5 MHz) or a sector probe, when the limb is particularly large-sized (Figure 1). Specifically, evaluation of the femoral veins should be done with the lower limb in extra-rotation, while other veins are studied in supine position with flexed knee. CUS with Doppler is the choice diagnostic test in patients with suspected DVT and the sensitivity and specificity of proximal CUS is greater than 95% (Figure 2). However, proximal CUS suffers from limitations[13]: (1) Calf vein thrombus, that are harder to assess than proximal veins; and (2) Iliac veins thrombus, that cannot be assessed for compressibility and thus these veins should be assessed with venography.
Figure 1.
Study optimization.
Figure 2.
Compression ultrasound example.
Pretest evaluation
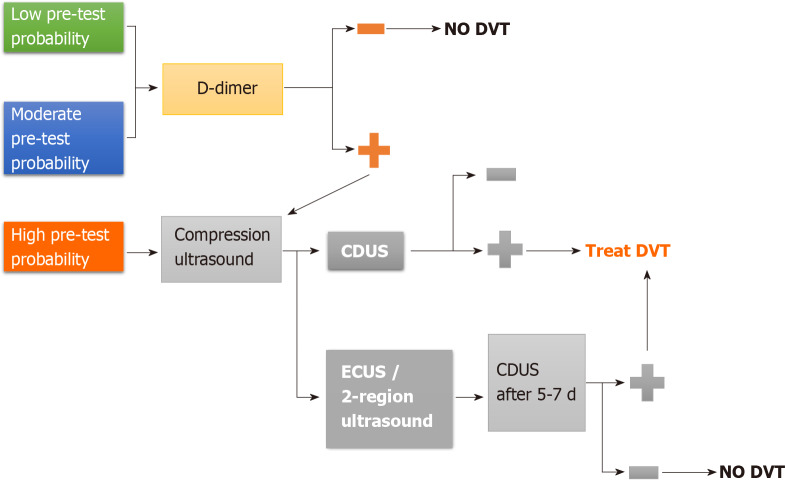
First step in DVT assessment is probability estimation according to Wells Score[14]. In case of low pretest probability, a negative D-Dimer can rule out DVT without the need for ultrasound confirmation. If, conversely, pretest probability by Wells Score is high, VU is recommended[15,16] (Figure 3). Therefore, the role of D-Dimer in this diagnostic process is limited: it is a degradation product of cross-linked fibrin and it is elevated in nearly all patients with acute DVT (high sensitivity), but it is non-specific since high levels are found in many other conditions (i.e. malignancy, sepsis, recent surgery or trauma, pregnancy, renal failure).
Figure 3.
Pretest evaluation algorithm. CDUS: Complete doppler ultrasound; DVT: Deep venous thrombosis; ECUS: Extended compression ultrasound.
Protocols
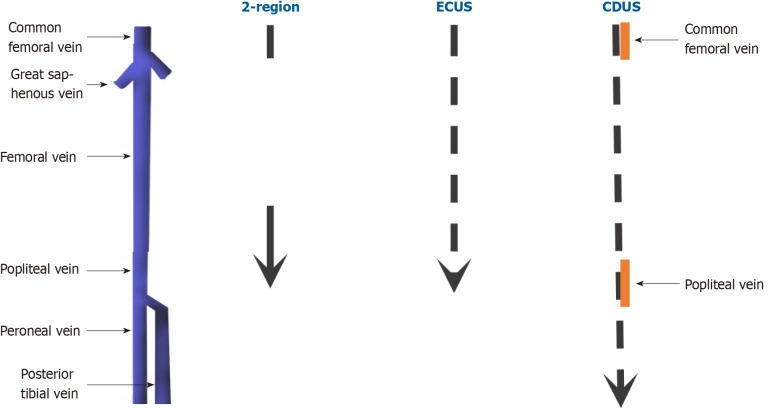
Complete doppler ultrasound: Is the preferred one and it includes bilateral compressions from inguinal ligament, passing through calf veins, to ankle (compressions are separated by 2 cm intervals); it also provides for bilateral common femoral and popliteal vein color doppler images and spectral doppler waveforms, in order to verify possible asymmetries[17,18]. CUS false positivity in calf evaluation is extremely uncommon (its specificity in calf district reaches about 97.8%)[19], and the risk of excessive treatment related to calf DVTs represents the principal argument against this protocol. Calf assessment may also provide alternative findings, like musculoskeletal abnormalities[20]. Prospective studies have demonstrated that lack of compressibility of a vein with the ultrasound probe is the most sensitive (> 95%) and specific (> 95%) sonographic sign for proximal vein thrombosis. The addition of color flow Doppler does not improve the sensitivity but can provide supportive evidence of thrombus and help to identify calf veins. Variation of venous size with the Valsalva maneuver has a low sensitivity and specificity for the diagnosis and is no longer performed in many centers[21-24]. In case of negative result, the risk of DVT after 3 mo is estimated about 0.57% (95%CI, 0.25%–0.89%) (Figure 4)[25].
