Abstract
BACKGROUND
Chlamydia psittaci (C. psittaci) is a gram-negative intracellular parasitic pathogenic bacterium that can infect avian and mammalian hosts, including humans. The detection of C. psittaci infections typically relies on traditional antigen-based immunoassays or serological testing that often lack sensitivity and/or specificity. Metagenomic next generation sequencing (mNGS) is an emerging tool for diagnosis.
AIM
To demonstrate that mNGS represents a valuable tool for rapid, sensitive, and accurate pathogen detection including C. psittaci infections.
METHODS
Four cases of psittacosis pneumonia and one case of pediatric psittacosis meningitis were diagnosed between December 2019 and May 2020 using mNGS at Changzhou Second People’s Hospital affiliated to Nanjing Medical University. Patients’ clinical characteristics, manifestations, and treatment histories were retrospectively evaluated.
RESULTS
All five patients had a history of exposure to wild (psittacine or other birds) or domesticated birds (chickens). All patients had a high fever (> 39℃) and three of them (60%) experienced organ insufficiency during the disease. The laboratory data showed normal to slightly increased leucocyte and neutrophil counts, and elevated procalcitonin levels in all five cases, and very high C-reactive protein levels in psittacosis pneumonia patients. mNGS identified a potential pathogen, C. psittaci, in patients’ bronchoalveolar lavage fluid or cerebrospinal fluid. Computed tomography revealed lung air-space consolidation, pleural thickening, and effusion fluid buildup in psittacosis pneumonia cases, and an arachnoid cyst in the right temporal lobe of the pediatric psittacosis meningitis patient. All patients experienced complete recovery following the administration of targeted anti-chlamydia therapy.
CONCLUSION
This study not only demonstrated that mNGS represents a valuable tool for rapid, sensitive, and accurate pathogen detection, but also raised public health concerns over C. psittaci infections.
Keywords: Chlamydia psittaci, Pneumonia, Meningitis, Metagenomic next generation sequencing, Etiology, Infectious disease
Core Tip: Detection of Chlamydia psittaci (C. psittaci) infections relies on traditional antigen-based immunoassays or serological testing that often lack sensitivity and/or specificity. Our data not only reinforce that metagenomic next generation sequencing represents a valuable tool for rapid, sensitive, and accurate pathogen detection, including the identification of C. psittaci, but also raise public health concerns over C. psittaci infections.
INTRODUCTION
Chlamydia are gram-negative intracellular parasitic pathogenic bacteria that can infect avian and mammalian hosts, including humans. Chlamydia can be parasitic in bird tissues, blood and feces, and is also a zoonotic disease with bird breeders, in particular, at increased risk of developing chlamydia infections.
Poultry infections are typically asymptomatic; however, once humans are infected with Chlamydia, it may cause multiple diseases, including those of the respiratory tract, gastrointestinal tract, cerebrospinal fluid (CSF), eyes, and serous cavity. Such infections may also induce pneumonia, gastroenteritis, encephalitis, arteriosclerosis, macular degeneration, and damage to multiple organs such as the liver, spleen, kidney, and myocardium. Although there have been many reports on Chlamydia[1,2], there is still a lack of sufficient attention to Chlamydia infections in humans’ daily lives and in clinical practice. Moreover, adequate detection methods with high sensitivity and specificity are also lacking in clinical practice; thus, leading to delays in diagnosis and an aggravation of disease[3].
Unlike traditional antigen-based immunoassays or serological testing, metagenomic next generation sequencing (mNGS) is an emerging technique to support pathogen detection[4-6]. mNGS can not only overcome the limitations of routine clinical microbial pre-culture methods but can also identify new or unexpected pathogens. Herein, we report a series of five patients whose bronchoalveolar lavage fluid (BALF) or CSF underwent mNGS testing and were diagnosed with Chlamydia infections. Consequently, the patients received targeted antibiotic therapy and recovered quickly.
