Abstract
The first two complete mitogenomes of the leafhopper genus Cladolidia (C. biungulata and C. robusta) were sequenced and annotated to further explore the phylogeny of Cladolidia. Both the newly sequenced mitogenomes have a typical circular structure, with lengths of 15,247 and 15,376 bp and A + T contents of 78.2% and 78%, respectively. We identified a highly conserved genome organization in the two Cladolidia spp. through comparative analysis that included the following assessments: genome content, gene order, nucleotide composition, codon usage, amino acid composition, and tRNA secondary structure. Moreover, we detected the base heterogeneity of Cicadellidae mitogenomic data and constructed phylogenetic trees using the nucleotide alignments of 12 subfamilies of 58 leafhopper species. We noted a weak heterogeneity in the base composition among the Cicadellidae mitogenomes. Phylogenetic analyses showed that the monophyly of each subfamily was generally well supported in the family Cicadellidae; the main topology was as follows: (Deltocephalinae + (Treehoppers + ((Megophthalminae + (Macropsinae + (Hylicinae + (Coelidiinae +Iassinae)) + (Idiocerinae + (Cicadellinae + (Typhlocybinae + (Mileewinae + (Evacanthinae +Ledrinae)))))))))). Within Coelidiinae, phylogenetic analyses revealed that C. biungulata and C. robusta belong to Coelidiinae and the monophyly of Cladolidia is well supported. In addition, on the basis of complete mitogenome phylogenetic analysis and the comparison of morphological characteristics, we further confirm the genus Olidiana as a paraphyletic group, suggesting that the genus may need taxonomic revisions.
Keywords: Cicadellidae, heterogeneity, leafhopper, mitochondrial phylogenomics, structure
We gathered 63 mitochondrial genome sequences for leafhopper representing all 12 subfamilies, including 2 newly sequenced mitochondrial genomes from Coelidiinae. The phylogenetic relationship of each subfamily of Cicadellidae was further confirmed.
1. INTRODUCTION
In contrast to nuclear DNA, the mitogenome has a maternal mode of inheritance and is usually minimally recombinogenic; it carries genes with comparatively rapid evolutionary rates (Ballard & Whitlock, 2004; Cameron, 2014; Moritz & Brown, 1987; Wolstenholme, 1992). The entire mitogenome is a valuable source of extensive information compared with single genes. Moreover, it exhibits genome‐level characteristics, including gene content, base composition, gene organization, and gene secondary structure. These characteristics have been widely used for species identification as well as phylogenetic, phylogeographic, and genomic evolution studies (Anderson et al., 1981; Chuan et al., 2012; Nelson et al., 2012).
Leafhoppers are the members of a larger group of hemipterans and comprise >22,000 species (Dietrich, 2005). Recently, an increasing number phylogenic studies have been conducted on leafhoppers using mitogenomic data (Du, Dai, et al., 2017; Du, Zhang, et al., 2017; Du et al., 2019; Li et al., 2017; Song et al., 2017, 2019; Wang et al., 2020). So far, the data of 143 complete or near‐complete mitogenomes of Cicadellidae have been published in the National Center for Biotechnology Information (NCBI) database. Most of these organisms belong to the following subfamilies: Deltocephalinae (58), Cicadellinae (18), and Typhlocybinae (29). However, despite its vast diversity (>1,400 species), knowledge on the mitogenome of Coelidiinae is limited (Li & Fan, 2017; Nielson, 2015; Viraktamath & Meshram, 2019; Wang et al., 2018, 2021; Wang, Fan, et al., 2019; Zhang, 1990). Therefore, sequencing the mitogenomes of Coelidiinae may help enrich population genetics and phylogenetic studies regarding Cicadellidae (Hemiptera).
Most previous studies on Coelidiinae relationships have focused on morphological characteristics. However, the phylogeny of Coelidiinae remains to be explored using mitogenomic data. The lack of mitogenome sequences has limited the expansion of knowledge regarding the molecular evolution and population genetic diversity of this subfamily. Nielson (2015) removed C. biungulata, C. robusta, and five other species from Calodia and created the genus Cladolidia based primarily on the differences in the processes of aedeagus between these groupings. However, the position of the genus Cladolidia within the subfamily is yet to be ascertained (Nielson, 2015).
In the present study, we sequenced two complete mitogenomes of the genus Cladolidia (C. biungulata and C. robusta) using high‐throughput sequencing; C. biungulata and C. robusta are the first and second species, respectively, that have been described for this genus. In addition, we described their molecular phylogenetic relationships with 58 leafhopper and 5 treehopper species. Furthermore, this study provides an insight into the identification, phylogeny, conservation genetics, and evolution of Cladolidia and its related species.
2. MATERIALS AND METHODS
2.1. Sample collection and DNA extraction
Detailed information on the specimens collected is presented in Table S1. The collected specimens were identified based on their morphological characteristics, as described previously (Li & Fan, 2017; Zhang, 1990). After the species were accurately identified, the specimens were preserved in absolute ethanol and stored at −20°C until genomic DNA extraction. Genomic DNA was extracted from the whole body of adult males after removing the abdomen using DNeasy® Blood & Tissue Kit. In brief, the samples were incubated at 56°C for 6 hr to lyse the cells completely and the total genomic DNA was eluted in 100‐μl double‐distilled water. The subsequent steps were performed according to the manufacturer's instructions. After evaluating the extracted genomic DNA quality using 1% agarose gel electrophoresis, it was stored at −20°C until further use. Both the voucher specimens with male genitalia and DNA samples have been deposited at the Institute of Entomology, Guizhou University, Guiyang, China.
2.2. Sequence analysis
The two complete mitogenomes of C. biungulata and C. robusta were sequenced by Berry Genomics on the HiSeq 2500 platform (Illumina) with 150‐bp paired‐end reads. The average insert length was 350 bp, and 6 GB of clean data were obtained. Each mitogenome was assembled using Geneious Prime 2019.2.1 software and based on a mitochondrial reference sequence of Olidiana ritcheriina (MK738125) (Wang, Wang, et al., 2019). The assembled mitochondrial gene sequences were compared with the homologous sequences of O. ritcheriina (MK738125) and Taharana fasciana (KY886913) (Wang et al., 2017; Wang, Wang, et al., 2019), which were retrieved from GenBank and identified via BLAST searches on NCBI to confirm sequence accuracy. We used the MITOS web server and BLAST searches on NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to annotate the assembled sequences using invertebrate genetic codes (Altschul et al., 1997; Bernt et al., 2013) as well as the search server tRNAscan‐SE 1.21 to identify the locations and predict the secondary structure of 22 typical tRNAs (Laslett & Canbäck, 2008; Schattner et al., 2005; Tamura et al., 2013). All rRNA genes were identified based on the locations of adjacent tRNA genes and comparisons with sequences of other leafhopper mitogenomes deposited in NCBI. ORF Finder in Geneious Prime was used to predict 13 protein‐coding gene (PCG) locations using invertebrate genetic codes. The mitogenomic map and comparative analysis were performed using CGView comparison tool (Stothard et al., 2017). Furthermore, the relative synonymous codon usage (RSCU) values and codon numbers were calculated using the MEGA version 7.0 program (Sudhir et al., 2016). Finally, chain asymmetry was calculated using the following formulas: AT skew = (A − T)/(A + T) and GC skew = (G − C)/(G + C) (Perna & Kocher, 1995).
