Abstract
Background –
There are few data on sex differences in suspected cardiac sarcoidosis.
Methods –
Consecutive patients with histologically proven sarcoidosis and suspected cardiac involvement were studied. We investigated sex differences in presenting features, cardiac involvement, and the long-term incidence of a primary composite endpoint of all-cause death or significant ventricular arrhythmia, and secondary endpoints of all-cause death and significant ventricular arrhythmia.
Results –
Among 324 patients, 163 (50.3%) were female and 161 (49.7%) were male patients. Female patients had a greater prevalence of chest pain (37.4% vs. 23.6%; p=0.010) and palpitations (39.3% vs. 26.1%; p=0.016) than male patients, but not dyspnea, presyncope, syncope, or arrhythmias at presentation. Female patients had a lower prevalence of late gadolinium enhancement on cardiovascular magnetic resonance imaging (20.2% vs. 35.4%; p=0.003) and less often met criteria for a clinical diagnosis of cardiac sarcoidosis (Heart Rhythm Society consensus criteria, 22.7% vs. 36.0%; p=0.012, and 2016 Japanese Circulation Society guideline criteria, 8.0% vs. 19.3%; p=0.005), indicating lesser cardiac involvement. However, the long-term incidence of all-cause death or significant ventricular arrhythmia was not different between female and male patients (23.2% vs. 23.2%; p=0.46). Among the secondary endpoints, the incidence of all-cause death was not different between female and male patients (20.7% vs. 14.3%; p=0.51), while female patients had a lower incidence of significant ventricular arrhythmia compared with male patients (4.3% vs. 13.0%; p=0.022). On multivariable analyses, sex was not associated with the primary endpoint (hazard ratio for female patients 1.36; 95% confidence interval 0.77–2.43; p=0.29).
Conclusions –
We observed distinct sex differences in patients with suspected cardiac sarcoidosis. A paradox was identified wherein female patients had a greater prevalence of chest pain and palpitations than male patients, but lesser cardiac involvement, and a similar long-term incidence of all-cause death or significant ventricular arrhythmia.
Keywords: Sarcoidosis, Cardiac Sarcoidosis, Ventricular Arrhythmia, Prognosis, Sex Differences
INTRODUCTION
Sarcoidosis is a multisystem, granulomatous disorder of unknown cause. Cardiac involvement is increasingly recognized as an important cause of heart failure, arrhythmia, and mortality in sarcoidosis.1 The increasing use of advanced cardiac imaging such as cardiac magnetic resonance (CMR) and positron emission tomography (PET) has led to greater recognition of cardiac sarcoidosis.2, 3 However, many clinical characteristics of cardiac sarcoidosis remain poorly defined.4, 5
Sex differences in clinical characteristics, imaging findings, and clinical outcomes have been described in many cardiovascular conditions including atherosclerotic heart disease, myocardial infarction without obstructive coronary artery disease, spontaneous coronary artery dissection, and stress cardiomyopathy. Sex differences have also been described in sarcoidosis, with a higher incidence among female patients.6–8 Data from the United States National Inpatient Sample also showed a significant preponderance of female patients among sarcoidosis patients who were hospitalized.9 However, sex differences in patients with suspected cardiac sarcoidosis have not been systematically studied. Investigating sex differences in the clinical presentation of patients with suspected cardiac sarcoidosis, cardiac involvement on imaging, and long-term outcomes could, thus, uncover differences with clinical, public health, and policy implications.
In this retrospective cohort study, we investigated sex differences in patients with histologically proven sarcoidosis who were suspected to have cardiac involvement. Specifically, we investigated differences in their clinical presentation, CMR findings, and long-term clinical outcomes.
METHODS
De-identified data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Cohort
We included consecutive adult patients with histologically proven sarcoidosis at the University of Minnesota who had CMR for evaluation of cardiac sarcoidosis suspected based on symptoms or electrocardiographic abnormalities suggestive of cardiac sarcoidosis.10 To avoid the confounding effects of sex differences related to coronary artery disease, patients with known coronary artery disease (defined as prior myocardial infarction, percutaneous intervention, coronary artery bypass surgery, or known obstructive coronary artery disease) were excluded from this study. Demographic data, medical history including symptoms around the time of the CMR, comorbidities, medications, and outcome data were collected blinded to CMR data as previously described.11, 12 This retrospective cohort study was approved by the University of Minnesota’s Institutional Review Board with a waiver of informed consent.
Cardiovascular Magnetic Resonance Imaging Protocol
CMR was performed on clinical 1.5 Tesla scanners (Siemens Avanto or Siemens Aera, Malvern, Pennsylvania) according to standard recommendations, as previously described.11, 12 CMR was done using a CMR protocol consisting of localizers to identify the cardiac position, cine CMR for anatomic and functional assessment using a steady-state free precession sequence in short-axis (every 10 mm to cover the entire left ventricle [LV] from the mitral valve plane through the apex), and 3 (2-, 3-, and 4-chamber) long-axis views, and late gadolinium enhancement (LGE) CMR performed 10 to 15 min after gadolinium contrast administration, using a 2-dimensional segmented inversion-recovery sequence, in views identical to the cine CMR images.
Cardiovascular Magnetic Resonance Imaging Analyses
CMR analyses were performed blinded to clinical information as previously described.11, 12 LV and right ventricular (RV) ejection fractions (EF) were determined by quantitative analysis according to standard recommendations. LGE was identified visually. In patients with LV LGE, the extent was quantified using the signal threshold versus reference myocardium approach using a >5 standard deviation (SD) threshold for LGE.11, 12
Diagnosis of Cardiac Sarcoidosis
Cardiac sarcoidosis was diagnosed by the Heart Rhythm Society (HRS) consensus criteria10 and separately by the 2016 Japanese Circulation Society (JCS) guideline criteria13 (both criteria are listed in the Supplemental Material). We used both criteria because there is no consensus on the best way to diagnose cardiac sarcoidosis and we wished to investigate whether a sex difference in cardiac involvement was related to the criteria used for diagnosis. By both criteria, a histological diagnosis can be made by the identification of non-caseating epithelioid granulomas in cardiac tissue, or a clinical diagnosis can be made in those not undergoing or with a negative cardiac biopsy when non-caseating epithelioid granulomas have been identified in organ(s) other than the heart, and clinical features (arrhythmia, electrocardiographic, and/or cardiac imaging features) are suggestive of cardiac involvement.10, 13 Since our objective was to study patients with extracardiac sarcoidosis and suspected cardiac involvement, we did not include patients with isolated cardiac sarcoidosis.