Figure 4.
Different protocols. Dotted arrows correspond to ultrasound scans separated by 2 cm intervals. Yellow segments instead represent doppler points. CDUS: Complete doppler ultrasound; ECUS: Extended compression ultrasound; 2-region: Two-region ultrasound.
Extended compression ultrasound: This protocol is a point-of-care examination that consists of compressions from thigh to knee and is principally utilized when complete doppler ultrasound (CDUS) is not quickly viable[26]. However, if it results negative, a confirmatory CDUS after 5-7 d is recommended, in order to exclude calf involvement (evaluating to start anticoagulation if this is not possible)[27-30]. (Figure 4).
Two-region ultrasound: The compressions are limited to femoral and popliteal areas. As in extended compression ultrasound (ECUS), also in this case a negative response should be followed by a CDUS examination 5-7 d later, because both the previous two protocols do not comprehend calf veins[27,31,32]. D-Dimer after a negative ECUS or two-region ultrasound does not affect the follow-up unless it results negative[33] (Figure 4).
Thrombosis sides and related approach
The management of DVT is also related to its side, in particular two sides are more controversial (Figure 5): (1) Ileocaval: Being in a blind side for ultrasound sonography, it could be underdiagnosed. Nevertheless, although a normal CUS could be present, asymmetrical or continuous femoral doppler waveforms or whole-leg swelling may indicate an upstream impediment. In these cases, it is reasonable to think about pelvic ultrasound, CT or magnetic resonance venography to rule out this possibility. It has been estimated that this side is involved in 1.6% of DVTs[34]. Because the accuracy of duplex ultrasound for ileocaval DVT is not established, the threshold for CT or magnetic resonance venography should be low; (2) Calf veins: Even if calf district examination is just included in CDUS protocol, calf involvement management is subject to debate. If the physician chooses a wait-and-see approach without treating, it is recommended to repeat ultrasound at 1 wk. If new scan shows proximal progression, then start anticoagulation (progression occurs in 9%-21.4% of cases and is usually associated to symptoms perseverance or exacerbation)[35]; if instead clot remains stable, you should scan again at 2 wk. If thrombus is not more observable at 1 wk or does not show significant evolution at 2 wk, or if you have begun treating, you can stop its follow-up. You could consider a new assessment in case of therapeutic changes[35,36]. More investigations will be needed to clarify prevalence and risk factors of progression in non-anticoagulated calf thromboses[37]. It is important to point out that short calf areas of non-compressibility are not significative[38], further scans or D-dimer may supply in these cases, although positive D-dimer has demonstrated to be not discriminatory[39]. Moreover, presence of calf DVT could be important in risk stratification about different fields like chronic venous insufficiency, mortality and cancer diagnosis or recurrent DVT occurrence[40,41]. The current American College of Radiology/American Institute of Ultrasound in Medicine/ Society of Radiologists in Ultrasound guidelines include selective calf imaging for the subset of patients with calf symptoms not explained by the proximal scan; and (3) Upper limbs: Old statistics referred an incidence of upper limbs thrombosis about 2%-3%, mainly in young patients and in right arm, especially after hard physical effort or in thoracic outlet syndrome.
