Abstract
Exophiala is a genus comprising several species of opportunistic black yeasts, which belongs to Ascomycotina. It is a rare cause of fungal infections. However, infections are often chronic and recalcitrant, and while the number of cases is steadily increasing in both immunocompromised and immunocompetent people, detailed knowledge remains scarce regarding infection mechanisms, virulence factors, specific predisposing factors, risk factors, and host response. The most common manifestations of Exophiala infection are skin infections, and the most frequent type of deep infection is pulmonary infection due to inhalation. The invasive disease ranges from cutaneous or subcutaneous infection to systemic dissemination to internal organs. The final identification of the causative organism should be achieved through a combination of several methods, including the newly introduced diagnostic analysis, matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry, together with sequencing of the ribosomal ribonucleic acid internal transcribed spacer region of the fungi, and histological and culture findings. Regarding treatment, because anti-infective agents and natural compounds exhibited poor antibiofilm activity, few treatments have ultimately been found to be effective for specific antifungal therapy, so the optimal antifungal therapy and duration of therapy for these infections remain unknown. Therefore, most forms of disease caused by Exophiala dermatitidis require aggressive combination therapies: Both surgical intervention and aggressive antifungal therapy with novel compounds and azoles are necessary for effective treatment.
Keywords: Exophiala dermatitidis, Black yeast, Immunocompromised host, Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry, Ribosomal ribonucleic acid, Antifungal drugs
Core Tip: Exophiala is a genus comprising several species of opportunistic black yeasts, which belongs to Ascomycotina, and it is a rare cause of fungal infections. The final identification of this organism should be achieved through a combination of several methods, including the newly introduced diagnostic analysis, matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry, together with sequencing of the ribosomal ribonucleic acid internal transcribed spacer region of the fungi, and histological and culture findings. Most forms of disease caused by this pathogen require aggressive combination therapies: both surgical intervention and aggressive antifungal therapy with novel compounds and azoles are necessary for effective treatment. Today, detailed knowledge regarding this pathogen is scarce, so further studies are required in order to collect evidence.
INTRODUCTION
Exophiala is a genus comprising several species of opportunistic black yeasts, which belongs to Ascomycotina, in the order Chaetothyriales[1,2]. Exophiala dermatitidis (E. dermatitidis) is a melanized, thermophilic, dimorphic, dematiaceous, and hyphal growth state fungus with multiple conidial forms, and it can be found in abundance in extreme natural habitats; in hot, moist artificial environments that are rich in toxic hydrocarbons, such as steam baths and bathrooms; in decaying organic matter; in creosote-treated oak railway ties in regions near sea level; and in dishwashers[3-12]. It is a polyextremophilic opportunistic human pathogen, which has frequently been isolated in tropical regions of the world[8,13]. Its growth characteristics include a polymorphic life cycle, its ability to grow in both high and low temperatures in broadly varying pH environments, its ability to survive high sodium chloride concentrations, and its ability to survive high amounts of both ultraviolet and radioactive radiation[2,10]. However, despite many ecological studies done worldwide, little remains known about the natural habitat of this fungus[2].
This dematiaceous (brown-pigmented) fungus is a large, heterogenous group of molds that can cause a wide range of diseases, including phaeohyphomycosis, chromoblastomycosis, and eumycotic mycetoma; however, it is rarely the cause of fungal infection, primarily in patients who already have certain predisposing factors[3,8,14-17]. It is known to generate a biofilm in a strain-specific manner, with invasive E. dermatitidis isolates forming biofilms with the greatest biomass[16,18]. In addition, melanin, which forms part of the cell wall of black yeasts, is known to be a major contributing factor to the pathogenicity of E. dermatitidis, and to its increased resistance against both host defense and anti-infective therapeutics[16]. Additionally, clinically, it is considered to be the most virulent species, and potentially the cause of disseminated infections, as both produce extracellular capsule-like material[19].
SYMPTOMS
E. dermatitidis is a rare cause of fungal infections, and the infection is often chronic and recalcitrant; however, much is still unknown about the mechanisms of infection[2,7,17,20]. In addition, while the number of cases is steadily increasing in both immunocompromised and immunocompetent people, detailed knowledge remains scarce regarding infection mechanisms, virulence factors, specific predisposing factors, risk factors, and host response[8,17,21].
Altered immune status is known to affect the progress of infectious diseases[14]. While more and more cases have been seen in immunocompromised patients, there is still little knowledge regarding infections, virulence factors, and host response[11]. Immunocompromised patients, including patients undergoing steroid therapy, patients who have received transplants, and patients with cancer and/or AIDS, are especially at risk of contracting fungal infections[14,21]. For instance, cutaneous, subcutaneous, and corneal infections with dematiaceous fungi naturally occur worldwide, and tropical and subtropical climates lend themselves to increased incidence of these infections, particularly because they are seen mainly as opportunistic infections in immune-deficient patients, and can cause cutaneous and subcutaneous phaeohyphomycosis in healthy individuals[4]. Disseminated infections are rare, but becoming more common, particularly in individuals who are immunocompromised, such as chemotherapy patients[3,22,23]. On the other hand, this fungus may potentially be a cause of disseminated infection in humans who otherwise appear to be healthy[24].