Clinical Follow-Up and Endpoints
Follow-up data were assembled through a review of electronic medical records. The primary endpoint was a composite of all-cause death or significant ventricular arrhythmia, defined as sustained ventricular tachycardia (duration >30 s) or appropriate implantable cardioverter-defibrillator (ICD) therapy (shock or antitachycardia pacing). Secondary endpoints were all-cause death and significant ventricular arrhythmia. The appropriateness of ICD therapies was adjudicated by cardiac electrophysiologists as part of the patients’ clinical care using intracardiac electrograms recorded by the ICD, and based on tachycardia rate, onset, stability, atrioventricular association, and the QRS morphology. Mortality status and death dates were also cross verified with the Minnesota State Department of Health’s Office of Vital Records. For patients who died outside the hospital, death certificates were reviewed to determine the cause of death.
Statistical Analyses
Continuous variables were compared between female and male patients using the t-test and presented as means with standard deviations. Non-normal continuous data were compared with the Mann-Whitney tests and presented as medians with interquartile ranges. Categorical variables were compared with chi-square or Fisher’s exact tests and presented as counts with proportions. The cumulative incidence of the primary endpoint was estimated using the Kaplan-Meier method and hazard ratios (HR) were calculated using Cox proportional hazards regression and presented with their associated 95% confidence intervals (CIs). Multivariable Cox proportional hazards regression modeling was done to account for relevant confounders of the association of sex with the primary outcome. The covariates of age, clinical diagnosis of cardiac sarcoidosis by the HRS consensus criteria, LVEF, RVEF, and LGE extent were chosen a priori based on prior published literature and our clinical experience with cardiac sarcoidosis. The Cox proportional hazards assumption was tested using Schoenfeld residuals. Statistical significance was defined as a two-tailed p value of <0.05. Statistical analyses were done in RStudio version 1.2.5042 (RStudio, Boston, Massachusetts and R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics (Table 1)
Table 1.
Patient Characteristics at the CMR – Overall and Stratified by Sex
| Overall Cohort (n = 324) |
Female patients (n = 163) |
Male patients (n = 161) |
P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 52.3 (12.2) | 53.5 (13.2) | 51.2 (11.0) | 0.09 |
| Body mass index (kg/m2) | 31.0 (7.29) | 30.8 (7.6) | 31.1 (6.9) | 0.67 |
| Race | ||||
| White | 253 (78.1) | 129 (79.1) | 124 (77.0) | 0.74 |
| Black | 60 (18.5) | 31 (19.0) | 29 (18.0) | 0.93 |
| Comorbidities | ||||
| Hypertension | 156 (48.1) | 82 (50.3) | 74 (46.0) | 0.50 |
| Diabetes mellitus | 64 (19.8) | 37 (22.7) | 27 (16.8) | 0.23 |
| Dyslipidemia | 136 (42.0) | 71 (43.6) | 65 (40.4) | 0.64 |
| Tobacco use | ||||
| Current use | 31 (9.6) | 13 (8.0) | 18 (11.2) | 0.43 |
| Former use | 126 (38.9) | 56 (34.4) | 70 (43.5) | 0.12 |
| Pulmonary hypertension | 47 (14.5) | 24 (14.7) | 23 (14.3) | >0.99 |
| Extracardiac sarcoidosis involvement | ||||
| Lung | 293 (90.4) | 143 (87.7) | 150 (93.2) | 0.14 |
| Skin | 35 (10.8) | 22 (13.5) | 13 (8.1) | 0.16 |
| Eyes | 23 (7.1) | 11 (6.7) | 12 (7.5) | 0.98 |
| Liver | 21 (6.5) | 13 (8.0) | 8 (5.0) | 0.38 |
| Central nervous system | 13 (4.0) | 7 (4.3) | 6 (3.7) | 1.00 |
| Clinical symptoms | ||||
| Any | 255 (78.7) | 135 (82.8) | 120 (74.5) | 0.092 |
| Chest pain | 99 (30.6) | 61 (37.4) | 38 (23.6) | 0.010 |
| Palpitations | 106 (32.7) | 64 (39.3) | 42 (26.1) | 0.016 |
| Dyspnea | 88 (27.2) | 39 (23.9) | 49 (30.4) | 0.23 |
| Presyncope | 66 (20.4) | 37 (22.7) | 29 (18.0) | 0.36 |
| Syncope | 19 (5.9) | 7 (4.3) | 12 (7.5) | 0.33 |
| Arrhythmia | ||||
| Ventricular arrhythmia | 99 (30.6) | 50 (30.7) | 49 (30.4) | 0.96 |
| Premature ventricular complexes | 89 (27.5) | 49 (30.1) | 40 (24.8) | 0.35 |
| Non-sustained ventricular tachycardia | 29 (9.0) | 10 (6.1) | 19 (11.8) | 0.11 |
| Sustained ventricular tachycardia | 9 (2.8) | 3 (1.8) | 6 (3.7) | 0.33 |
| Ventricular fibrillation/cardiac arrest | 3 (0.9) | 2 (1.2) | 1 (0.6) | 1.00 |
| Supraventricular tachycardia | 28 (8.6) | 13 (8.0) | 15 (9.3) | 0.82 |
| Atrial fibrillation/flutter | 38 (11.7) | 13 (8.0) | 25 (15.5) | 0.052 |
| Atrioventricular block ≥2nd degree | 15 (4.6) | 5 (3.1) | 10 (6.2) | 0.28 |
| Medications | ||||
| Aspirin | 87 (26.9) | 41 (25.2) | 46 (28.6) | 0.57 |
| Statins | 85 (26.2) | 47 (28.8) | 38 (23.6) | 0.35 |
| ACE-I/ARB | 84 (25.9) | 38 (23.3) | 46 (28.6) | 0.34 |
| Beta-blockers | 91 (28.1) | 46 (28.2) | 45 (28.0) | >0.99 |
| Steroids | 115 (35.5) | 56 (34.4) | 59 (36.6) | 0.75 |
| Non-steroid immunomodulators | 56 (17.3) | 25 (15.3) | 31 (19.3) | 0.43 |
Values are n (%), mean ± SD, or median (interquartile range).
ACE-I = Angiotensin Converting Enzyme-Inhibitor; ARB = Angiotensin Receptor Blocker; BMI = Body Mass Index
This study included 324 patients with histologically proven sarcoidosis and suspected cardiac involvement, of whom, 163 (50.3%) were female and 161 (49.7%) were male patients. The mean age of the overall cohort was 52.3 ± 12.2 years. There were no differences in the demographics and the prevalence of comorbidities between female and male patients.
