Abstract
This study explored the feasibility of electrostatic spray drying for producing a monoclonal antibody (mAb) powder formulation at lower drying temperatures than conventional spray drying and its effect on protein stability. A mAb formulation was dried by either conventional spray drying or electrostatic spray drying with charge (ESD). The protein powders were then characterized using solid-state Fourier transform infrared spectroscopy (ssFTIR), differential scanning calorimetry (DSC), size exclusion chromatography (SEC), and solid-state hydrogen/deuterium exchange with mass spectrometry (ssHDX-MS). Particle characterizations such as BET surface area, particle size distribution, and particle morphology were also performed. Conventional spray drying of the mAb formulation at the inlet temperature of 70°C failed to generate dry powders due to poor drying efficiency; electrostatic spray drying at the same temperature at 5kV enabled the formation of powder formulation with satisfactory moisture contents. Deconvoluted peak areas of deuterated samples from the ssHDX-MS study showed a good correlation with the loss of the monomeric peak area measured by size exclusion chromatography in the 90-day accelerated stability study conducted at 40°C. Low-temperature (70°C inlet temperature) drying with an electrostatic charge (5kV) led to better protein physical stability as compared with the samples spray-dried at the high temperature (130°C inlet temperature) without charge. This study shows that electrostatic spray drying can produce solid monoclonal antibody formulation at lower inlet temperature than traditional spray drying with better physical stability.
Keywords: Electrostatic spray drying, protein structure, solid formulation, physical stability, solid-state hydrogen/deuterium exchange with mass spectrometric analysis (ssHDX-MS)
Graphical Abstract

1. Introduction
Generally, protein formulations in the solid form have better physical and chemical stability than those in the solution state (Cicerone et al., 2015). In the pharmaceutical industry, lyophilization is the mainstay drying technique for manufacturing protein solid products (Carpenter et al., 2002). However, lyophilization is a batch process and time- and energy-consuming (Langford et al., 2018). The cake-like solid form produced using this technique is not suitable for applications that require specific particle sizes such as inhalation therapies; additional steps such as milling are needed to generate particles with a required size for these therapeutic applications (Burkoth et al., 1999; Costantino et al., 2000). The mechanical stresses induced by milling may result in the degradation of proteins (Johnson, 1997).
Spray drying is another technique for producing solid-state protein formulations with advantages such as high throughput, and the ability to obtain desired flowability and/or aerosolization (Maa and Prestrelski, 2000; Zhang et al., 2021). During spray-drying, a solution is atomized to form small droplets that are then exposed to a drying gas to produce solid particles (Ameri and Maa, 2006). Solvent is removed by evaporation during this process and therefore resulting in dried particles (Lin et al., 2015). There is an increasing interest to produce biological solids using spray drying (Cun et al., 2021). For example, pharmaceutical products such as Exubera® (inhalable insulin) and Raplixa® (a blend of thrombin and fibrinogen powder) are spray-dried (Lee, 2002; White et al., 2005). While spray drying offers advantages in terms of material properties and processing times, the process stresses such as high temperature, atomization stresses, and air-liquid interfacial stresses may have an adverse effect on protein stability (Abdul-Fattah et al., 2007; Chen et al., 2021; Manning et al., 2010). Such effects of the spray drying process on the stability of the formulation are dependent on the protein type and formulation (Bowen et al., 2012; Bowen et al., 2013).
Electrostatic spray drying (ESD) is a technique that uses an electrostatic charge to the feed solution in order to dry at lower temperatures than conventional spray drying. The application of an electronic charge results in the polar solvent picking up more electrons than the solute. This results in presence of higher amounts of solvents at the surface during droplet formation thus resulting in better drying efficiency at lower temperatures. The concept of applying an electrostatic charge to spray drying feed solution has been previously attempted to produce nanoparticles (Gomez et al., 1998), nanosuspensions (Thakkar and Misra, 2020), and microspheres for encapsulation (Wang et al., 2019). This study aimed to explore the feasibility of producing protein solid formulations by electrostatic spray drying at a lower drying temperature than traditional spray drying. Secondary structure and physical stability were compared among formulations produced by traditional spray drying and electrostatic spray drying. A monoclonal antibody formulation (mAb) was obtained from Genentech, Inc. (South San Francisco, USA). The characterization of the solid formulations was done using ssFTIR, powder X-ray diffraction, modulated differential scanning calorimetry, and ssHDX-MS. Particle characterization techniques such as Karl-Fischer coulometric titration for moisture content analysis, laser diffraction for particle size distribution, scanning electron microscopy (SEM) for particle morphology, and BET for surface area measurement were also conducted for the dried powders. Physical stability was determined by measuring the loss in the monomeric peak area of samples stored at 40°C over 90-days using size exclusion chromatography (SEC).