The most common manifestations of the infection are skin infections; in addition, less commonly, extracutaneous diseases from traumatic implantation have been reported, including the eyes, heart, gastrointestinal tract, lungs, and bones[3,4,14,22,25,26]. The fungus can actively grow and penetrate the skin; consequently, it is more frequently isolated from superficial, cutaneous, subcutaneous, and deep seated infections, including visceral infections, with central line associated bloodstream infection (CLABSI) or endocarditis seen on rare occasions[11,25,27-29]. Regarding these skin infections, mycetoma is a tumorous skin growth, notable for the black granules that form within tissue[14]. Chromoblastomycosis is a superficial or subcutaneous skin infection, which is histologically characterized by pigmented sclerotic bodies, as well as pseudoepitheliomatous hyperplasia[14]. The invasive disease, known as phaeohyphomycosis, varies significantly: from cutaneous or subcutaneous infections to systemic dissemination to the internal organs[4,14]. According to a previous report, this is the third most common species among the reported causes of disseminated phaeohyphomycosis, at 7%, and is among the most significant human pathogens of the dematiaceous fungi[3].
The most common forms of deep infections are respiratory system infections, due to inhalation, and pulmonary infections; the latter are mostly not invasive, but it seems likely that subclinical colonization may be involved in Europe or Latin America, as rates can vary from 1% to 19%[3,16,25,30]. This pathogen is known to cause deep or localized phaeohyphomycosis in immunocompromised people worldwide, and is regularly found in the lungs of cystic fibrosis (CF) patients, but only very rarely is it found in respiratory specimens from patients who do not suffer from CF[2,18,31]. In one study, about 75% of CF patients were found to have been colonized by yeasts, chiefly among these Candida albicans (38%) and C. dubliniensis (12%). Additionally, Aspergillus spp. (Aspergillus fumigatus: 29%) was detected in 35% of patients, followed by E. dermatitidis and Scedosporium/Lomentospora complex isolates, each found in 4% of patients[32]. Conversely, the most common form of systemic phaeohyphomycosis is cerebral infection, though only in Asian patients; other localized deep forms of the disease have also been reported, including arthritis and endocarditis[3,25]. This suggests the potential possibility of race-dependent virulence[25]. Cerebral phaeohyphomycosis is a central nervous infection that is sporadic, but often fatal[7,16,33]. Although cases of cerebral phaeohyphomycosis are known to have occurred in immunocompromised persons, it has most commonly been found in immunocompetent individuals who have no obvious risk factors[3].
EXAMINATION
Identification of Exophiala at the species level has conventionally been based on morphological observations, complemented by molecular tests[34]. Tissue cultures can provide a definitive contribution to differential diagnosis of mold infections[14]. In one study, 100% of E. dermatitidis was correctly identified when using Sabouraud glucose agar, and 92.9% of strains were correctly identified at the species level when using Columbia agar[35]. In addition, Burkholderia cepacia selective agar can prove useful for recovery from CF patients’ sputum samples[36]. Here, we show a sputum sample from our institution, from a patient with pneumonia due to E. dermatitidis. Black, yeast-like colonies grown on the Chromogenic Agar CHROMagar® Candida plate can be observed (Figure 1). We extracted and cultured the bacteria on potato dextrose agar using the specimen at 37 °C, yielding a huge colony on the plate 21 days later (Figure 2). In addition, the diphasic form was observed under a differential interference contrast microscope (Figure 3), bacterial growth was confirmed below 40 °C, and morphological characteristics were confirmed with a stereoscopic microscope (Figure 4). All of these characteristics were also compatible with E. dermatitidis. However, this is a slow-growing fungus, which can ordinarily take up to two weeks to culture in a microbiology laboratory setting; therefore, it is slow to detect it conventionally, leading to a risk of bacterial overgrowth due to common cohabiting pan- and multi-resistant bacterial pathogens from sputum, specifically Pseudomonas aeruginosa and B. cepacia complex organisms[37]. As mentioned above, conventional mycological identification, premised on recognizing morphological characteristics, can prove problematic[38].
Figure 1.