Sex Differences in the Clinical Presentation (Table 1)
Overall, 135 female (82.8%) and 120 male (74.5%) patients presented with one or more symptoms of chest pain, palpitations, dyspnea, presyncope, or syncope (p = 0.092). Dyspnea, chest pain and palpitations were common presenting symptoms. Female patients reported having a greater prevalence of chest pain (37.4% vs. 23.6%; p = 0.010) and palpitations (39.3% vs. 26.1%; p = 0.016) than male patients. There were no differences in the prevalence of dyspnea, presyncope, or syncope between female and male patients. There were no differences in the prevalence of ventricular or supraventricular arrhythmias or atrioventricular block between female and male patients. Similarly, there was no difference in the use of cardiovascular or immunosuppressant medications between female and male patients. At the time of evaluation for cardiac sarcoidosis, 35.5% of the overall cohort was receiving steroids and 17.3% of the overall cohort was receiving non-steroid immunosuppressants, without differences between the sexes.
Sex Differences in Cardiovascular Magnetic Resonance Imaging Findings (Table 2)
Table 2.
CMR Characteristics – Overall and Stratified by Sex
| Overall Cohort (n = 324) |
Female patients (n = 163) |
Male patients (n = 161) |
P value | |
|---|---|---|---|---|
| LVEF (%) | 56.9 (51.4, 60.9) | 58.4 (54.7, 62.0) | 55.3 (48.4, 59.6) | <0.001 |
| LVEDVI (mL/m2) | 60.7 (49.7, 72.8) | 58.5 (48.7, 69.6) | 63.3 (52.3, 77.3) | 0.005 |
| LVESVI (mL/m2) | 26.3 (20.2, 33.2) | 24.3 (18.6, 29.3) | 28.5 (21.5, 35.9) | <0.001 |
| RVEF (%) | 52.9 (49.9, 57.9) | 55.3 (51.5, 60.1) | 50.8 (46.1, 55.9) | <0.001 |
| RVEDVI (mL/m2) | 62.1 (52.3, 72.0) | 57.4 (49.0, 67.2) | 66.1 (56.0, 76.7) | <0.001 |
| RVESVI (mL/m2) | 27.6 (22.8, 35.0) | 25.4 (21.1, 30.0) | 31.6 (25.9, 39.9) | <0.001 |
| LV LGE presence | 90 (27.8) | 33 (20.2) | 57 (35.4) | 0.003 |
| RV LGE presence | 18 (5.6) | 7 (4.3) | 11 (6.8) | 0.45 |
| LV LGE extent* (%) | 5.3 (2.5, 12.1) | 4.8 (2.7, 10.0) | 5.5 (2.3, 13.3) | 0.65 |
Values are n (%), mean ± SD, or median (interquartile range).
Among patients with LGE.
CMR = Cardiovascular Magnetic Resonance; EDVI = End-Diastolic Volume Index, EF = Ejection Fraction; ESVI = End-Systolic Volume Index; LGE = Late Gadolinium Enhancement; LV = Left Ventricle; RV = Right Ventricle
Female patients had higher LVEF (58.4% vs. 55.3%; p <0.001) and RVEF (55.3% vs. 50.8%; p <0.001) than male patients. Female patients had smaller LV and RV volumes than male patients even after indexing to body surface area. Female patients had a lower prevalence of LV LGE than male patients (20.2% vs. 35.4%; p = 0.003). Among those with LGE, the LGE extent was not different between female and male patients (4.8% vs. 5.5%; p = 0.65).
Sex Differences in the Diagnosis of Cardiac Sarcoidosis (Table 3)
Table 3.
Diagnosis of Cardiac Sarcoidosis – Overall and Stratified by Sex
| Overall Cohort (n = 324) |
Female patients (n = 163) |
Male patients (n = 161) |
P value | |
|---|---|---|---|---|
| Histological diagnosis | 11 (3.4) | 4 (2.5) | 7 (4.3) | 0.53 |
| Clinical diagnosis by HRS consensus criteria | 95 (29.3) | 37 (22.7) | 58 (36.0) | 0.012 |
| Clinical diagnosis by 2016 JCS guideline criteria | 44 (13.6) | 13 (8.0) | 31 (19.3) | 0.005 |
Values are n (%).
HRS = Heart Rhythm Society; JCS = Japanese Circulation Society
Among the overall cohort, 3.4% had a histological diagnosis of cardiac sarcoidosis, while 29.3% met the HRS consensus criteria and 13.6% met the 2016 JCS guideline criteria for the clinical diagnosis of cardiac sarcoidosis. The difference in the proportions of patients meeting the two criteria for the clinical diagnosis of cardiac sarcoidosis may be explained by the fact that the HRS consensus criteria require fulfillment of ≥1 of 7 criteria for clinical involvement while the 2016 JCS guideline criteria require either ≥2 of 5 major criteria, or 1 major and ≥2 of 3 minor criteria (both sets of criteria are listed in the Supplemental Material). There were no differences between the proportions of female and male patients who had a histological diagnosis of cardiac sarcoidosis (2.5% vs. 4.3%; p = 0.53), but a smaller proportion of female patients met the criteria for the clinical diagnosis of cardiac sarcoidosis; female patients were 0.6 and 0.4 times as likely as male patients to have clinical cardiac sarcoidosis using the HRS consensus criteria and the 2016 JCS guideline criteria, respectively. Thus, the sex difference in cardiac involvement did not differ based on the criteria used for diagnosis.
Sex Differences in Clinical Outcomes
During the study period, 10 (6.1%) female and eight (5.0%) male patients received permanent pacemakers (p = 0.83), and 17 (10.4%) female and 31 (19.3%) male patients received ICDs (p = 0.038). Of those who received permanent pacemakers, three (30%) female and four (50%) male patients had their devices changed to ICDs during the study period. At a median follow-up of 3.9 years (interquartile range 1.9–6.0 years), 53 patients reached the primary composite endpoint. The total follow-up was 1337.4 patient-years. Thirty-five patients died and 22 had significant ventricular arrhythmias. Of the patients experiencing the primary endpoint, 25 were female and 28 were male. Among the secondary endpoints, 20 female and 15 male patients died, while 6 female and 16 male patients had significant ventricular arrhythmias. Of the 35 deaths, the causes were known for 31; they were cardiac in 7 (3 in female and 4 in male patients) and non-cardiac in 24 (15 in female and 9 in male patients). Of the 4 patients with deaths of undetermined causes, 2 were female and 2 were male patients.