2. Materials and Methods
2.1. Materials
A Monoclonal Antibody (mAb) formulation (containing 27% w/w trastuzumab) formulated with trehalose dihydrate (approximately 65% w/w), sodium phosphate buffer (approximately 8% w/w), and polysorbate 20 (approximately 0.4% w/w) was obtained from Genentech, Inc., (South San Francisco, CA, USA) as a frozen solution. The frozen solution was thawed at 4°C for 24 h, mixed by gentle inversion, filtered using a 0.22 μm filter, and then subjected to lyophilization or electrostatic spray drying. Sodium phosphate monobasic and sodium phosphate dibasic heptahydrate were purchased from Millipore Sigma (St. Louis, MO, USA). A supersaturated solution of lithium chloride (Thermo Fisher Scientific, Waltham, MA, USA) in D2O (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) was used to maintain the RH at 11% in the desiccator for HDX studies.
2.2. Electrostatic Spray Drying
A laboratory-scale electrostatic spray dryer (PolarDry® Model 0.1, Fluid Air, Naperville, IL, USA) was employed to perform electrostatic spray drying. To save the mAb formulation, a surrogate formulation with BSA was prepared with the same excipients and same total solid content to the mAb formulation in the preliminary study to optimize the operating parameters for electrostatic spray drying. BSA was considered as a surrogate protein due to its susceptibility to thermal stresses compared to other model proteins that were studied previously such as lysozyme and myoglobin. The parameters chosen based on the optimization study are listed in Table 1. Usually, lab-scale spray drying has been done at inlet temperatures between 120–140°C (Bowen et al., 2012; Chen et al., 2021). In this study, inlet temperatures of 130°C and 70°C (40–50% less than 130°C) were chosen to investigate the effects of low-temperature drying. The preliminary data showed that traditional spray drying with an inlet temperature of 70°C did not form dry powders due to insufficient drying with droplets dripping to the bottom of the drying chamber. However, electrostatic spray drying with a 5kV charge at the inlet temperature of 70°C successfully formed the powder which was collected in the filter sock. The spray drying using the same machine without charge is deemed as traditional/conventional spray drying in this study.
Table 1:
Electrostatic spray drying conditions for mAb formulations
| Electrostatic spray drying conditions | Inlet Temperature (°C) | Drying gas flow rate (Nm3/hr) | Atomizing Gas pressure (kPa) | Flow rate (rpm)/(ml/min) | Voltage (kV) |
|---|---|---|---|---|---|
| Condition 1 | 130 | 4.5 | 250 | 20/2.5 | 0 |
| Condition 2 | 130 | 4.5 | 250 | 20/2.5 | 5 |
| Condition 3 | 70 | 4.5 | 250 | 10/1.25 | 5 |
2.3. Karl Fischer Titration for Moisture Content Analysis
A 917 KF Coulometer (Metrohm, Riverview, FL, USA) was used to perform a coulometric titration and determine the moisture content of the dried powders. The samples were reconstituted in one mL of anhydrous methanol (septum sealed bottle DriSolv®, Millipore Sigma St. Louis, MO, USA) and injected into the cell. The titration endpoint was determined using the Riedel-de Haën Hydranal® Coulomat reagent (Honeywell Research Chemicals, Seelze, Germany). The weight of the injected amount and moisture content in ppm were recorded and converted to w/w%.
2.4. X-ray Powder Diffraction (PXRD)
A Rigaku SmartLab X-ray diffractometer (The Woodlands, TX, USA) consisting of a Cu Kα X-ray source and Bragg-Brentano geometry was applied to identify the presence of crystallinity in the dried powders. A scanning rate of 4°/min with a step size of 0.02° between 4 and 40 degrees was used to obtain the diffraction intensity as a function of 2θ.
2.5. Solid-State Fourier Transform Infrared Spectroscopy (ssFTIR)
Attenuated total reflectance (ATR) mode on a Nicolet Nexus spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with Smart iTR accessory was used to obtain the ssFTIR measurements for the protein secondary structural analysis. A range of 800 to 4000 cm−1 in absorbance mode at 4 cm−1 resolution was used to collect the data. All spectra were corrected for moisture content and CO2. OPUS 6.5 analysis software (Bruker, Billerica, MA, USA) was used to process the spectra with baseline correction, smoothing, normalization, and second derivatization.
2.6. Modulated Differential Scanning Calorimetry (mDSC)
Inside a nitrogen glovebox maintained at an RH of less than 10%, 2 – 4 mg of the dried powders were loaded into Tzero aluminum pans (TA Instruments, New Castle, DE, USA) and sealed using Tzero hermetic lids (TA Instruments, New Castle, DE, USA). The sample pans were loaded into a Discovery Series DSC 25 differential scanning calorimeter (TA Instruments, New Castle, DE, USA). A heating ramp rate of 2°C /min from −5°C to 180°C with modulation of ±1°C every 60 s was used. Glass transition temperature (Tg) was determined using the in-built TRIOS software package (v4.3.0, TA instruments, New Castle, DE, USA).