MATERIALS AND METHODS
Patients
Patients were admitted to the Department of Respiratory and Critical Care Medicine and the Department of Pediatrics at Changzhou Second People’s Hospital affiliated to Nanjing Medical University between December 2019 and May 2020. An etiologic diagnosis was not known at the time of admission. Patients’ clinical characteristics, manifestations, and treatment history were retrospectively evaluated. The treating physicians were informed about the research-based mNGS results through a reporting mechanism approved by the Institutional Review Board of Changzhou Second People’s Hospital. Written informed consent was obtained from each patient prior to sample collection.
mNGS protocol
mNGS was performed in a Clinical Laboratory Improvement Amendments-certified and College of American Pathologists-accredited laboratory (Nanjing Geneseeq Technology, Jiangsu Province, China). BALF and CSF samples were collected from patients as previously described[7]. Genomic DNA was extracted using the TIANamp Magnetic DNA Kit (Tiangen) according to the manufacturer’s protocols. The quantity and quality of DNA were assessed using Qubit (Thermo Fisher Scientific) and NanoDrop (Thermo Fisher Scientific) instruments, respectively. DNA libraries were prepared using the KAPA Hyper Prep Kit (KAPA Biosystems) according to the manufacturer’s protocols. An Agilent 2100 was used for quality control and DNA libraries were 75 bp single-end sequenced on an Illumina NextSeq 550Dx (Illumina).
Bioinformatics analysis
An in-house bioinformatics pipeline was developed for pathogen detection. Briefly, Trimmomatic v.0.36 software[8] was used to generate high-quality sequencing data by removing low quality reads, adapter contamination, duplicated and short (length < 36 bp) reads. Human host sequences were filtered by mapping to the human reference genome (hs37d5) using Bowtie2 software[9]. Kraken 2 (v2.0.7) software[10] was used to assign nonhuman sequences to the microbial genome database, which contains more than 20,000 genome sequences of bacteria, fungi, viruses, and parasites for sequence alignment (ftp://ftp.ncbi.nlm.nih.gov/genomes/genbank/). To distinguish putative pathogens from contaminating microbial sequences derived from skin, collection tubes, laboratory agents, or the environment, negative ‘no-template’ control (NTC) samples were analyzed in parallel. Unique nonhuman sequences were identified only if the detected reads were > 10-fold higher than that in the NTC samples. For bacteria (excluding Mycobacterium, Nocardia and Legionella pneumophila), fungi, viruses, and parasites, the result was considered positive if a species detected by mNGS had at least three non-overlapping reads.
Data interpretation
A final sequencing list of suspected pathogenic microorganisms was obtained after removal of common background microorganisms compared to the NTCs. The top-ranked taxa were considered with respect to the clinical features of the patient. Microbes with known pathogenicity that could cause a clinical phenotype concordant with the clinical presentation were considered the potential infectious agents.
RESULTS
Patient characteristics
Four adult patients (three males and one female) and one male pediatric patient were retrospectively studied. The characteristics of all patients are summarized in Table 1. All patients had avian exposure history and were admitted to the hospital due to remittent fever of higher than 39°C. Other symptoms were also observed, including coughing in three adult patients and dizziness, vomiting, and convulsions in the pediatric patient (patient 5). Four patients developed chills, chest tightness, and asthma, and three patients developed dry cough and chest pain. The clinical symptoms were heterogeneous and non-specific, including listlessness, mild cyanosis of the lips, red and swollen pharynx, and thick breath sounds in both sides of the lungs or the right lower lung lobe only. Of note, patient 5 developed Brudzinski-positive and Babinski-positive symptoms, with a capillary filling time of less than 3 s.
Table 1.