On Kaplan-Meier analyses (Figure 1), the cumulative incidence of the primary endpoint at 8 years was not significantly different between female and male patients (23.2% vs. 23.2%; log-rank p = 0.46). Similarly, the cumulative incidence of all-cause death at 8 years was not significantly different between female and male patients (20.7% vs. 14.3%; log-rank p = 0.51) (Figure 2). There was no difference in the cause of death between female and male patients (p = 0.62). However, female patients had a significantly lower cumulative incidence of significant ventricular arrhythmia compared with male patients (4.3% vs. 13.0%; log-rank p = 0.022) (Figure 3).
Figure 1. Kaplan-Meier Analyses for the Primary Composite Endpoint.
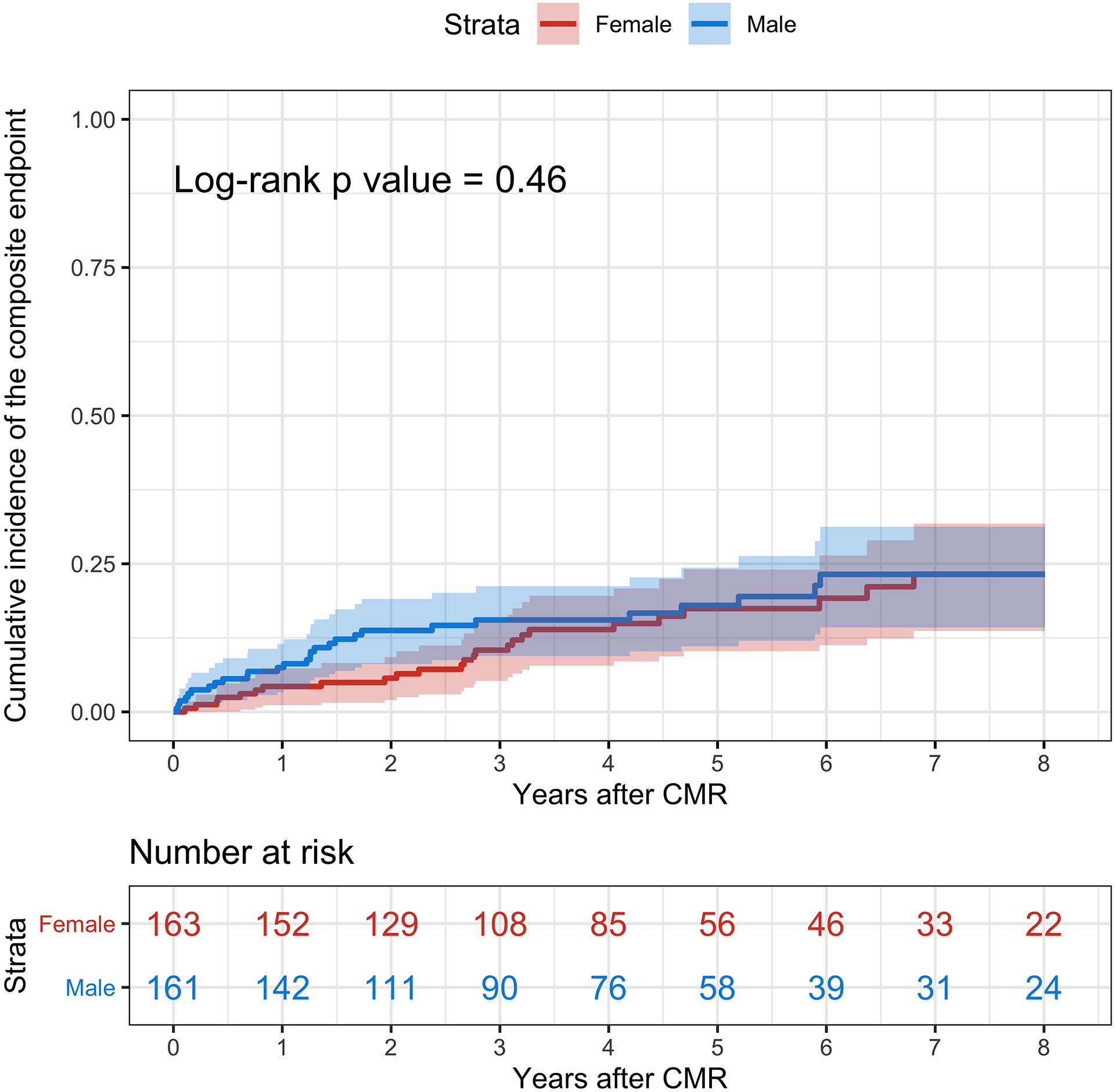
The cumulative incidence of the primary composite endpoint of all-cause death and significant ventricular arrhythmias is represented in red for female patients and blue for male patients. The shaded areas represent the 95% confidence interval. CMR = Cardiovascular Magnetic Resonance.
Figure 2. Kaplan-Meier Analyses for All-cause Death.
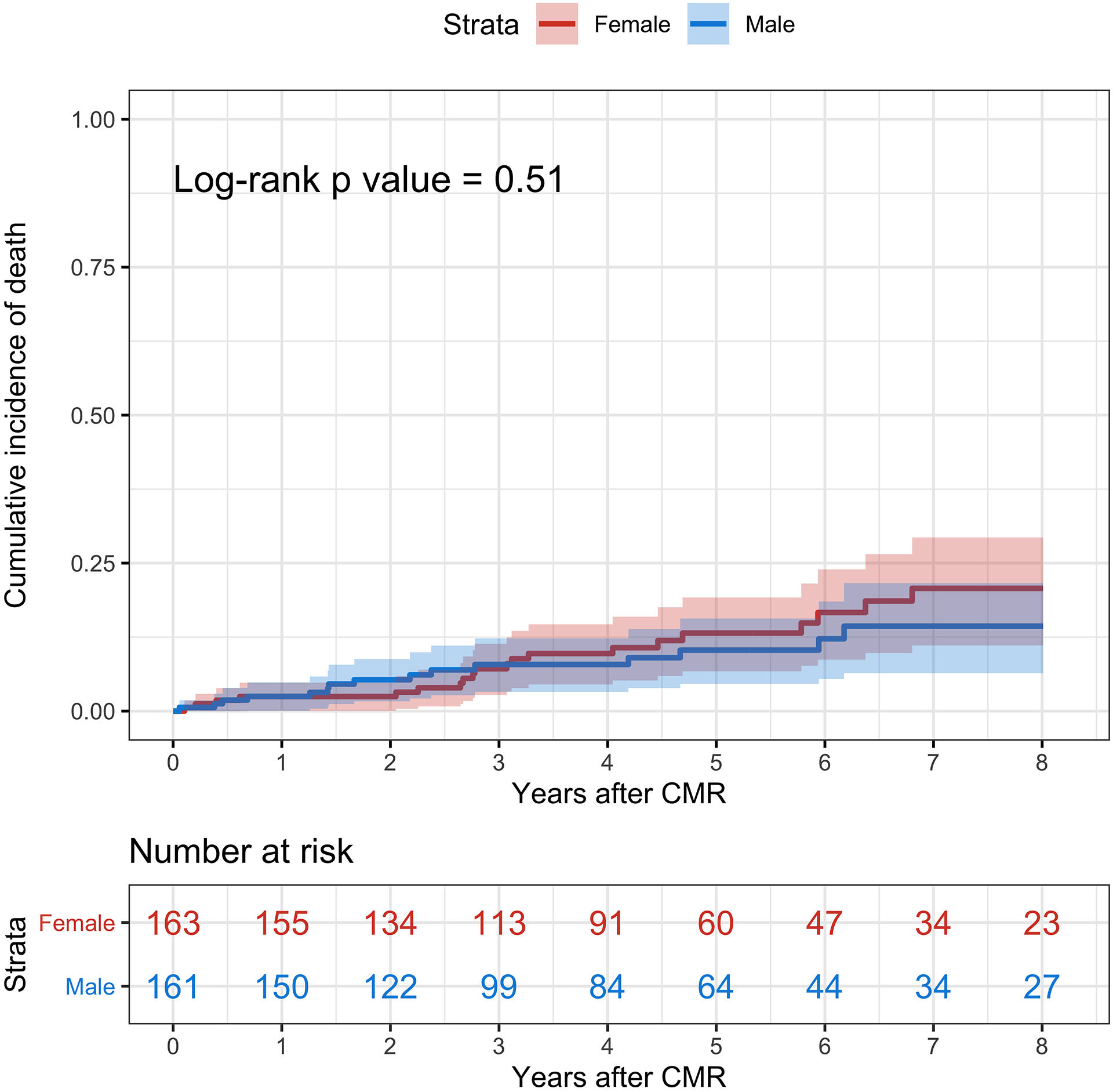
