Abstract
With the continuous development of digital medicine, minimally invasive precision and safety have become the primary development trends in hepatobiliary surgery. Due to the specificity and complexity of hepatobiliary surgery, traditional preoperative imaging techniques such as computed tomography and magnetic resonance imaging cannot meet the need for identification of fine anatomical regions. Imaging-based three-dimensional (3D) reconstruction, virtual simulation of surgery and 3D printing optimize the surgical plan through preoperative assessment, improving the controllability and safety of intraoperative operations, and in difficult-to-reach areas of the posterior and superior liver, assistive robots reproduce the surgeon’s natural movements with stable cameras, reducing natural vibrations. Electromagnetic navigation in abdominal surgery solves the problem of conventional surgery still relying on direct visual observation or preoperative image assessment. We summarize and compare these recent trends in digital medical solutions for the future development and refinement of digital medicine in hepatobiliary surgery.
Keywords: Hepatobiliary surgery, Three-dimensional visualization, Three-dimensional printing, Electromagnetic tracking, Real-time navigation, Robot-assisted surgery
Core Tip: This paper analyzes the latest trends in three-dimensional visualization, robot-assisted surgery, and electromagnetic intraoperative navigation in hepatobiliary surgery and summarizes the advantages and limitations of existing technologies and potential solution strategies. It also analyzes existing real-time intraoperative navigation, compares optical tracking navigation to electromagnetic tracking navigation with a focus on the advantages and existing limitations, and attempts to improve the program as an educational learning tool for new physicians. Additionally, it aims to popularize hepatobiliary surgery as digital medicine and tries to illustrate a direction for the advancement and development of digital medicine in hepatobiliary surgery.
INTRODUCTION
The safety and effectiveness of hepatobiliary surgery is based on knowledge of the detailed anatomy of the hepatobiliary structures, but the structure of the liver is complex and the vascularity and anatomy of the bile ducts at the hilum are prone to variation[1]. Traditional surgery based on two-dimensional (2D) images to visualize the three-dimensional (3D) spatial relationships of anatomical structures in the mind in order to complete the operation, but it’s a significant challenge for new inexperienced surgeons. 3D visualization, digital imaging and 3D printing can clearly show the 3D spatial relationship of the lesion site, which can help with difficult intrahepatic vein reconstruction and blood supply assessment as well as biliary vein drainage problems, enabling surgeons to better plan their operations and pushing surgery towards precision and minimally invasive surgery. The development of robot-assisted surgery can overcome the disadvantages of traditional laparoscopy in hepatobiliary surgery, such as inadequate depth perception, inevitable hand tremors, and the surgeon's greater susceptibility to fatigue after prolonged surgery, helping surgeons to be more flexible in operating on delicate sites[2,3]. Intraoperative navigation reduces the practical uncertainty of the operation and the deformation and displacement of tissues, and evolving digital medicine is helping surgeons to optimize preoperative planning, perform precise and safe intraoperative procedures and carry out accurate postoperative analysis[4,5].
THREE-DIMENSINAL VISUALIZATION IMAGES AND THEIR EXTENSION
The segmental anatomy of the liver and the anatomy of the blood vessels and bile ducts are diverse and the presence of various anatomical variants requires individualized surgical plans to ensure that the operation is carried out safely. Experienced surgeons can sketch a 3D image in their minds based on preoperative 2D images such as computed tomography (CT) plane magnetic resonance imaging (MRI) to complete the operation successfully, but it is a significant challenge for surgeons new to the profession[6,7]. 3D visualization and 3D printing technologies can clearly show the specific spatial anatomy of a lesion and can help young surgeons optimize their surgical plans, which can be used for liver resection, liver transplantation, radiofrequency ablation, transjugular intrahepatic portosystemic shunts, gallstones, gallbladder cancer and many other diseases[4,8,9]. Especially in the case of malignant liver tumor resection, the application of 3D visualization and 3D printing technology allows for accurate preoperative assessment, simulation and optimization of the surgical plan to ensure that the operation is carried out safely[10].
3D visualization
With the rapid development of digital medicine, 3D visualization images are increasingly used in the diagnosis and treatment of hepatobiliary diseases, and more and more companies are developing 3D visualization software for medical use, such as Liversim, Mint Liver, etc.[11]. A large amount of fine stereoscopic data helps surgeons to clearly identify the anatomical relationship of the lesion before surgery, helping the team to share accurate 3D surgical images[12]. Particularly for surgery on hepatobiliary malignancies, 3D visualization technology also allows for a comprehensive assessment of the vasculature and evaluation of variants, which helps the surgery to unfold safely[13,14].
As shown in Table 1, Miyamoto et al[15] used 3D visualization images to diagnose parabile ducts in patients with cholangiocarcinoma that could not be detected by multilayer spiral CT and magnetic resonance cholangiopancreatography. Zeng et al[16] conducted a retrospective study of patients with type-III hilar cholangiocarcinoma using 3D modelling, demonstrating the safety and efficacy of 3D visualization. Nakayama et al[13] retrospectively analyzed 240 consecutive patients undergoing liver resection and demonstrated the effectiveness of 3D simulation to help surgeons effectively reduce operative time. Lin et al[17] explored the value of 3D visualization in pancreatic resection and validated the effectiveness of 3D visualization images to help surgeons plan surgery.
Table 1.
Three-dimensional visualization and robot-assisted surgery in recent years
| Surgical site | Sample size | Patient type of disease | Imaging systems | Incidence of complications (%) | Summary of technology | Ref. | |
| 3D visualization | Bile duct department | 1 | Extrahepatic cholangiocarcinoma combined with paracolic bile duct | Synapse Vincent | 0 | Accuracy and reliability | Miyamoto et al[15], 2014 |
| Hepatic portal | 47 | Type-III cholangiocarcinoma of the porta hepatis | MI-3DVS | Safety, effectiveness, and feasibility | Zeng et al[16], 2016 | ||
| Liver | 120 | Hepatocellular carcinoma, bile duct cancer, liver transplantation | Synapse Vincent | 10.8 | Time savings | Nakayama et al[13], 2017 | |
| Pancreas | 64 | Pancreatic cancer, biliary tract cancer, neuroendocrine tumors, IPMN | Synapse Vincent | 14 | Safety, effectiveness, and feasibility | Miyamoto et al[100], 2018 | |
| Pancreas | 44 | Pancreatic cancer | MVT | Safety, effectiveness, and feasibility | Lin et al[17], 2020 | ||
| Robot-assisted | Major and minor liver resections | 40 | Hemangioma, HCC, hydatid cyst, cholangiocarcinoma | da Vinci Surgical System | 12.5 | Safety and feasibility | Troisi et al[37], 2013 |
| Major liver resection | 25 | Fatty liver, hepatic hemangioma, giant adenoma, HCC, secondary liver carcinoma | da Vinci Surgical System | 9.3 | Safety and feasibility | Spampinato et al[33], 2014 | |
| Wedge resection of the liver | 20 | HCC, secondary liver carcinoma, hepatic hemangioma, liver stones | da Vinci Surgical System | 9.5 | Safety and feasibility | Felli et al[47], 2015 | |
| Cholecystectomy | 38 | Benign biliary disease | da Vinci Surgical System | 0 | Safety and effectiveness | Gustafson et al[51], 2016 | |
| Cholecystectomy | 1833 | Benign gallbladder disease | da Vinci Surgical System, Zeus system, AESPO | 9.3 | No superiority over laparoscopy | Han et al[101], 2018 | |
| Major and minor liver resections | 1312 | Liver tumors | da Vinci Surgical System | 17.8 | No superiority over laparoscopy | Zhang et al[2], 2020 |
MVT: A three-dimensional multi-touch visualization table introduced by Sectra in 2010 at the Radiological Society of North America. IPMN: Intraductal papillary mucinous neoplasm; HCC: Hepatocellular carcinoma; 3D: Three-dimensional.
Advantages and limitations of 3D visualization
The development and application of visualization images has changed the paradigm of surgery and can also help inexperienced surgeons to learn with simulation, improved safety, reduced intraoperative risk and to some extent reduced postoperative complications[12,17,18].
However, the current 3D visualization techniques still have some limitations[18]. First, the process of medical image reconstruction mainly includes image data pre-processing, segmentation and annotation, alignment and fusion, 3D reconstruction, visual image display, etc. Each step of the process affects the results of 3D reconstruction, and the quality of the raw data acquired during the process and the different capabilities of the various reconstruction software applications also affect the outcome of 3D reconstruction. Second, although the reconstructed images produced by current visualization software are generally better than the image post-processing software that comes with CT or MRI, they are based on secondary processing of the original CT or MRI images, which inevitably results in partial loss of the original data during the image processing, thus affecting the fineness and clarity of the reconstructed images[18]. Future research should maximize the preservation of raw data, optimize the algorithms of various reconstruction techniques, improve the fidelity of the reconstruction and increase the accuracy of the 3D visualized images. Third, the reconstruction of images is currently time-consuming, taking at least one to two hours, future research could be technically optimized to reduce the reconstruction time[19]. Fourth, soft tissue organs such as the liver surface, intrahepatic structures and the bile duct tree are usually deformed intraoperatively due to changes in position and surgical procedures[20]. Although studies have also described calibration algorithms based on deformed organs, the currently available DIR algorithms still have limitations when dealing with complex deformations including volume changes, and optimization solutions for variable organ alignment remain a difficult area for future research, and further development and testing studies are needed in the future[21].
3D printing
3D printing is an extension and expansion of 3D visualization technology. High-fidelity 3D printed models can realistically reflect the 3D spatial relationships of fine anatomical areas such as lesion sites and blood vessels, allowing for multi-dimensional predictions of surgical procedures before surgery, achieving a leap from 3D images to solid 3D physical models[22,23].