Figure 5.
Reference points and respective sonogram images. A: Artery; GS: Great saphenous vein; V: vein.
Thrombus types and classification
The thrombus evolution is characterized by several phases, each with different embolic risk: (1) Early stage (1-6 d): Clot shows hypoechoic structure and low adherence to vessel walls; (2) 2nd stage (7-14 d): Inhomogeneous structure with alternation of echogenic and hypo/an-echoic areas; and (3) 3rd and 4th stage (> 14 d): Organization and recanalization phases: Flow appearance inside thrombus to color doppler.
The first two phases have the highest PE risk.
Basing on echogenic characteristics and appearance of thrombus, we can classify lesions as follows (Table 2): (1) Acute Venous thrombosis: Noncompressible, but the clot is not stiff and gets deformed under probe push; thrombus presents a regular profile, and the respective vein is dilated; (2) Chronic post-thrombotic change: It shows residual findings after an acute venous thrombosis. In this case clot is noncompressible, fixed and resists to pressure deformation; moreover, its profile is often non-uniform, as well as non-uniform and thickened could look vessel wall after thrombus incorporation or recanalization, while vein does not present dilated rather its caliber may be reduced (scarring setting). Sometimes it is associated to thick adhesions (synechiae), as effect of retraction forces exerted by thrombotic material, and less often to calcification sides. Echogenicity does not reflect how old thrombus is[42]. It is important to keep in mind that this persisting lesion is not a thrombus and anticoagulation therapy is not required in this case[43]; (3) Subacute thrombus: This term shouldn’t be commonly used since it refers to a typical and unusual situation in which ultrasound shows a change in acute thrombus aspect few weeks apart, not includable in chronic post thrombotic change definition. These changes should occur no later than 6 mo after clot formations[44,45] (thrombus usually progresses or heals within 6 mo from its generation); (4) Scarring: It is a process that can follow a not completely recovered acute thrombosis, due to fibroblasts action on thrombus and consequent fibrosis with its effects on wall thickening and synechiae production. It can determine an uncomplete stenosis which could endure for years[44,46]. Scarring has to be more correctly considered part of “chronic post thrombotic change” definition; (5) Indeterminate (equivocal): Definition utilized when it is not possible to clearly classify the lesion; (6) and Recurrent DVT: It is a thrombus formation on a chronic post thrombotic change region or a new acute venous thrombosis in a patient with a former thrombosis episode in same or contralateral leg[47-50]. It is a quite common eventuality[49], especially in patients with scarring lesions[51]. It could be not easy to recognize a new acute thrombus occurring in a chronic post thrombotic change zone[47,51,52]. Various criteria have been advanced to support diagnosis, in particular increments in compressed vein size > 4 mm or in D-dimer values, while no modifications in ultrasound scans at 1-3 d and at 7-10 d as exclusion criterion; however, their efficiency is still not clear[35,47,53-55]. Magnetic resonance also has been considered to assist differential diagnosis of recurrent DVTs from simple scars[55].
Table 2.
Lesion types
|
Lesion definition
|
Characteristics
|
| Acute thrombus | Noncompressible; deformable under probe push; regular profile; dilated vein |
| Subacute thrombus | Change in acute thrombus aspect few weeks apart, not includable in chronic post-thrombotic change definition (no later than 6 mo after clot formation) |
| Chronic post-thrombotic change | Noncompressible; resists to pressure deformation; non-uniform profile; reduced/normal vein caliber |
Serial scanning or D-dimer may be helpful in cases where the ultrasound does not detect clear new abnormalities, or the findings are difficult to interpret. Equivocal ultrasound findings may require serial imaging after 1-3 and 7-10 d to determine if there are any acute changes that would indicate recurrent DVT. D-dimer may also be helpful to establish if recurrent DVT is present.