The cumulative incidence of all-cause death is represented in red for female patients and blue for male patients. The shaded areas represent the 95% confidence interval. CMR = Cardiovascular Magnetic Resonance.
Figure 3. Kaplan-Meier Analyses for Significant Ventricular Arrhythmias.
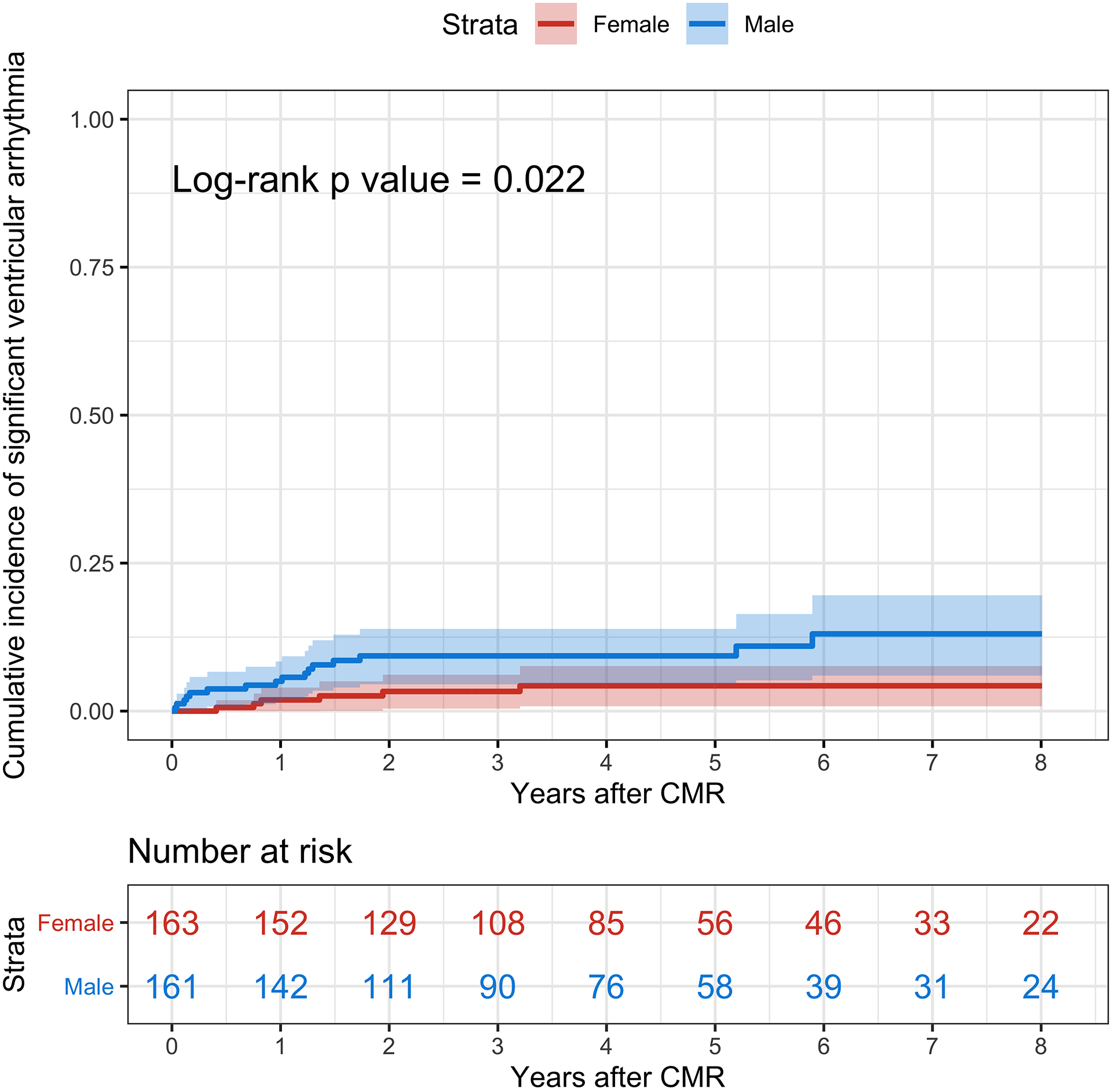
The cumulative incidence of significant ventricular arrhythmias is represented in red for female patients and blue for male patients. The shaded areas represent the 95% confidence interval. CMR = Cardiovascular Magnetic Resonance.
On unadjusted Cox proportional hazards regression analyses, sex was not associated with the primary endpoint (HR 0.82 for female compared to male patients, 95% CI, 0.48–1.40; p = 0.46). On adjusted Cox proportional hazards regression analyses (Table 4), sex was not associated with the primary endpoint (HR 1.36 for female compared to male patients, 95% CI, 0.77–2.43; p = 0.29), after adjustment for age, clinical diagnosis of cardiac sarcoidosis by the HRS consensus criteria, LVEF, RVEF, and LGE extent. Independent predictors of the primary endpoint were a clinical diagnosis of cardiac sarcoidosis by the HRS consensus criteria, RVEF, and LGE extent. The proportional hazards assumption was valid for all the model covariates and the global model (p = 0.22).