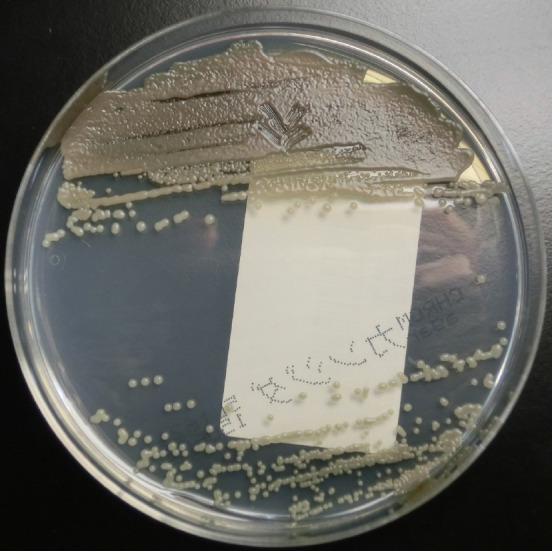
Colonies of Exophiala dermatitidis on Chromogenic Agar CHROMagar® Candida plate. Sputum sample of pneumonia patient from our institution. Many black, yeast-like colonies were observed.
Figure 2.
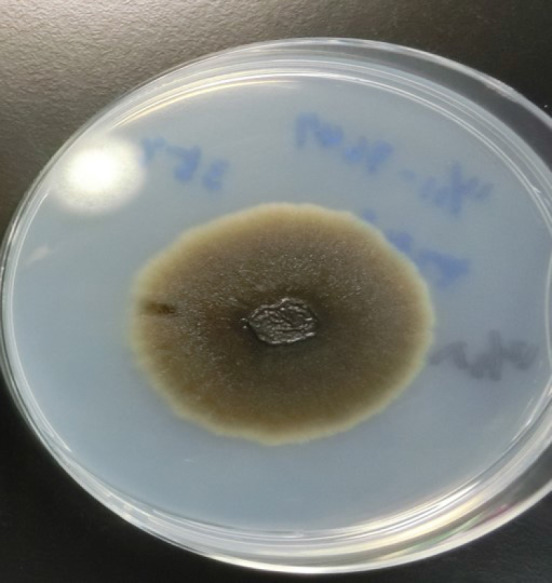
A colony of Exophiala dermatitidis on potato dextrose agar. Sputum sample of pneumonia patient from our institution, 21 d after cultivation. A very large black colony was yielded.
Figure 3.
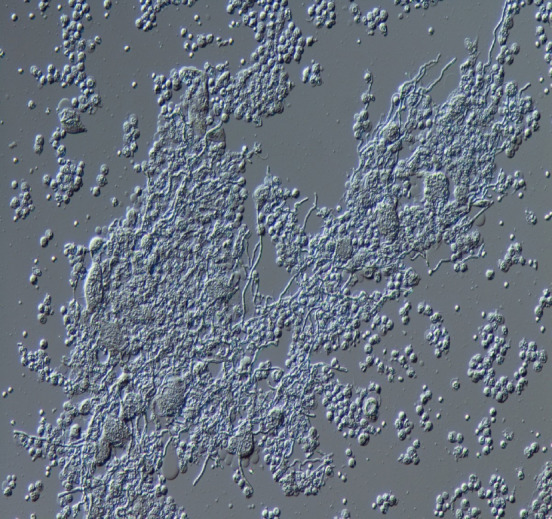
Appearance of Exophiala dermatitidis under differential interference contrast microscope. Sputum sample of pneumonia patient from our institution. The diphasic form was observed.
Figure 4.
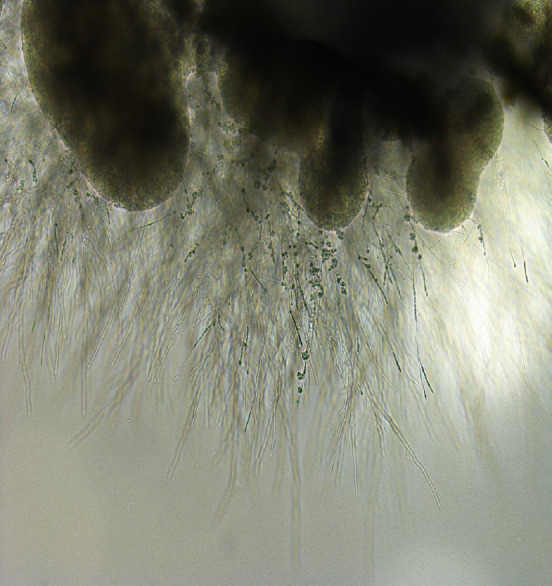
Appearance of Exophiala dermatitidis under stereoscopic microscope. Sputum sample of pneumonia patient from our institution. Melanized, dimorphic, dematiaceous, and hyphal-growth-state fungus, with multiple conidial forms, was confirmed.