FOLLOW-UP
During anticoagulant therapy, ultrasound follow up is not necessary. For example, in the early stages of treatment, there may be minimal progression of thrombotic material, but this is not an indication to change anticoagulant or to insert a caval filter. Therefore, to evaluate “response” of venous clot to therapy does not alter treatment[36,56,57].
Ultrasound at the end of treatment may be helpful to get a clear picture of the venous district for future assessment[54].
It is unclear whether the persistence of thrombotic abnormalities can guide on a possible prolongation of anticoagulant therapy. Further studies will be needed to define the correlation between residual risk and therapy[58].
A separate mention deserves isolated distal DVT, that-as stated above-sometimes resolves or does not extend proximally without treatment and is associated with less severe complications. Thus, routine use of whole leg ultrasonography has the potential to lead to the diagnosis of DVT that does not necessarily need to be treated. Data on distal DVT remain unclear. It is not yet known who patients are at risk and how long any anticoagulation therapy should be[59].
CONCLUSION
DVT is an often-misrecognized pathology that can cause serious clinical conditions, such as PE. Actually, ultrasound evaluation of the lower venous district can give essential information for a rapid diagnosis, especially in conditions of hemodynamic instability or when second level examinations are not readily available. Hence, compressive ultrasonography is one of the most effective tools in the emergency department in the hand of physicians. It is a non-invasive, low-cost diagnostic methodology that does not expose the patient to ionizing radiation; therefore, it is a rapid examination that should be part of the diagnostic flow chart for PE.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: March 15, 2021
First decision: April 6, 2021
Article in press: July 5, 2021
Specialty type: Critical care medicine
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, MBChB DC S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
Contributor Information
Alessandro Di Vilio, Unit of Cardiology and Intensive Coronary Care, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy. adivilio56@gmail.com.
Andrea Vergara, Unit of Cardiology and Intensive Coronary Care, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy.
Alfonso Desiderio, Unit of Cardiology and Intensive Coronary Care, Umberto I Hospital, Nocera Inferiore 84014, Italy.
Franco Iodice, Unit of Cardiology and Intensive Coronary Care, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy.
Alessandro Serio, Human Anatomy and Sport Medicine Division, Department of Public Health, University of Naples “Federico II”, Naples 80131, Italy.
Stefano Palermi, Human Anatomy and Sport Medicine Division, Department of Public Health, University of Naples “Federico II”, Naples 80131, Italy.
Francesco Gambardella, Human Anatomy and Sport Medicine Division, Department of Public Health, University of Naples “Federico II”, Naples 80131, Italy.
Simona Sperlongano, Unit of Cardiology and Intensive Coronary Care, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy.
Renato Gioia, Department of Medicine, Surgery, and Dentistry, University of Salerno, Salerno 84084, Italy.
Maria Acitorio, Unit of Cardiology and Intensive Coronary Care, Umberto I Hospital, Nocera Inferiore 84014, Italy.
Antonello D'Andrea, Unit of Cardiology and Intensive Coronary Care, Umberto I Hospital, Nocera Inferiore 84014, Italy.