The use of 3D printing in liver surgery has become widespread, and studies have shown good results with negative margins for using this technique in the treatment of small liver cancers[24]. Joo et al[25] applied a 3D-printed transparent liver model. The 3D technique was also applied by Fang et al[26] in surgeries on liver diseases such as intrahepatic bile duct stones and liver malignancies. He et al[27] also applied 3D printing in liver resection and autologous liver transplantation for vesicular encapsulation disease with satisfactory surgical results. Yang et al[28] used HepaRG cells and bioink to construct 3D bioprinted hepatic-like biotin, demonstrating that 3D bioprinting can be used to generate human liver tissue as an alternative transplant donor for therapy.
As shown in Table 2, current 3D printing enables the adjustment and placement of 3D printed models in optimal anatomical positions, facilitating both the placement of surgical instruments and the intuitive real-time navigation of key steps in surgery. It also allows rapid identification and precise positioning of key sites, optimizing the plane of surgical resection, the separation of important vessels and the precise removal of lesions, thereby improving surgical precision and safety and reducing surgical risk[29]. A number of studies have shown that 3D printing can produce implant shapes that precisely match their anatomical characteristics, ensuring that implant surgery is carried out safely[30,31].
Table 2.
Advantages and current limitations of existing three-dimensional printing
|
Advantages
|
Limitations
|
| (1) Realistic spatially dissected views | (1) Time-consuming production |
| (2) Intuitive real-time navigation for rapid identification and location | (2) Rigid model with poor soft tissue compliance |
| (3) Improved surgical safety | (3) Fragility |
| (4) Less time consumed and fewer complications | (4) High cost |
| (5) Novel educational techniques | (5) Issues of specificity, safety, and sustainability of implantable 3D-printed products |
3D: Three-dimensional.
Despite these advantages, 3D printing has a number of limitations. First, 3D printing devices take longer to plan and produce, often delays surgery and therefore are unsuitable for emergency surgery. Second, the issue of the material of the model is also a key point to be examined, as the visceral soft tissue organs are deformable and rigid models cannot reproduce the compliance of the tissue[32,33]. Fragile models are also unsuitable for surgery, and certain models cannot be handled by the surgeon during surgery because the particular material cannot be sterilized[34,35]. Third, the design and manufacture of 3D models for transplantation is more challenging, requiring consideration not only of the specificity of soft tissue organs, but also of the safety and sustainability of 3D printed products. Fourth, the high additional cost is also one of the disadvantages of current3D printing that cannot be ignored, of course, it is believed that with the development of bioprinting technology, these issues may be addressed to some extent[34].
ROBOT-ASSISTED HPATOBILIARY SURGERY
Precision and minimally invasive surgery have long been the pursuit of surgical procedures, and with the development of surgical anatomy and perioperative care, enhanced imaging modalities such as 3D visualization, and advances in laparoscopic surgery and robotic devices, minimally invasive surgery is becoming the gold standard in specific areas of gastrointestinal surgery[36-38]. However, the straight instruments of the laparoscope allow only four degrees of freedom, and the surgeon's inevitable physical hand tremors are magnified by the long laparoscopic tube. These factors, combined with the 2D field of view, the narrow space and the lack of depth perception, add to the difficulty of laparoscopic surgery, and prolonged procedures are more likely to lead to surgeon fatigue[2]. The robot-assisted surgical system offers many advantages over laparoscopic surgery, including the filtering out of physiological hand tremors based on simulated surgeon wrist movements, a stable camera platform, a 3D surgical field of view and visual magnification, seven degrees of freedom of dexterity, and reduced surgical fatigue for the surgeon[39].
Operation of robotic surgery: Indications and contraindications
Currently, most robot-assisted minimally invasive surgery is performed using the Da Vinci Si Surgical System telesurgery system, in which the surgeon sits at a console and operates several master robots, with intraoperative manipulation and view capture performed by three robotic instrument arms and one camera arm[40]. The stable platform's 3D field of view and flexible robot arm help surgeons better expose anatomical structures for selective control, dissection, and handling[39]. The robotic platform also enables near-infrared fluorescence imaging using indocyanine green (ICG) to assess tissue perfusion and identify lymphatic structures, distinguishing between healthy liver and tumor tissue[41]. The use of ICG fluorescence imaging also improves the discrimination between biliary tract and vascular structures, facilitating the identification of resection lines and helping the surgeon to maintain an accurate resection plane intraoperatively[42]. The use of these devices together allows for better control of the vascular system and fine structures such as the bile ducts, reducing intraoperative risks and intraoperative complications.
According to the available guidelines, indications for robotic hepatectomy include malignant tumors of the liver such as primary liver cancer, secondary liver cancer, and other rare malignant tumors of the liver, as well as benign diseases including adenomas, cavernous hemangiomas with symptoms or over 10 cm in diameter, focal nodular hyperplasia, cystic diseases such as hepatic echinococcosis, and intrahepatic bile duct stones requiring hepatic resection involving combined organ resection[43]. Indications for machine bile duct resection include intra- and extra-hepatic bile duct stones requiring combined hepatic segmental surgery or lobectomy for gallbladder cancer without abdominal implant metastases or large vessel invasion, type I, II and III cholangiocarcinoma of the porta hepatis, etc.[44-46]. Contraindications for robotic surgery include, in addition to the same contraindications as for open hepatobiliary resection, severe cardiopulmonary disease that does not tolerate pneumoperitoneum, intra-abdominal adhesions that are difficult to separate and reveal the lesion in two or more operations, lesions that are close to or that directly invade large blood vessels, invasion of the hilum, invasion of the portal vein, hepatic artery and other blood vessels, or lesions that require extensive hilar lymph node dissection[32].
Robotic surgery in hepatobiliary surgery
As shown in Table 1, Troisi et al[37] reviewed liver resections in 40 patients, comparing robot-assisted surgery with laparoscopic surgery, where the robotic platform provided some reduction in complications compared to laparoscopic surgery, and in difficult posterior and superior segments, robot-assisted surgery appeared to be more advantageous and confirmed the safety and feasibility of robot-assisted surgery[37]. Spampinato et al[33] conducted a retrospective analysis of the perioperative outcomes of robot-assisted major hepatectomy vs laparoscopic major hepatectomy, which confirmed the safety of robot-assisted surgery. Felli et al[47] demonstrated the safety of robotic surgery through initial experience with 20 consecutive robotic liver resections. Zhang et al[2] conducted a meta-analysis in which robot-assisted surgery had advantages over laparoscopic hepatectomy in major hepatectomy. It has also been shown that the proportion of major resections was higher in the more difficult posterior epigastric group than in the laparoscopic group, and that surgeons subjectively preferred robot-assisted surgery[3]. Kamiński et al[48] compared laparoscopic cholecystectomy with robotic cholecystectomy and showed no statistical difference between the two groups in terms of operative time and major bleeding complications, and found that the robotic approach may help in the management of bile duct injuries.
In addition, single-incision robotic cholecystectomy recapitulates the advantages of single-incision surgery, which is based on the same principles as multi-port laparoscopic cholecystectomy and offers the advantages of high definition and stereoscopic vision[49]. It overcomes some of the limitations of conventional laparoscopy through a clear 3D view, redistribution of instruments and optimized engineering design, making it safe and feasible to operate on different gallbladder lesions[49-51]. Gustafson et al[51] compared 38 laparoscopic procedures with 44 robotic single-incision cholecystectomies and found no significant differences between the two groups in terms of either transit rate, length of stay, incidence of incisional hernias requiring repair, or intraoperative and postoperative complications.
Advantages
With the growing trend towards minimally invasive surgery continues to develop, robot-assisted surgery is increasingly being used in hepatobiliary surgery, where it offers potential advantages over other techniques, and studies have shown its advantages in facilitating bile duct reconstruction and vascular anastomosis, and large hepatectomy, and resection of lesions located in highly complex areas[52-54].
First, robotic-assisted technology has more precise resolution, greater magnification, smaller instruments and greater mobility, making it more advantageous in delicate areas such as the liver portal[3], studies have shown that robotic surgery can reduce abdominal wall trauma and improve post-operative diaphragm function, thereby reducing respiratory complications, among other things. Second, the robotic system reproduces the surgeon's natural movements through a steady camera, reducing surgeon fatigue and filtering out physiological tremors, improving precision, accuracy and safety in surgery[43]. Third, the flexible robotic arm can help surgeons perform more precise and safer dissections and sutures, especially in the event of acute bleeding, and the resting position of the robotic arm to stop bleeding allows for safer transfer of open surgery[47]. Previous studies have also shown that intraoperative blood loss is reduced in robotic surgery compared to traditional laparoscopic or open techniques[52]. Fourth, improved venous drainage and reduced bile duct injury are both potential advantages of robotic surgery, which can reduce postoperative pain and complications such as ascites bile duct injury in cirrhotic patients and effectively improve their postoperative quality of life[55]. Fifth, robotic surgery can be used in conjunction with fluoroscopic techniques, with the robotic console providing fluoroscopic cholangiograms that are more conducive to a safe procedure[49].
In recent years, the use of two important phases of minimally invasive hepatectomy — hilar resection and hepatic cavity resection — has improved with the spread of robotic surgery and surgeons’ increased level of experience[49]. As surgeons gain experience, the learning curve for robotic surgical approaches is likely to decrease[56]. In addition, as robotic surgery and open surgery share a common skill principle, even new surgeons with less experience may have a shorter learning curve on the operating table and a correspondingly shorter operating time[57].