Table 4.
Multivariable Regression Analyses for the Primary Composite Endpoint
| HR | 95% CI | P value | |
|---|---|---|---|
| Female patients (vs. male patients) | 1.36 | 0.77–2.43 | 0.29 |
| Age (per 5 years increase) | 1.07 | 0.93–1.22 | 0.35 |
| Clinical diagnosis of cardiac sarcoidosis by HRS consensus criteria | 2.69 | 1.49–4.84 | <0.001 |
| LV EF (per 5% decrease) | 0.96 | 0.85–1.10 | 0.59 |
| RV EF (per 5% decrease) | 1.26 | 1.09–1.46 | 0.002 |
| LV LGE extent (per 5% increase) | 1.61 | 1.38–1.89 | <0.001 |
CI = Confidence Interval; EF = Ejection Fraction; HR = Hazard Ratio; HRS = Heart Rhythm Society; LGE = Late Gadolinium Enhancement; LV = Left Ventricle, RV = Right Ventricle
DISCUSSION
Our analysis provides novel insights into sex differences in presenting symptoms, CMR findings, and long-term clinical outcomes in a large cohort of patients with histologically proven sarcoidosis and suspected cardiac involvement. Female patients had a greater prevalence of chest pain and palpitations at presentation than male patients, but female patients were less likely to meet the criteria for a clinical diagnosis of cardiac sarcoidosis. On CMR, female patients had higher EFs, smaller volumes, and a lower prevalence of LGE, indicating lesser cardiac involvement. There were no differences between female and male patients in the long-term incidence of all-cause death or significant ventricular arrhythmia, although female patients had a lower incidence of significant ventricular arrhythmia compared with male patients.
Investigating sex differences in patients with suspected cardiac sarcoidosis rather than in those with diagnosed cardiac sarcoidosis makes sense for two important reasons. First, while sarcoidosis affects an estimated 200,000 patients in the United States, symptomatic cardiac involvement occurs in only around 5% of patients.14 However, a larger proportion of sarcoidosis patients are suspected of having cardiac involvement and undergo testing. Thus, substantially more patients are suspected of having cardiac sarcoidosis than are diagnosed with cardiac sarcoidosis. Second, there is no consensus on the best approach to diagnose cardiac sarcoidosis in the absence of histopathological examination of cardiac tissue. The currently used criteria are based primarily on expert consensus, not validated using histologically proven cardiac sarcoidosis or long-term clinical outcomes, and poorly concordant as seen in this and other studies.15, 16
Female patients in our study had a higher prevalence than male patients of chest pain and palpitations. A greater prevalence of chest pain in female than male patients was also noted in a survey of 1,026 Dutch sarcoidosis patients with 33.3% of female patients reporting chest pain compared with 20.8% of male patients (p<0.001).17 Extensive epidemiologic and clinical evidence shows that female patients are at increased risk for acute and chronic pain than male patients.18 This difference may have multiple biological, psychological, and social mechanisms involving sex hormones, genetic factors, endogenous opioid function, pain coping, gender roles, and others.18 Alternatively, chest pain and palpitations could be caused by pulmonary sarcoidosis or other noncardiac causes related or unrelated to sarcoidosis.
Female patients in our study had a lower prevalence of cardiac involvement as defined by the HRS consensus criteria, the 2016 JCS guideline criteria, and the presence of LGE. This sex difference in cardiac involvement was also seen in previous studies.
Among 1,815 sarcoidosis patients at the University of Cincinnati, Zhou et al. found that female patients had lower than half the rate of cardiac involvement compared with male patients, based on the refined World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) criteria of highly probable and probable (2.6% vs. 6.6%, p<0.001).19 Among 1,017 Caucasian sarcoidosis patients, Darlington et al. diagnosed cardiac sarcoidosis at a rate of 1.8% in female patients compared with 2.6% in male patients20, using the original Japanese Ministry of Health and Welfare guidelines.21 Finally, among 1,375 Polish sarcoidosis patients, Martusewicz-Boros et al. diagnosed 64 with cardiac sarcoidosis based on LGE, and the prevalence of cardiac involvement was 2.8% in female patients compared with 6.4% in male patients (p = 0.002).22 While the sex differences in cardiac involvement in these studies mirror our findings, only a small minority of patients in each of these cohorts had suspected cardiac involvement or CMR to look for cardiac involvement, unlike in our cohort where all had CMRs. In contrast to our findings, Tuominen et al.23 noted a higher prevalence of abnormal fluorodeoxyglucose (18F-FDG) uptake on PET in female compared with male patients (35% in female vs. 16% in male patients) among 137 patients with suspected cardiac sarcoidosis. It is notable that among the patients in the study, only 19.7% had pulmonary sarcoidosis, and data on histological proof of extracardiac sarcoidosis were not provided. Since the lungs are involved in over 90% of sarcoidosis patients as noted in many large studies14, 19, 24, Tuominen et al.’s cohort appears to be composed mostly of patients who were suspected of having an inflammatory cardiomyopathy, but not specifically cardiac sarcoidosis. This differs from our cohort where all patients had histological proof of sarcoidosis and the prevalence of pulmonary involvement was 90.4%.
Our paradoxical finding of a greater symptom burden in female patients despite lesser cardiac involvement compared with male patients is similar to the findings of greater angina burden in female patients despite less extensive coronary artery disease noted in a large Mayo Clinic cohort study,25 the BARI 2D,26 COURAGE,27 and most recently, ISCHEMIA28 trials. While the mechanisms underlying the paradox are not well understood, microvascular coronary artery dysfunction defined by endothelial dysfunction and limited coronary flow reserve has been implicated as the most likely explanation for ischemia and chest pain in female patients without obstructive coronary artery disease. Many female patients in our cohort were middle-aged and had cardiovascular risk factors; microvascular coronary artery dysfunction may explain the greater prevalence of chest pain in female patients.