2.7. Particle Size Distribution
A Mastersizer 3000 (Malvern Panalytical, Malvern, United Kingdom) equipped with an air dispersion unit - Aero S was used to measure the particle size distribution of the dried samples. Each sample (approximately 10–20 mg) was dispersed through the measuring zone using compressed air at 4 bar. The sizes below which 10% (D10), 50% (D50), and 90% (D90) of the material are contained along with the surface moment mean D[3,2] and volume moment mean D [4.3] were determined by the built-in software.
2.8. Scanning Electron Microscopy (SEM)
The morphology of the dried samples was visualized using a Teneo Volumescope (Fei, Hillsboro, OR, USA). Powders mounted on sample holders were sputter-coated with platinum for 60 s and analyzed under vacuum using a voltage of 2 kV and a working distance of 8.9 – 10 mm to determine morphological properties.
2.9. Surface Area Measurement
The surface area of the dried samples was measured using a Tristar II 3020 (Micromeritics, Norcross, GA, USA). The residual moisture in the samples was removed by degassing them with nitrogen for 12 hours. A 6-point BET method (Brunauer et al., 1938) using helium adsorption was applied to determine the surface area.
2.10. Stability Studies by Size Exclusion Chromatography (SEC)
SEC was used to measure protein aggregation after storage at the accelerated stability conditions. The samples were weighed (2 – 4 mg per vial), sealed, and stored at 40°C. Each sample was reconstituted to obtain a protein concentration of 1 mg/mL at different time points (15, 30, 60, and 90 days). 100μL of each reconstituted sample was analyzed in a 1260 Infinity II series high-performance liquid chromatography (HPLC) system (Agilent Technologies, Santa Clara, CA, USA) equipped with a TSK gel G2000SWXL column (Tosoh Bioscience LLC, King of Prussia, PA, USA) and operated at a flow rate of 1 mL/min with a mobile phase of sodium phosphate buffer (100 mM concentration at pH 6.8, made with sodium phosphate monobasic and sodium phosphate dibasic heptahydrate). The percentage loss of area under the curve for the monomeric peak was used to determine the physical instability of the sample.
2.11. Solid-State Hydrogen Deuterium Exchange with Mass Spectrometric Analysis (ssHDX-MS)
Deuterium incorporation into formulations has been used to measure protein conformation and protein interactions with other components in the solid matrix. (Iyer et al., 2016; Sophocleous et al., 2012). Excipients, temperature, and processing method, and conditions are known to be some of the factors that influence the deuterium incorporation which can be measured using a mass spectrometer.
Uncapped vials with 2–4 mg of the samples were stored at 25°C in a sealed desiccator maintained at 11% RH using a saturated lithium chloride in D2O solution. Once the samples were exposed to D2O vapor for specific times (4, 12, 24, 48, 120, and 240 h), three vials of each sample were removed from the desiccator and capped. To prevent back exchange, the temperature of the samples in the vials was reduced rapidly by submerging the vials in liquid nitrogen and storing them at −80°C until analysis.
The dried samples subjected to hydrogen-deuterium exchange were reconstituted in 1 mL of ice-cold quench buffer (a solution containing 0.1% formic acid in MS water with a pH of 2.5). Ten μL of the reconstituted solution was injected into a protein microtrap (Michrom Bioresources, Inc., Auburn, CA, USA). Desalting of the sample was done using a 0.1% formic acid in acetonitrile and water in a 1:9 ratio for 2 min in a high-pressure liquid chromatography system (1200 series, Agilent Technologies, Santa Clara, CA, USA) followed by elution over 11 min with a gradient of 0.1% formic acid in acetonitrile and water in a 9:1 ratio. The protein microtrap was kept in a custombuilt refrigeration unit held at 4°C (Keppel et al., 2011) to limit the back exchange of the deuterons within the samples. A 6230 TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA) was operated in the mass range of 200–3200 m/z to obtain the mass spectra of the sample. The spectra were analyzed using the MassHunter Workstation software (Agilent Technologies, Santa Clara, CA, USA) to obtain the deconvoluted peaks, protein mass, and the change in the protein mass. The deuterium incorporation data obtained were fitted to a mono-exponential kinetics model:
| (1) |
where D(t) the total amount of deuterons incorporated at time t, Dmax is the maximum number of deuterons that can be incorporated in the sample as determined experimentally, and k is the observed hydrogen-deuterium exchange rate constant. Prism GraphPad software Version 9 (GraphPad, La Jolla, CA, USA) was used to fit the kinetics data of ssHDX-MS.