Limitations
Current robotic surgery is not mature and still has many limitations. First, compared to laparoscopic techniques, robotic surgery is not as resource efficient, as robotic surgery lacks compression options to control acute bleeding, it usually requires at least two experienced hepatobiliary surgeons to interact with coordination at the console and around the patient for safety reasons. In this regard, there is a need for a technical solution for simpler and faster instrument changes that can be performed independently by the surgeon at the console, thus increasing the efficiency of surgical resources[3]. Second, tactile sensitivity is also one of the primary issues facing surgeons, as the robotic arm has no tactile feedback, in order to avoid tissue damage, the instruments should always be in the surgeon's field of view as blind movements of the instruments can cause damage to surrounding organs and structures, so robotic surgery requires higher quality intraoperative images[47]. Okuda et al[58] have developed new forceps with force sensors that can analyze the gripping force generated by forceps during laparoscopic surgery and display it graphically on a laptop display, providing real-time feedback to the surgical staff. Experiments have shown that this measurement is accurate and feasible and that this new device with force sensors will also provide real-world feedback during endoscopic surgery, providing practical haptic feedback to aid robot-assisted surgical systems (such as the da Vinci Surgical System) is expected to overcome the lack of haptic feedback in robotic surgery. Third, the choice of anatomical approach is one of the limitations of current robotic surgery. Although the bipolar-based "vascular closure" has multiple degrees of freedom, their branches are too wide for precise and substantial dissection, and the longer time required to change instruments and applicators in robotic surgery compared to open and laparoscopic liver surgery also contributes to the longer operative times, and in these areas there is still a need for some technical adjustments to be made[36,59]. The restricted placement of casing needles is also an issue of concern and solution. Ideally, for optimal setup in the cross-section, four 8 mm robotic trocar needles should be placed in a hypothetical straight line at a distance of approximately 7 cm from each other; however, for setup of the upper segment, especially for severe underlying lesions such as large steatotic livers, trocar needle placement may be limited, increasing the postoperative complications of robotic surgery[60]. Fourth, the long operating time remains a drawback of robotic surgery as the preoperative assembly of the robotic system is very time-consuming[2]. The learning curve for robotic surgery inevitably leads to some increased operative times and the need for resident involvement in all procedures, although the learning curve for robots appears to be faster than for laparoscopy, training in advanced laparoscopic techniques is still required before starting robotic hepatobiliary surgery[60]. Reports of robotic hepatectomy at this stage may be somewhat selective and there may be serious adverse events that are not published. The next step is also the need for standardized training in robotic surgery, such as dedicated robotic surgery training using virtual reality training tables or robotic dual consoles, which is the basis for establishing a successful robotic surgery[61,62]. One of the problems with robotic surgery is its high cost, as many hospitals cannot afford this new technology due to the high cost of purchasing and maintaining robots, but in recent years, as surgeons have gained experience, operating times have been reduced and patient lengths of stay have become less decisive in terms of cost[56]. Despite these reports, more prospective randomized studies are needed to assess the true costs of robotic surgery in different procedures, combining robotic surgery with accelerated recovery and perioperative care could theoretically significantly reduce the patient’s length of stay and therefore offset the existing high costs[3]. In addition, there is a lack of communication between clinicians and those developing the technology. Clinicians should communicate fully with technicians to inform them of their needs and the advantages and disadvantages of the existing technology so that they can target improvements to facilitate continuous technological progress, optimization of image processing, develop new computer interfaces to facilitate interfacing, and even add modules with sensory haptics to overcome the lack of tactile feedback and assess pathology based on accurate 3D reconstruction, etc.
REAL-TIME NAVIGATION
Accurate surgical navigation, which can better guide surgeons and improve surgical safety, has received widespread attention with the development of computer science and imaging technology. Surgical navigation refers to the use of medical imaging equipment and computer image processing methods to visualize the patient's preoperative multimodal image data before surgery, to precisely match the patient's anatomy during surgery using rapid alignment procedures, and to obtain and display the position of surgical instruments in space in real time using a 3D positioning system[63-65].
The accuracy of the tracking technique is an important basis for the reliability of the navigation procedure, and the accuracy of the tracking system largely reflects the quality and performance of the surgical navigation system. To date, optical tracking system (OTS) and electromagnetic tracking system (EMT) are the two main tracking techniques used in surgical navigation. Table 3 compares some of the basic characteristics of OTS and EMT.
Table 3.
Comparison of optical and electromagnetic tracking navigation
|
Item
|
Optical tracking
|
Electromagnetic tracking
|
| Tracking accuracy | High | Low |
| Robustness relative to environmental conditions | High | Low |
| Visible line of sight | Need for | No need for |
| Tracking of rigid objects | Suitable for | Unsuitable for |
| Electromagnetic field | No need for | Need for |
| Interference from magnetic field | Nothing | Notable |
| Common uses in the surgeries: | ||
| Neurosurgery | + | |
| Orthopedic | + | |
| Endoscopic abdominal | + |
The OTS is used to locate visual markers by means of a camera. Its high tracking accuracy and robustness are widely used to estimate the position of surgical tools relative to the target area, with great accuracy and tracking volume, but its main limitation is that a visible line of sight between the intraoperative marker site and the camera is required. Without a line of sight, optical tracking cannot be achieved, and the tip of the knife is usually the location to be tracked and typically needs to be placed near the end of a rigid instrument. As only rigid instruments can be used due to the possibility of tip shift of the tracker, the use of optical tracking is limited, so optical navigation systems are mainly used to track rigid objects, for example in orthopedic surgery[66,67].
The EMT uses a known magnetic field geometry to determine the attitude of the sensor measuring the magnetic flux or field to achieve attitude measurement and dynamic tracking of the target, with the advantages of real-time positioning, high accuracy and no fear of obstruction[68]. EMT provides a solution for precise positioning when line of sight cannot be established, enabling small electromagnetic (EM) sensors to be positioned independently of line of sight in a given EM field, facilitating fast and accurate tracking[66], this avoids the limitations of line of sight establishment problems, and the small size of the sensor allows it to be embedded in the tip of the surgical instrument, reducing tracking errors caused by the large distance between the sensor and the tip of the positioning instrument[66]. Therefore, EM surgical navigation systems are commonly used in endoscopic surgery and abdominal surgery[69].
Real-time navigation and the applications mediated by EM tracking
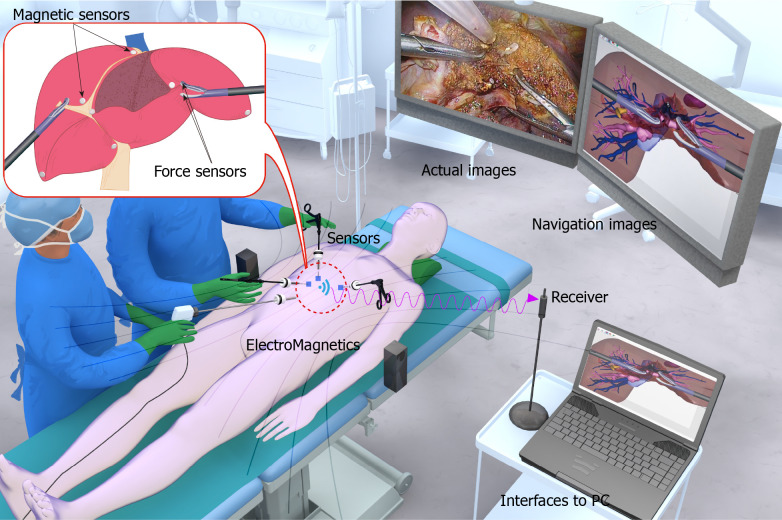
The implementation of EM tracking-mediated real-time navigation consists of three important steps: (1) Preoperative acquisition of 3D visualization images of organ tissues; (2) Alignment of the virtual 3D visualization images with the real intraoperative images using real-time EM tracking technology and tracking and matching of the virtual 3D images with the changing real images; and (3) Overlay of the virtual images with the real images through augmented reality so that the real environment and virtual images are superimposed on the same screen in real time. As shown in Figure 1, these superimposed virtual images materialize and visualize the intraoperative hepatobiliary structures, helping the surgeon to better judge their spatial relationships and thus making the operation run more smoothly[70-72]. Augmented reality allows the 3D visualization of the hepatobiliary model to be projected onto the surgical area for precise alignment of the coverage area, avoiding hand-eye coordination problems for the surgeon in traditional laparoscopic surgery[73].
Figure 1.
Intraoperative schematic of the electromagnetic tracking procedure. It shows a schematic diagram based on electromagnetic tracking navigation under a developing work by the Ohkohchi team which is used to track the position of the micro electro mechanical system (MEMS) within the magnetic field in real time without the need for line of sight and send the real-time information to a computer workstation, fuse the real-time intraoperative actual procedure and visual images with the preoperative computed tomography or magnetic resonance imaging pictures to form a three-dimensional reconstruction image, and display the real intraoperative actual procedure and visual images and the corresponding reconstruction images side-by-side on a TV monitor to achieve real-time navigation of the surgical site (this is the project of “Development of Real-time Navigation System for Laparoscopic Hepatectomy”, University of Tsukuba, Japan, 2017.4-2020.3).
A real-time ultrasound and preoperative CT or MRI image fusion system has been developed in recent years to construct preoperative CT or MRI image datasets as tomographic images and fuse them with real-time acquired ultrasound images with high precision and dynamics[1]. Although this method of navigation is feasible, the inevitable problem of poor accuracy when tracking and locating ultrasound is due to the effect of temperature and air displacement on ultrasonic positioning.
Today, as EM navigation procedures continue to evolve, a number of manufacturers have developed different stand-alone EM tracking systems for medical applications, with the main commercial EM tracking devices currently used in clinical applications being the NDI Aurora (NDI Medical, Canada), the Polhemus Fastack (Polhemus, Canada), and the Ascension MiniBIRD (Ascension Technologies, United States)[74].
Song et al[75] proposed a magnetic tracking-based planar shape-sensing and navigation system for a flexible surgical robot applied to transoral surgery. The permanent magnets were mounted at the distal end of the robot to provide 3D localization and 2D orientation estimation, so there was no need to mount the sensors on the robot. Navigation validation on an experimental platform showed that the approach was feasible and can work in the surgical environment, despite localization errors within the tracking system and the robot[75].