Patients’ demographic and clinical characteristics
|
Patient No.
|
1
|
2
|
3
|
4
|
5
|
| Sex | M | M | M | F | M |
| Age (yr) | 75 | 73 | 44 | 66 | 5 |
| Symptoms | Fever, cough | Fever | Fever, cough | Fever, cough | Fever, dizziness, vomiting, convulsions |
| Chronic disease | Hypertension, diabetes, coronary heart disease, lacunar infarction, prostatic hyperplasia, amoebic dysentery | Hepatitis B | No history | No history | No history |
| Exposure history | Birds | Chickens | Birds | Psittacine birds | Birds |
| Fever | 39.7℃ | 40.9℃ | 40.1℃ | 40.0℃ | 39.9℃ |
| Leucocytes | 8.46 × 109/L | 2.98 × 109/L | 14.51 × 109/L | 2.93 × 109/L | 24.01 × 109/L |
| Neutrophils | 83.20% | 83.20% | 85.20% | 49.90% | 88.20% |
| Platelet count | 106.00 × 109/L | 106.00 × 109/L | 191.00 × 109/L | 286.00 × 109/L | 301.00 × 109/L |
| Procalcitonin | 1.65 ng/mL | 0.49 ng/mL | 0.01 ng/mL | 0.19 ng/mL | 4.28 ng/mL |
| C-reactive protein | 241.70 mg/L | 169.00 mg/L | 225.40 mg/L | 96.30 mg/L | 6.30 mg/L |
| Aspartate aminotransferase | 53.5 U/L | Normal | Normal | 65.1 U/L | Normal |
| Glutamate aminotransferase | Normal | Normal | 124.2 U/L | 172.3 U/L | Normal |
| Urea nitrogen | 14.5 mmol/L | Normal | Normal | Normal | Normal |
| Creatinine | 353.0 μmol/L | Normal | Normal | Normal | Normal |
| Mycoplasma pneumoniae IgG titer | 1:40 | Negative | 1:40 | Negative | 1:160 |
| CSF culture | N/A | N/A | N/A | N/A | Streptococcus pneumoniae |
| Diagnosis | Psittacosis pneumonia | Psittacosis pneumonia | Psittacosis pneumonia | Psittacosis pneumonia | Purulent meningitis, Psittacosis meningitis |
| Antibiotics | Piperacillin, tazobactam, etimicin, moxifloxacin | Ganciclovir, tigecycline | Piperacillin, tazobactam, moxifloxacin, tigecycline | Piperacillin, tazobactam, moxifloxacin | Ceftazidime, vancomycin, ceftriaxone sodium, azithromycin, rifampicin |
| Hospitalization (d) | 13 | 11 | 11 | 10 | 23 |
CSF: Cerebrospinal fluid; N/A: Not applicable.
Clinical investigations
All patients underwent physical examinations and blood tests upon hospital admission. The evaluations were indicative of inflammation, including a white blood cell count ranging from 2.93 × 109 /L to 24.01 × 109 /L, a neutrophil percentage ranging from 49.9% to 88.2%, a platelet count ranging from 106.00 × 109 /L to 301.00 × 109 /L, the clinical inflammatory factor procalcitonin (PCT) level ranging from 0.01 to 4.28 ng/mL, and a C-reactive protein level ranging from 6.3 mg/L to 225.4 mg/L (Table 1). In addition, three patients had increased levels of aspartate aminotransferase and/or glutamate aminotransferase (Table 1).