2.12. Statistical Analysis
A two-way ANOVA with Turkey’s test was used for comparisons among groups with a p-value of 0.05 (Prism software version 9, GraphPad, La Jolla, CA, USA).
3. Results
3.1. Preliminary optimization Study
For the the process optimization of the PolarDry® Model 0.1, three operation parameters such as temperature, feed rate and voltage have been considered and the responses such as yield, moisture content and outlet temperature were reported in Table 2. The inlet temperatures and flow rates were selected based on literature as well as previous studies. The flow rate for the low temperature drying has been adjusted based on drying efficiency. The equipment is designed to operate between 0–20 kV hence the entire range of the applicable voltage has been tested in a increment of 5 kV. The atomizing pressure and gas flow rate have been kept constant.
Table 2:
Process optimization study results for the PolarDry® Model 0.1electrostatic spray drying
| Formulation | Varying Factor 1 | Varying Factor 2 | Varying Factor 3 | Response 1 | Response 2 | Response 3 | |
|---|---|---|---|---|---|---|---|
| Inlet Temperature (°C) | Flow rate (rpm)/(ml/min) | Voltage (kV) | Yield (%) | % Moisture content (n=3) | Outlet Temperature (°C) | ||
| Average | S. D | ||||||
| BSA Formulation (BSA + Trehalose + surfactant − 48 mg/ml) | 130 | 25 / 3.5 | 0 | 47.37 | 3.04 | 0.04 | 67–74 |
| 5 | 43.97 | 3.01 | 0.20 | 67–74 | |||
| 10 | 38.45 | 2.91 | 0.28 | 67–74 | |||
| 15 | 29.72 | 3.35 | 0.21 | 67–74 | |||
| 20 | 26.14 | 3.95 | 0.43 | 67–74 | |||
| 100 | 20 / 2.5 | 0 | 53.42 | 5.24 | 0.55 | 48–56 | |
| 5 | 51.47 | 5.77 | 0.77 | 48–56 | |||
| 10 | 46.49 | 7.20 | 1.62 | 48–56 | |||
| 15 | 27.47 | 6.93 | 1.43 | 48–56 | |||
| 20 | 21.42 | 5.59 | 0.99 | 48–56 | |||
| 70 | 20/2.5 | 0 | - | - | - | - | |
| 5 | - | - | - | - | |||
| 10 | - | - | - | - | |||
| 15 | - | - | - | - | |||
| 20 | - | - | - | - | |||
| 15 / 1.875 | 0 | - | - | - | - | ||
| 5 | 50.12 | 5.20 | 0.60 | 32–37 | |||
| 10 | 46.75 | 4.89 | 0.21 | 32–37 | |||
| 15 | 27.91 | 5.07 | 0.12 | 32–37 | |||
| 20 | 21.58 | 5.45 | 0.14 | 32–37 | |||
| 10 / 1.2 | 0 | - | - | - | - | ||
| 2 | 54.54 | 5.66 | 0.20 | 32–37 | |||
| 5 | 50.73 | 4.97 | 0.61 | 32–37 | |||
| 10 | 41.47 | 4.83 | 0.43 | 32–37 | |||
It has been observed that the application of charge over 5 kV resulted in a reduced yield without adding any benefit in terms of the moisture content or outlet temperature. This is due to the particles retaining charge and sticking to the drying chamber. Therefore, voltages over 5kV have not been used for the mAb formulation. For those samples that dry powder was not formed, results are reported as blank responses.
3.2. Moisture Content
The moisture content of the low-temperature spray drying showing a slightly higher moisture content than the high-temperature drying conditions. However, no statistically significant difference (p > 0.05) in moisture content among the three spray-dried samples has been observed (Table 3).
Table 3:
Moisture content, glass transition temperature, particle size distribution, and surface area of dried samples (Mean ± SD, n=3)
| Processing Condition | Moisture content (%w/w) | Glass Transition Temperature (Tg in °C) | Particle size (μm) | Surface area (m2/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | S.D. | Average | S.D. | D10 | D50 | D90 | Span | Average | S.D. | |
| ESD −130°C, 0kv | 5.24 | 0.03 | 58.37 | 0.98 | 0.28 | 2.56 | 10.20 | 3.88 | 1.13 | 0.33 |
| ESD −130°C, 5kv | 4.95 | 0.24 | 58.08 | 0.59 | 0.28 | 2.50 | 7.21 | 2.77 | 1.08 | 0.02 |
| ESD −70°C, 5kv | 5.64 | 0.43 | 42.15 | 0.16 | 0.27 | 2.37 | 7.54 | 3.07 | 1.79 | 0.03 |
3.3. mDSC Analysis
Glass transition temperature (Tg) data are presented in Table 3. The Tg values of the high-temperature drying are observed to be similar i.e., ~58 °C with no statistically significant difference observed between them. The electrostatic spray-dried sample processed at 70°C and 5kv exhibited significantly lower Tg values of ~42 °C than high-temperature drying samples (p<0.001).