Kok et al[76] evaluated the feasibility and safety of an internally developed EM navigation system for real-time rectal tumor tracking using the NDI Aurora V2 EM tracking system (Northern Digital Inc, Waterloo, Ontario, Canada), employing a patient tracker with an EM sensor (Philips Traxtal/Percunav, Philips, Best, the Netherlands) patient tracker to determine the patient's position during surgery and to place tracking sensors on the tumor to adjust for real-time tumor motion, providing continuous interpretable navigation data for rectal surgery, this prospective study demonstrates that real-time tumor tracking with EM navigation is feasible, safe and accurate and provides direction for wider clinical implementation and contributes to further research to improve workflow and demonstrate clinical benefit[76].
The Ohkochi team at the University of Tsukuba, Japan, in collaboration with LEXI at University of Tokyo, have developed a new forceps with a powerful sensor that connects a micro electromechanical systems triaxial pressure sensor to the forceps tip to measure the pressure exerted by an endoscopic surgical forceps, the gripping force generated by the forceps with the pressure sensor during laparoscopic surgery was measured and analyzed in real time using quantitative data with temperature-compensated triaxial forces displayed graphically on a laptop computer display, providing real-time feedback to the surgical staff on pressure changes due to complex movements, the results show that this measurement is accurate and feasible, and this is the first study to report on the measurement of complex movements during actual surgery, Okuda et al[58] are working on the development of a position sensor system by combining it with a pressure-sensing system, when the data obtained from the device with the pressure sensor is combined with the real-time navigation system, it can display the magnitude of the grip force based on the information provided about the position of the operating site, helping the surgeon to control the intraoperative operation when the pressure is too high and causes damage to the internal soft tissue organs. This will be a breakthrough in traditional navigation surgery, overcoming the lack of tactile feedback from existing navigation[58].
Advantages
Real-time navigation based on EM tracking offers the possibility of navigation in minimally invasive abdominal surgery without the line-of-sight interference problems of optical systems. It provides real-time accurate spatial 3D measurements in the presence of obstruction, allowing real-time unobstructed tracking of miniaturized sensors embedded in surgical tools, probes, needles, guidewires and catheters, which can even be placed at the tips of flexible machinery, helping surgeons to achieve real-time precise navigation of the surgical area and improve the safety of the procedure[66].
EM tracking in surgical navigation provides a non-invasive, radiation-free way to navigate intraoperatively in real-time without any invasive procedures such as portal venipuncture or hepatic dissection, showing the fine anatomy of the lesion in real time, combines flexibly with surgical instruments, solves the surgeon's hand-eye coordination problem, and improves the accuracy and controllability of surgical navigation[77]. In addition, real-time navigation allows the intraoperative surgical team to share intraoperative information to ensure that the operation is carried out safely.
Limitations
First, EMT are highly sensitive to EM interference and magnetic field distortions[66]. Second, existing EM tracking systems do not provide for accurate position tracking at longer distances from the source, some current studies have confirmed that the stability of EM navigation systems needs further improvement, these systems can operate with a limited amount of tracking but their accuracy decreases as the distance between the transmitter and receiver increases, the accuracy of AR navigation decreases when the EM sensor is far from the magnetic field generator and it is difficult to have systems that can track small sensors with a volume greater than 1 cubic metre[78]. Third, in addition to technical shortcomings, EM tracking technology lacks environmental robustness and accuracy compared to optical tracking navigation, and the robustness of EM tracking can be a problem in some environments, so all systems need to be carefully evaluated in clinical practice[79]. The development of customized systems for different environments and applications may offer some solutions for increasing the robustness of EM tracking technology[66]. Fourth, although the ideal navigation system is easy to use for those unfamiliar with intrahepatic anatomy, current navigation systems sometimes require manual intraoperative adjustment, which takes time and requires an in-depth knowledge of hepatobiliary anatomy, and therefore still requires the surgeon to be very familiar with the anatomy in order to ensure a smooth operation[80]. Fifthly, the issue of alignment in real-time surgery has always been a challenge[70]. Sixth, the time-consuming problem of superimposing reconstructed images onto real-time intraoperative images is also a current technical challenge[81]. The construction of superimposed images is still time-consuming and labor-intensive in routine use, and although currently available simulation software programs have reduced surgery time by up to one hour, skilled surgeons still need three to four hours to construct overlay images[82]. There is therefore an urgent need to develop new techniques to reduce the time taken to superimpose images, and in the future it is also hoped that technicians will be able to provide more information on pathological or biological conditions in addition to the superimposed images to enrich the usefulness of the navigation system. Seventh, in terms of image display technology, although various methods are used in navigational surgery, such as monitor-based video fluoroscopic and projection-based systems, there are still problems to overcome such as limited resolution, overlapping distorted images and cumbersome operation[83]. Eighth, the cost of navigation equipment is relatively high and it is believed that as the price of equipment decreases it will be able to drive more hospitals to perform procedures with real-time navigation and more surgeons to participate. However, many clinicians are not aware of the advances in augmented reality technology, so there needs to be a full exchange of information and communication of needs between clinicians and technicians in the clinical setting to develop technology that meets clinicians' expectations, which will help create new inventions and facilitate the advancement and development of navigation[84].
Problem analysis and anticipation of improvements
The accuracy and distortion of EM tracking has been a central issue of research. The accuracy of EM tracking is affected by a variety of factors, and existing EM tracking systems have multiple sources of error, physical laws, design limitations, and manufacturing imperfections or environmental noise can all lead to positioning errors. The intraoperative alignment of deformed organs is also another challenge in navigation technology due to the effects of intraoperative manipulation and respiratory activity, and the clarity and resolution of reconstructed images based on real-time intraoperative images is also of concern to researchers. Despite some attempts to compensate for the tracking, alignment and reconstruction of images, there are still some issues to be resolved[85,86].
Accuracy and distortion: In EMT, errors can be classified as (1) inherent system errors, (2) field distortion errors, and (3) motion-induced errors. Inherent system errors are static errors that can occur when the sensor is placed at a fixed point or when the system is updated; distortion errors refer to disturbances in the secondary and unwanted magnetic fields which can be caused by eddy currents induced by ferromagnetic or conductive materials or by external currents, and they can also originate from the FG field generator and sensor design; motion-induced errors can be caused by changes in the speed of the sensor and the environment during the measurement[79,87].
Upgrading the system to avoid eddy currents and performing a system calibration function can help to some extent with inherent system errors[79,88]. Static pre-calibration processes are cumbersome and ineffective for most dynamic clinical procedures, and often require too many EM sensors to compensate for field distortion in dynamic environments, making them inefficient. A fusion-based approach has also been applied that combines measurements from multiple redundant EM sensors with the motion model of the instrument being tracked, which uses both localization and mapping (SLAM) algorithms to create field distortion maps and compensate for EM tracking errors in real time, however, it requires a large surgical space to complement the tracking technique or an excessive number of redundant sensors, and increases time of calibration. Too many devices also have an impact on the surgeon's surgical space, and their computational complexity, convergence and performance in dynamic environments and spaces still need to be considered by technicians in the future[89].
Alignment errors: The main problem with navigational surgery in hepatobiliary surgery are the accuracy, complexity and time-consuming nature of alignment. First, the EM transmitter should be placed as close as possible to the operating table to avoid interference with alignment accuracy caused by longer distances, second, a suitable probe point should be selected. For transabdominal scans, the ideal location for the probe point is below the glabella, whereas for intraoperative scans, the probe point should be set on the surface of the liver, preferably in the easily recognizable round ligament at the inferior edge of the fissure, the tip of the main portal vein near the tumor can also be used as an important intraoperative landmark, as can the branching vessels near the tumor for precise intraoperative adjustment[90]. In addition, intraoperative control of ventilation or reduction of tidal volume can reduce respiratory-related alignment errors to some extent, and deformed livers can also be monitored in real time using respiratory gating techniques to compensate for errors in the tracking position of EM sensors[91]. It is also necessary to calibrate the camera and the spatial relationship between the camera lens and the solenoid and to manually verify the reference boundary markers, all of the aforementioned techniques can help to improve the accuracy of the alignment[92].
Image reconstruction has also been investigated using surface data obtained from a flexible liver model that simulates deformation, and this data was then used to construct a sample library to predict liver displacement and deformation in alignment, including changes in the shape and internal relative position of the internal structures of the liver[93,94]. However, due to the movement of the diaphragm during breathing and the pulling of instruments can lead to changes in the position and shape of the liver and the occurrence of biliary tract deformities, this leads to incorrect positioning in the navigation system[90]. Although interactive and automated alignment systems have been developed that allow for periodically repeated real-time image acquisition to accommodate alignment difficulties caused by liver deformation and displacement, these systems require a hybrid operating room with CT or MRI equipment and have not been performed in human hepatobiliary surgery[95]. There are also reports of proposed 3D dense surface reconstruction algorithms that can localize hidden structures in intestinal surgery and gallbladder surgery, as well as enhanced block mapping algorithms and reimage mapping techniques that facilitate the implementation of dynamic alignment and aid in alignment studies of variable organs, although there are reports of these studies using existing engineering techniques and mathematical algorithms to solve organ deformation problems, the required methods and algorithms are complex and still need to be simplified and optimized[95].