On the other hand, in the past decade, matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) has come into use at medical centers internationally, as a diagnostic technique[35]. This technique takes advantage of taxon-specific mass patterns that have been recorded from abundant cell proteins, directly from whole-cell preparations[38]. The relationships observed between the ITS (internal transcribed spacer) strains, from the MALDI-TOF MS profiling analyses, were supported by DNA-sequence-based analyses of the ITS1 and ITS2 regions of ribonucleic acid[38]. The ribosomal RNA (rRNA) genes (small and large subunits), rRNA ITS regions, and protein-coding genes, including the genes for translation elongation factor 1-α and β-tubulin, have served as fungal identification targets[38]. There was extremely little intra-species sequence diversity in medically important dematiaceous fungi, such as E. dermatitidis: In some cases, no changes were detected at all in Exophiala species[15]. Furthermore, the D1/D2 domain is sufficiently variable to identify pathogenic dematiaceous fungi and other relevant species[15]. Therefore, phylogenetic analysis of the ITS region sequence provided information that was useful for not only the identification of new species, but also for the subtyping and origin of Exophiala species[39]. The data demonstrated that MALDI-TOF MS can serve a rapid, reliable, and cost-effective way to identify isolates of E. dermatitidis, as well as other clinically relevant fungi and yeasts that can ordinarily be difficult to identify using conventional methods[38,40]. This represents a major breakthrough in medical mycology diagnostics, and will lead to improved fungal pathogen identification rates, including rare and nonsporulating fungi, in ordinary clinical laboratory settings[35]. In addition, this is expected to simplify the routine identification of bacteria, mycobacteria, and fungi, compared to using traditional diagnostic methods; one exception, however, is viruses, which, due to their relatively low protein content, remain difficult to detect, even with this technique[35,38]. Consequently, this technique seems promising for use in species-level identification of Exophiala, alongside ITS sequencing, which remains the gold standard[41]. On the other hand, there is still very little generated data available on any black yeast species; as a result, these databases are often incomplete or entirely absent[35,41]. However, a recent study has confirmed that this technique is able to identify organisms such as black yeasts, which are ordinarily comparatively difficult to identify, down to the species level, with a discrimination rate of E. dermatitidis using MALDI-TOF MS of at least 80.6% — impressive, but unfortunately still not 100%[35]. The identification results for E. dermatidis is based on the database provided with the system. In this study, mass spectrometry was performed using the Microflex LT model (Bruker Daltonik GmbH) MALDI-TOF instrument. Also, the linear positive-ion mode was used within a mass range of 2000-20000 Da for microbial identification, and the instrument was equipped with a 60-Hz nitrogen laser and for each spectrum, 240 Laser shots from different positions of the sample spot were accumulated and analyzed. Accurate, quality-controlled reference databases are still necessary for both the ITS-based and MALDI-TOF MS-based identification modalities, and it understanding each approach’s limitations is key[35]. In particular, the low-quality mass spectra caused by bad material processing and insufficient database entries for some fungal isolates can hinder MALDI-TOF MS-based identification; additionally, only a very small percentage of fungi have been sequenced in the ITS region, and the taxonomic reliability of fungal ITS sequences available in public repositories remains inadequate[35]. Another major issue currently associated with MALDI-TOF MS-based identification of fungi is similarities in molecular components, which can make it impossible to distinguish between sister species[41]. As shown here, there are several limitations to the MALDI-TOF MS technique[41].
A high-resolution melting assay has been developed, which can be serve to help ascertain the identity of E. dermatitidis, which may vary in its clinical significance, with a high degree of accuracy[1]. For species-level identification of Exophiala isolates, the ligase-dependent reaction assay was found to be both less expensive and more reliable than rolling circle amplification[42]. Antifungal susceptibility tests demonstrated that triazole compounds were the most active agents against Exophiala in vitro[34]. In addition, triazoles, especially itraconazole (ITCZ) and posaconazole (PSCZ), were found to have minimum inhibitory concentration (MIC) values below those of amphotericin B (AMB), caspofungin (CPFG), and flucytosine (5-FC)[40]. This suggests that, in the future, it may be possible to use this in order to identify species directly, in clinical samples and/or in environmental niches[1].