References
- 1.Girard P, Musset D, Parent F, Maitre S, Phlippoteau C, Simonneau G. High prevalence of detectable deep venous thrombosis in patients with acute pulmonary embolism. Chest. 1999;116:903–908. doi: 10.1378/chest.116.4.903. [DOI] [PubMed] [Google Scholar]
- 2.Blebea J, Ewald S. Asymptomatic pulmonary embolism complicating deep venous thrombosis. JAMA. 1994;272:517; author reply 517–517; author reply 518. doi: 10.1001/jama.272.7.517b. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost. 2017;117:57–65. doi: 10.1160/TH15-08-0686. [DOI] [PubMed] [Google Scholar]
- 4.Hirsh J, Hull RD, Raskob GE. Clinical features and diagnosis of venous thrombosis. J Am Coll Cardiol. 1986;8:114B–127B. doi: 10.1016/s0735-1097(86)80013-4. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36:76–77. [PMC free article] [PubMed] [Google Scholar]
- 6.Guérin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, Pontal D, Guégan M, Simonneau G, Meyer G, Sanchez O. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014;112:598–605. doi: 10.1160/TH13-07-0538. [DOI] [PubMed] [Google Scholar]
- 7.Wells PS, Hirsh J, Anderson DR, Lensing AW, Foster G, Kearon C, Weitz J, D'Ovidio R, Cogo A, Prandoni P. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–1330. doi: 10.1016/s0140-6736(95)92535-x. [DOI] [PubMed] [Google Scholar]
- 8.Ye YP, Li YY, Chen J, Zheng G, Ma X, Peng XX, Yang YH. [The diagnostic values of Wells score and modified Geneva score for pretesting acute pulmonary embolism: a prospective study] Zhonghua Nei Ke Za Zhi. 2012;51:626–629. [PubMed] [Google Scholar]
- 9.Penaloza A, Melot C, Motte S. Comparison of the Wells score with the simplified revised Geneva score for assessing pretest probability of pulmonary embolism. Thromb Res. 2011;127:81–84. doi: 10.1016/j.thromres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Guo XJ, Liu M, Guo YM, Ma HX, Guo YL, Zhu L, Wang JG, Yang YH, Wang C. [A comparison of the predictive values of three clinical scoring systems for suspected acute pulmonary embolism based on multidetector CT angiography] Zhonghua Jie He He Hu Xi Za Zhi. 2009;32:119–123. [PubMed] [Google Scholar]
- 11.Shen JH, Chen HL, Chen JR, Xing JL, Gu P, Zhu BF. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482–492. doi: 10.1007/s11239-015-1250-2. [DOI] [PubMed] [Google Scholar]
- 12.Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14:592–598. doi: 10.1197/j.aem.2007.03.1356. [DOI] [PubMed] [Google Scholar]
- 13.Habscheid W, Höhmann M, Wilhelm T, Epping J. Real-time ultrasound in the diagnosis of acute deep venous thrombosis of the lower extremity. Angiology. 1990;41:599–608. doi: 10.1177/000331979004100803. [DOI] [PubMed] [Google Scholar]
- 14.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 15.Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M, Pauker SG, Makdissi R, Guyatt GH. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e351S–e418S. doi: 10.1378/chest.11-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qaseem A, Snow V, Barry P, Hornbake ER, Rodnick JE, Tobolic T, Ireland B, Segal J, Bass E, Weiss KB, Green L, Owens DK Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57–62. doi: 10.1370/afm.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society for Vascular Ultrasound. SVU Vascular Technology Professional Performance Guidelines. Lower Extremity Venous Duplex Evaluation. Updated 2019. [cited 20 January 2021]. Available from: https://www.svu.org/practice-resources/professional-performance-guidelines/
- 18.Talbot SR, Oliver MA. Techniques of Venous Imaging. Pasadena, CA: Appleton Davies, Inc. 1992: 59-118. [Google Scholar]