Superimposed images: Reconstructed images can be displayed in a variety of ways, either video-based or projection-based. Video-based reconstructed image display is commonly used for laparoscopic, robotic and endoscopic procedures[57]. The external video monitor displays the actual surgical scene, and the virtual 3D reconstructed image in the video has poor resolution, requiring tracking and correction of multiple anatomical structures to compensate for changes in the surgeon's field of view and changes in the projected image due to changes in the curvature of the surface of the organ being tracked, this adds to the complexity of constructing the image[1,96,97]. The projection-based reconstructed image also interferes with the surgeon's depth perception, as the image is disturbed and lost when the projector's beam is interrupted by the surgeon's body or robotic arm, and the constructed image is distorted when the beam is not projected on a flat area[98,99]. The development of 3D future holographic projection technology may address the issues of overlapping image interference and diminished depth perception, thereby improving projection-based displays in intraoperative navigation[70]. There is also a transparent display in use that reflects the image in a translucent mirror, allowing the surgeon to view the reconstructed image while also looking directly into the surgical field. It does not require additional video compositing, making it more convenient than conventional video displays and avoids the problem of distortion of the projected image due to changes in the curvature of the object's surface, in addition it does not require special glasses or sensing devices, future research will require improved transparent display methods and more advanced naked eye 3D to provide doctors with a more accurate display of spatial images[29].
CONCLUSION
In Table 4, we summarize the advantages and existing limitations of the latest trends in existing digital healthcare such as 3D visualization of images as well as robot-assisted surgery and real-time EM-based intraoperative navigation. Visualization techniques are more widely used in clinical practice, providing a 3D view of the lesion area and clearer spatial anatomical relationships through the preoperative sharing of accurate 3D surgical images. By creating conditions for complex and precise procedures, such techniques also help surgeons to optimize their surgical plans before surgery and to carry out preoperative simulations through software, which not only reduces surgery time but also reduces intraoperative risks and postoperative complications, improves patient prognosis, and can be used as a new teaching technique for new doctors. However, it is still time consuming and costly to plan and produce 3D models, and rigid models do not reproduce the compliance of soft tissues, implantable organs, and the specificity of 3D-printed products. The specificity, safety, and sustainability of 3D-printed products remain to be addressed. Robotic surgery, which is more minimally invasive than traditional laparoscopic or open surgery, with smaller instruments and a greater degree of motion, a clearer 3D field of view, more precise resolution, and greater magnification. Additionally, it offers filtering out of natural tremors, better ergonomics for the operator, the advantage of highly complex site resections, and improved venous drainage to reduce postoperative complications and help improve patients' quality of life. However, at this stage, robotic surgery is not mature and still has many limitations. Current limitations of robotic surgery include inefficient surgical resources, lack of tactile feedback, limited choice of anatomical approach, limitations in trocar placement, excessive operative time, long assembly time of the robotic system, time-consuming docking procedures, potential tendency to prolong pulmonary portal Pringle surgery, potential bleeding from the clamp squeeze technique, and high costs. Real-time navigation based on EM tracking has the advantage of not requiring any invasive operations and is not limited by line of sight, allowing for real-time intraoperative tracking and navigation, sharing of intraoperative information in real time, display of intraoperative fine anatomy, identification of lesions that cannot be detected by the naked eye, and lessening of hand-eye coordination issues during laparoscopic surgery. The development of sensors is expected to improve the accuracy of navigation for the safe unfolding of hepatobiliary surgery, but at this stage there are also problems with EM navigation systems that are not very stable, as well as low tracking accuracy, poor robustness to environmental conditions, magnetic field interference and tracking errors, poor navigation accuracy, a time-consuming reconstructed image superimposition process, low resolution of reconstructed images, large distortion, and intraoperative variable organ alignment problems. There are problems to be solved, and insufficient information exchange between the technician and the clinician remains problematic.
Table 4.
Advantages and limitations of three-dimensional visualization, robot-assisted surgery, and electromagnetic tracking navigation
|
|
Advantages
|
Limitations
|
| 3D visualization | Realistic spatially dissected views | Complex and time-consuming reconstruction process |
| Accurate 3D preoperative images | Possible loss of raw data due to operational errors | |
| Possibility of complicated surgery | Distortion in reconstructed images | |
| Optimization of preoperative assessment | Poor accuracy of reconstructed images | |
| Time-saving simulation | Complex algorithms and imperfect display techniques | |
| Less time consumed and fewer complications | Registration of mutable organs | |
| Novel educational techniques | High cost | |
| Robot-assisted | Better micro-invasiveness | Inefficient surgical resources |
| Smaller equipment for wider scope | Lack of tactile feedback | |
| Larger and clearer 3D views | Limitations in the choice of anatomical methods | |
| Micro-invasiveness | Restrictions on the placement of casing needles | |
| Improved venous drainage | Time-consuming operation | |
| More accurate resolution and greater magnification | Prolonged Pringle operation in the hilar region | |
| Filtering of natural tremor | Potential bleeding tendency of the clamping and squeezing technique | |
| Better ergonomics of the operator | High cost | |
| Electromagnetic tracking real-time navigation | No requirement for any other invasive operations | Magnetic field interference and tracking errors |
| No line of sight restrictions | Low tracking accuracy and robustness relative to environmental conditions | |
| Real-time intraoperative tracking and navigation | Low stability of electromagnetic navigation system | |
| Display of intraoperative fine anatomy | High cost | |
| Improved safety of surgical operations | Registration of mutable organs | |
| Identification of lesions that are not visually detectable | Accuracy of navigation issues | |
| Simultaneous sharing of intraoperative information | Time-consuming reconstruction image overlay | |
| Increased hand-eye coordination for doctors | Low resolution and distortion of the reconstructed image | |
| Insufficient communication between technicians and surgeons | ||
| Tedious operation |
3D: Three-dimensional.
It is noteworthy that in previous studies we have found that surgeons tend to focus on the surgical procedure to the neglect of post-operative care. The final healing after surgery is the result of a combination of factors such as the quality of surgery, intraoperative blood loss, the size of the resected lesion, the patient's underlying preoperative disease or comorbidities, and the patient's physical condition. The concept of Enhanced Recovery After Surgery was first developed by Danish surgeon Henrik Kehlet, based on the principles of reducing the stress of surgery, shortening the length of hospital stay and reducing perioperative complications, leading to rapid recovery. The concept is also considered to be a safe and effective treatment combining existing surgical options with accelerated recovery perioperative care. This could theoretically significantly reduce the length of a patient's hospital stay, which could to some extent offset the existing high costs. The price of various technologies — be it robotic surgery, 3D printing or EM navigation tracking — will certainly come down in the future. This will require a concerted effort and adequate communication between the entire healthcare industry, corporate bodies and technicians in order to target technological improvements and facilitate the continued progress of digital healthcare. Despite the opportunities and challenges, digital healthcare is sure to flourish in the future.
ACKNOWLEDGEMENTS
We thank Dr. Kim J of University of Tsukuba Faculty of Medicine for designing the figure image.
Footnotes
Conflict-of-interest statement: The authors declare no potential financial interests.
Manuscript source: Invited manuscript
Peer-review started: February 14, 2021
First decision: April 6, 2021
Article in press: August 2, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaya B, Yang HX S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
Contributor Information
Yun Wang, Institute of Regenerative Medicine, and Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Di Cao, Institute of Regenerative Medicine, and Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Si-Lin Chen, Institute of Regenerative Medicine, and Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Yu-Mei Li, Institute of Regenerative Medicine, and Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Yun-Wen Zheng, Institute of Regenerative Medicine, and Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China; Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Ibaraki, Japan; Guangdong Provincial Key Laboratory of Large Animal Models for Biomedicine, and School of Biotechnology and Heath Sciences, Wuyi University, Jiangmen 529020, Guangdong Province, China; School of Medicine, Yokohama City University, Yokohama 234-0006, Kanagawa, Japan. ywzheng@md.tsukuba.ac.jp.
Nobuhiro Ohkohchi, Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Ibaraki, Japan.