3.4. Particle Size Distribution
Samples have similar D10 and D50 values in their particle size distributions; the spray-dried sample without electrostatic charge showed a slightly higher span as compared to the samples spray-dried with an application of electrostatic charge (Table 3). This is due to the higher D90 value observed in the samples produced through high-temperature drying without the application of charge.
3.5. Surface Area Measurements
The surface area of the high-temperature drying samples is observed to be similar i.e., ~1.1 m2/g. However, the samples dried using low-temperature drying showed a significantly higher surface area of ~1.8 m2/g (p<0.0001) than the samples dried at higher temperatures (Table 3).
3.6. X-ray Powder Diffraction
All dried mAb formulations were amorphous without apparent crystalline peaks in their XRD patterns (Fig. S1).
3.7. Secondary Structural Analysis by ssFTIR
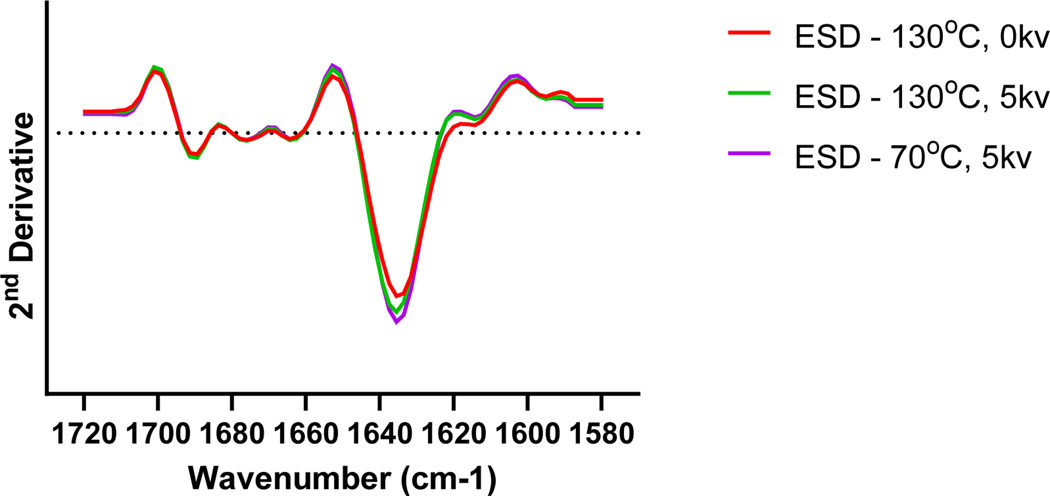
Amide I region of the ssFTIR spectrum was analyzed for each formulation (Fig. 1). FTIR bands were observed at ~1635 cm−1, suggesting the presence of extended loops (Fu et al., 1994; Kumosinski and Farrell, 1993) that are generally associated with monoclonal antibodies. While a reduction of peak intensity has been observed for high-temperature drying with charge as compared to other conditions, no difference in peak locations within the ssFTIR spectra has been observed between the dried samples.
Figure 1:
Solid-state FTIR spectra of dried mAb formulations
3.8. Particle Morphology
The morphology of the dried samples was visualized using scanning electron microscopy (Fig. S2). All spray-dried samples were spherical with a smoother surface irrespective of the processing conditions used.
3.9. ssHDX-MS
The amount of deuterium uptake by the dried protein formulations as a function of time was used to measure protein conformation and interactions between proteins and excipients in the solid-state. The deuterium incorporation was compared among the processing conditions for the mAb formulation using a mono-exponential model (Eqn. 1) (Fig. 2). Spray-dried samples with charge showed no significant difference in deuterium uptake kinetics (p>0.05) and were significantly different from the spray-dried samples without charge (p < 0.05). The rate of deuterium uptake (k) and the maximum deuterium uptake (Dmax) values determined from the mono-exponential model are reported in Table 4.
Figure 2:
Hydrogen/deuterium exchange kinetics in the solid-state for the mAb formulations (n = 3, mean ± SD; error bars cannot be shown when smaller than the height of the symbol.)
Table 4:
Deuterium incorporation kinetics fitted to the mono-exponential model (Mean ± SD, n=3)
| Processing condition | k (h−1) | Dmax | ||
|---|---|---|---|---|
| Average | S. D. | Average | S. D | |
| ESD - 130°C, 0kV | 0.02742 | 0.00072 | 154.5 | 2.0 |
| ESD - 130°C, 5kV | 0.02688 | 0.00113 | 150.4 | 0.4 |
| ESD - 70°C, 5kV | 0.02657 | 0.00081 | 150.0 | 1.7 |
The deconvoluted mass spectra of the mAb formulations were examined after exposing the samples to D2O for 240 h (Fig 3). The spectra were compared with the undeuterated mAb formulation that was not subjected to any drying. The sample spray-dried at the higher temperature without charge showed a slightly higher degree of peak broadening than other samples. The broadened peaks indicate a broader distribution of deuterated states, suggesting the presence of a heterogeneous population of protein conformational states in the formulation.