Despite lesser cardiac involvement in female than male patients in our study, the incidence of our primary outcome was not different in female compared with male patients. However, the incidence of sustained ventricular arrhythmia was indeed lower in female patients, in concordance with the lower prevalence of cardiac involvement by LGE. We and others have shown that LGE is an excellent predictor of ventricular arrhythmic outcomes in sarcoidosis.11, 12, 29 While the incidence of all-cause death was numerically greater in female patients compared with male patients, it was not statistically different. This suggests that the lower prevalence of cardiac involvement may have little impact on overall mortality in sarcoidosis, of which pulmonary and cardiac disease are the two leading causes.1 Of note, population studies of decedents with sarcoidosis in the United States have identified greater age-adjusted all-cause death rates in female than male patients.30, 31
Our data have important implications. We provide the first systematic data identifying sex differences in the clinical symptoms, CMR findings, and long-term clinical outcomes among patients with suspected cardiac sarcoidosis. These observations lay the groundwork for additional studies to fully understand the interplay between these aspects of the disease and the paradox between symptom burden, cardiac involvement, and long-term clinical outcomes. For instance, the hypothesis that microvascular coronary artery dysfunction explains the greater prevalence of chest pain in female patients with suspected cardiac sarcoidosis could be investigated using vasodilator stress CMR32 or PET. Our findings also make a strong argument for the routine and systematic inclusion of sex-specific analyses in sarcoidosis research. Such practices could eventually lead to an improved understanding of sex differences in the diagnosis, treatment, and prognostication of patients with suspected cardiac sarcoidosis, and promote improved outcomes in both sexes.
Limitations
We studied sarcoidosis patients that were clinically referred for suspected cardiac involvement based on physician judgment, and thus, referral bias is inevitable. Our cohort consists of sarcoidosis patients seen at a single tertiary care academic medical center for evaluation of cardiac sarcoidosis. Eighty percent of our cohort is white. Thus, our findings may not be generalizable to all-comers with sarcoidosis and need replication in a multicenter, more racially diverse cohort. Sex differences in cardiovascular testing could have influenced who was included in our study. We had a modest number of secondary endpoints, which precluded multivariable analyses.
18F-FDG PET was selectively used only in patients with abnormal CMRs to determine the presence and the extent of myocardial inflammation as recommended,33, 34 and therefore, these data were not available in all patients for the study of sex differences. LGE in patients with extracardiac sarcoidosis may not always represent cardiac sarcoidosis. Cardiac monitoring was not universally used; thus, self-limited ventricular arrhythmias could have been underrecognized. Finally, we did not investigate the burden or the severity of extracardiac sarcoidosis, which may influence overall mortality.
CONCLUSIONS
We observed distinct sex differences in patients with histologically proven sarcoidosis who were suspected to have cardiac involvement. A paradox was identified wherein female patients had a greater prevalence of chest pain and palpitations at presentation than male patients, but lesser cardiac involvement based on clinical diagnosis of cardiac sarcoidosis and the presence of LGE. The long-term incidence of all-cause death or significant ventricular arrhythmia was not different between female and male patients.
Supplementary Material
What Is Known
Sex differences in clinical characteristics, imaging findings, and clinical outcomes have been described in many cardiovascular conditions.
Sex differences have also been described in sarcoidosis, with a higher incidence among women compared with men.
What the Study Adds
In this study – the first on the topic – we observed distinct sex differences among patients with histologically proven sarcoidosis and suspected cardiac involvement.
A paradox was identified wherein female patients had a greater prevalence of chest pain and palpitations than male patients, but lesser cardiac involvement, and a similar long-term incidence of all-cause death or significant ventricular arrhythmia.
FUNDING SOURCES
This work was supported by National Institutes of Health grant K23HL132011, a University of Minnesota Clinical and Translational Science Institute KL2 Scholars Career Development Program Award (National Institutes of Health grant KL2TR000113-05), a University of Minnesota Clinical and Translational Science Institute K-R01 Transition to Independence Grant (supported by the National Institutes of Health grant UL1TR002494), and a Lillehei Heart Institute Red Heart Soiree Seed Grant, all awarded to Chetan Shenoy.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- 18F-FDG
fluorodeoxyglucose
- CI
confidence interval
- CMR
cardiovascular magnetic resonance imaging
- EDVI
End-Diastolic Volume Index
- EF
ejection fraction
- ESVI
End-Systolic Volume Index
- HR
hazard ratio
- HRS
Heart Rhythm Society
- ICD
implantable cardiac defibrillator
- JCS
Japanese Circulation Society
- LGE
late gadolinium enhancement
- LV
left ventricle
- PET
positron emission tomography
- RV
right ventricle
- SD
standard deviation
Footnotes
DISCLOSURE STATEMENT
Henri Roukoz has received consulting fees from Boston Scientific and speaking fees from Medtronic. Lisa von Wald has received speaking fees from Medtronic. All other authors have nothing to disclose.
SUPPLEMENTAL MATERIALS
Supplemental Methods.
REFERENCES
- 1.Sauer WH, Stern BJ, Baughman RP, Culver DA and Royal W. High-Risk Sarcoidosis. Current Concepts and Research Imperatives. Ann Am Thorac Soc. 2017;14(Supplement_6):S437–S444. Epub 2017/10/27. doi: 10.1513/AnnalsATS.201707-566OT. [DOI] [PubMed] [Google Scholar]
- 2.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–32. Epub 2014/12/21. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 3.Birnie DH, Nery PB, Ha AC and Beanlands RS. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68(4):411–21. Epub 2016/07/23. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 4.Okasha O, Kazmirczak F, Chen KA, Farzaneh-Far A and Shenoy C. Myocardial Involvement in Patients With Histologically Diagnosed Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis of Gross Pathological Images From Autopsy or Cardiac Transplantation Cases. J Am Heart Assoc. 2019;8(10):e011253. Epub 2019/05/10. doi: 10.1161/JAHA.118.011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trivieri MG, Spagnolo P, Birnie D, Liu P, Drake W, Kovacic JC, Baughman R, Fayad ZA and Judson MA. Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(16):1878–1901. Epub 2020/10/17. doi: 10.1016/j.jacc.2020.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybicki BA, Major M, Popovich J Jr., Maliarik MJ and Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–41. Epub 1997/02/01. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, Oritsu M and Nukiwa T. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31(2):372–9. Epub 2007/10/26. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 8.Brito-Zerón P, Kostov B, Baughman RP and Ramos-Casals M. Geoepidemiology of Sarcoidosis. In: Baughman RP and Valeyre D, eds. Sarcoidosis Philadelphia: Elsevier; 2019: 1–21. [Google Scholar]