TREATMENT
Due to the rarity of infections caused by this fungus, little is known about its treatment[17]. Various fungi are able to colonize surfaces and form biofilms[18]. Due to resistance to antifungal drugs, infections associated with fungal biofilms are frequently refractory to targeted treatment[18]. This leads to a variety of illnesses in humans that are always refractory to the available treatment modalities[43]. Data on in vitro susceptibility remains scarce, and seemingly correlates poorly with in vivo drug efficacy[44]. More information remains necessary on activities against species of Exophiala[44]. Furthermore, although antifungal susceptibility tests have been developed, there is still very little in vitro data available for dematiaceous fungi, and comparisons are difficult because the previous literature is often inconsistent regarding methodology[44]. To date, most forms of disease caused by E. dermatitidis have required therapy combining surgery with medical treatment that uses novel compounds and azoles, in order to achieve effective results[3,20,25]. In particular, aggressive antifungal therapy is vital, in combination with surgical intervention[45]. However, because anti-infective agents and natural compounds have demonstrated poor antibiofilm activity, few treatments have ultimately been found to be effective when it comes to specific antifungal therapy; as a result, it is still unknown what the optimal antifungal therapy and duration of therapy are for these infections[18,21,25,44]. MIC results for all E. dermatitidis strains have revealed that CPFG has the widest range and the highest MICs (range 1–16 mg/L, geometric mean 4.912 mg/L), while PSCZ has the lowest MICs (range 0.016–0.031 mg/L, geometric mean 0.061 mg/L)[10]. However, their clinical effectiveness remains unknown for treatment of infections caused by this fungus[10]. Additionally, European Society of Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi, which was published in 2014, do not indicate AMB for treatment[34]. Therefore, combination therapies that use novel compounds and azoles could prove to be an effective solution[20,21].
A recent study has reported that crucial stress responses, mechanisms of cell wall repair, and antifungal resistance in pathogenic fungi are governed by Hsp90[43]. Consequently, targeting Hsp90 with specific inhibitors holds considerable promise as a combination strategy[43]. In addition, the compounds were detected to have the greatest impact when added prior to cell adhesion; these findings suggest that, ultimately, prevention may prove more effective than treatment for biofilm-associated infections caused by this fungus[22]. Furthermore, in cases of suspected CLABSI, the most effective treatment seems to be promptly removing the central line, followed by treatment with AMB or an azole[29]. According to another study, pyrvinium pamoate may exert antifungal activity on its own, and may demonstrate a favorable synergy with azoles against planktonic E. dermatitidis[20,21]. The underlying mechanisms could perhaps be elucidated by inducing apoptosis/necrosis, inhibiting drug efflux pumps, and signaling pathways that are related to stress response and growth control[20,21]. More in-depth investigation into the mechanism of the antifungal properties of pyrvinium, and the innovation of novel formulation of pyrvinium, may help establish novel antifungal strategies. In addition, lonafarnib, an orally bioavailable nonpeptide tricyclic farnesyltransferase inhibitor, which has excellent pharmacokinetic and safety profiles, could serve to enhance the in vitro antifungal activity of ITCZ, PSCZ, and voriconazole against E. dermatitidis; this suggests that azoles, particularly ITCZ and PSCZ, combined with a farnesyltransferase inhibitor, might provide a potential strategy for managing infections caused by this fungus[44].
PROGNOSIS
Diseases reported to have been caused by this fungus range from benign cutaneous infections to systemic infections with a 40% fatality rate[8,11]. In particular, while systemic infections are exceedingly rare, they are associated with both significant morbidity and mortality[9]. A positive outcome was achieved in a disseminated E. dermatitidis infection in an immunodeficient patient, through a combination of aggressive surgical intervention and prolonged treatment using broad-spectrum antifungal agents[9]. In addition, it is suspected that melanin may dramatically reduce the invasiveness and virulence of E. dermatitidis during the initial days of the skin infection[8].
CONCLUSION
There are clearly still many uncertainties, mainly regarding accurate diagnosis and treatment. Because of the difficulty of morphological distinction in culture between E. dermatitidis and other species, and because no individual technique is sufficient for identification on its own, the final identification of the causative organism should be achieved through a combination of several methods, including the newly introduced diagnostic analysis MALDI-TOF MS, together with sequencing of the rDNA ITS region of the fungi, and histological and culture findings. Furthermore, due to the rarity of infections caused by this fungus, little is known about its treatment. Therefore, further studies are required in order to collect evidence.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: March 5, 2021
First decision: March 25, 2021
Article in press: August 24, 2021
Specialty type: Mycology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Uchida-Fujii E S-Editor: Gong ZM L-Editor: A P-Editor: Liu JH
Contributor Information
Daisuke Usuda, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan. d.usuda.qa@juntendo.ac.jp.
Toshihiro Higashikawa, Department of Geriatric Medicine, Kanazawa Medical University Himi Municipal Hospital, Himi-shi 935-8531, Toyama-ken, Japan.
Yuta Hotchi, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Kenki Usami, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Shintaro Shimozawa, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Shungo Tokunaga, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Ippei Osugi, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Risa Katou, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Sakurako Ito, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Toshihiko Yoshizawa, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Suguru Asako, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Kentaro Mishima, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Akihiko Kondo, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Keiko Mizuno, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Hiroki Takami, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Takayuki Komatsu, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Jiro Oba, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Tomohisa Nomura, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
Manabu Sugita, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima-ku 177-8521, Tokyo, Japan.