2.3. Phylogenetic analysis
In total, 58 leafhopper and 5 treehopper species were selected to construct the phylogenetic tree after the removal of sequences that were unverified, lacked an accurate scientific name, and were repetitive. Phylogenetic analysis was performed using alignments of the 13 PCGs of leafhopper species with the other complete or near‐complete mitogenomes of the treehopper species. The two species of Cosmoscarta bispecularis (KP064511) and Tettigades auropilosa (KM000129) (Yan & Zu, 2019) were used as the outgroup (Table S2). Each PCG was aligned using the TranslatorX online tool, employing MAFFT to perform protein alignment (Abascal et al., 2010; Castresana, 2000; Katoh et al., 2017). Then, the resulting 13 alignments were assessed and manually corrected using the MEGA version 7.0 program (Sudhir et al., 2016). The best schemes for partition and substitution models (Table S3) were determined in PartitionFinder version 2.1.1 using the Akaike information criterion and the greedy search algorithm (Lanfear et al., 2017). For phylogenetic analyses, the maximum likelihood (ML) and the Bayesian inference (BI) methods were used to construct the ML and BI trees based on two datasets (13PCG12, first and second codons of 13 PCGs [6,676 bp]; the amino acid [AA] sequences of 13 PCGs [3,338 bp]; these datasets were deposited in Dryad: https://doi.org/10.5061/dryad.zkh1893b3). The third codon positions may suffer from mutation saturation, which can lead to noise in the phylogenetic analysis (Blouin et al., 1998; Breinholt & Kawahara, 2013). Hence, the third codons were discarded from the phylogenetic analysis. The heterogeneity of sequence divergence within the two datasets was analyzed using AliGROOVE, with the default sliding window size (Kück et al., 2014).
ML analysis was performed with 1,000 rapid bootstrapping replicates using iqtree (Suchard & Huelsenbeck, 2012), whereas BI analysis was performed in MrBayes 3.2.7a with 4 chains and sampling of the chains every 1,000 generations (Nguyen et al., 2014). Two independent runs of 10 million generations were performed. After the average standard deviation of split frequencies fell to <0.001, the initial 25% of the samples was discarded as burn‐in and the remaining trees were used to generate a consensus tree and calculate the posterior probabilities. The BI and ML analyses were performed on the CIPRES Science Gateway (https://www.phylo.org) website. The phylogenetic trees were visualized using FigTree 1.4.2.
3. RESULTS
3.1. General features of Cladolidia mitogenome
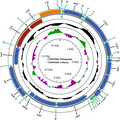
The annotations of the mitogenomes of the two Cladolidia species and the circular maps are shown in Table 1 and Figure 1, respectively. The two complete mitogenome sequences of C. biungulata (MW406474) and C. robusta (MW406475) are closed‐circular molecules, with lengths of 15,247 and 15,376 bp, respectively. These completely sequenced mitogenomes are medium‐sized in length and within the range of those of other Cicadellidae species (14,805 bp of Nephotettix cincticeps to 17,562 bp of Parazyginella tiani) (Wang et al., 2018). The two mitogenomes contained a typical set of 37 mitochondrial genes (13 PCGs, 22 tRNAs, and 2 rRNAs) along with a control region. Of these 37 genes, 23 are present on the heavy strand (J‐strand), whereas 14 are located on the light strand (N‐strand) (Figure 1, Table 1). The gene order of these two mitogenomes is identical to that of all previously published mitogenomes of Cicadellidae and the ancestral Drosophila yakuba (Clary & Wolstenholme, 1985). These two mitogenomes of Cladolidia contain 10 nucleotides that are dispersed among six intergenic spacers (ranging from 1 to 4 bp), and the longest spacer sequence (4 bp) is located between trnH and nad5, trnA, and trnR. There are a total of 14 overlapping regions (ranging from 1 to 11 bp), and the conserved 11‐bp overlapping nucleotide sequence between trnW and trnC is extremely common in Cicadellidae (Du, Zhang, et al., 2017; Du et al., 2019; Wang et al., 2017, 2018, 2020, 2021; Wang, Wang, et al., 2019).
TABLE 1.
Organization of the Cladolidia robusta/C. biungulata mitogenome
| Gene | Direction | Length (bp) | Start | Stop | Anticodon | Intergenic nucleotides | AT content (%) |
|---|---|---|---|---|---|---|---|
| trnI | J | 62 | – | – | GAT | 77.4 | |
| trnQ | N | 67 | – | – | TTG | 1 | 79.1/77.6 |
| trnM | J | 68/66 | – | – | CAT | −1/0 | 75/74.2 |
| nad2 | J | 955/957 | ATT | T/TAA | – | 0 | 82.1/82.7 |
| trnW | J | 62 | – | – | TCA | 0/−2 | 80.680.6 |
| trnC | N | 57/65 | – | – | GCA | −8/−11 | 84.2/84.6 |
| trnY | N | 63/62 | – | – | GTA | 0/−5 | 79.4/79 |
| cox1 | J | 1,536 | ATG | TAA | – | 2 | 71.5/72.5 |
| trnL1(UUR) | J | 67/68 | – | – | TAA | 0 | 82.1/82.4 |
| cox2 | J | 676 | ATT | T | – | 0 | 76.5/75.9 |
| trnK | J | 71 | – | – | CTT | 0 | 76.1/77.5 |
| trnD | J | 64 | – | – | GTC | −1/0 | 85.9/84.4 |
| atp8 | J | 150 | ATA | TAA | – | 1/0 | 82/82.7 |
| atp6 | J | 636 | ATA | TAA | – | −1 | 76.3/77.7 |
| cox3 | J | 778 | ATG | T | – | 0 | 73.5/73.8 |
| trnG | J | 61/63 | – | – | TCC | 0/−2 | 75.4/79.4 |
| nad3 | J | 354 | ATA | TAG | – | 0 | 79.1/80.4 |
| trnA | J | 61 | – | – | TGC | −2 | 80.3 |
| trnR | J | 59/63 | – | – | TCG | 4/1 | 74.6 |
| trnN | J | 64 | – | – | GTT | −1/−2 | 78.1/76.6 |
| trnS1 | J | 62 | – | – | GCT | −1 | 7,169.4 |
| trnE | J | 63 | – | – | TTC | −1 | 87.3/87.3 |
| trnF | N | 67 | – | – | GAA | −1 | 82.1/83.6 |
| nad5 | N | 1,674 | ATT | TAA | – | −1 | 77.4/77.9 |
| trnH | N | 60 | – | – | GTG | 0 | 75/78.3 |
| nad4 | N | 1,308 | ATT/ ATG | TAA | – | −1 | 77.8/78 |
| nad4l | N | 276 | ATG | TAA/TAG | – | 2 | 83.7/84.4 |
| trnT | J | 65 | – | – | TGT | 2 | 87.7 |
| trnP | N | 62 | – | – | TGG | 0 | 74.2/75.8 |
| nad6 | J | 474 | ATT | TAA | – | 4/2 | 82.3/80.8 |
| cob | J | 1,122/1,126 | ATA/ATC | TAA | – | 0 | 73.5/74.1 |
| trnS2(UCN) | J | 61/64 | – | – | TGA | −1/−1 | 82/79.7 |
| nad1 | N | 939 | ATT | TAA | – | −4/−7 | 77.2/78.4 |
| trnL2(CUN) | N | 68 | – | – | TAG | 0 | 75/79.4 |
| rrnL | N | 1,186/1,182 | – | – | – | 0 | 82/82.2 |
| trnV | N | 60 | – | – | TAC | 0 | 73.3/75 |
| rrnS | N | 779/730 | – | – | 0 | 81.6/81.5 | |
| CR | 1,016/1,199 | – | – | – | 0 | 84/82.5 |
FIGURE 1.