- 19.Goodacre S, Sampson F, Thomas S, van Beek E, Sutton A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6. doi: 10.1186/1471-2342-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Righini M. Is it worth diagnosing and treating distal deep vein thrombosis? J Thromb Haemost. 2007;5 Suppl 1:55–59. doi: 10.1111/j.1538-7836.2007.02468.x. [DOI] [PubMed] [Google Scholar]
- 21.Mattos MA, Londrey GL, Leutz DW, Hodgson KJ, Ramsey DE, Barkmeier LD, Stauffer ES, Spadone DP, Sumner DS. Color-flow duplex scanning for the surveillance and diagnosis of acute deep venous thrombosis. J Vasc Surg. 1992;15:366–75; discussion 375. doi: 10.1067/mva.1992.33847. [DOI] [PubMed] [Google Scholar]
- 22.Monreal M, Montserrat E, Salvador R, Bechini J, Donoso L, MaCallejas J, Foz M. Real-time ultrasound for diagnosis of symptomatic venous thrombosis and for screening of patients at risk: correlation with ascending conventional venography. Angiology. 1989;40:527–533. doi: 10.1177/000331978904000603. [DOI] [PubMed] [Google Scholar]
- 23.Lensing AW, Doris CI, McGrath FP, Cogo A, Sabine MJ, Ginsberg J, Prandoni P, Turpie AG, Hirsh J. A comparison of compression ultrasound with color Doppler ultrasound for the diagnosis of symptomless postoperative deep vein thrombosis. Arch Intern Med. 1997;157:765–768. [PubMed] [Google Scholar]
- 24.Davidson BL, Elliott CG, Lensing AW. Low accuracy of color Doppler ultrasound in the detection of proximal leg vein thrombosis in asymptomatic high-risk patients. The RD Heparin Arthroplasty Group. Ann Intern Med. 1992;117:735–738. doi: 10.7326/0003-4819-117-9-735. [DOI] [PubMed] [Google Scholar]
- 25.Johnson SA, Stevens SM, Woller SC, Lake E, Donadini M, Cheng J, Labarère J, Douketis JD. Risk of deep vein thrombosis following a single negative whole-leg compression ultrasound: a systematic review and meta-analysis. JAMA. 2010;303:438–445. doi: 10.1001/jama.2010.43. [DOI] [PubMed] [Google Scholar]
- 26.Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, Clement C, Robinson KS, Lewandowski B. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 27.Lewiss RE, Kaban NL, Saul T. Point-of-Care Ultrasound for a Deep Venous Thrombosis. Glob Heart. 2013;8:329–333. doi: 10.1016/j.gheart.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Poley RA, Newbigging JL, Sivilotti ML. Estimated effect of an integrated approach to suspected deep venous thrombosis using limited-compression ultrasound. Acad Emerg Med. 2014;21:971–980. doi: 10.1111/acem.12459. [DOI] [PubMed] [Google Scholar]
- 29.Langan EM 3rd, Coffey CB, Taylor SM, Snyder BA, Sullivan TM, Cull DL, Youkey JR, Gray BH. The impact of the development of a program to reduce urgent (off-hours) venous duplex ultrasound scan studies. J Vasc Surg. 2002;36:132–136. doi: 10.1067/mva.2002.125021. [DOI] [PubMed] [Google Scholar]
- 30.Bauld DL, Kovacs MJ. Dalteparin in emergency patients to prevent admission prior to investigation for venous thromboembolism. Am J Emerg Med. 1999;17:11–15. doi: 10.1016/s0735-6757(99)90004-9. [DOI] [PubMed] [Google Scholar]
- 31.Hannula O, Vanninen R, Rautiainen S, Mattila K, Hyppölä H. Teaching limited compression ultrasound to general practitioners reduces referrals of suspected DVT to a hospital: a retrospective cross-sectional study. Ultrasound J. 2021;13:1. doi: 10.1186/s13089-021-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birdwell BG, Raskob GE, Whitsett TL, Durica SS, Comp PC, George JN, Tytle TL, McKee PA. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1–7. doi: 10.7326/0003-4819-128-1-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi E, Camporese G, Büller HR, Siragusa S, Imberti D, Berchio A, Ghirarduzzi A, Verlato F, Anastasio R, Prati C, Piccioli A, Pesavento R, Bova C, Maltempi P, Zanatta N, Cogo A, Cappelli R, Bucherini E, Cuppini S, Noventa F, Prandoni P Erasmus Study Group. Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: a randomized controlled trial. JAMA. 2008;300:1653–1659. doi: 10.1001/jama.300.14.1653. [DOI] [PubMed] [Google Scholar]