Figure 3:
Deconvoluted mass spectra of mAb formulations
For the mAb formulation, there has been no difference observed between samples dried with the application of charge while high-temperature drying without any charge showed a significantly greater peak area than other processing conditions. This indicates that the population heterogeneity within the formulation is the highest for the high-temperature spray-dried formulations.
3.10. Physical Stability
Accelerated stability studies were conducted at 40°C over 90 days (Fig. 4). The spray-dried sample at a higher temperature without any charge, in general, showed higher monomer loss as compared with the sample dried at a lower temperature with charge.
Figure 4:
Physical stability of mAb formulations on storage at 40°C (n = 5, mean ± SD; error bars cannot be shown when smaller than the height of the symbol.)
3.11. Correlation of solid-state characterization with physical stability
A correlation of the 90-day time point of the stability data with solid-state characterization was performed. The band intensity of the peaks observed at 1635 cm−1from the ssFTIR showed a rank ordering of the formulations as ESD samples at 70°C, 5kV > SD samples at 130°C, 0kV > ESD samples at 130°C, 5kV while Tg showed a rank ordering of the dried formulations to be SD samples at 130°C, 0kV > ESD samples at 130°C, 5kV > ESD samples at 70°C, 5kV (Fig. 5). These rank orderings of the formulations by ssFTIR and Tg are not the same as observed in the stability results (Fig. 4). However, ssHDX-MS results, such as the peak areas of deconvoluted spectra, showed better correlation to stability and had the same rank ordering as the stability results as shown in Fig. 5C.
Figure 5:
Correlation of 90-day physical stability results with ssFTIR band intensity (A), Tg (B), and % peak area of the deuterated samples measured by ssHDX (C) (n = 3, mean ± SD; error bars cannot be shown when smaller than the height of the symbol)
4. Discussion
In this study, a mAb formulation was conventional spray-dried (without charge) and electrostatic spray-dried (with charge) to compare the techniques. The impact of solid-state properties on the accelerated storage stability of these samples was examined. High-temperature spray drying may be aggressive for thermal sensitive biologics, which may disrupt protein conformation and impact shelf-life (Ameri and Maa, 2006; Wu et al., 2019). The SEC (Fig. 5) and ssHDX-MS results have shown that the low-temperature spray-dried samples have slightly better physical stability than the spray-dried samples, which could be due to reduced thermal stresses during drying. The key finding of this study is that electrostatic spray drying can produce dry powder of the mAb formulation at the low inlet temperature of 70°C with a satisfactory moisture content, while spray drying without charge is unable to generate dry powder at the same temperature. The result indicates that electrostatic spray drying has much higher drying efficiency as compared with the samples dried at the high drying temperature without charge (Table 3), which is beneficial to those thermo-sensitive biological products. Such an improved drying efficiency is attributable to the application of electrostatic charge that resulted in an increased movement of the solvent towards the surface during the droplet formation. Within the electrostatic spray-dried samples, those samples dried at the low-temperature had higher moisture content and lower Tg but showed better physical stability than the samples dried at the high-temperature. This indicates that the effect of drying temperature on protein stability is more significant than residual moisture content and Tg. Thus, electrostatic spray drying provides a viable way to manufacture powder formulation of thermo-sensitive biological products.
ssFTIR showed little to no difference in the peak positions for the formulations processed under different conditions. No structural perturbation was observed for all tested samples. Traditionally, the secondary structure measured by ssFTIR is used to predict the stability of the samples as the perturbations observed in the secondary structure could be related to the denaturation of the proteins. In this study, ssFTIR could not distinguish the secondary structure differences in the formulations processed under different conditions.
Glass transition temperature by mDSC is another parameter that has been previously used to identify the difference in formulations as well as predict the stability of the samples. It was reported that the increased mobility of the proteins contributes to the aggregation when stored at temperatures above its glass transition temperature (Duddu and Dal Monte, 1997). However, in this study, the correlations between Tg and stability results are poor (Fig. 5).
ssHDX-MS is a characterization technique used to examine the population heterogeneity of the dried protein. It is used to measure the differences in the intramolecular and intermolecular hydrogen bonding networks of the samples in the dried state. The intramolecular and intermolecular hydrogen bonds comprise the bonds that contribute to the structure of the protein formulation. The deconvoluted peaks (Fig. 3) of the samples after 240 h of deuteration were analyzed and a peak shift, as well as peak broadening of the deconvoluted peaks was observed with an increase in deuteration of the samples. This is due to the difference in deuteration states of the species in the sample. A higher peak shift indicates an increase in deuterium incorporation into the samples and a larger peak broadening suggests the presence of higher population heterogeneity within the sample. Among the spray-dried samples, the samples dried at higher temperatures showed larger peak broadening, suggesting that the low-temperature drying resulted in more homogeneous samples as compared to high-temperature drying. ssHDX-MS results such as peak areas (Fig. 5C) showed a clear correlation with physical stability for the dried mAb formulations, which is consistent with the previous findings (Moorthy et al., 2014; Moorthy et al., 2018; Mutukuri et al., 2021; Wilson et al., 2019).