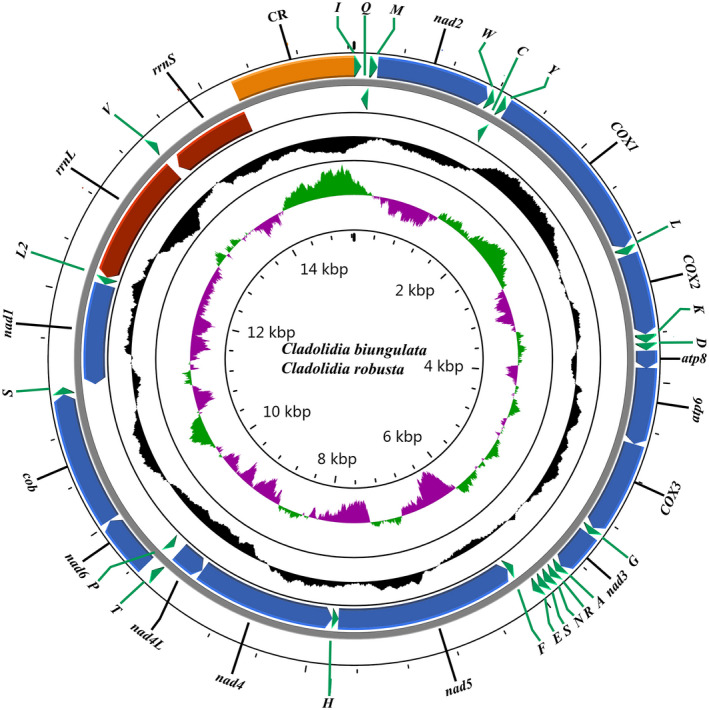
Mitogenome map of Cladolidia spp
The nucleotide composition of the two Cladolidia species reveals a strong A + T bias in the entire mitogenome, and the A + T contents between C. biungulata and C. robusta are nearly equal (78.2% in C. biungulata and 78.4% in C. robusta). As with other Coelidiinae, the nucleotide composition of the two mitogenomes is clearly biased toward A/T nucleotides, with 13 PCGs, 22 tRNAs, <2 rRNAs, and a control region. This phenomenon to some extent is due to the damage or accumulation of mutations in the mitochondrial DNA (Martin, 1995).
3.2. PCGs and codon usage of Cladolidia mitogenome
A total of 13 PCGs were identified in each of the two Cladolidia mitogenomes. In both mitogenomes, all PCGs use the canonical initiation codon ATN and the canonical stop codon TAA/TAG, except for cox2 and cox3. C. biungulata also harbors nad2, which uses an incomplete stop codon T‐‐. This phenomenon has also been noted in other Coelidiinae insects (Wang et al., 2017; Wang, Wang, et al., 2019). The incomplete stop codons are modified into complete TAA codons via posttranscriptional polyadenylation during mRNA maturation (Perna & Kocher, 1995). Of note, cox1, cox3, and atp6 in each species have the same start and stop codons. The longest PCG is nad5 (1,674 bp), and the shortest is atp8 (150 bp). Only four genes (nad5, nad4, nad4l, and nad1) are present on the N‐strand. The other nine genes (cox1, cox2, cox3, atp8, atp6, nad2, nad3, nad6, and cob) are located on the J‐strand (Figure 1, Table 1), which is similar to the mitogenome structure of most other Coelidiinae insects (Wang et al., 2017, 2021; Wang, Wang, et al., 2019).
The RSCU values and codon number for C. robusta (very similar to C. biungulata) are shown in Figure 2. The most frequently used codon is AUA (Met, N = 367), followed by AUU (Ile, N = 340), UUA (Leu, N = 333), and UUU (Phe, N = 290). However, in previous studies, the most frequently used codon was UUU (Phe) (Wang et al., 2017, 2021; Wang, Wang, et al., 2019). Moreover, the majority of frequently used codons end with A or U (Figure 2). These two factors appear to contribute to the high A + T content of PCGs and the AT bias of the whole mitogenome.
FIGURE 2.
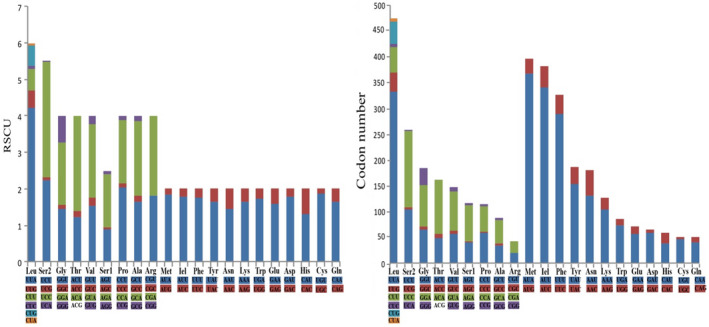
Relative synonymous codon usage (RSCU) and codon number of Cladolidia robusta
Comparative analysis revealed that the mitogenome of Coelidiinae is a conservative poly‐T (with 28–31 bp) structure (Figure 3). Such a large poly‐T structure is not found in the mitogenomes of other leafhoppers; hence, we hypothesized that this particular structure serves as a DNA barcode for the subfamily.