- 34.Ouriel K, Green RM, Greenberg RK, Clair DG. The anatomy of deep venous thrombosis of the lower extremity. J Vasc Surg. 2000;31:895–900. doi: 10.1067/mva.2000.105956. [DOI] [PubMed] [Google Scholar]
- 35.Michiels JJ, Gadisseur A, Van Der Planken M, Schroyens W, De Maeseneer M, Hermsen JT, Trienekens PH, Hoogsteden H, Pattynama PM. A critical appraisal of non-invasive diagnosis and exclusion of deep vein thrombosis and pulmonary embolism in outpatients with suspected deep vein thrombosis or pulmonary embolism: how many tests do we need? Int Angiol. 2005;24:27–39. [PubMed] [Google Scholar]
- 36.Society for Vascular Medicine. Five things physicians and patients should question. Choosing Wisely. February 21, 2013. [cited 1 December 2017]. Available from http://www.choosingwisely.org/societies/society-for-vascular-medicine/
- 37.Masuda EM, Kistner RL. The case for managing calf vein thrombi with duplex surveillance and selective anticoagulation. Dis Mon. 2010;56:601–613. doi: 10.1016/j.disamonth.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Norén A, Ottosson E, Sjunnesson M, Rosfors S. A detailed analysis of equivocal duplex findings in patients with suspected deep venous thrombosis. J Ultrasound Med. 2002;21:1375–83; quiz 1384. doi: 10.7863/jum.2002.21.12.1375. [DOI] [PubMed] [Google Scholar]
- 39.Linkins LA, Bates SM, Lang E, Kahn SR, Douketis JD, Julian J, Parpia S, Gross P, Weitz JI, Spencer FA, Lee AY, O'Donnell MJ, Crowther MA, Chan HH, Lim W, Schulman S, Ginsberg JS, Kearon C. Selective D-dimer testing for diagnosis of a first suspected episode of deep venous thrombosis: a randomized trial. Ann Intern Med. 2013;158:93–100. doi: 10.7326/0003-4819-158-2-201301150-00003. [DOI] [PubMed] [Google Scholar]
- 40.Seinturier C, Bosson JL, Colonna M, Imbert B, Carpentier PH. Site and clinical outcome of deep vein thrombosis of the lower limbs: an epidemiological study. J Thromb Haemost. 2005;3:1362–1367. doi: 10.1111/j.1538-7836.2005.01393.x. [DOI] [PubMed] [Google Scholar]
- 41.Galanaud JP, Arnoult AC, Sevestre MA, Genty C, Bonaldi M, Guyard A, Giordana P, Pichot O, Colonna M, Quéré I, Bosson JL OPTIMEV-SFMV Investigators. Impact of anatomical location of lower limb venous thrombus on the risk of subsequent cancer. Thromb Haemost. 2014;112:1129–1136. doi: 10.1160/TH14-04-0351. [DOI] [PubMed] [Google Scholar]
- 42.Murphy TP, Cronan JJ. Evolution of deep venous thrombosis: a prospective evaluation with US. Radiology. 1990;177:543–548. doi: 10.1148/radiology.177.2.2217798. [DOI] [PubMed] [Google Scholar]
- 43.Comerota AJ, Oostra C, Fayad Z, Gunning W, Henke P, Luke C, Lynn A, Lurie F. A histological and functional description of the tissue causing chronic postthrombotic venous obstruction. Thromb Res. 2015;135:882–887. doi: 10.1016/j.thromres.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Markel A. Origin and natural history of deep vein thrombosis of the legs. Semin Vasc Med. 2005;5:65–74. doi: 10.1055/s-2005-871743. [DOI] [PubMed] [Google Scholar]
- 45.Piovella F, Crippa L, Barone M, Viganò D'Angelo S, Serafini S, Galli L, Beltrametti C, D'Angelo A. Normalization rates of compression ultrasonography in patients with a first episode of deep vein thrombosis of the lower limbs: association with recurrence and new thrombosis. Haematologica. 2002;87:515–522. [PubMed] [Google Scholar]