5. Conclusions
This study demonstrated that electrostatic spray drying could produce dry powder of the mAb formulation with satisfactory moisture content at the low inlet temperature of 70°C, which could not be achieved using the same spray drying setting without charge. The electrostatic spray dried mAb formulation at 70°C and 5kV had superior storage physical stability than those produced at 130°C without charge, as predicted by the ssHDX-MS data. The results indicate that electrostatic spray drying at low temperatures is a feasible option for producing thermal-sensitive protein solid formulations. Further studies are warranted to test other thermo-sensitive biological products.
Supplementary Material
Acknowledgment
This study was partially supported by a grant from Genentech. Qi (Tony) Zhou and Tarun Tejasvi Mutukuri were also supported by the National Institute of Allergy and Infectious Diseases of the National Institute of Health under Award Number R01AI146160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The authors would like to acknowledge the access and support of PolarDry® Model 0.1 as provided by Fluid Air, a division of Spraying Systems Co. We are grateful for the helpful discussion and MS access from Dr. Elizabeth M. Topp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdul-Fattah AM, Kalonia DS, Pikal MJ, 2007. The Challenge of Drying Method Selection for Protein Pharmaceuticals: Product Quality Implications. Journal of Pharmaceutical Sciences 96, 1886–1916. [DOI] [PubMed] [Google Scholar]
- Ameri M, Maa Y-F, 2006. Spray drying of biopharmaceuticals: stability and process considerations. Drying technology 24, 763–768. [Google Scholar]
- Bowen M, Armstrong N, Maa Y. f., 2012. Investigating high-concentration monoclonal antibody powder suspension in nonaqueous suspension vehicles for subcutaneous injection. Journal of pharmaceutical sciences 101, 4433–4443. [DOI] [PubMed] [Google Scholar]
- Bowen M, Turok R, Maa Y-F, 2013. Spray drying of monoclonal antibodies: investigating powder-based biologic drug substance bulk storage. Drying Technology 31, 1441–1450. [Google Scholar]
- Brunauer S, Emmett PH, Teller E, 1938. Adsorption of Gases in Multimolecular Layers. Journal of the American Chemical Society 60, 309–319. [Google Scholar]
- Burkoth TL, Bellhouse BJ, Hewson G, Longridge DJ, Muddle AG, Sarphie DF, 1999. Transdermal and transmucosal powdered drug delivery. Critical Reviews in Therapeutic Drug Carrier Systems 16, 331–384. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Chang BS, Garzon-Rodriguez W, Randolph TW, 2002. Rational design of stable lyophilized protein formulations: theory and practice. Pharm Biotechnol 13, 109–133. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mutukuri TT, Wilson NE, Zhou Q, 2021. Pharmaceutical protein solids: Drying technology, solid-state characterization and stability. Advanced Drug Delivery Reviews 172, 211–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone MT, Pikal MJ, Qian KK, 2015. Stabilization of proteins in solid form. Adv Drug Deliv Rev 93, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino HR, Firouzabadian L, Hogeland K, Wu C, Beganski C, Carrasquillo KG, Cordova M, Griebenow K, Zale SE, Tracy MA, 2000. Protein spray-freeze drying. Effect of atomization conditions on particle size and stability. Pharm Res 17, 1374–1383. [DOI] [PubMed] [Google Scholar]
- Cun D, Zhang C, Bera H, Yang M, 2021. Particle engineering principles and technologies for pharmaceutical biologics. Advanced Drug Delivery Reviews 174, 140–167. [DOI] [PubMed] [Google Scholar]
- Duddu SP, Dal Monte PR, 1997. Effect of Glass Transition Temperature on the Stability of Lyophilized Formulations Containing a Chimeric Therapeutic Monoclonal Antibody. Pharmaceutical Research 14, 591–595. [DOI] [PubMed] [Google Scholar]
- Fu F-N, Deoliveira DB, Trumble WR, Sarkar HK, Singh BR, 1994. Secondary Structure Estimation of Proteins Using the Amide III Region of Fourier Transform Infrared Spectroscopy: Application to Analyze Calcium-Binding-Induced Structural Changes in Calsequestrin. Applied Spectroscopy 48, 1432–1441. [Google Scholar]
- Gomez A, Bingham D, de Juan L, Tang K, 1998. Production of protein nanoparticles by electrospray drying. Journal of Aerosol Science 29, 561–574. [Google Scholar]