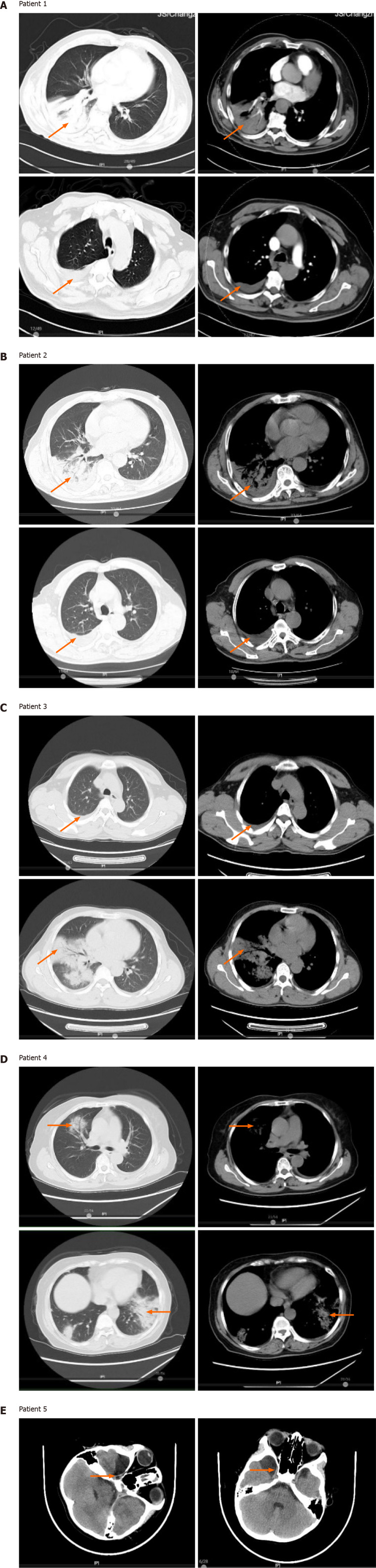
Computed tomography (CT) imaging showed varying degrees of lung inflammation in all patients. Patient 1 had chronic bronchial emphysema in both lungs, air-space consolidation in the lower right lung lobe, thickening of the left pleura, and an enlarged spleen (Figure 1A). Patient 2 had bilateral pleural effusion fluid buildup and nodules in the lower left lobe (Figure 1B). Patient 3 had inflammation with pleural effusion fluid buildup in the right lung (Figure 1C). Patient 4 had inflammation of the upper right lobe and air-space consolidation in the lower lobes of both lungs. Patient 4 also had small nodules in the lower right lobe as well as left pleural cavity and interlobular effusion (Figure 1D). The pediatric patient, patient 5, underwent a brain CT, which showed an arachnoid cyst in the right temporal lobe (Figure 1E).
Figure 1.
Chest and brain imaging of patients at diagnosis. A: Chest computed tomography (CT) images of patient 1, the red arrows indicate changes of chronic bronchitis emphysema in both lungs, the right lower lung space, a thickened left pleura, and splenomegaly; B: Chest CT images of patient 2, the red arrows indicate inflammation of the right lung, bilateral pleural effusion fluid buildup, and small nodules in the lower left lobe; C: Chest CT images of patient 3, the red arrows indicate right lung inflammation with pleural effusion fluid buildup; D: Chest CT images of patient 4, the red arrows indicate air-space consolidation in the upper and lower lobes of the right lung, small nodules in the right lower lobe, and effusion fluid buildup in the left thoracic cavity and between the lobes; E: Brain magnetic resonance imaging images of patient 5, the red arrows indicate an arachnoid cyst in the right temporal lobe.
Treatment
Patient 1 was initially treated with piperacillin, tazobactam, and etimicin, but no benefit was observed. A fiberoptic bronchoscopy was then performed and BALF was immediately subjected to mNGS testing. The mNGS results revealed the presence of Chlamydia psittaci (C. psittaci) (Table 2). The patient then received moxifloxacin and recovered rapidly, as shown by chest radiographs (Figure 2A).
Table 2.
Pathogenic microorganisms detected using metagenomic next generation sequencing
| Patient No. | Total reads |
Chlamydia psittaci
|
Other microbes detected
|
||
|
Reads
|
Genome coverage rate
|
Reads
|
Genome coverage rate
|
||
| 1 | 22128603 | 231 | 1.45% | - | - |
| 2 | 33738995 | 305 | 1.81% | 207 | 0.11% (Chlamydia albicans) |
| 3 | 50359059 | 2 | 0.01% | - | - |
| 4 | 41903841 | 6 | 0.02% | - | - |
| 5 | 18745727 | 2137 | 7.76% | 388 | 0.73% (Streptococcus pneumonia) |
Genome coverage rate was defined as matched reads length per genome length.