References
- 1.Miyata A, Arita J, Kawaguchi Y, Hasegawa K, Kokudo N. Simulation and navigation liver surgery: an update after 2,000 virtual hepatectomies. Glob Health Med. 2020;2:298–305. doi: 10.35772/ghm.2020.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Yuan Q, Xu Y, Wang W. Comparative clinical outcomes of robot-assisted liver resection vs laparoscopic liver resection: A meta-analysis. PLoS One. 2020;15:e0240593. doi: 10.1371/journal.pone.0240593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmelzle M, Krenzien F, Schöning W, Pratschke J. [Possibilities and limits of robotic liver surgery - Current status 2020] Chirurg. 2021;92:107–114. doi: 10.1007/s00104-020-01300-w. [DOI] [PubMed] [Google Scholar]
- 4.Fang C, Zhang P, Qi X. Digital and intelligent liver surgery in the new era: Prospects and dilemmas. EBioMedicine. 2019;41:693–701. doi: 10.1016/j.ebiom.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochanski RB, Lombardi JM, Laratta JL, Lehman RA, O'Toole JE. Image-Guided Navigation and Robotics in Spine Surgery. Neurosurgery. 2019;84:1179–1189. doi: 10.1093/neuros/nyy630. [DOI] [PubMed] [Google Scholar]
- 6.Mathew RP, Venkatesh SK. Liver vascular anatomy: a refresher. Abdom Radiol (NY) 2018;43:1886–1895. doi: 10.1007/s00261-018-1623-z. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda M, Hariyama M, Oshiro Y, Suzuki S. Development of new software enabling automatic identification of the optimal anatomical liver resectable region, incorporating preoperative liver function. Oncol Lett. 2019;18:6639–6647. doi: 10.3892/ol.2019.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su L, Dong Q, Zhang H, Zhou X, Chen Y, Hao X, Li X. Clinical application of a three-dimensional imaging technique in infants and young children with complex liver tumors. Pediatr Surg Int. 2016;32:387–395. doi: 10.1007/s00383-016-3864-7. [DOI] [PubMed] [Google Scholar]
- 9.Witowski J, Budzyński A, Grochowska A, Ballard DH, Major P, Rubinkiewicz M, Złahoda-Huzior A, Popiela TJ, Wierdak M, Pędziwiatr M. Decision-making based on 3D printed models in laparoscopic liver resections with intraoperative ultrasound: a prospective observational study. Eur Radiol. 2020;30:1306–1312. doi: 10.1007/s00330-019-06511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuda Y, Taura K, Seo S, Yasuchika K, Nitta T, Ogawa K, Hatano E, Uemoto S. Usefulness of operative planning based on 3-dimensional CT cholangiography for biliary malignancies. Surgery. 2015;158:1261–1271. doi: 10.1016/j.surg.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Pianka F, Baumhauer M, Stein D, Radeleff B, Schmied BM, Meinzer HP, Müller SA. Liver tissue sparing resection using a novel planning tool. Langenbecks Arch Surg. 2011;396:201–208. doi: 10.1007/s00423-010-0734-y. [DOI] [PubMed] [Google Scholar]
- 12.Fang CH, Zhang P, Zhou WP, Zhou J, Dai CL, Liu JF, Jia WD, Liang X, Zeng SL, Wen S. [Efficacy of three-dimensional visualization technology in the precision diagnosis and treatment for primary liver cancer: a retrospective multicenter study of 1 665 cases in China] Zhonghua Wai Ke Za Zhi. 2020;58:375–382. doi: 10.3760/cma.j.cn112139-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K, Oshiro Y, Miyamoto R, Kohno K, Fukunaga K, Ohkohchi N. The Effect of Three-Dimensional Preoperative Simulation on Liver Surgery. World J Surg. 2017;41:1840–1847. doi: 10.1007/s00268-017-3933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W, He SS, Zeng SL, Zhang P, Yang J, Xiang N, Zeng N, Fan YF, Wen S, Fang CH, Zhang K. [Three-dimensional visual assessment and virtual reality study of centrally located hepatocellular carcinoma on the axis of blood vessels] Zhonghua Waike Zazhi. 2019;57:358–365. doi: 10.3760/cma.j.issn.0529-5815.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto R, Oshiro Y, Hashimoto S, Kohno K, Fukunaga K, Oda T, Ohkohchi N. Three-dimensional imaging identified the accessory bile duct in a patient with cholangiocarcinoma. World J Gastroenterol. 2014;20:11451–11455. doi: 10.3748/wjg.v20.i32.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng N, Tao H, Fang C, Fan Y, Xiang N, Yang J, Zhu W, Liu J, Guan T, Xiang F. Individualized preoperative planning using three-dimensional modeling for Bismuth and Corlette type III hilar cholangiocarcinoma. World J Surg Oncol. 2016;14:44. doi: 10.1186/s12957-016-0794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C, Gao J, Zheng H, Zhao J, Yang H, Lin G, Li H, Pan H, Liao Q, Zhao Y. Three-Dimensional Visualization Technology Used in Pancreatic Surgery: a Valuable Tool for Surgical Trainees. J Gastrointest Surg. 2020;24:866–873. doi: 10.1007/s11605-019-04214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang C, An J, Bruno A, Cai X, Fan J, Fujimoto J, Golfieri R, Hao X, Jiang H, Jiao LR, Kulkarni AV, Lang H, Lesmana CRA, Li Q, Liu L, Liu Y, Lau W, Lu Q, Man K, Maruyama H, Mosconi C, Örmeci N, Pavlides M, Rezende G, Sohn JH, Treeprasertsuk S, Vilgrain V, Wen H, Wen S, Quan X, Ximenes R, Yang Y, Zhang B, Zhang W, Zhang P, Zhang S, Qi X. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int. 2020;14:437–453. doi: 10.1007/s12072-020-10052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada Y, Matsumoto S, Mori H, Takaji R, Kiyonaga M, Hijiya N, Tanoue R, Tomonari K, Tanoue S, Hongo N, Ohta M, Seike M, Inomata M, Murakami K, Moriyama M. Periportal lymphatic system on post-hepatobiliary phase Gd-EOB-DTPA-enhanced MR imaging in normal subjects and patients with chronic hepatitis C. Abdom Radiol (NY) 2017;42:2410–2419. doi: 10.1007/s00261-017-1155-y. [DOI] [PubMed] [Google Scholar]
- 20.Chen-Yoshikawa TF, Hatano E, Yoshizawa A, Date H. Clinical application of projection mapping technology for surgical resection of lung metastasis. Interact Cardiovasc Thorac Surg. 2017;25:1010–1011. doi: 10.1093/icvts/ivx247. [DOI] [PubMed] [Google Scholar]
- 21.Sen A, Anderson BM, Cazoulat G, McCulloch MM, Elganainy D, McDonald BA, He Y, Mohamed ASR, Elgohari BA, Zaid M, Koay EJ, Brock KK. Accuracy of deformable image registration techniques for alignment of longitudinal cholangiocarcinoma CT images. Med Phys. 2020;47:1670–1679. doi: 10.1002/mp.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Lopez V, Robles-Campos R, García-Calderon D, Lang H, Cugat E, Jiménez-Galanes S, Férnandez-Cebrian JM, Sánchez-Turrión V, Fernández-Fernández JM, Barrera-Gómez MÁ, de la Cruz J, Lopez-Conesa A, Brusadin R, Gomez-Perez B, Parrilla-Paricio P. Applicability of 3D-printed models in hepatobiliary surgey: results from "LIV3DPRINT" multicenter study. HPB (Oxford) 2021;23:675–684. doi: 10.1016/j.hpb.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Lin S, Xie Q, Ouyang W, Tan T, Li J, Chen Z, Yang J, Wu H, Pan J, Hu C, Zou Y. Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg Endosc. 2019;33:411–417. doi: 10.1007/s00464-018-6308-8. [DOI] [PubMed] [Google Scholar]
- 24.Igami T, Nakamura Y, Hirose T, Ebata T, Yokoyama Y, Sugawara G, Mizuno T, Mori K, Nagino M. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38:3163–3166. doi: 10.1007/s00268-014-2740-7. [DOI] [PubMed] [Google Scholar]
- 25.Joo I, Kim JH, Park SJ, Lee K, Yi NJ, Han JK. Personalized 3D-Printed Transparent Liver Model Using the Hepatobiliary Phase MRI: Usefulness in the Lesion-by-Lesion Imaging-Pathologic Matching of Focal Liver Lesions-Preliminary Results. Invest Radiol. 2019;54:138–145. doi: 10.1097/RLI.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 26.Fang C, Fang Z, Fan Y, Li J, Xiang F, Tao H. [Application of 3D visualization, 3D printing and 3D laparoscopy in the diagnosis and surgical treatment of hepatic tumors] Nanfang Yike Daxue Xuebao. 2015;35:639–645. [PubMed] [Google Scholar]
- 27.He YB, Bai L, Li T, Ji XW, Tuerganaili A, Jiang Y, Zhao JM, Shao YM, Liu WY, Wen H. [Application of three-dimensional visualization technology in surgical treatment for patients with hepatic alveolar echinococcosis] Zhonghua Wai Ke Za Zhi. 2016;54:704–709. doi: 10.3760/cma.j.issn.0529-5815.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Sun L, Pang Y, Hu D, Xu H, Mao S, Peng W, Wang Y, Xu Y, Zheng YC, Du S, Zhao H, Chi T, Lu X, Sang X, Zhong S, Wang X, Zhang H, Huang P, Sun W, Mao Y. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut. 2021;70:567–574. doi: 10.1136/gutjnl-2019-319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang R, Ma L, Li A, Yu L, Rong Z, Zhang X, Xiang C, Liao H, Dong J. Choledochoscopic Examination of a 3-Dimensional Printing Model Using Augmented Reality Techniques: A Preliminary Proof of Concept Study. Surg Innov. 2018;25:492–498. doi: 10.1177/1553350618781622. [DOI] [PubMed] [Google Scholar]
- 30.Suh YJ, Lim TH, Choi HS, Kim MS, Lee SJ, Kim SH, Park CH. 3D Printing and NIR Fluorescence Imaging Techniques for the Fabrication of Implants. Materials (Basel) 2020;13 doi: 10.3390/ma13214819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Doremalen RFM, van der Linde RA, Kootstra JJ, van Helden SH, Hekman EEG. Can 3D-printing avoid discomfort-related implant removal in midshaft clavicle fractures? Arch Orthop Trauma Surg. 2020 doi: 10.1007/s00402-020-03654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, He J, Boggi U, Troisi RI, Efanov M, Azoulay D, Panaro F, Pessaux P, Wang XY, Zhu JY, Zhang SG, Sun CD, Wu Z, Tao KS, Yang KH, Fan J, Chen XP. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 2019;25:1432–1444. doi: 10.3748/wjg.v25.i12.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spampinato MG, Coratti A, Bianco L, Caniglia F, Laurenzi A, Puleo F, Ettorre GM, Boggi U. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc. 2014;28:2973–2979. doi: 10.1007/s00464-014-3560-4. [DOI] [PubMed] [Google Scholar]