- 46.Teichman JMH. Editorial Comment on: In Vitro Comparison of Stone Fragmentation When Using Various Settings with Modern Variable Pulse Holmium Lasers by Bell et al. (From: Bell JR, Penniston KL, Nakada SY, J Endourol 2017;31:1067-1072) J Endourol. 2017;31:1345–1346. doi: 10.1089/end.2017.0711. [DOI] [PubMed] [Google Scholar]
- 47.Schellong SM. Diagnosis of recurrent deep vein thrombosis. Hamostaseologie. 2013;33:195–200. doi: 10.5482/HAMO-13-06-0029. [DOI] [PubMed] [Google Scholar]
- 48.Prandoni P, Lensing AW, Prins MH, Bernardi E, Marchiori A, Bagatella P, Frulla M, Mosena L, Tormene D, Piccioli A, Simioni P, Girolami A. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137:955–960. doi: 10.7326/0003-4819-137-12-200212170-00008. [DOI] [PubMed] [Google Scholar]
- 49.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prandoni P, Prins MH, Lensing AW, Ghirarduzzi A, Ageno W, Imberti D, Scannapieco G, Ambrosio GB, Pesavento R, Cuppini S, Quintavalla R, Agnelli G AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150:577–585. doi: 10.7326/0003-4819-150-9-200905050-00003. [DOI] [PubMed] [Google Scholar]
- 51.Tan M, Mos IC, Klok FA, Huisman MV. Residual venous thrombosis as predictive factor for recurrent venous thromboembolim in patients with proximal deep vein thrombosis: a sytematic review. Br J Haematol. 2011;153:168–178. doi: 10.1111/j.1365-2141.2011.08578.x. [DOI] [PubMed] [Google Scholar]
- 52.Cronan JJ, Leen V. Recurrent deep venous thrombosis: limitations of US. Radiology. 1989;170:739–742. doi: 10.1148/radiology.170.3.2644660. [DOI] [PubMed] [Google Scholar]
- 53.Watson HG. RVO--real value obscure. J Thromb Haemost. 2011;9:1116–1118. doi: 10.1111/j.1538-7836.2011.04296.x. [DOI] [PubMed] [Google Scholar]
- 54.Le Gal G, Kovacs MJ, Carrier M, Do K, Kahn SR, Wells PS, Anderson DA, Chagnon I, Solymoss S, Crowther M, Righini M, Perrier A, White RH, Vickars L, Rodger M. Validation of a diagnostic approach to exclude recurrent venous thromboembolism. J Thromb Haemost. 2009;7:752–759. doi: 10.1111/j.1538-7836.2009.03324.x. [DOI] [PubMed] [Google Scholar]
- 55.Tan M, Mol GC, van Rooden CJ, Klok FA, Westerbeek RE, Iglesias Del Sol A, van de Ree MA, de Roos A, Huisman MV. Magnetic resonance direct thrombus imaging differentiates acute recurrent ipsilateral deep vein thrombosis from residual thrombosis. Blood. 2014;124:623–627. doi: 10.1182/blood-2014-04-566380. [DOI] [PubMed] [Google Scholar]
- 56.van Ramshorst B, van Bemmelen PS, Hoeneveld H, Faber JA, Eikelboom BC. Thrombus regression in deep venous thrombosis. Quantification of spontaneous thrombolysis with duplex scanning. Circulation. 1992;86:414–419. doi: 10.1161/01.cir.86.2.414. [DOI] [PubMed] [Google Scholar]
- 57.Meissner MH, Caps MT, Bergelin RO, Manzo RA, Strandness DE Jr. Propagation, rethrombosis and new thrombus formation after acute deep venous thrombosis. J Vasc Surg. 1995;22:558–567. doi: 10.1016/s0741-5214(95)70038-2. [DOI] [PubMed] [Google Scholar]
- 58.Donadini MP, Ageno W, Antonucci E, Cosmi B, Kovacs MJ, Le Gal G, Ockelford P, Poli D, Prandoni P, Rodger M, Saccullo G, Siragusa S, Young L, Bonzini M, Caprioli M, Dentali F, Iorio A, Douketis JD. Prognostic significance of residual venous obstruction in patients with treated unprovoked deep vein thrombosis: a patient-level meta-analysis. J Thromb Haemost. 2014;111:172–179. doi: 10.1160/TH13-04-0336. [DOI] [PubMed] [Google Scholar]
- 59.Palareti G. Do isolated calf deep vein thrombosis need anticoagulant treatment? J Thorac Dis. 2016;8:E1691–E1693. doi: 10.21037/jtd.2016.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]