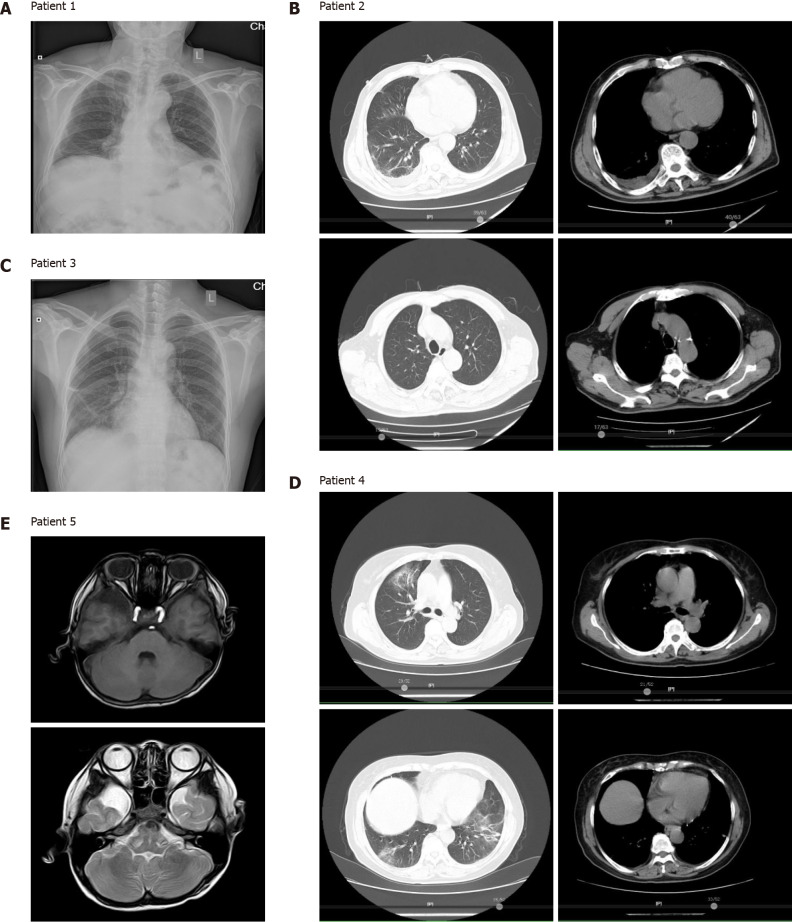
Figure 2.
Chest and brain imaging showed lesion absorption following anti-Chlamydia treatment in patients. A: Chest X-ray image of patient 1; B: Chest computed tomography (CT) images of patient 2; C: Chest X-ray image of patient 3; D: Chest CT images of patient 4; E: Brain magnetic resonance imaging image of patient 5. All patients experienced complete recovery after treatment with anti-Chlamydia antibiotics.
Patient 2 had self-administered acetaminophen prior to hospital admission and was initially treated with ganciclovir plus tigecycline. The patient’s BALF was subjected to mNGS and was diagnosed with a co-infection of C. psittaci and Candida albicans (Table 2). After receiving ganciclovir plus tigecycline for 10 d, CT scans revealed that the patient’s lung lesions were significantly absorbed (Figure 2B).
Patient 3 had taken acetaminophen prior to hospital admission and was initially treated with piperacillin, tazobactam, and moxifloxacin. However, no benefits from these therapies were observed. mNGS testing of the patient’s BALF was performed and revealed two C. psittaci-specific reads, but no other potential pathogenic microbe sequences (Table 2). After receiving tigecycline, the patient’s body temperature decreased and his lung lesions were significantly absorbed, as shown on chest radiographs (Figure 2C).
Patient 4 had self-administered acetaminophen, and was initially administered piperacillin, tazobactam, and moxifloxacin following hospital admission. The patient’s BALF underwent mNGS after her body temperature decreased, and she was diagnosed with a C. psittaci infection (Table 2). The patient rapidly recovered after receiving targeted antibiotic treatment, and her lung lesions were significantly absorbed, as shown on CT images (Figure 2D).