References
- 1.Bahadori T, Didehdar M, Khansarinezhad B, Shokohi T. Identification of opportunistic and nonopportunistic Exophiala species using high resolution melting analysis. Med Mycol. 2020;58:938–945. doi: 10.1093/mmy/myz136. [DOI] [PubMed] [Google Scholar]
- 2.Babič MN, Zupančič J, Gunde-Cimerman N, de Hoog S, Zalar P. Ecology of the Human Opportunistic Black Yeast Exophiala dermatitidis Indicates Preference for Human-Made Habitats. Mycopathologia. 2018;183:201–212. doi: 10.1007/s11046-017-0134-8. [DOI] [PubMed] [Google Scholar]
- 3.Brandt ME, Warnock DW. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother. 2003;15 Suppl 2:36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 4.Döğen A, Kaplan E, Ilkit M, de Hoog GS. Massive contamination of Exophiala dermatitidis and E. phaeomuriformis in railway stations in subtropical Turkey. Mycopathologia. 2013;175:381–386. doi: 10.1007/s11046-012-9594-z. [DOI] [PubMed] [Google Scholar]
- 5.Zalar P, Novak M, de Hoog GS, Gunde-Cimerman N. Dishwashers--a man-made ecological niche accommodating human opportunistic fungal pathogens. Fungal Biol. 2011;115:997–1007. doi: 10.1016/j.funbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Lavrin T, Konte T, Kostanjšek R, Sitar S, Sepčič K, Prpar Mihevc S, Žagar E, Župunski V, Lenassi M, Rogelj B, Gunde Cimerman N. The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death. Cells. 2020;9 doi: 10.3390/cells9040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Xing H, Jiang X, Zeng J, Liu Z, Chen J, Wu Y. Cerebral Phaeohyphomycosis Caused by Exophiala dermatitidis in a Chinese CARD9-Deficient Patient: A Case Report and Literature Review. Front Neurol. 2019;10:938. doi: 10.3389/fneur.2019.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poyntner C, Mirastschijski U, Sterflinger K, Tafer H. Transcriptome Study of an Exophiala dermatitidis PKS1 Mutant on an ex Vivo Skin Model: Is Melanin Important for Infection? Front Microbiol. 2018;9:1457. doi: 10.3389/fmicb.2018.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang R, Minion J, Skinner S, Wong A. Disseminated Exophiala dermatitidis causing septic arthritis and osteomyelitis. BMC Infect Dis. 2018;18:255. doi: 10.1186/s12879-018-3171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdanparast SA, Mohseni S, De Hoog GS, Aslani N, Sadeh A, Badali H. Consistent high prevalence of Exophiala dermatitidis, a neurotropic opportunist, on railway sleepers. J Mycol Med. 2017;27:180–187. doi: 10.1016/j.mycmed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Poyntner C, Blasi B, Arcalis E, Mirastschijski U, Sterflinger K, Tafer H. The Transcriptome of Exophiala dermatitidis during Ex-vivo Skin Model Infection. Front Cell Infect Microbiol. 2016;6:136. doi: 10.3389/fcimb.2016.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zupančič J, Novak Babič M, Zalar P, Gunde-Cimerman N. The Black Yeast Exophiala dermatitidis and Other Selected Opportunistic Human Fungal Pathogens Spread from Dishwashers to Kitchens. PLoS One. 2016;11:e0148166. doi: 10.1371/journal.pone.0148166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaram M, Nagao H. First Report of Environmental Isolation of Exophiala spp. in Malaysia. Curr Microbiol. 2020;77:2915–2924. doi: 10.1007/s00284-020-02109-w. [DOI] [PubMed] [Google Scholar]
- 14.Myoken Y, Sugata T, Fujita Y, Kyo T, Fujihara M, Katsu M, Mikami Y. Successful treatment of invasive stomatitis due to Exophiala dermatitidis in a patient with acute myeloid leukemia. J Oral Pathol Med. 2003;32:51–54. doi: 10.1034/j.1600-0714.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 15.Abliz P, Fukushima K, Takizawa K, Nishimura K. Identification of pathogenic dematiaceous fungi and related taxa based on large subunit ribosomal DNA D1/D2 domain sequence analysis. FEMS Immunol Med Microbiol. 2004;40:41–49. doi: 10.1016/S0928-8244(03)00275-X. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhoff L, Olsowski M, Rath PM, Steinmann J. Exophiala dermatitidis: Key issues of an opportunistic fungal pathogen. Virulence. 