- 9.Patel N, Kalra R, Doshi R, Arora H, Bajaj NS, Arora G and Arora P. Hospitalization Rates, Prevalence of Cardiovascular Manifestations, and Outcomes Associated With Sarcoidosis in the United States. J Am Heart Assoc. 2018;7(2). Epub 2018/01/24. doi: 10.1161/JAHA.117.007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–23. Epub 2014/05/14. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Kazmirczak F, Chen KA, Adabag S, von Wald L, Roukoz H, Benditt DG, Okasha O, Farzaneh-Far A, Markowitz J, Nijjar PS, et al. Assessment of the 2017 AHA/ACC/HRS Guideline Recommendations for Implantable Cardioverter-Defibrillator Implantation in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12(9):e007488. Epub 2019/08/23. doi: 10.1161/CIRCEP.119.007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velangi PS, Chen KA, Kazmirczak F, Okasha O, von Wald L, Roukoz H, Farzaneh-Far A, Markowitz J, Nijjar PS, Bhargava M, et al. Right Ventricular Abnormalities on Cardiovascular Magnetic Resonance Imaging in Patients With Sarcoidosis. JACC Cardiovasc Imaging. 2020;13(6):1395–1405. Epub 2020/01/20. doi: 10.1016/j.jcmg.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi-Ueda H, Eishi Y, Kitakaze M, et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis- Digest Version. Circ J. 2019;83(11):2329–2388. Epub 2019/10/11. doi: 10.1253/circj.CJ-19-0508. [DOI] [PubMed] [Google Scholar]
- 14.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr., Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–9. Epub 2001/12/06. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro Neto ML, Jellis C, Hachamovitch R, Wimer A, Highland KB, Sahoo D, Khabbaza JE, Pande A, Bindra A, Southern BD, et al. Performance of diagnostic criteria in patients clinically judged to have cardiac sarcoidosis: Is it time to regroup? Am Heart J. 2020;223:106–109. Epub 2020/04/03. doi: 10.1016/j.ahj.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Ueberham L, Jahnke C, Paetsch I, Klingel K, Kuehl M, Hindricks G and Dinov B. Current Diagnostic Criteria Show a Substantial Disagreement in Classification of Patients With Suspected Cardiac Sarcoidosis. JACC Clin Electrophysiol. 2021;7(4):538–539. Epub 2021/04/24. doi: 10.1016/j.jacep.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 17.De Vries J, Van Heck GL and Drent M. Gender differences in sarcoidosis: symptoms, quality of life, and medical consumption. Women Health. 1999;30(2):99–114. Epub 2000/07/06. doi: 10.1300/j013v30n02_07. [DOI] [PubMed] [Google Scholar]
- 18.Bartley EJ and Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–8. Epub 2013/06/26. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Lower EE, Li HP, Costea A, Attari M and Baughman RP. Cardiac Sarcoidosis: The Impact of Age and Implanted Devices on Survival. Chest. 2017;151(1):139–148. Epub 2016/09/11. doi: 10.1016/j.chest.2016.08.1457. [DOI] [PubMed] [Google Scholar]
- 20.Darlington P, Gabrielsen A, Sorensson P, Cederlund K, Eklund A and Grunewald J. Cardiac involvement in Caucasian patients with pulmonary sarcoidosis. Respir Res. 2014;15:15. Epub 2014/02/11. doi: 10.1186/1465-9921-15-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiraga H, Yuwai K and Hiroe M. The Japanese Ministry of Health and Welfare. Guideline for the diagnosis of cardiac sarcoidosis: study report on diffuse pulmonary diseases (in Japanese) Tokyo, Japan. 1993:23–24. doi. [Google Scholar]
- 22.Martusewicz-Boros MM, Boros PW, Wiatr E, Kempisty A, Piotrowska-Kownacka D and Roszkowski-Sliz K. Cardiac Sarcoidosis: Is it More Common in Men? Lung. 2016;194(1):61–6. Epub 2015/09/29. doi: 10.1007/s00408-015-9805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuominen H, Haarala A, Tikkakoski A, Korkola P, Kahonen M, Nikus K and Sipila K. (18)F-FDG-PET in Finnish patients with clinical suspicion of cardiac sarcoidosis: Female sex and history of atrioventricular block increase the prevalence of positive PET findings. J Nucl Cardiol. 2019;26(2):394–400. Epub 2017/06/07. doi: 10.1007/s12350-017-0940-x. [DOI] [PubMed] [Google Scholar]
- 24.Judson MA, Boan AD and Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):119–27. Epub 2013/03/07. doi. [PubMed] [Google Scholar]
- 25.Bell MR, Berger PB, Holmes DR Jr., Mullany CJ, Bailey KR and Gersh BJ. Referral for coronary artery revascularization procedures after diagnostic coronary angiography: evidence for gender bias? J Am Coll Cardiol. 1995;25(7):1650–5. Epub 1995/06/01. doi: 10.1016/0735-1097(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 26.Tamis-Holland JE, Lu J, Bittner V, Magee MF, Lopes N, Adler DS, Kip KE, Schwartz L, Groenewoud YA, Jacobs AK, et al. Sex, clinical symptoms, and angiographic findings in patients with diabetes mellitus and coronary artery disease (from the Bypass Angioplasty Revascularization Investigation [BARI] 2 Diabetes trial). Am J Cardiol. 2011;107(7):980–5. Epub 2011/02/01. doi: 10.1016/j.amjcard.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Acharjee S, Teo KK, Jacobs AK, Hartigan PM, Barn K, Gosselin G, Tanguay JF, Maron DJ, Kostuk WJ, Chaitman BR, et al. Optimal medical therapy with or without percutaneous coronary intervention in women with stable coronary disease: A pre-specified subset analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation (COURAGE) trial. Am Heart J. 2016;173:108–17. Epub 2016/02/28. doi: 10.1016/j.ahj.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, Picard MH, Kwong RY, Bairey-Merz CN, Cyr DD, et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020;5(7):773–786. Epub 2020/04/01. doi: 10.1001/jamacardio.2020.0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman GC, Shaw PW, Balfour PC Jr., Gonzalez JA, Kramer CM, Patel AR and Salerno M. Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovasc Imaging. 2017;10(4):411–420. Epub 2016/07/28. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gideon NM and Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100(4):423–7. Epub 1996/04/01. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 31.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D and Brown KK. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–30. Epub 2011/02/19. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Indorkar R, Kwong RY, Romano S, White BE, Chia RC, Trybula M, Evans K, Shenoy C and Farzaneh-Far A. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovasc Imaging. 2019;12(8 Pt 2):1686–1695. Epub 2018/11/10. doi: 10.1016/j.jcmg.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Slart R, Glaudemans A, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, Jaber WA, Russell R, Gimelli A, Rouzet F, et al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol. 2018;25(1):298–319. Epub 2017/10/19. doi: 10.1007/s12350-017-1043-4. [DOI] [PubMed] [Google Scholar]
- 34.Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, Abston E, Bernstein RC, Blankstein R, Chen ES, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(8):e26–e51. Epub 2020/04/16. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