The pediatric patient (patient 5) had no history of medication. He was initially treated with ceftazidime after hospital admission, with very little benefit. He was then switched to vancomycin and ceftriaxone; however, no benefit was observed. The patient’s CSF was then collected for mNGS, which revealed the presence of C. psittaci and Streptococcus pneumoniae (S. pneumoniae) (Table 2). The patient received azithromycin, rifampicin, and vancomycin, after which, he recovered quickly, as shown on brain magnetic resonance imaging (Figure 2E).
None of the patients had any symptoms such as fever or cough after hospital discharge.
DISCUSSION
In this study, we found that all five patients had a history of exposure to birds (psittacine or other birds) or poultry (chicken). This finding emphasized the necessity for physicians to inquire about patients’ living environments and animal contact histories to identify suspicious cases. In addition, our patient cohort consisted of male and female patients with ages ranging from 5 to 75 years. Three of the cases displayed organ function insufficiency, and all of the cases exhibited common features of Chlamydia infection, which targets individuals regardless of their age or sex. Following anti-Chlamydia treatment, the symptoms significantly improved, and the patient outcomes were further confirmed using CT imaging, thus, suggesting the effectiveness of the treatments.
Another interesting finding of this study was that Chlamydia was not only detected in BALF but also in CSF, a finding that has not been widely reported and requires further investigation. Furthermore, we demonstrated the clinical utility of mNGS to sensitively detect microbial infections, including those containing different bacteria, viruses, and fungi. mNGS revealed that two patients in our cohort were also positive for C. albicans (patient 2) and S. pneumoniae (patient 5), in addition to C. psittaci. For instance, mNGS results revealed the co-occurrence of C. psittaci and S. pneumoniae in the CSF specimen of patient 5, while the genome coverage rate of C. psittaci (7.76%) was much greater than that of S. pneumoniae (0.73%) indicative of a higher pathogen load of C. psittaci. The patient fully recovered following anti-Chlamydia treatment; however, it did not rule out possible co-infection as S. pneumonia can also cause meningitis and induce inflammation resulting in blood-brain barrier disruption[11,12]. Those findings emphasize the complexity of diseases related to microbial infections and highlight the importance of treating patients using personalized anti-infective drug combinations.
This study also raised public health concerns over C. psittaci infections. C. psittaci research began in the 1930s when psittacosis infections were spreading globally. Sir Frank Macfarlane Burnet, a famous Australian virologist, immunologist, and Nobel laureate, was the first person to study this bacterium[13]. Infections with C. psittaci, a zoonotic pathogen, are rarely reported in humans, and approximately 1% of community-acquired pneumonia may be caused by Chlamydia infections[1]. The clinical manifestations of C. psittaci infections vary from asymptomatic to mild respiratory symptoms, while some severe cases affect multiple organs[14]. As patients with asymptomatic and mild symptoms are prone to misdiagnosis or self-healing, the actual incidence C. psittaci infections in humans may be higher[15]. Currently, conventional diagnostic approaches, including culturing, serologic assays, and PCR are used to detect C. psittaci infections; however, none of these methods provide timely and accurate results, which may delay detection and exacerbate the disease[16,17]. Therefore, alternative detection approaches, such as mNGS should be considered for the diagnosis of infectious diseases.
Despite the complexity of different infectious diseases, mNGS technology can provide rapid and comprehensive diagnoses. mNGS detects all microbial nucleic acids in a sample and identifies suspected pathogenic microorganisms. Current mNGS technologies can detect 20000 types of bacteria (including 400 types of Mycobacteria and 400 types of Mycoplasma/Chlamydia/Rickettsia), 4600 types of viruses, 1800 types of fungi, and 100 types of parasites[18]. Moreover, when coupled with machine learning analyses, mNGS can also identify suspected drug resistance genes, which broadens the application of mNGS in disease diagnosis. Overall, our findings demonstrated that mNGS represents a valuable tool for rapid, sensitive, and accurate pathogen detection.