2019;10:984–998. doi: 10.1080/21505594.2019.1596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanuskova D, Horakova J, Buzassyova D, Poczova M, Bodova I, Svec P, Chocholova A, Adamcakova J, Sykora T, Pozdechova M, Geczova L, Kolenova A. A case of Exophiala dermatitidis infection in a child after allogeneic stem cell transplantation: case report and literature review of paediatric cases. JMM Case Rep. 2017;4:e005102. doi: 10.1099/jmmcr.0.005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff L, Olsowski M, Zilmans K, Dittmer S, Haase G, Sedlacek L, Steinmann E, Buer J, Rath PM, Steinmann J. Biofilm formation of the black yeast-like fungus Exophiala dermatitidis and its susceptibility to antiinfective agents. Sci Rep. 2017;7:42886. doi: 10.1038/srep42886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y, da Silva NM, Weiss VA, Vu D, Moreno LF, Vicente VA, Li R, de Hoog GS. Comparative Genomic Analysis of Capsule-Producing Black Yeasts Exophiala dermatitidis and Exophiala spinifera, Potential Agents of Disseminated Mycoses. Front Microbiol. 2020;11:586. doi: 10.3389/fmicb.2020.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Gao L, Yuan M, Yuan L, Yang J, Zeng T. In vitro and in vivo Study of Antifungal Effect of Pyrvinium Pamoate Alone and in Combination With Azoles Against Exophiala dermatitidis. Front Cell Infect Microbiol. 2020;10:576975. doi: 10.3389/fcimb.2020.576975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagiya H, Maeda T, Kusakabe S, Kawasaki K, Hori Y, Kimura K, Ueda A, Yoshioka N, Sunada A, Nishi I, Morii E, Kanakura Y, Tomono K. A fatal case of Exophiala dermatitidis disseminated infection in an allogenic hematopoietic stem cell transplant recipient during micafungin therapy. J Infect Chemother. 2019;25:463–466. doi: 10.1016/j.jiac.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez-Velez M, Hoang A, Weinstein J, Harkins K, Shah R. Endogenous Exophiala dermatitidis endophthalmitis. Am J Ophthalmol Case Rep. 2020;19:100774. doi: 10.1016/j.ajoc.2020.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida T, Tachita T, Fujinami H, Oshima Y, Sasaki H, Marumo Y, Narita T, Ito A, Ri M, Kusumoto S, Ishida T, Komatsu H, Iida S. Exophiala dermatitidis Fungemia Diagnosed Using Time-of-flight Mass Spectrometry during Chemotherapy for Malignant Lymphoma and Successful Treatment with Voriconazole. Intern Med. 2019;58:2219–2224. doi: 10.2169/internalmedicine.2082-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y, Laureijssen-van de Sande WWJ, Moreno LF, Gerrits van den Ende B, Li R, de Hoog S. Comparative Ecology of Capsular Exophiala Species Causing Disseminated Infection in Humans. Front Microbiol. 2017;8:2514. doi: 10.3389/fmicb.2017.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol. 2007;45:3713–3720. doi: 10.1128/JCM.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwahashi C, Eguchi H, Hotta F, Uezumi M, Sawa M, Kimura M, Yaguchi T, Kusaka S. Orbital abscess caused by Exophiala dermatitidis following posterior subtenon injection of triamcinolone acetonide: a case report and a review of literature related to Exophiala eye infections. BMC Infect Dis. 2020;20:566. doi: 10.1186/s12879-020-05294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila A, Jahan C, Rivero C, Amadio C, Ampuero A, Pagella H. Central line associated blood stream infection (CLABSI) due to Exophiala dermatitidis in an adult patient: Case report and review. Med Mycol Case Rep. 2019;24:33–36. doi: 10.1016/j.mmcr.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger JS, Cusumano LR, Derose JJ, Sarwar UN. Exophiala (Wangiella) dermatitidis Prosthetic Aortic Valve Endocarditis and Prosthetic Graft Infection in an Immune Competent Patient. Case Rep Infect Dis. 2017;2017:4839314. doi: 10.1155/2017/4839314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Nandakumar A, Nair S, Singh A, Shashindran N, Thulasidharan S, Subhash K, Ramachandran A, Chowdhary A. Exophiala dermatitidis as a cause of central line associated bloodstream infection in an infant: Case report and literature review. Rev Iberoam Micol. 2021;38:12–15. doi: 10.1016/j.riam.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Devoto TB, Alava KSH, Pola SJ, Pereda R, Rubeglio E, Finquelievich JL, Cuestas ML. Molecular epidemiology of Aspergillus species and other moulds in respiratory samples from Argentinean patients with cystic fibrosis. Med Mycol. 2020;58:867–873. doi: 10.1093/mmy/myz133. [DOI] [PubMed] [Google Scholar]