- 34.Huber T, Huettl F, Tripke V, Baumgart J, Lang H. Experiences With Three-dimensional Printing in Complex Liver Surgery. Ann Surg. 2021;273:e26–e27. doi: 10.1097/SLA.0000000000004348. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda S, Kihara T, Akita Y, Kobayashi T, Nikawa H, Ohdan H. Simulation and navigation of living donor hepatectomy using a unique three-dimensional printed liver model with soft and transparent parenchyma. Surg Today. 2020;50:307–313. doi: 10.1007/s00595-019-01868-9. [DOI] [PubMed] [Google Scholar]
- 36.Gavriilidis P, Roberts KJ, Aldrighetti L, Sutcliffe RP. A comparison between robotic, laparoscopic and open hepatectomy: A systematic review and network meta-analysis. Eur J Surg Oncol. 2020;46:1214–1224. doi: 10.1016/j.ejso.2020.03.227. [DOI] [PubMed] [Google Scholar]
- 37.Troisi RI, Patriti A, Montalti R, Casciola L. Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Int J Med Robot. 2013;9:160–166. doi: 10.1002/rcs.1495. [DOI] [PubMed] [Google Scholar]
- 38.Milone M, Manigrasso M, Burati M, Velotti N, Milone F, De Palma GD. Surgical resection for rectal cancer. Is laparoscopic surgery as successful as open approach? PLoS One. 2018;13:e0204887. doi: 10.1371/journal.pone.0204887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lafaro KJ, Stewart C, Fong A, Fong Y. Robotic Liver Resection. Surg Clin North Am. 2020;100:265–281. doi: 10.1016/j.suc.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Fahrner R, Rauchfuß F, Bauschke A, Kissler H, Settmacher U, Zanow J. Robotic hepatic surgery in malignancy: review of the current literature. J Robot Surg. 2019;13:533–538. doi: 10.1007/s11701-019-00939-w. [DOI] [PubMed] [Google Scholar]
- 41.Achterberg FB, Sibinga Mulder BG, Meijer RPJ, Bonsing BA, Hartgrink HH, Mieog JSD, Zlitni A, Park SM, Farina Sarasqueta A, Vahrmeijer AL, Swijnenburg RJ. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann Transl Med. 2020;8:1448. doi: 10.21037/atm-20-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pesce A, La Greca G. Is it still reasonable to raise doubts on ICG-fluorescence cholangiography during laparoscopic cholecystectomy? Updates Surg. 2020;72:1285–1286. doi: 10.1007/s13304-020-00830-6. [DOI] [PubMed] [Google Scholar]
- 43.Di Benedetto F, Petrowsky H, Magistri P, Halazun KJ. Robotic liver resection: Hurdles and beyond. Int J Surg. 2020;82S:155–162. doi: 10.1016/j.ijsu.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 44.Nota CLMA, Smits FJ, Woo Y, Borel Rinkes IHM, Molenaar IQ, Hagendoorn J, Fong Y. Robotic Developments in Cancer Surgery. Surg Oncol Clin N Am. 2019;28:89–100. doi: 10.1016/j.soc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Na KJ, Kang CH. Robotic thymectomy for advanced thymic epithelial tumor: indications and technical aspects. J Thorac Dis. 2020;12:63–69. doi: 10.21037/jtd.2019.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanford DE. An Update on Technical Aspects of Cholecystectomy. Surg Clin North Am. 2019;99:245–258. doi: 10.1016/j.suc.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Felli E, Santoro R, Colasanti M, Vennarecci G, Lepiane P, Ettorre GM. Robotic liver surgery: preliminary experience in a tertiary hepato-biliary unit. Updates Surg. 2015;67:27–32. doi: 10.1007/s13304-015-0285-4. [DOI] [PubMed] [Google Scholar]
- 48.Kamiński JP, Bueltmann KW, Rudnicki M. Robotic vs laparoscopic cholecystectomy inpatient analysis: does the end justify the means? J Gastrointest Surg. 2014;18:2116–2122. doi: 10.1007/s11605-014-2673-3. [DOI] [PubMed] [Google Scholar]
- 49.Escobar-Dominguez JE, Hernandez-Murcia C, Gonzalez AM. Description of robotic single site cholecystectomy and a review of outcomes. J Surg Oncol. 2015;112:284–288. doi: 10.1002/jso.23931. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez A, Murcia CH, Romero R, Escobar E, Garcia P, Walker G, Gallas M, Dickens E, McIntosh B, Norwood W, Kim K, Rabaza J, Parris D. A multicenter study of initial experience with single-incision robotic cholecystectomies (SIRC) demonstrating a high success rate in 465 cases. Surg Endosc. 2016;30:2951–2960. doi: 10.1007/s00464-015-4583-1. [DOI] [PubMed] [Google Scholar]
- 51.Gustafson M, Lescouflair T, Kimball R, Daoud I. A comparison of robotic single-incision and traditional single-incision laparoscopic cholecystectomy. Surg Endosc. 2016;30:2276–2280. doi: 10.1007/s00464-015-4223-9. [DOI] [PubMed] [Google Scholar]
- 52.Guerra F, Di Marino M, Coratti A. Robotic Surgery of the Liver and Biliary Tract. J Laparoendosc Adv Surg Tech A. 2019;29:141–146. doi: 10.1089/lap.2017.0628. [DOI] [PubMed] [Google Scholar]
- 53.Zhao ZM, Yin ZZ, Meng Y, Jiang N, Ma ZG, Pan LC, Tan XL, Chen X, Liu R. Successful robotic radical resection of hepatic echinococcosis located in posterosuperior liver segments. World J Gastroenterol. 2020;26:2831–2838. doi: 10.3748/wjg.v26.i21.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nota CL, Woo Y, Raoof M, Boerner T, Molenaar IQ, Choi GH, Kingham TP, Latorre K, Borel Rinkes IHM, Hagendoorn J, Fong Y. Robotic Versus Open Minor Liver Resections of the Posterosuperior Segments: A Multinational, Propensity Score-Matched Study. Ann Surg Oncol. 2019;26:583–590. doi: 10.1245/s10434-018-6928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giulianotti PC, Bianco FM, Daskalaki D, Gonzalez-Ciccarelli LF, Kim J, Benedetti E. Robotic liver surgery: technical aspects and review of the literature. Hepatobiliary Surg Nutr. 2016;5:311–321. doi: 10.21037/hbsn.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez-Ciccarelli LF, Quadri P, Daskalaki D, Milone L, Gangemi A, Giulianotti PC. Robotic approach to hepatobiliary surgery. Chirurg. 2017;88:19–28. doi: 10.1007/s00104-016-0223-0. [DOI] [PubMed] [Google Scholar]
- 57.Becker F, Morgül H, Katou S, Juratli M, Hölzen JP, Pascher A, Struecker B. Robotic Liver Surgery - Current Standards and Future Perspectives. Z Gastroenterol. 2021;59:56–62. doi: 10.1055/a-1329-3067. [DOI] [PubMed] [Google Scholar]
- 58.Okuda Y, Nakai A, Sato T, Kurata M, Shimoyama I, Oda T, Ohkohci N. New device with force sensors for laparoscopic liver resection - investigation of grip force and histological damage. Minim Invasive Ther Allied Technol. 2020:1–6. doi: 10.1080/13645706.2020.1755313. [DOI] [PubMed] [Google Scholar]
- 59.Ban D, Ishikawa Y, Tanabe M. Can robotic liver resection compensate for weaknesses of the laparoscopic approach? Hepatobiliary Surg Nutr. 2020;9:385–387. doi: 10.21037/hbsn.2019.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmelzle M, Schöning W, Pratschke J. [Liver Surgery - Setup, Port Placement, Structured Surgical Steps - Standard Operating Procedures in Robot-Assisted Liver Surgery] Zentralbl Chir. 2020;145:246–251. doi: 10.1055/a-1135-9162. [DOI] [PubMed] [Google Scholar]
- 61.Lai ECH, Tang CN. Training robotic hepatectomy: the Hong Kong experience and perspective. Hepatobiliary Surg Nutr. 2017;6:222–229. doi: 10.21037/hbsn.2017.01.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang RS, Ambani SN. Robotic Surgery Training: Current Trends and Future Directions. Urol Clin North Am. 2021;48:137–146. doi: 10.1016/j.ucl.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 63.Quero G, Lapergola A, Soler L, Shahbaz M, Hostettler A, Collins T, Marescaux J, Mutter D, Diana M, Pessaux P. Virtual and Augmented Reality in Oncologic Liver Surgery. Surg Oncol Clin N Am. 2019;28:31–44. doi: 10.1016/j.soc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Schoeb DS, Schwarz J, Hein S, Schlager D, Pohlmann PF, Frankenschmidt A, Gratzke C, Miernik A. Mixed reality for teaching catheter placement to medical students: a randomized single-blinded, prospective trial. BMC Med Educ. 2020;20:510. doi: 10.1186/s12909-020-02450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tarassoli SP. Artificial intelligence, regenerative surgery, robotics? Ann Med Surg (Lond) 2019;41:53–55. doi: 10.1016/j.amsu.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorriento A, Porfido MB, Mazzoleni S, Calvosa G, Tenucci M, Ciuti G, Dario P. Optical and Electromagnetic Tracking Systems for Biomedical Applications: A Critical Review on Potentialities and Limitations. IEEE Rev Biomed Eng. 2020;13:212–232. doi: 10.1109/RBME.2019.2939091. [DOI] [PubMed] [Google Scholar]
- 67.O'Donoghue K, Jaeger HA, Cantillon-Murphy P. A Radiolucent Electromagnetic Tracking System for Use with Intraoperative X-ray Imaging. Sensors (Basel) 2021;21 doi: 10.3390/s21103357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner M, Gondan M, Zöllner C, Wünscher JJ, Nickel F, Albala L, Groch A, Suwelack S, Speidel S, Maier-Hein L, Müller-Stich BP, Kenngott HG. Electromagnetic organ tracking allows for real-time compensation of tissue shift in image-guided laparoscopic rectal surgery: results of a phantom study. Surg Endosc. 2016;30:495–503. doi: 10.1007/s00464-015-4231-9. [DOI] [PubMed] [Google Scholar]