CONCLUSION
Our study highlights the risk of bird/poultry exposure and C. psittaci infections, as they may underlie a potential public health problem. mNGS represents a valuable tool for rapid, sensitive, and accurate pathogen detection, including the detection of C. psittaci; thus, enabling clinicians to adopt personalized and appropriate treatments.
ARTICLE HIGHLIGHTS
Research background
Chlamydia psittaci (C. psittaci) is a gram-negative intracellular parasitic pathogenic bacterium that can infect avian and mammalian hosts, including humans. The detection of C. psittaci infections typically relies on traditional antigen-based immunoassays or serological testing that often lack sensitivity and/or specificity.
Research motivation
This study provides evidence to illustrate metagenomic next generation sequencing (mNGS) as a promising clinical-microbiology tool for pathogen detection.
Research objectives
This study aimed to demonstrate that mNGS represents a valuable tool for rapid, sensitive, and accurate pathogen detection including C. psittaci infections.
Research methods
Four cases of psittacosis pneumonia and one case of pediatric psittacosis meningitis were diagnosed using mNGS. Patients’ clinical characteristics, manifestations, and treatment histories were retrospectively evaluated.
Research results
All five patients had a history of exposure to wild (psittacine or other birds) or domesticated birds (chickens). All patients had a high fever and three of them experienced organ insufficiency during the disease. The laboratory data showed normal to slightly increased leucocyte and neutrophil counts, and elevated procalcitonin levels in all five cases, and very high C-reactive protein levels in psittacosis pneumonia patients. mNGS identified a potential pathogen, C. psittaci, in patients’ bronchoalveolar lavage fluid or cerebrospinal fluid. Computed tomography revealed lung air-space consolidation, pleural thickening, and effusion fluid buildup in psittacosis pneumonia cases, and an arachnoid cyst in the right temporal lobe of the pediatric psittacosis meningitis patient. All patients experienced complete recovery following the administration of targeted anti-Chlamydia therapy.
Research conclusions
Our data not only reinforce that mNGS represents a valuable tool for rapid, sensitive, and accurate pathogen detection, including C. psittaci, but also raise public health concerns over C. psittaci infections.
Research perspectives
Despite the complexity of different infectious diseases, mNGS technology can provide rapid and comprehensive diagnoses. The application of mNGS in disease diagnosis can be further broadened by coupling with machine learning algorithms in various aspects including the identification of suspected drug resistance genes.
ACKNOWLEDGEMENTS
We would like to thank the patients and their families for providing consent for publication. We also thank the research staff and co-investigators involved in this study.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Changzhou Second People’s Hospital affiliated to Nanjing Medical University, China (approval No. 2020KY268-01).
Informed consent statement: All study participants provided informed consent prior to sample collection according to the institutional guidelines and gave permissions to the use of their clinical data and accompanying images.
Conflict-of-interest statement: Ou QX and Shao Y are employees of Nanjing Geneseeq Technology Inc; Liu J is an employee of Dinfectome Inc. The remaining authors have nothing to disclose.
Manuscript source: Unsolicited manuscript
Peer-review started: February 18, 2021
First decision: April 13, 2021
Article in press: July 2, 2021
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan X S-Editor: Yan JP L-Editor: Webster JR P-Editor: Yuan YY
Contributor Information
Xiao-Wei Yin, Department of Respiratory and Critical Care Medicine, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China.
Zheng-Dao Mao, Department of Respiratory and Critical Care Medicine, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China.
Qian Zhang, Department of Respiratory and Critical Care Medicine, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China.
Qiu-Xiang Ou, Research & Development, Nanjing Geneseeq Technology Inc., Nanjing 210032, Jiangsu Province, China.
Jia Liu, Research & Development, Dinfectome Inc., Nanjing 213164, Jiangsu Province, China.
Yang Shao, Research & Development, Nanjing Geneseeq Technology Inc., Nanjing 210032, Jiangsu Province, China; School of Public Health, Nanjing Medical University, Nanjing 213164, Jiangsu Province, China.