- Iyer LK, Sacha GA, Moorthy BS, Nail SL, Topp EM, 2016. Process and Formulation Effects on Protein Structure in Lyophilized Solids Using Mass Spectrometric Methods. Journal of pharmaceutical sciences 105, 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, 1997. Preparation of peptide and protein powders for inhalation. Adv Drug Deliv Rev 26, 3–15. [DOI] [PubMed] [Google Scholar]
- Keppel TR, Jacques ME, Young RW, Ratzlaff KL, Weis DD, 2011. An efficient and inexpensive refrigerated LC system for H/D exchange mass spectrometry. Journal of the American Society for Mass Spectrometry 22, 1472–1476. [DOI] [PubMed] [Google Scholar]
- Kumosinski TF, Farrell HM, 1993. Determination of the global secondary structure of proteins by Fourier transform infrared (FTIR) spectroscopy. Trends in Food Science & Technology 4, 169–175. [Google Scholar]
- Langford A, Bhatnagar B, Walters R, Tchessalov S, Ohtake S, 2018. Drying technologies for biopharmaceutical applications: Recent developments and future direction. Drying Technology 36, 677–684. [Google Scholar]
- Lee G, 2002. Spray-drying of proteins. Pharm Biotechnol 13, 135–158. [DOI] [PubMed] [Google Scholar]
- Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT, 2015. Powder production and particle engineering for dry powder inhaler formulations. Current pharmaceutical design 21, 3902–3916. [DOI] [PubMed] [Google Scholar]
- Maa YF, Prestrelski SJ, 2000. Biopharmaceutical powders: particle formation and formulation considerations. Curr Pharm Biotechnol 1, 283–302. [DOI] [PubMed] [Google Scholar]
- Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS, 2010. Stability of protein pharmaceuticals: an update. Pharm Res 27, 544–575. [DOI] [PubMed] [Google Scholar]
- Moorthy BS, Schultz SG, Kim SG, Topp EM, 2014. Predicting Protein Aggregation during Storage in Lyophilized Solids Using Solid State Amide Hydrogen/Deuterium Exchange with Mass Spectrometric Analysis (ssHDX-MS). Molecular Pharmaceutics 11, 1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy BS, Zarraga IE, Kumar L, Walters BT, Goldbach P, Topp EM, Allmendinger A, 2018. Solid-State Hydrogen–Deuterium Exchange Mass Spectrometry: Correlation of Deuterium Uptake and Long-Term Stability of Lyophilized Monoclonal Antibody Formulations. Molecular Pharmaceutics 15, 1–11. [DOI] [PubMed] [Google Scholar]
- Mutukuri TT, Wilson NE, Taylor LS, Topp EM, Zhou QT, 2021. Effects of drying method and excipient on the structure and physical stability of protein solids: Freeze drying vs. spray freeze drying. International Journal of Pharmaceutics 594, 120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophocleous AM, Zhang J, Topp EM, 2012. Localized hydration in lyophilized myoglobin by hydrogen-deuterium exchange mass spectrometry. 1. Exchange mapping. Molecular pharmaceutics 9, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar S, Misra M, 2020. Electrospray drying of docetaxel nanosuspension: A study on particle formation and evaluation of nanocrystals thereof. Journal of Drug Delivery Science and Technology 60, 102009. [Google Scholar]
- Wang J, Helder L, Shao J, Jansen JA, Yang M, Yang F, 2019. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: Effect of polymer end groups. Int J Pharm 564, 1–9. [DOI] [PubMed] [Google Scholar]
- White S, Bennett DB, Cheu S, Conley PW, Guzek DB, Gray S, Howard J, Malcolmson R, Parker JM, Roberts P, 2005. EXUBERA®: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes technology & therapeutics 7, 896–906. [DOI] [PubMed] [Google Scholar]
- Wilson NE, Topp EM, Zhou QT, 2019. Effects of drying method and excipient on structure and stability of protein solids using solid-state hydrogen/deuterium exchange mass spectrometry (ssHDX-MS). Int J Pharm 567, 118470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wu L, Wan F, Rantanen J, Cun D, Yang M, 2019. Effect of thermal and shear stresses in the spray drying process on the stability of siRNA dry powders. International journal of pharmaceutics 566, 32–39. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davis DA, AboulFotouh K, Wang J, Williams D, Bhambhani A, Zakrewsky M, Maniruzzaman M, Cui Z, Williams RO, 2021. Novel formulations and drug delivery systems to administer biological solids. Advanced Drug Delivery Reviews 172, 183–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.