FIGURE 3.
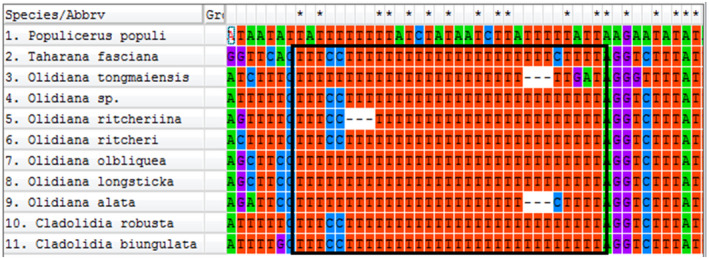
Poly‐T structure of ND5 in the subfamily Coelidiinae
3.3. tRNAs and rRNAs of Cladolidia mitogenome
All 22 tRNAs of C. biungulata and C. robusta mitogenomes were identified; they ranged from 57 to 68 bp in length. Among the tRNA genes, 14 are located on the J‐strand and 8 on the N‐strand, which is the coding pattern observed in almost all Cicadellidae mitogenomes (Du, Zhang, et al., 2017; Du et al., 2019; Wang et al., 2017, 2018, 2020, 2021; Wang, Wang, et al., 2019). The 22 tRNA genes in the two Cladolidia species were identified, and their secondary structures are shown in Figure 4. All these gene products are folded into the typical cloverleaf secondary structure, except trnS1, which lacks the dihydrouridine (DHU) arm; the loss of the DHU arm in trnS1 is a typical feature in Cicadellidae mitogenomes (Wang et al., 2017, 2018; Wang, Wang, et al., 2019). The combined length of tRNA genes of C. biungulata and C. robusta is 1,411 bp and 1,394 bp, with A + T contents of 79.4% and 78.9%, respectively. rrnS is located between trnL2 (CUN) and trnV, whereas rrnL is flanked by trnV and the control region (Figure 1, Table 2). Two rRNA genes, rrnS and rrnL, in C. biungulata and C. robusta have the same total length (2,222 bp). In Cladolidia, the A + T (81.8%) contents are the same and AT skews can be either positive or negative. The 22 tRNA and 2 rRNA genes are highly conserved, particularly trnI, trnA, trnR, and trnE, and the secondary structures are exactly the same between C. biungulata and C. robusta.
FIGURE 4.
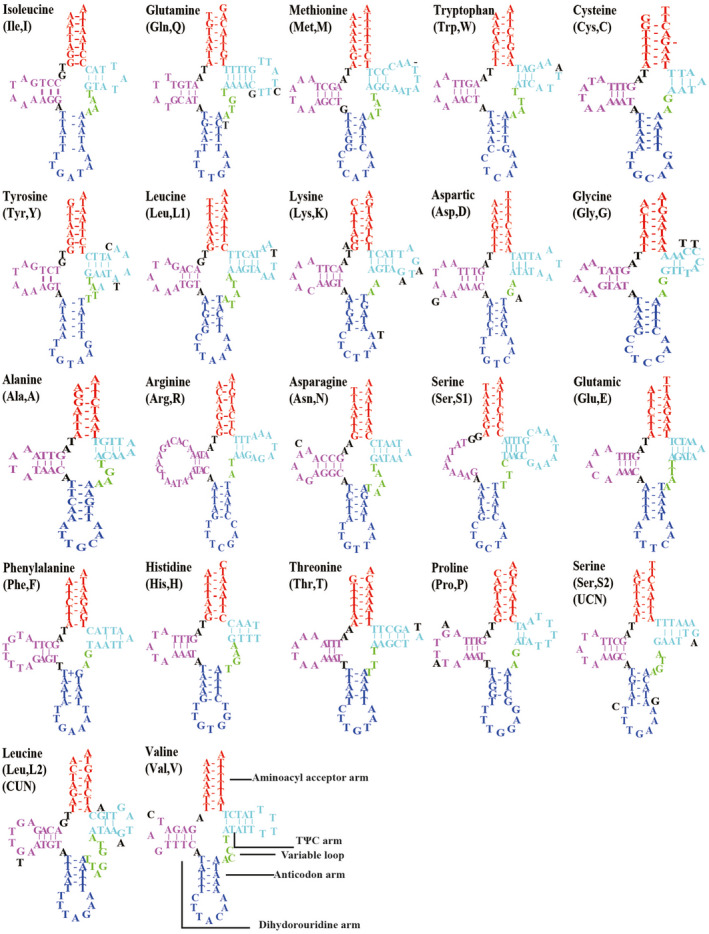
Predicted secondary structures of the 22 tRNAs of Cladolidia biungulata mitogenome. “‐” indicates the sites without a codon in Cladolidia robusta
TABLE 2.
Nucleotide composition and skewness of Cladolidia mitogenomes
| Region | Length | AT content% | AT skew | GC skew | |
|---|---|---|---|---|---|
| Cladolidia biungulata | Whole | 15,376 | 78.2 | 0.16 | −0.24 |
| 13 PCGs | 1,411 | 79.4 | 0.18 | −0.26 | |
| 22 tRNAs | 1,394 | 78.9 | 0.12 | −0.14 | |
| 2 rRNAs | 1,972 | 81.8 | 0.18 | −0.27 | |
| Control region | 1,199 | 83 | −0.02 | −0.01 | |
| Cladolidia robusta | Whole | 15,247 | 78.4 | 0.16 | −0.24 |
| 13 PCGs | 1,394 | 78.9 | 0.18 | −0.25 | |
| 22 tRNAs | 1,402 | 79.5 | 0.11 | −0.10 | |
| 2 rRNAs | 1,956 | 81.8 | 0.17 | −0.27 | |
| Control region | 1,016 | 82.5 | 0.01 | −0.09 |
Abbreviation: PCG, protein‐coding gene.
3.4. Control region of Cladolidia mitogenome
The control regions are located between rrnS and trnI, with lengths of 1,016 (C. biungulata) and 1,199 bp (C. robusta), respectively. The control region has the highest A + T content (83% and 82.5%) among the two complete C. biungulata and C. robusta mitogenomes (Table 2). Comparative analysis of the base composition of every component of the Coelidiinae mitogenomes indicated that the control regions have the highest A + T content, ranging from 82.5% (C. robusta) to 85.9% (O. obliqua). In the control region, both AT and GC skew are negative, indicating that T and C are more abundant than A and G. The GC content was the most significant factor in determining the codon bias among organisms, which is consistent with the general tendency of the complete mitogenome.