Zhi-Guang Liu, Department of Respiratory and Critical Care Medicine, Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, Changzhou 213164, Jiangsu Province, China. 545485079@qq.com.
Data sharing statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- 1.Hogerwerf L, DE Gier B, Baan B, VAN DER Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145:3096–3105. doi: 10.1017/S0950268817002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogerwerf L, Holstege MMC, Benincà E, Dijkstra F, van der Hoek W. Temporal and spatial analysis of psittacosis in association with poultry farming in the Netherlands, 2000-2015. BMC Infect Dis. 2017;17:519. doi: 10.1186/s12879-017-2608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieuwenhuizen AA, Dijkstra F, Notermans DW, van der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. 2018;18:442. doi: 10.1186/s12879-018-3317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann B, Tappe D, Höper D, Herden C, Boldt A, Mawrin C, Niederstraßer O, Müller T, Jenckel M, van der Grinten E, Lutter C, Abendroth B, Teifke JP, Cadar D, Schmidt-Chanasit J, Ulrich RG, Beer M. A Variegated Squirrel Bornavirus Associated with Fatal Human Encephalitis. N Engl J Med. 2015;373:154–162. doi: 10.1056/NEJMoa1415627. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MR, O'Donovan BD, Gelfand JM, Sample HA, Chow FC, Betjemann JP, Shah MP, Richie MB, Gorman MP, Hajj-Ali RA, Calabrese LH, Zorn KC, Chow ED, Greenlee JE, Blum JH, Green G, Khan LM, Banerji D, Langelier C, Bryson-Cahn C, Harrington W, Lingappa JR, Shanbhag NM, Green AJ, Brew BJ, Soldatos A, Strnad L, Doernberg SB, Jay CA, Douglas V, Josephson SA, DeRisi JL. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol. 2018;75:947–955. doi: 10.1001/jamaneurol.2018.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang B, Wei X, Wang X, Tang Y, Zhu J, Zheng X, Zhang C, Li S. Metagenomic next-generation sequencing of viruses, bacteria, and fungi in the epineurium of the facial nerve with Bell's palsy patients. J Neurovirol. 2020;26:727–733. doi: 10.1007/s13365-020-00892-7. [DOI] [PubMed] [Google Scholar]
- 8.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iovino F, Orihuela CJ, Moorlag HE, Molema G, Bijlsma JJ. Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS One. 2013;8:e68408. doi: 10.1371/journal.pone.0068408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012;7:383–394. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer KF, Eddie B, Stevens IM. Recent Studies on Psittacosis. Am J Public Health Nations Health. 1935;25:571–579. doi: 10.2105/ajph.25.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco G, Corrente M, Martella V. Detection of Chlamydophila psittaci in asymptomatic animals. J Clin Microbiol. 2005;43:5410–1; author reply 5410. doi: 10.1128/JCM.43.10.5410-5411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rane V, Khailin K, Williams J, Francis M, Kotsanas D, Korman TM, Graham M. Underdiagnosis of Chlamydia trachomatis and Chlamydia psittaci revealed by introduction of respiratory multiplex PCR assay with Chlamydiaceae family primers. Diagn Microbiol Infect Dis. 2018;90:163–166. doi: 10.1016/j.diagmicrobio.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA, Korman TM, Holmes PW, Christiansen KJ, Waterer GW, Pierce RJ, Mayall BC, Armstrong JG, Catton MG, Nimmo GR, Johnson B, Hooy M, Grayson ML Australian CAP Study Collaboration. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46:1513–1521. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 17.de Gier B, Hogerwerf L, Dijkstra F, van der Hoek W. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. 2018;146:303–305. doi: 10.1017/S0950268817003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong GL, MacCannell DR, Taylor J, Carleton HA, Neuhaus EB, Bradbury RS, Posey JE, Gwinn M. Pathogen Genomics in Public Health. N Engl J Med. 2019;381:2569–2580. doi: 10.1056/NEJMsr1813907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.