- 31.Grenouillet F, Cimon B, Pana-Katatali H, Person C, Gainet-Brun M, Malinge MC, Le Govic Y, Richaud-Thiriez B, Bouchara JP. Exophiala dermatitidis Revealing Cystic Fibrosis in Adult Patients with Chronic Pulmonary Disease. Mycopathologia. 2018;183:71–79. doi: 10.1007/s11046-017-0218-5. [DOI] [PubMed] [Google Scholar]
- 32.Ziesing S, Suerbaum S, Sedlacek L. Fungal epidemiology and diversity in cystic fibrosis patients over a 5-year period in a national reference center. Med Mycol. 2016;54:781–786. doi: 10.1093/mmy/myw035. [DOI] [PubMed] [Google Scholar]
- 33.Deng S, Pan W, Liao W, de Hoog GS, Gerrits van den Ende AH, Vitale RG, Rafati H, Ilkit M, Van der Lee AH, Rijs AJ, Verweij PE, Seyedmousavi S. Combination of Amphotericin B and Flucytosine against Neurotropic Species of Melanized Fungi Causing Primary Cerebral Phaeohyphomycosis. Antimicrob Agents Chemother. 2016;60:2346–2351. doi: 10.1128/AAC.02526-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva WC, Gonçalves SS, Santos DW, Padovan AC, Bizerra FC, Melo AS. Species diversity, antifungal susceptibility and phenotypic and genotypic characterisation of Exophiala spp. infecting patients in different medical centres in Brazil. Mycoses. 2017;60:328–337. doi: 10.1111/myc.12597. [DOI] [PubMed] [Google Scholar]
- 35.Özhak-Baysan B, Öğünç D, Döğen A, Ilkit M, de Hoog GS. MALDI-TOF MS-based identification of black yeasts of the genus Exophiala. Med Mycol. 2015;53:347–352. doi: 10.1093/mmy/myu093. [DOI] [PubMed] [Google Scholar]
- 36.Raclavsky V, Novotny R. Burkholderia cepacia selective agar can be useful for recovery of Exophiala dermatitidis from sputum samples of cystic fibrosis patients. J Cyst Fibros. 2016;15:e19. doi: 10.1016/j.jcf.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Nagano Y, Elborn JS, Millar BC, Goldsmith CE, Rendall J, Moore JE. Development of a novel PCR assay for the identification of the black yeast, Exophiala (Wangiella) dermatitidis from adult patients with cystic fibrosis (CF) J Cyst Fibros. 2008;7:576–580. doi: 10.1016/j.jcf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Kondori N, Erhard M, Welinder-Olsson C, Groenewald M, Verkley G, Moore ER. Analyses of black fungi by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS): species-level identification of clinical isolates of Exophiala dermatitidis. FEMS Microbiol Lett. 2015;362:1–6. doi: 10.1093/femsle/fnu016. [DOI] [PubMed] [Google Scholar]
- 39.Suh MK, Lee HC, Kim DM, Ha GY, Choi JS. Molecular phylogenetics of exophiala species isolated from Korea. Ann Dermatol. 2012;24:287–294. doi: 10.5021/ad.2012.24.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Almeida JN Júnior, Sztajnbok J, da Silva AR Junior, Vieira VA, Galastri AL, Bissoli L, Litvinov N, Del Negro GM, Motta AL, Rossi F, Benard G. Rapid identification of moulds and arthroconidial yeasts from positive blood cultures by MALDI-TOF mass spectrometry. Med Mycol. 2016;54:885–889. doi: 10.1093/mmy/myw044. [DOI] [PubMed] [Google Scholar]
- 41.Ergin Ç, Gök Y, Bayğu Y, Gümral R, Özhak-Baysan B, Döğen A, Öğünç D, Ilkit M, Seyedmousavi S. ATR-FTIR Spectroscopy Highlights the Problem of Distinguishing Between Exophiala dermatitidis and E. phaeomuriformis Using MALDI-TOF MS. Microb Ecol. 2016;71:339–346. doi: 10.1007/s00248-015-0670-z. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan E, Ilkit M, de Hoog GS. Comparison of the rolling circle amplification and ligase-dependent reaction methods for the identification of opportunistic Exophiala species. Med Mycol. 2018;56:759–769. doi: 10.1093/mmy/myx095. [DOI] [PubMed] [Google Scholar]
- 43.Gao L, Sun Y, He C, Li M, Zeng T. In vitro interactions between 17-AAG and azoles against Exophiala dermatitidis. Mycoses. 2018;61:853–856. doi: 10.1111/myc.12824. [DOI] [PubMed] [Google Scholar]
- 44.Qiao J, Sun Y, Gao L, He C, Zheng W. Lonafarnib synergizes with azoles against Aspergillus spp. and Exophiala spp. Med Mycol. 2018;56:452–457. doi: 10.1093/mmy/myx072. [DOI] [PubMed] [Google Scholar]
- 45.Homa M, Manikandan P, Saravanan V, Revathi R, Anita R, Narendran V, Panneerselvam K, Shobana CS, Aidarous MA, Galgóczy L, Vágvölgyi C, Papp T, Kredics L. Exophiala dermatitidis Endophthalmitis: Case Report and Literature Review. Mycopathologia. 2018;183:603–609. doi: 10.1007/s11046-017-0235-4. [DOI] [PubMed] [Google Scholar]