3.5. Phylogenetic relationship
By detecting the base heterogeneity of mitogenome datasets used for constructing a phylogenetic tree, we can determine whether the base heterogeneity of each dataset will cause a major error in the tree construction process (Li et al., 2015; Liua et al., 2018; Morgan et al., 2013; Sheffield et al., 2009; Song et al., 2016; Timmermans et al., 2015). On the basis of the calculation results obtained from the AliGROOVE (Kück et al., 2014) software, the heterogeneity of PCG12 and AA datasets in the mitogenomic data of Cicadellidae is weak (Figure 5). Hence, the two datasets could be used to construct a phylogenetic tree.
FIGURE 5.
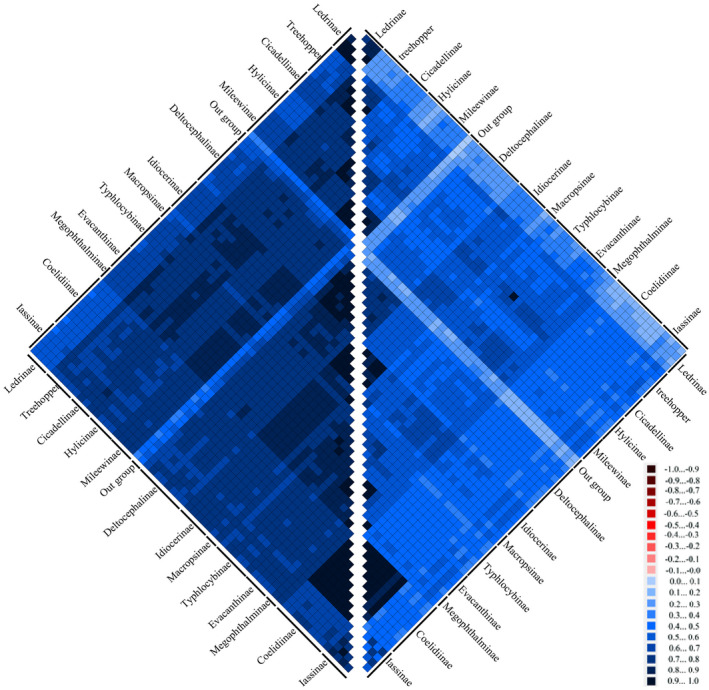
Heterogeneity of amino acids (left) and PCG12 (right) in the mitogenome of Cicadellidae. Differences in heterogeneity between sequences are represented by color, with dark red (−1) to dark blue (+1) representing differences from heavy to light. PCG, protein‐coding gene
BI and ML analyses using 13PCGs12 and the AA datasets generated phylogenic trees with two topologies (Figures 6, 7, S8, and S9). The monophyly of each subfamily was generally well supported in the family Cicadellidae, which is consistent with the findings of some previous molecular phylogenetic studies (Du, Zhang, et al., 2017; Du et al., 2019; Wang et al., 2017, 2018, 2020, 2021; Wang, Wang, et al., 2019). However, this finding is different from that of the studies by (Xue et al., 2020) and (Dietrich et al., 2017). They supported the inclusion of Macropsini and Idiocerini as the tribes of Eurymelinae. The phylogenetic relationships determined in our study do not support this inclusion, possibly owing to the use of molecular data different from those of previous studies and the limited mitogenomic data evaluation in our study; therefore, multiple gene types and more taxa should be sampled in the future to resolve this issue. Our analyses confirm that Iassinae is a sister group of Coelidiinae. Nine species of Coelidiinae are clustered together, and all phylogenetic relationships demonstrated a high nodal support in both ML (bootstrap support [BS] > 90) and BI (posterior probabilities [PP] = 1.00) analyses. These results provide substantial support for these two species (C. biungulata and C. robusta) being the members of the Coelidiinae subfamily and Cicadellidae family.
FIGURE 6.
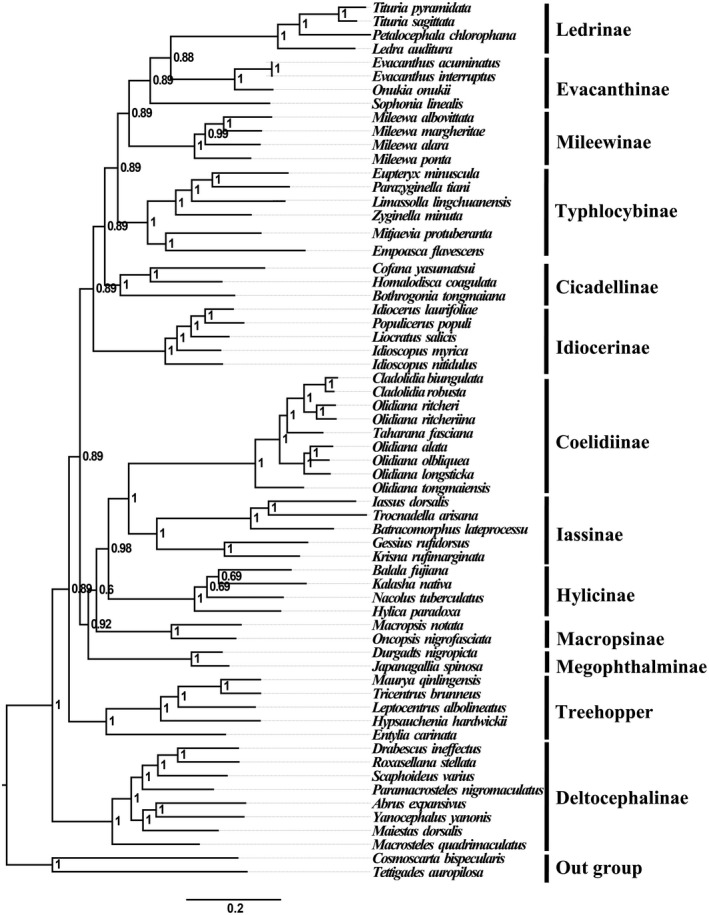
Phylogenetic tree of Cicadellidae species inferred via Bayesian analyses of the amino acid datasets
FIGURE 7.