- 69.Leong F, Garbin N, Natali CD, Mohammadi A, Thiruchelvam D, Oetomo D, Valdastri P. Magnetic Surgical Instruments for Robotic Abdominal Surgery. IEEE Rev Biomed Eng. 2016;9:66–78. doi: 10.1109/RBME.2016.2521818. [DOI] [PubMed] [Google Scholar]
- 70.Tang R, Ma LF, Rong ZX, Li MD, Zeng JP, Wang XD, Liao HE, Dong JH. Augmented reality technology for preoperative planning and intraoperative navigation during hepatobiliary surgery: A review of current methods. Hepatobiliary Pancreat Dis Int. 2018;17:101–112. doi: 10.1016/j.hbpd.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Zhu W, Yang J, Xiang N, Zeng N, Hu H, Jia F, Fang C. Augmented Reality Navigation for Stereoscopic Laparoscopic Anatomical Hepatectomy of Primary Liver Cancer: Preliminary Experience. Front Oncol. 2021;11:663236. doi: 10.3389/fonc.2021.663236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bari H, Wadhwani S, Dasari BVM. Role of artificial intelligence in hepatobiliary and pancreatic surgery. World J Gastrointest Surg. 2021;13:7–18. doi: 10.4240/wjgs.v13.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cong X, Li T. Design and Development of Virtual Medical System Interface Based on VR-AR Hybrid Technology. Comput Math Methods Med. 2020;2020:7108147. doi: 10.1155/2020/7108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attivissimo F, Lanzolla AML, Carlone S, Larizza P, Brunetti G. A novel electromagnetic tracking system for surgery navigation. Comput Assist Surg (Abingdon) 2018;23:42–52. doi: 10.1080/24699322.2018.1529199. [DOI] [PubMed] [Google Scholar]
- 75.Song S, Zhang C, Liu L, Meng MQ. Preliminary study on magnetic tracking-based planar shape sensing and navigation for flexible surgical robots in transoral surgery: methods and phantom experiments. Int J Comput Assist Radiol Surg. 2018;13:241–251. doi: 10.1007/s11548-017-1672-8. [DOI] [PubMed] [Google Scholar]
- 76.Kok END, Eppenga R, Kuhlmann KFD, Groen HC, van Veen R, van Dieren JM, de Wijkerslooth TR, van Leerdam M, Lambregts DMJ, Heerink WJ, Hoetjes NJ, Ivashchenko O, Beets GL, Aalbers AGJ, Nijkamp J, Ruers TJM. Accurate surgical navigation with real-time tumor tracking in cancer surgery. NPJ Precis Oncol. 2020;4:8. doi: 10.1038/s41698-020-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krumb H, Hofmann S, Kügler D, Ghazy A, Dorweiler B, Bredemann J, Schmitt R, Sakas G, Mukhopadhyay A. Leveraging spatial uncertainty for online error compensation in EMT. Int J Comput Assist Radiol Surg. 2020;15:1043–1051. doi: 10.1007/s11548-020-02189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andria G, Attivissimo F, Di Nisio A, Lanzolla AML, Ragolia MA. Assessment of Position Repeatability Error in an Electromagnetic Tracking System for Surgical Navigation. Sensors (Basel) 2020;20 doi: 10.3390/s20040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franz AM, Haidegger T, Birkfellner W, Cleary K, Peters TM, Maier-Hein L. Electromagnetic tracking in medicine--a review of technology, validation, and applications. IEEE Trans Med Imaging. 2014;33:1702–1725. doi: 10.1109/TMI.2014.2321777. [DOI] [PubMed] [Google Scholar]
- 80.Robu MR, Edwards P, Ramalhinho J, Thompson S, Davidson B, Hawkes D, Stoyanov D, Clarkson MJ. Intelligent viewpoint selection for efficient CT to video registration in laparoscopic liver surgery. Int J Comput Assist Radiol Surg. 2017;12:1079–1088. doi: 10.1007/s11548-017-1584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oldhafer KJ, Peterhans M, Kantas A, Schenk A, Makridis G, Pelzl S, Wagner KC, Weber S, Stavrou GA, Donati M. [Navigated liver surgery : Current state and importance in the future] Chirurg. 2018;89:769–776. doi: 10.1007/s00104-018-0713-3. [DOI] [PubMed] [Google Scholar]
- 82.Liu W, Sawant A, Ruan D. Prediction of high-dimensional states subject to respiratory motion: a manifold learning approach. Phys Med Biol. 2016;61:4989–4999. doi: 10.1088/0031-9155/61/13/4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yasuda J, Okamoto T, Onda S, Fujioka S, Yanaga K, Suzuki N, Hattori A. Application of image-guided navigation system for laparoscopic hepatobiliary surgery. Asian J Endosc Surg. 2020;13:39–45. doi: 10.1111/ases.12696. [DOI] [PubMed] [Google Scholar]
- 84.Okamoto T, Onda S, Yanaga K, Suzuki N, Hattori A. Clinical application of navigation surgery using augmented reality in the abdominal field. Surg Today. 2015;45:397–406. doi: 10.1007/s00595-014-0946-9. [DOI] [PubMed] [Google Scholar]
- 85.Kügler D, Krumb H, Bredemann J, Stenin I, Kristin J, Klenzner T, Schipper J, Schmitt R, Sakas G, Mukhopadhyay A. High-precision evaluation of electromagnetic tracking. Int J Comput Assist Radiol Surg. 2019;14:1127–1135. doi: 10.1007/s11548-019-01959-5. [DOI] [PubMed] [Google Scholar]
- 86.Krumb H, Das D, Chadda R, Mukhopadhyay A. CycleGAN for interpretable online EMT compensation. Int J Comput Assist Radiol Surg. 2021;16:757–765. doi: 10.1007/s11548-021-02324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gherardini M, Clemente F, Milici S, Cipriani C. Localization accuracy of multiple magnets in a myokinetic control interface. Sci Rep. 2021;11:4850. doi: 10.1038/s41598-021-84390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrews CM, Henry AB, Soriano IM, Southworth MK, Silva JR. Registration Techniques for Clinical Applications of Three-Dimensional Augmented Reality Devices. IEEE J Transl Eng Health Med. 2021;9:4900214. doi: 10.1109/JTEHM.2020.3045642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadjadi H, Hashtrudi-Zaad K, Fichtinger G. Simultaneous Electromagnetic Tracking and Calibration for Dynamic Field Distortion Compensation. IEEE Trans Biomed Eng. 2016;63:1771–1781. doi: 10.1109/TBME.2015.2502138. [DOI] [PubMed] [Google Scholar]
- 90.Lv A, Li Y, Qian HG, Qiu H, Hao CY. Precise Navigation of the Surgical Plane with Intraoperative Real-time Virtual Sonography and 3D Simulation in Liver Resection. J Gastrointest Surg. 2018;22:1814–1818. doi: 10.1007/s11605-018-3872-0. [DOI] [PubMed] [Google Scholar]
- 91.Hostettler A, Nicolau SA, Rémond Y, Marescaux J, Soler L. A real-time predictive simulation of abdominal viscera positions during quiet free breathing. Prog Biophys Mol Biol. 2010;103:169–184. doi: 10.1016/j.pbiomolbio.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 92.Zhu H, Rohling RN, Salcudean SE. Hand-eye coordination-based implicit re-calibration method for gaze tracking on ultrasound machines: a statistical approach. Int J Comput Assist Radiol Surg. 2020;15:837–845. doi: 10.1007/s11548-020-02143-w. [DOI] [PubMed] [Google Scholar]
- 93.Heiselman JS, Jarnagin WR, Miga MI. Intraoperative Correction of Liver Deformation Using Sparse Surface and Vascular Features via Linearized Iterative Boundary Reconstruction. IEEE Trans Med Imaging. 2020;39:2223–2234. doi: 10.1109/TMI.2020.2967322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heiselman JS, Miga MI. Strain Energy Decay Predicts Elastic Registration Accuracy From Intraoperative Data Constraints. IEEE Trans Med Imaging. 2021;40:1290–1302. doi: 10.1109/TMI.2021.3052523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miyata A, Arita J, Shirata C, Abe S, Akamatsu N, Kaneko J, Kokudo N, Hasegawa K. Quantitative Assessment of the Accuracy of Real-Time Virtual Sonography for Liver Surgery. Surg Innov. 2020;27:60–67. doi: 10.1177/1553350619875301. [DOI] [PubMed] [Google Scholar]
- 96.Ivashchenko OV, Kuhlmann KFD, van Veen R, Pouw B, Kok NFM, Hoetjes NJ, Smit JN, Klompenhouwer EG, Nijkamp J, Ruers TJM. CBCT-based navigation system for open liver surgery: Accurate guidance toward mobile and deformable targets with a semi-rigid organ approximation and electromagnetic tracking of the liver. Med Phys. 2021;48:2145–2159. doi: 10.1002/mp.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo H, Yin D, Zhang S, Xiao D, He B, Meng F, Zhang Y, Cai W, He S, Zhang W, Hu Q, Guo H, Liang S, Zhou S, Liu S, Sun L, Guo X, Fang C, Liu L, Jia F. Augmented reality navigation for liver resection with a stereoscopic laparoscope. Comput Methods Programs Biomed. 2020;187:105099. doi: 10.1016/j.cmpb.2019.105099. [DOI] [PubMed] [Google Scholar]
- 98.Onda S, Okamoto T, Kanehira M, Fujioka S, Suzuki N, Hattori A, Yanaga K. Short rigid scope and stereo-scope designed specifically for open abdominal navigation surgery: clinical application for hepatobiliary and pancreatic surgery. J Hepatobiliary Pancreat Sci. 2013;20:448–453. doi: 10.1007/s00534-012-0582-y. [DOI] [PubMed] [Google Scholar]
- 99.Eck U, Winkler A. [Display technologies for augmented reality in medical applications] Unfallchirurg. 2018;121:278–285. doi: 10.1007/s00113-018-0463-1. [DOI] [PubMed] [Google Scholar]
- 100.Miyamoto R, Oshiro Y, Sano N, Inagawa S, Ohkohchi N. Three-dimensional surgical simulation of the bile duct and vascular arrangement in pancreatoduodenectomy: A retrospective cohort study. Ann Med Surg (Lond) 2018;36:17–22. doi: 10.1016/j.amsu.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han C, Shan X, Yao L, Yan P, Li M, Hu L, Tian H, Jing W, Du B, Wang L, Yang K, Guo T. Robotic-assisted vs laparoscopic cholecystectomy for benign gallbladder diseases: a systematic review and meta-analysis. Surg Endosc. 2018;32:4377–4392. doi: 10.1007/s00464-018-6295-9. [DOI] [PubMed] [Google Scholar]