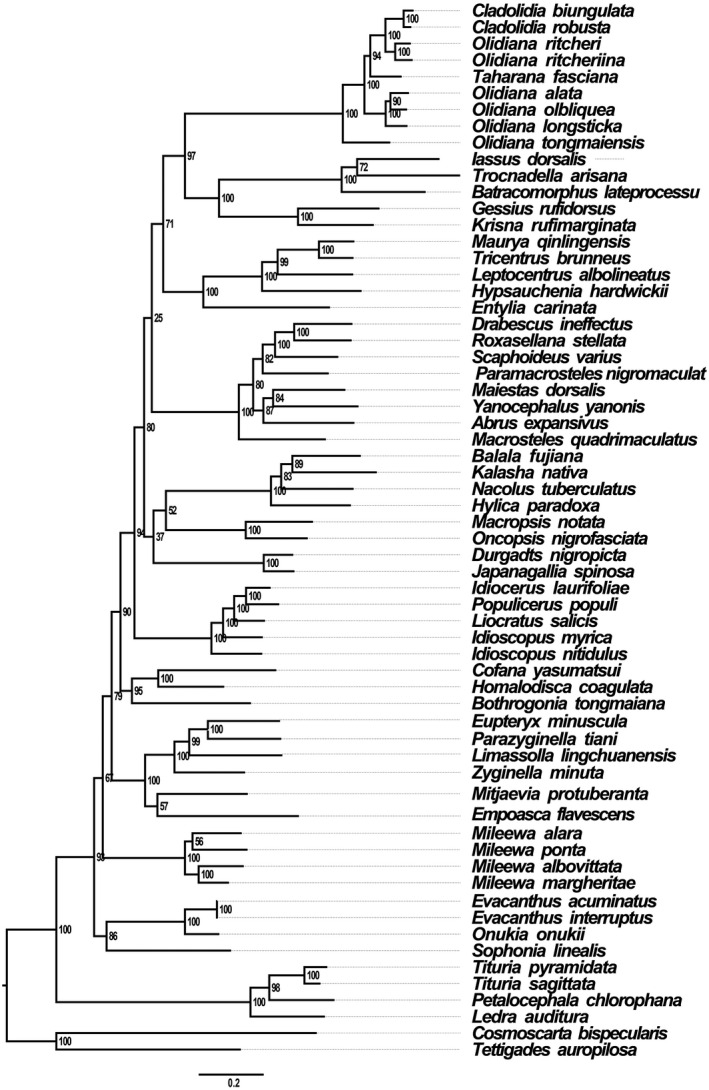
Phylogenetic tree Cicadellidae species inferred via maximum likelihood analyses of the amino acid datasets
4. DISCUSSION
The results of all analyses performed in the present study clearly support the 12 included cicadellidae subfamilies being monophyletic groups. The BI tree showed the following relationship within Membracoidea: (Deltocephalinae + (Treehoppers + ((Megophthalminae + (Macropsinae + (Hylicinae + (Coelidiinae +Iassinae)) + (Idiocerinae + (Cicadellinae + (Typhlocybinae + (Mileewinae + (Evacanthinae +Ledrinae))))))))) (Figure 6, S8, and S9). However, the ML tree showed the following phylogenetic relationships: (Ledrinae + (Evacanthinae + (Mileewinae + (Typhlocybinae + (Cicadellinae + (Idiocerinae + ((Macropsinae + (Megophthalminae +Hylicinae)+ (Deltocephalinae + ((Treehoppers + (Coelidiinae +Iassinae) +))))))))))) (Figure 7). In all BI analyses with higher approval ratings than ML analyses, this phenomenon is commonly noted in the analyses performed in previous studies; other recent analyses of relationships among some leafhopper subfamilies have yielded trees with low support for many deep internal branches (Wang et al., 2018, 2020). These two relationships of BI analyses and ML analyses differ primarily in the positions of Deltocephalinae and Ledrinae. In ML‐AA analysis, Ledrinae occupied the basal branch of leafhopper species in all phylogenetic analyses. This further confirms that the subfamily Ledrinae is an ancient group of leafhoppers, which is consistent with the findings of previous molecular phylogenetic studies (Du, Zhang, et al., 2017; Du et al., 2019; Wang et al., 2017, 2018, 2020, 2021; Wang, Wang, et al., 2019). However, Deltocephalinae, rather than Ledrinae, occupied the basal branch of leafhopper species in other (BI‐AA, BI/ML‐PCG12) phylogenetic analyses. Our analyses confirm that Iassinae and Coelidiinae are assigned to the sister groups of treehoppers, Macropsinae, and Megophthalminae with high approval ratings (ML, BS = 100; BI, PP = 1.00); this result is different from that observed in previous studies (Du et al., 2019; Wang et al., 2017, 2018, 2020, 2021; Wang, Wang, et al., 2019). In the present study, phylogenetic relationships showed that the subfamily Megophthalminae is a sister group of Macropsinae instead of treehoppers.
In all analyses, the two species of the genus Cladolidia also clustered closely with the genus Taharana; the results showed that the genus Cladolidia is a monophylic group. However, the genus Olidiana was not classified as monophyletic and can be divided into three branches. The three species O. ritcheriina, Olidiana sp., and O. ritcheri also clustered closely to the genus Taharana. The remaining species were split into two clades: one included O. longsticka, O. obliqua, and O. alata and the other included only one species (O. tongmaiensis). This conclusion was further confirmed based on significant differences in their morphological characteristics, which were characterized by body color, shape, and the position of the processes on the aedeagus shaft. Therefore, on the basis of the complete mitogenome phylogenetic analysis and the comparison of morphological characteristics, we propose that Olidiana is not monophyletic; hence, this genus may need taxonomic revisions. Future studies on both the morphological and molecular characteristics of additional species are warranted to reveal phylogenetic relationships within Coelidiinae.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Xianyi Wang: Formal analysis (equal); Investigation (lead); Methodology (lead); Writing‐original draft (equal); Writing‐review & editing (equal). Jiajia Wang: Conceptualization (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Renhuai Dai: Conceptualization (equal); Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (No. 31672342); the Program of Excellent Innovation Talents, Guizhou Province, China [Grant number 20206003]; and the Guizhou Province Graduate Research Fund YJSCXJH[2020]073. We also wish to thank Dr. Hongpin Zhang and Yalin Yao (Institute of Entomology, Guizhou University, Guiyang, China) for providing specimens in this study and two anonymous reviewers for reading the manuscript and making a lot of very valuable suggestion.
Wang, X., Wang, J., & Dai, R. (2021). Structural features of the mitogenome of the leafhopper genus Cladolidia (Hemiptera: Cicadellidae: Coelidiinae) and phylogenetic implications in Cicadellidae. Ecology and Evolution, 11, 12554–12566. 10.1002/ece3.8001
DATA AVAILABILITY STATEMENT
GenBank accession numbers: Cladolidia biungulata (MW406474) and Cladolidia robusta (MW406475). These two datasets 13PCG12 dataset (first and second codons of 13 PCGs, 6,676 bp); AA dataset, the amino acid sequences of 13 PCGs, 3,338 bp were deposited in Dryad: https://doi.org/10.5061/dryad.zkh1893b3).
REFERENCES
- Abascal, F., Zardoya, R., & Telford, M. J. (2010). TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Research, 38(Web Server), W7–W13. 10.1093/nar/gkq291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., & Lipman, D. J. (1997). Gapped BLAST and PSI‐BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H. L., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., Schreier, P. H., Smith, A. J. H., Staden, R., & Young, I. G. (1981). Sequence and organization of the human mitochondrial genome. Nature, 290, 457–465. 10.1038/290457a0 [DOI] [PubMed] [Google Scholar]
- Ballard, J. W., & Whitlock, M. C. (2004). The incomplete natural history of mitochondria. Molecular Ecology, 13, 729–744. 10.1046/j.1365-294X.2003.02063.x [DOI] [PubMed] [Google Scholar]
- Bernt, M., Donath, A., Jühling, F., Externbrink, F., Florentz, C., Fritzsch, G., Pütz, J., Middendorf, M., & Stadler, P. F. (2013). MITOS: Improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution, 69, 313–319. 10.1016/j.ympev.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Blouin, M. S., Yowell, C. A., Courtney, C. H., & Dame, J. B. (1998). Substitution bias, rapid saturation, and the use of mtDNA for nematode systematics. Molecular Biology and Evolution, 15, 1719–1727. 10.1093/oxfordjournals.molbev.a025898 [DOI] [PubMed] [Google Scholar]
- Breinholt, J. W., & Kawahara, A. Y. (2013). Phylotranscriptomics: Saturated third codon positions radically influence the estimation of trees based on next‐gen data. Genome Biology and Evolution, 5, 2082–2092. 10.1093/gbe/evt157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, S. L. (2014). Insect mitochondrial genomics: implications for evolution and phylogeny. Annual Review of Entomology, 59, 95–117. 10.1146/annurev-ento-011613-162007 [DOI] [PubMed] [Google Scholar]
- Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- Chuan, M. A., Yang, P., Jiang, F., Chapuis, M. P., Shali, Y., Sword, G. A., & Kang, L. E. (2012). Mitochondrial genomes reveal the global phylogeography and dispersal routes of the migratory locust. Molecular Ecology, 21, 4344–4358. 10.1111/j.1365-294X.2012.05684.x [DOI] [PubMed] [Google Scholar]
- Clary, D. O., & Wolstenholme, D. R. (1985). The mitochondrial DNA molecule of Drosophila yakuba: Nucleotide sequence, gene organization, and genetic code. Journal of Molecular Evolution, 22, 252–271. 10.1007/BF02099755 [DOI] [PubMed] [Google Scholar]
- Dietrich, C. H. (2005). Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Florida Entomologist, 88, 502–517. 10.1007/0-387-31311-7_34 [DOI] [Google Scholar]
- Dietrich, C. H., Allen, J. M., Lemmon, A. R., Lemmon, E. M., Takiya, D. M., Evangelista, O. W., & Johnson, K. P. (2017). Anchored hybrid enrichment‐based phylogenomics of leafhoppers and treehoppers (Hemiptera: Cicadomorpha: Membracoidea). Insect Systematics and Diversity, 1, 57–72. 10.1093/isd/ixx003 [DOI] [Google Scholar]
- Du, Y., Dai, W., & Dietrich, C. H. (2017). Mitochondrial genomic variation and phylogenetic relationships of three groups in the genus Scaphoideus (Hemiptera: Cicadellidae: Deltocephalinae). Scientific Reports, 7, 14197. 10.1038/s41598-017-17145-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. M., Dietrich, C. H., & Dai, W. (2019). Complete mitochondrial genome of Macrosteles quadrimaculatus (Matsumura) (Hemiptera: Cicadellidae: Deltocephalinae) with a shared tRNA rearrangement and its phylogenetic implications. International Journal of Biological Macromolecules, 122, 1027–1034. 10.1016/j.ijbiomac.2018.09.049 [DOI] [PubMed] [Google Scholar]
- Du, Y., Zhang, C., Dietrich, C. H., Zhang, Y., & Dai, W. (2017). Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Scientific Reports, 7, 14197. 10.1038/s41598-017-14703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K., Rozewicki, J., & Yamada, K. D. (2017). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 4, 1–7. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück, P., Meid, S. A., Groß, C., Wägele, J. W., & Misof, B. (2014). AliGROOVE – visualization of heterogeneous sequence divergence within multiple sequence alignments and detection of inflated branch support. BMC Bioinformatics, 15, 294. 10.1186/1471-2105-15-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., & Calcott, B. (2017). Partitionfinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34, 772–773. 10.1093/molbev/msw260 [DOI] [PubMed] [Google Scholar]
- Laslett, D., & Canbäck, B. (2008). ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics, 24, 172–175. 10.1093/bioinformatics/btm573 [DOI] [PubMed] [Google Scholar]
- Li, H., Leavengood, J. M., Chapman, E. G., Burkhardt, D., Song, F., Jiang, P., Liu, J. P., Zhou, X. G., & Cai, W. Z. (2017). Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proceedings of the Royal Society. Series B. Biological Sciences, 284, 20171223. 10.1098/rspb.2017.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Shao, R. F., Song, N., Song, F., Jiang, P., Li, Z. H., & Cai, W. Z. (2015). Higher‐level phylogeny of paraneopteran insects inferred from mitochondrial genome sequences. Scientific Reports, 5, 8527. 10.1038/srep08527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. Z., & Fan, Z. H. (2017). Coelidiinae (Hemiptera: Cicadellidae) from China (pp. 1–443). Guizhou Science and Technology Publishing House Press. [Google Scholar]
- Liua, Y. Q., Song, F., Jiang, P., Wilsonc, J. J., Cai, W. Z., & Li, H. (2018). Compositional heterogeneity in true bug mitochondrial phylogenomics. Molecular Phylogenetics and Evolution, 118, 135–144. 10.1016/j.ympev.2017.09.025 [DOI] [PubMed] [Google Scholar]
- Martin, A. P. (1995). Metabolic rate and directional nucleotide substitution in animal mitochondrial DNA. Molecular Biology and Evolution, 12, 1124–1131. 10.1006/jhev.1995.1071 [DOI] [PubMed] [Google Scholar]
- Morgan, C. C., Foster, P. G., Webb, A. E., Pisani, D., McInerney, J. O., & O'Connell, M. J. (2013). Heterogeneous models place the root of the placental mammal phylogeny. Molecular Biology and Evolution, 30, 2145–2156. 10.1093/molbev/mst1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, C., Dowling, T. E., & Brown, W. M. (1987). Evolution of animal mitochondrial DNA: Relevance for population biology and systematics. Annual Review of Ecology Evolution and Systematics, 18, 269–292. 10.1146/annurev.es.18.110187.001413 [DOI] [Google Scholar]
- Nelson, L. A., Lambkin, C. L., Batterham, P., Wallman, J. F., Dowton, M., Whiting, M. F., Yeates, D. K., & Cameron, S. L. (2012). Beyond barcoding: A mitochondrial genomics approach to molecular phylogenetics and diagnostics of blowflies (Diptera: Calliphoridae). Gene, 511, 131–142. 10.1016/j.gene.2012.09.103 [DOI] [PubMed] [Google Scholar]
- Nguyen, L. T., Schmidt, H. A., Arndt, V. H., & Minh, B. Q. (2014). IQ‐TREE: A fast and effective stochastic algorithm for estimating maximum‐likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson, M. W. (2015). A revision of the tribe Coelidiini of the Oriental, Palearctic and Australian biogeographical regions (Hemiptera: Cicadellidae: Coelidiinae). Insecta Mundi, 0410, 1–202. [Google Scholar]
- Perna, N. T., & Kocher, T. D. (1995). Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. Journal of Molecular Evolution, 41, 353–358. 10.1007/BF00186547 [DOI] [PubMed] [Google Scholar]
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner, P., Brooks, A. N., & Lowe, T. M. (2005). The tRNAscan‐SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research, 33, 686–689. 10.1093/nar/gki366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, N. C., Song, H. J., Cameron, S. L., & Whiting, M. F. (2009). Nonstationary evolution and compositional heterogeneity in beetle mitochondrial phylogenomics. Systematic Biology, 58, 381–394. 10.1093/sysbio/syp037 [DOI] [PubMed] [Google Scholar]
- Song, F., Li, H., Jiang, P., Zhou, X. G., Liu, J. P., Sun, C. H., Vogler, A. P., & Cai, W. Z. (2016). Capturing the phylogeny of holometabola with mitochondrial genome data and Bayesian site‐heterogeneous mixture models. Genome Biology and Evolution, 8, 1411–1426. 10.1093/gbe/evw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, N., Cai, W. Z., & Li, H. (2017). Deep‐level phylogeny of Cicadomorpha inferred from mitochondrial genomes sequenced by NGS. Scientific Reports, 7, 1–11. 10.1038/s41598-017-11132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, N., Zhang, H., & Zhao, T. (2019). Insights into the phylogeny of Hemiptera from increased mitogenomic taxon sampling. Molecular Phylogenetics and Evolution, 137, 236–249. 10.1016/j.ympev.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Stothard, P., Grant, J. R., & Van, D. G. (2017). Visualizing and comparing circular genomes using the CGView family of tools. Briefings in Bioinformatics, 20, 1576–1582. 10.1093/bib/bbx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhir, K., Glen, S., & Koichiro, T. (2016). Mega 7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Phylogenetics and Evolution, 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans, M. J., Barton, C., Haran, J., Ahrens, D., Culverwell, C. L., Ollikainen, A., Vogler, A. P., Dodsworth, S., Foster, P. G., Bocak, L., & Vogler, A. P. (2015). Family‐level sampling of mitochondrial genomes in Coleoptera: compositional heterogeneity and phylogenetics. Genome Biology and Evolution, 8, 161–175. 10.1093/gbe/evv241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viraktamath, C. A., & Meshram, N. M. (2019). Leafhopper tribe Coelidiini (Hemiptera: Cicadellidae: Coelidiinae) of the Indian subcontinent. Zootaxa, 4653, 1–91. 10.11646/zootaxa.4653.1.1 [DOI] [PubMed] [Google Scholar]
- Wang, J. J., Li, H., & Dai, R. H. (2017). Complete mitochondrial genome of Taharana fasciana (Insecta, Hemiptera: Cicadellidae) and comparison with other Cicadellidae insects. Genetica, 145, 593–602. 10.1007/s10709-017-9984-8 [DOI] [PubMed] [Google Scholar]
- Wang, J. J., Wu, Y. F., Dai, R. H., & Yang, M. F. (2020). Comparative mitogenomes of six species in the subfamily Iassinae (Hemiptera: Cicadellidae) and phylogenetic analysis. International Journal of Biological Macromolecules, 149, 1294–1303. 10.1016/j.ijbiomac.2020.01.270 [DOI] [PubMed] [Google Scholar]
- Wang, J.‐J., Yang, M.‐F., Dai, R.‐H., Li, H. U., & Wang, X.‐Y. (2018). Characterization and phylogenetic implications of the complete mitochondrial genome of Idiocerinae (Hemiptera: Cicadellidae). International Journal of Biological Macromolecules, 120, 2366–2372. 10.1016/j.ijbiomac.2018.08.191 [DOI] [PubMed] [Google Scholar]
- Wang, X. Y., Fan, Z. H., Li, Z. Z., & Dai, R. H. (2019). Key to genera of Chinese Coelidiinae leafhoppers, with description a new species of the leafhopper genus Baseprocessa (Hemiptera: Auchenorrhyncha: Cicadellidae). Zootaxa, 4701, 454–460. 10.11646/zootaxa.4701.5.5 [DOI] [PubMed] [Google Scholar]
- Wang, X. Y., Wang, J. J., & Dai, R. H. (2021). Mitogenomics of five Olidiana leafhoppers (Hemiptera: Cicadellidae: Coelidiinae) and their phylogenetic implications. PeerJ, 9, e11086. 10.7717/peerj.11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. Y., Wang, J. J., Fan, Z. H., & Dai, R. H. (2019). Complete mitogenome of Olidiana ritcheriina (Hemiptera: Cicadellidae) and phylogeny of Cicadellidae. PeerJ, 7, e8072. 10.7717/peerj.8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme, D. R. (1992). Animal Mitochondrial DNA: Structure and Evolution. International Review of Cytology, 141, 173–216. 10.1016/S0074-7696(08)62066-5 [DOI] [PubMed] [Google Scholar]
- Xue, Q. Q., Dietrich, C. H., & Zhang, Y. L. (2020). Phylogeny and classification of the leafhopper subfamily Eurymelinae (Hemiptera: Cicadellidae) inferred from molecules and morphology. Systematic Entomology, 45, 687–702. 10.1111/syen.12425 [DOI] [Google Scholar]
- Yan, C., & Zu, Z. (2019). The complete mitochondrial genome of Cosmoscarta dorsimacula . Mitochondrial DNA Part B, 4(1), 975–976. 10.1080/23802359.2019.1580158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. L. (1990). A taxonomic study of Chinese Cicadellidae (Homoptera) (pp. 1–218). Tianze Publishing House Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
GenBank accession numbers: Cladolidia biungulata (MW406474) and Cladolidia robusta (MW406475). These two datasets 13PCG12 dataset (first and second codons of 13 PCGs, 6,676 bp); AA dataset, the amino acid sequences of 13 PCGs, 3,338 bp were deposited in Dryad: https://doi.org/10.5061/dryad.zkh1893b3).


